Design, Characterization, and Antibacterial Performance of MAPLE-Deposited Coatings of Magnesium Phosphate-Containing Silver Nanoparticles in Biocompatible Concentrations
Abstract
:1. Introduction
2. Results and Discussion
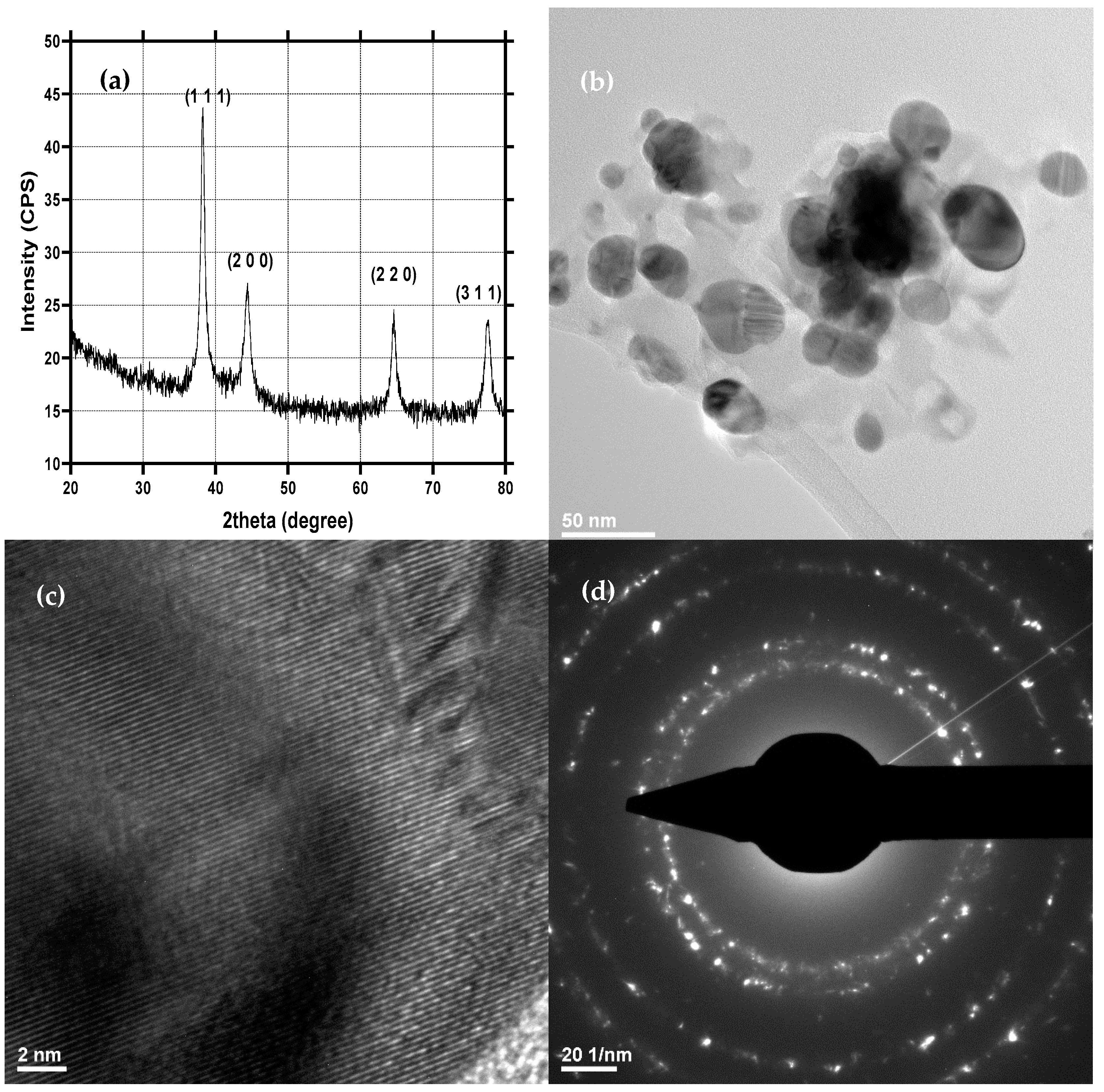
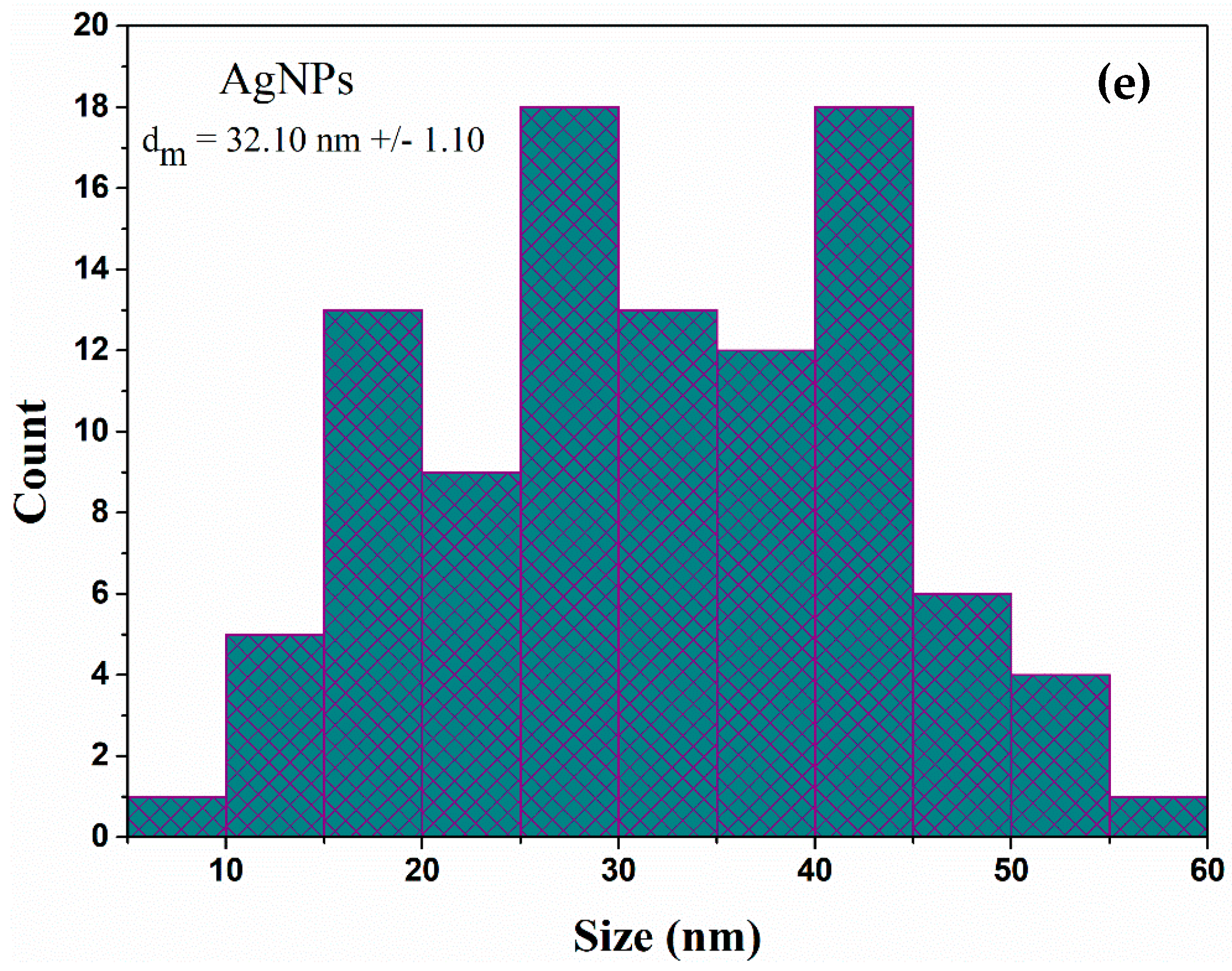
2.1. Physicochemical Characterization of AgNPs
2.2. Physicochemical Characterization of Mg3(PO4)2
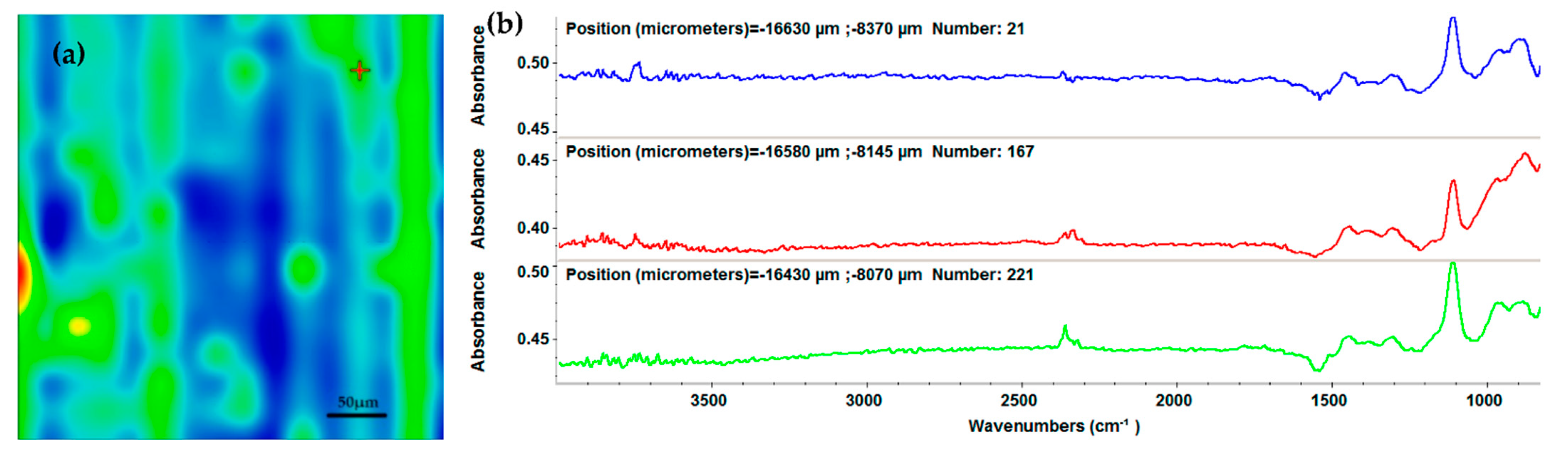
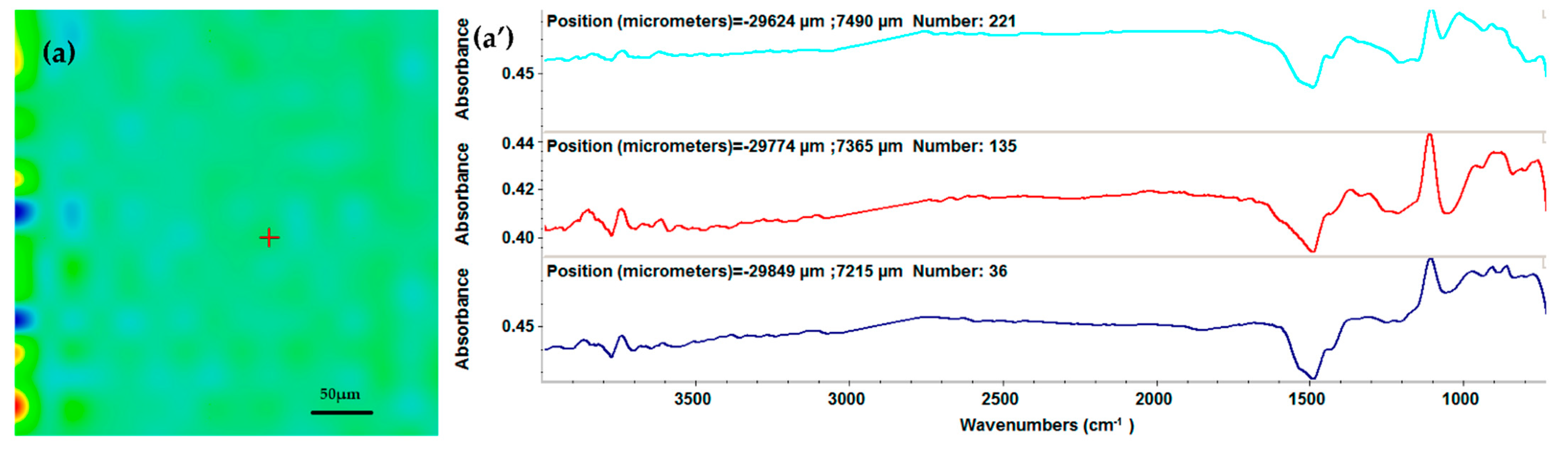
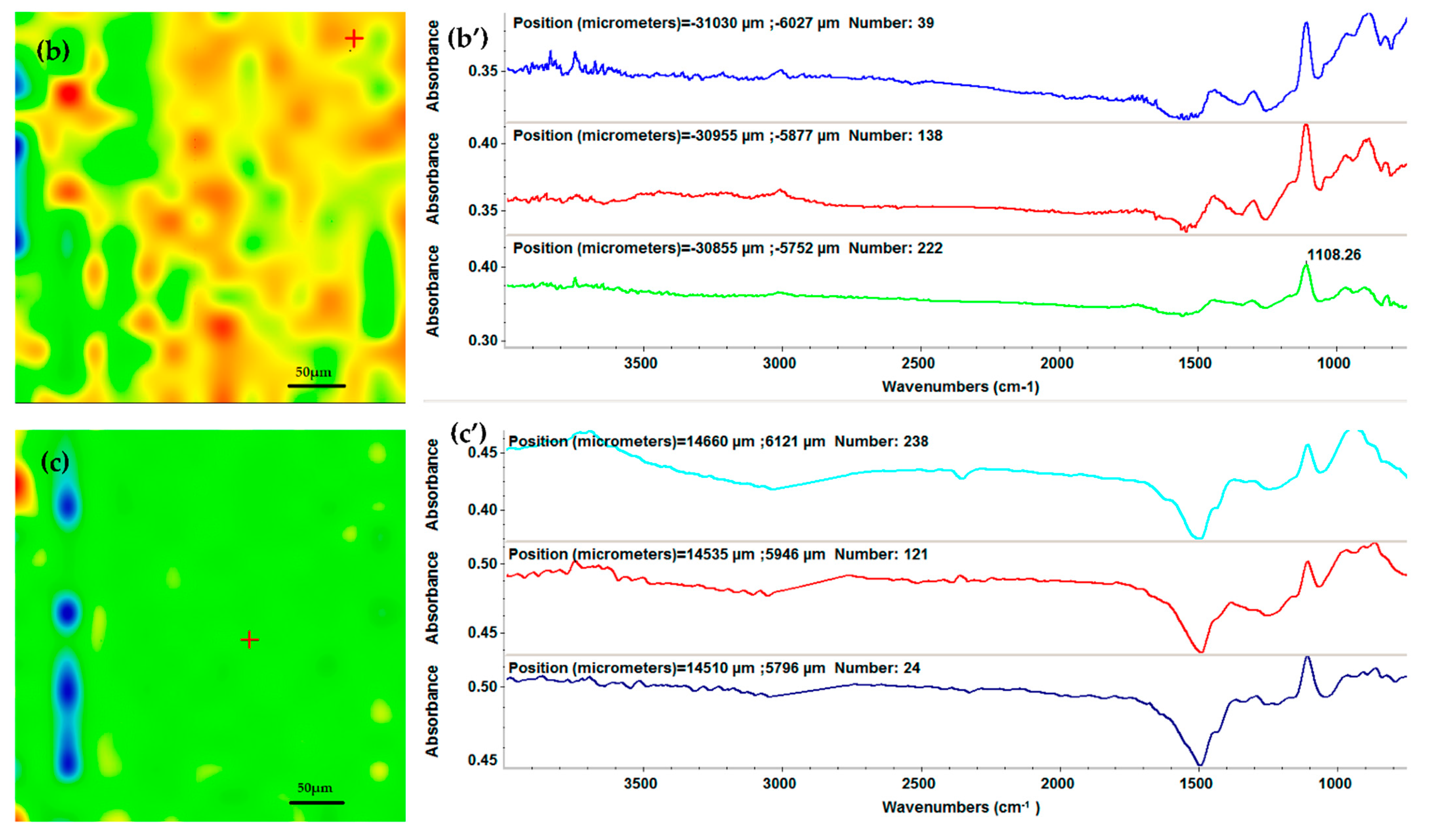
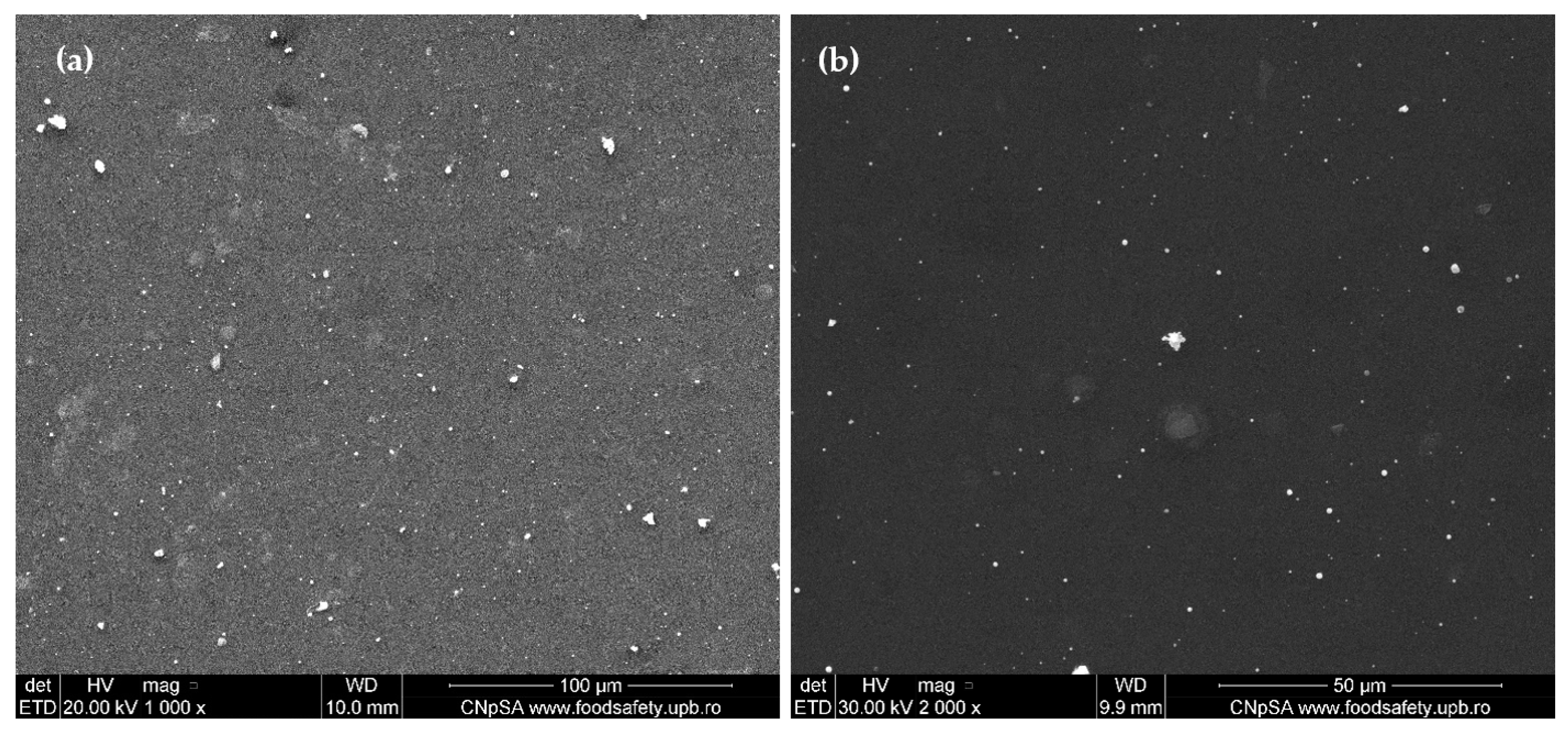
2.3. Physicochemical Characterization of the AgNPs/Mg3(PO4)2 Coatings
2.4. In Vitro Evaluation
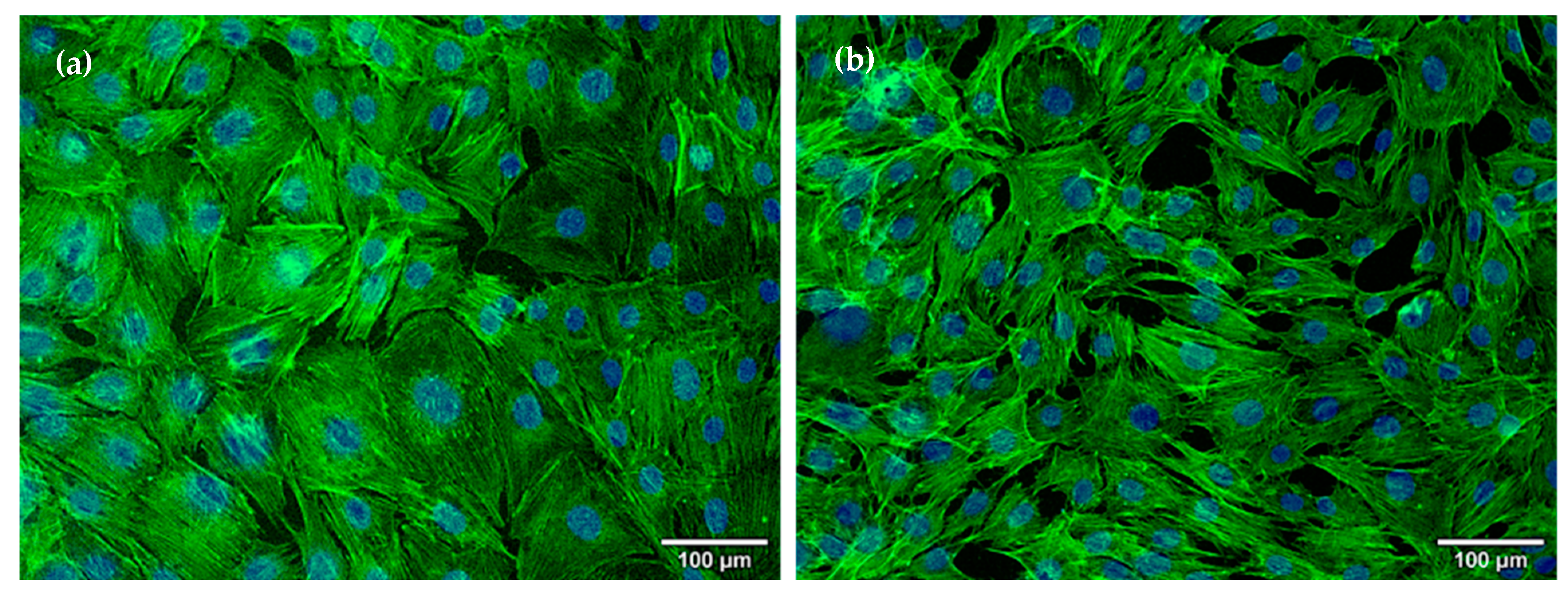
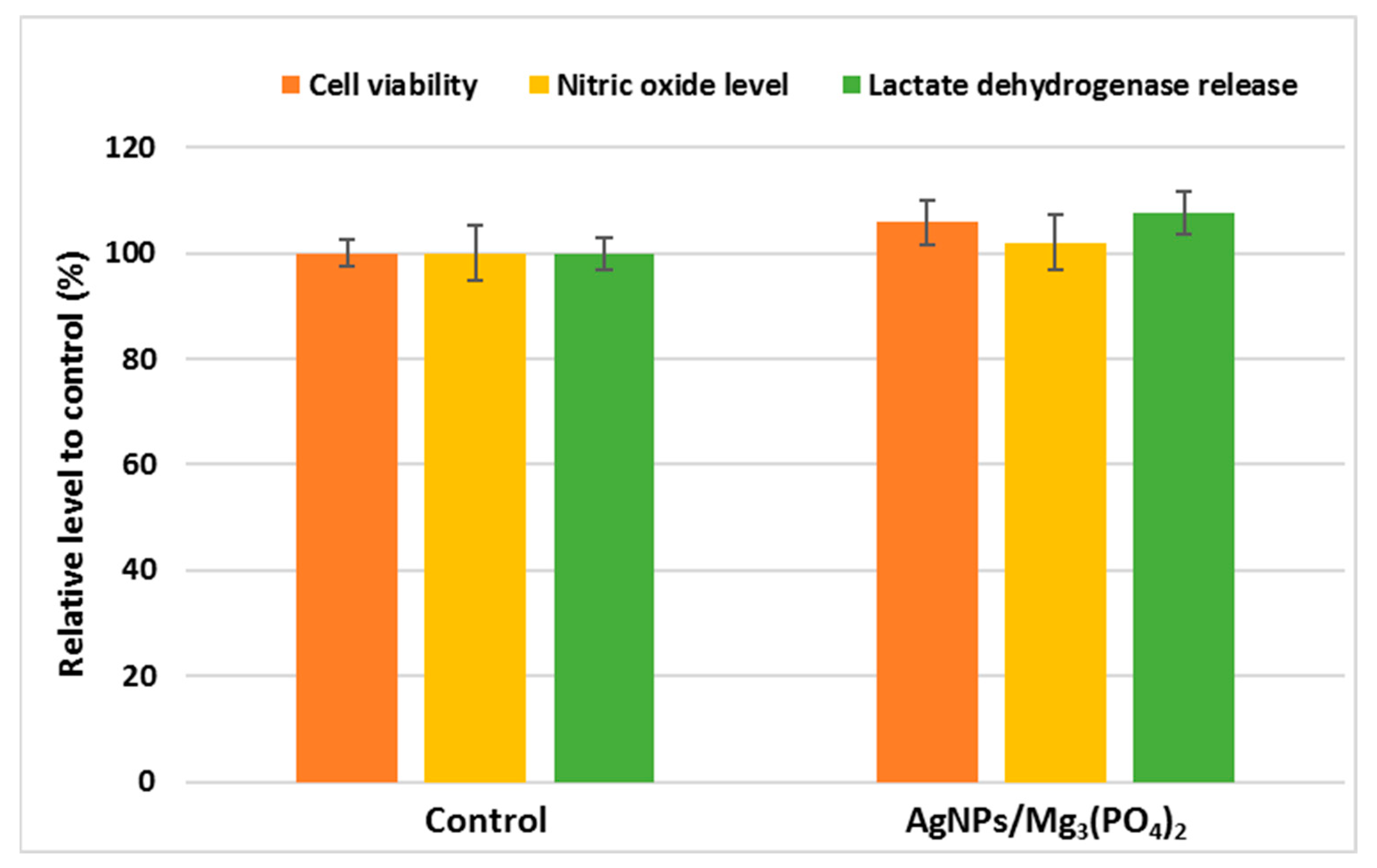
2.4.1. Osteoblasts Behavior
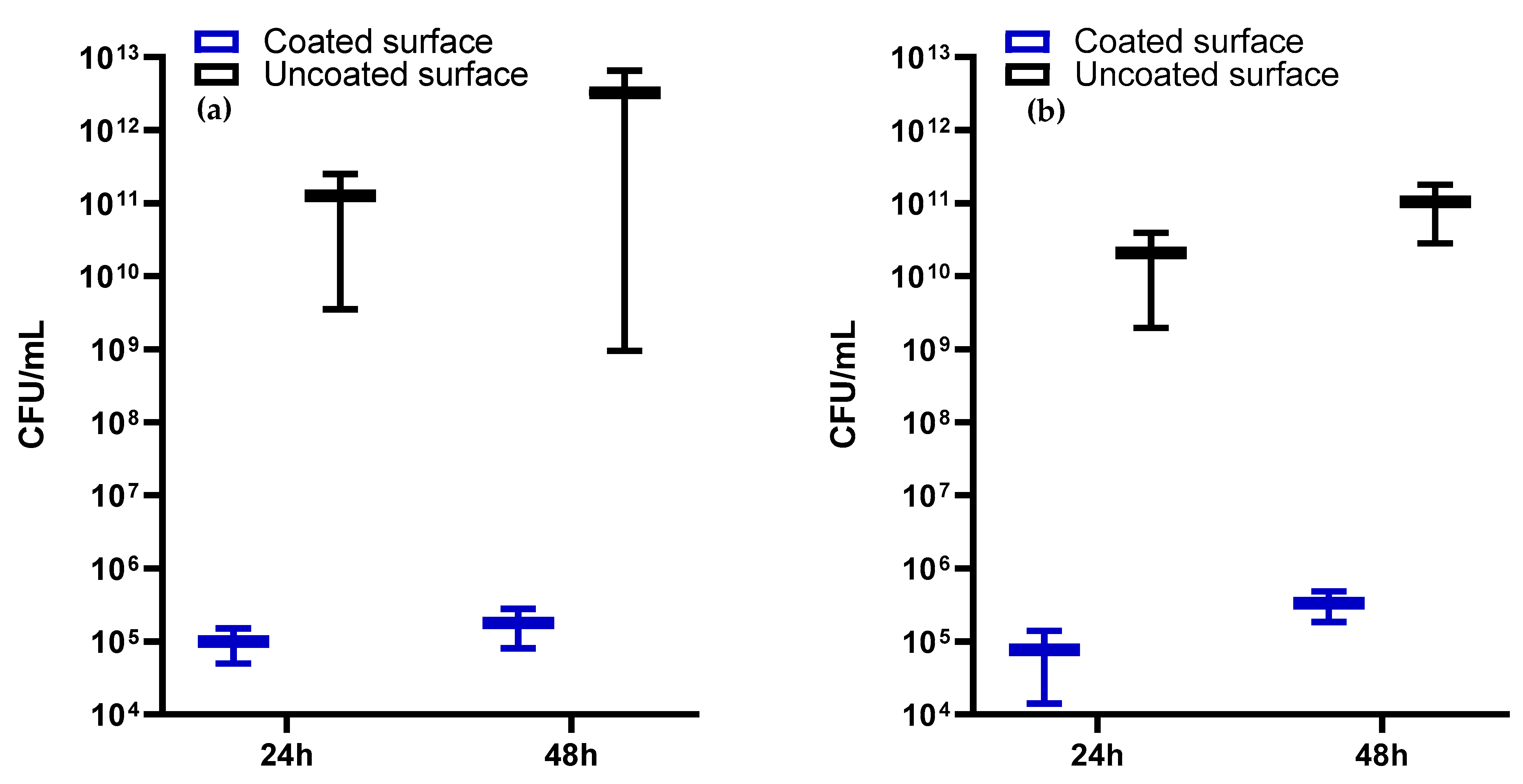
2.4.2. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis Methods
3.2.1. AgNPs Synthesis
3.2.2. Mg3(PO4)2 Synthesis
3.2.3. Coatings Fabrication by MAPLE Technique
3.3. Characterization Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Jayash, S.N.; Al-Namnam, N.M.; Shaghayegh, G. Osteoprotegerin (OPG) pathways in bone diseases and its application in therapeutic perspectives. Biointerface Res. Appl. Chem. 2020, 10, 5193–5200. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Herbaj, S.; Redmond, J.; McCarthy, H.O.; Dunne, N.J. Calcium Phosphate Nanoparticles-Based Systems for RNAi Delivery: Applications in Bone Tissue Regeneration. Nanomaterials 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [Green Version]
- van Vugt, T.A.; Geurts, J.A.P.; Arts, J.J.; Lindfors, N.C. 3—Biomaterials in treatment of orthopedic infections. In Management of Periprosthetic Joint Infections (PJIs); Arts, J.J.C., Geurts, J., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 41–68. [Google Scholar]
- Burnell, J.M.; Teubner, E.J.; Miller, A.G.J.C.T.I. Normal maturational changes in bone matrix, mineral, and crystal size in the rat. Calcif. Tissue Int. 1980, 31, 13–19. [Google Scholar] [CrossRef]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef]
- Faisal, N. Mechanical Behavior of Nano-Scaled Graphene Oxide Reinforced High-Density Polymer Ethylene for Orthopedic Implants. Biointerface Res. Appl. Chem. 2020, 10, 7223–7233. [Google Scholar] [CrossRef]
- Massit, A.; El Yacoubi, A.; Kholtei, A.; El Idrissi, B.C. XRD and FTIR Analysis of Magnesium Substituted Tricalcium Calcium Phosphate Using a Wet Precipitation Method. Biointerface Res. Appl. Chem. 2021, 11, 8034–8042. [Google Scholar] [CrossRef]
- Massit, A.; El Yacoubi, A.; Rezzouk, A.; El Idrissi, B.C. Thermal Behavior of Mg-Doped Calcium-Deficient Apatite and Stabilization of beta Tricalcium Phosphate. Biointerface Res. Appl. Chem. 2020, 10, 6837–6845. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef]
- Razzaque, M.S.J. Magnesium: Are we consuming enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinella, M.A. The refeeding syndrome and hypophosphatemia. J. Intensive Care Med. 2003, 61, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J.J.N. Magnesium in aging, health and diseases. Nutients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Tapia, F.; Boccaccini, A.R.; Strobel, L.A.J. Magnesium-containing bioactive glasses for biomedical applications. Int. J. Appl. Glass Sci. 2012, 3, 221–253. [Google Scholar] [CrossRef]
- Willumeit, R.; Möhring, A.; Feyerabend, F. Optimization of cell adhesion on mg based implant materials by pre-incubation under cell culture conditions. Int. J. Mol. Sci. 2014, 15, 7639–7650. [Google Scholar] [CrossRef] [Green Version]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G.J.B. Magnesium and its alloys as orthopedic biomaterials: A review. Int. J. Mol. Sci. 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Sikder, P.; Bhaduri, S.B.; Ong, J.L.; Guda, T. Silver (Ag) doped magnesium phosphate microplatelets as next-generation antibacterial orthopedic biomaterials. J. Biomed. Mater. Res. 2020, 108, 976–989. [Google Scholar] [CrossRef]
- Worthley, L.; Baker, S. The essentials of calcium, magnesium and phosphate metabolism: Part I. Physiology. Crit. Care Resusc. 2002, 4, 301–306. [Google Scholar]
- Florea, D.A.; Albuleț, D.; Grumezescu, A.M.; Andronescu, E. Surface modification—A step forward to overcome the current challenges in orthopedic industry and to obtain an improved osseointegration and antimicrobial properties. Mater. Chem. Phys. 2020, 243, 122579. [Google Scholar] [CrossRef]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Asp. Med. 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Aydın, M.; Azak, E.; Bilgin, H.; Menekse, S.; Asan, A.; Mert, H.T.E.; Yulugkural, Z.; Altunal, L.N.; Hatipoğlu, Ç.A.; Ertem, G.T.J.; et al. Changes in antimicrobial resistance and outcomes of health care–associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1737–1742. [Google Scholar] [CrossRef]
- Esmaili, F.; Sanei-Dehkordi, A.; Amoozegar, F.; Osanloo, M. A Review on the Use of Essential Oil-Based Nanoformulations in Control of Mosquitoes. Biointerface Res. Appl. Chem. 2021, 11, 12516–12529. [Google Scholar] [CrossRef]
- Gounden, S.; Daniels, A.; Singh, M. Chitosan-Modified Silver Nanoparticles Enhance Cisplatin Activity in Breast Cancer Cells. Biointerface Res. Appl. Chem. 2021, 11, 10572–10584. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Ruiz, R.D.; Selari, P.; de Araujo, W.L.; de Souza, A.O. Anti-Biofilm Action of Biological Silver Nanoparticles Produced by Aspergillus tubingensis and Antimicrobial Activity of Fabrics Carrying it. Biointerface Res. Appl. Chem. 2021, 11, 14764–14774. [Google Scholar] [CrossRef]
- Thiruvengadam, V.; Bansod, A.V. Characterization of Silver Nanoparticles Synthesized using Chemical Method and its Antibacterial Property. Biointerface Res. Appl. Chem. 2020, 10, 7257–7264. [Google Scholar] [CrossRef]
- Tyagi, P.K.; Mishra, R.; Khan, F.; Gupta, D.; Gola, D. Antifungal Effects of Silver Nanoparticles Against Various Plant Pathogenic Fungi and its Safety Evaluation on Drosophila melanogaster. Biointerface Res. Appl. Chem. 2020, 10, 6587–6596. [Google Scholar] [CrossRef]
- Samberg, M.; Monteiro-Riviere, N. Silver Nanoparticles in Biomedical Applications. In Nanotoxicology, 2nd ed.; Taylor & Francis Group: Abingdon, UK, 2014; pp. 405–422. ISBN 9780429171420. [Google Scholar]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Šileikaitė, A.; Prosyčevas, I.; Puišo, J.; Juraitis, A.; Guobienė, A.J.M.S. Analysis of silver nanoparticles produced by chemical reduction of silver salt solution. Mater. Sci. 2006, 12, 287–291. [Google Scholar]
- Vorobyova, S.; Lesnikovich, A.; Sobal, N.J.C.; Physicochemical, S.A.; Aspects, E. Preparation of silver nanoparticles by interphase reduction. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 375–379. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Qian, X.-F.; Yin, J.; Zhu, Z.-K. A simple method for selective immobilization of silver nanoparticles. Appl. Surf. Sci. 2005, 250, 109–116. [Google Scholar] [CrossRef]
- Jabbar, A.H.; Al-janabi, H.S.O.; Hamzah, M.; Mezan, S.; Tumah, A.; Ameruddin, A.J. Green Synthesis and Characterization of Silver Nanoparticle (AgNPs) using Pandanus Atrocarpus Extract. Int. J. Adv. Sci. Technol. 2020, 29, 4913–4922. [Google Scholar]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X.; et al. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: New insight into the antimicrobial action of silver. Sci. Rep. 2016, 6, 32699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqarni, A.S.; Hussin, R.; Alamri, S.N.; Ghoshal, S.K. Customized physical and structural features of phosphate-based glass-ceramics: Role of Ag nanoparticles and Ho3+ impurities. J. Taibah Univ. Sci. 2020, 14, 954–962. [Google Scholar] [CrossRef]
- Bee, S.-L.; Bustami, Y.; Ul-Hamid, A.; Lim, K.; Abdul Hamid, Z.A. Synthesis of silver nanoparticle-decorated hydroxyapatite nanocomposite with combined bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2021, 32, 106. [Google Scholar] [CrossRef]
- Tsuji, T.; Watanabe, N.; Tsuji, M. Laser induced morphology change of silver colloids: Formation of nano-size wires. Appl. Suf. Sci. 2003, 211, 189–193. [Google Scholar] [CrossRef]
- Stiff-Roberts, A.D.; Ge, W. Organic/hybrid thin films deposited by matrix-assisted pulsed laser evaporation (MAPLE). Appl. Phys. Rev. 2017, 4, 041303. [Google Scholar] [CrossRef]
- Radu, A.; Filipescu, M.; Dumitru, M.; Moldovan, A.; Dinescu, M.; Antohe, S.J.R.R.I.P. Physical properties of metal oxide nanoparticles processed as thin films by maple technique. Rom. Rep. Phys. 2020, 72, 503. [Google Scholar]
- Gherasim, O.; Popescu, R.C.; Grumezescu, V.; Mogoșanu, G.D.; Mogoantă, L.; Iordache, F.; Holban, A.M.; Vasile, B.Ș.; Bîrcă, A.C.; Oprea, O.-C.; et al. MAPLE Coatings Embedded with Essential Oil-Conjugated Magnetite for Anti-Biofilm Applications. Materials 2021, 14, 1612. [Google Scholar] [CrossRef]
- Grumezescu, V.; Negut, I.; Grumezescu, A.M.; Ficai, A.; Dorcioman, G.; Socol, G.; Iordache, F.; Truşcă, R.; Vasile, B.S.; Holban, A.M. MAPLE fabricated coatings based on magnetite nanoparticles embedded into biopolymeric spheres resistant to microbial colonization. Appl. Surf. Sci. 2018, 448, 230–236. [Google Scholar] [CrossRef]
- Grumezescu, V.; Holban, A.M.; Iordache, F.; Socol, G.; Mogoşanu, G.D.; Grumezescu, A.M.; Ficai, A.; Vasile, B.Ş.; Truşcă, R.; Chifiriuc, M.C.; et al. MAPLE fabricated magnetite@eugenol and (3-hidroxybutyric acid-co-3-hidroxyvaleric acid)–polyvinyl alcohol microspheres coated surfaces with antimicrobial properties. Appl. Surf. Sci. 2014, 306, 16–22. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.; Gherasim, O.; Grumezescu, V.; Socol, M.; Zgura, I.; Florica, C.; Popescu, R.C.; Savu, D.; Holban, A.M.; et al. Laser Processed Antimicrobial Nanocomposite Based on Polyaniline Grafted Lignin Loaded with Gentamicin-Functionalized Magnetite. Polymers 2019, 11, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negut, I.; Grumezescu, V.; Ficai, A.; Grumezescu, A.; Holban, A.; Popescu, R.C.; Savu, D.; Vasile, B.; Socol, G. MAPLE deposition of Nigella sativa functionalized Fe 3 O 4 nanoparticles for antimicrobial coatings. Appl. Surf. Sci. 2018, 455. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Saviuc, C. Magnetite Nanocomposites Thin Coatings Prepared by MAPLE to Prevent Microbial Colonization of Medical Surfaces; Springer: New Delhi, India, 2015. [Google Scholar]
- Grumezescu, V.; Andronescu, E.; Holban, A.M.; Mogoanu, L.i.; Mogq anu, G.D.; Grumezescu, A.M.; Stanculescu, A.I.; Socol, G.; Iordache, F.; Maniu, H.; et al. MAPLE fabrication of thin films based on kanamycin functionalized magnetite nanoparticles with anti-pathogenic properties. Appl. Surf. Sci. 2015, 336, 188–195. [Google Scholar] [CrossRef]
- Rădulescu, D.; Grumezescu, V.; Andronescu, E.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Oprea, A.E.; Rădulescu, M.; Surdu, A.; Trusca, R.; et al. Biocompatible cephalosporin-hydroxyapatite-poly(lactic-co-glycolic acid)-coatings fabricated by MAPLE technique for the prevention of bone implant associated infections. Appl. Surf. Sci. 2016, 374, 387–396. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Negut, I.; Dumitrescu, M.F.; Stan, M.S.; Nica, I.C.; Holban, A.M.; Socol, G.; Andronescu, E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics 2021, 10, 160. [Google Scholar] [CrossRef]
- Visan, A.; Stan, G.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Besleaga, C.; Luculescu, C.; Chifiriuc, M.; Hussien, M.D.; Marsan, O.; et al. Combinatorial MAPLE deposition of antimicrobial orthopedic maps fabricated from chitosan and biomimetic apatite powders. Int. J. Pharm. 2016, 511, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Rusen, L.; Brajnicov, S.; Neacsu, P.; Marascu, V.; Bonciu, A.; Dinescu, M.; Dinca, V.; Cimpean, A. Novel degradable biointerfacing nanocomposite coatings for modulating the osteoblast response. Surf. Coat. Technol. 2017, 325, 397–409. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J. Matrix-Assisted Pulsed Laser Evaporation (MAPLE) technique for deposition of hybrid nanostructures. Front. Nanosci. Nanotechnol. 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Cristescu, R.; Visan, A.; Socol, G.; Surdu, A.V.; Oprea, A.E.; Grumezescu, A.M.; Chifiriuc, M.C.; Boehm, R.D.; Yamaleyeva, D.; Taylor, M.; et al. Antimicrobial activity of biopolymeric thin films containing flavonoid natural compounds and silver nanoparticles fabricated by MAPLE: A comparative study. Appl. Surf. Sci. 2016, 374, 290–296. [Google Scholar] [CrossRef]
- Omran, B.; Nassar, H.; Fatthallah, N.; Hamdy, A.; El-Shatoury, E.; El-Gendy, N.S. Characterization and antimicrobial activity of silver nanoparticles mycosynthesized by Aspergillus brasiliensis. J. Appl. Microbiol. 2018, 125, 370–382. [Google Scholar] [CrossRef]
- Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.S.; Oprea, O.; Nicoară, A.I.; Yang, C.-H.; Huang, K.-S.; Andronescu, E. Synthesis of Magnetite Nanoparticles through a Lab-On-Chip Device. Materials 2021, 14, 5906. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Beherei, H.; Al-Rashidy, Z.; Farag, D.; Salem, Z. Dental pulp stem cell viability and osteogenic potential assessment of new Mg-phosphate magnetic bioceramic nanoparticles. J. Mater. Res. 2022, 37, 595–607. [Google Scholar] [CrossRef]
- Golafshan, N.; Vorndran, E.; Zaharievski, S.; Brommer, H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Gbureck, U.; van Weeren, R.; Castilho, M.; Malda, J. Tough magnesium phosphate-based 3D-printed implants induce bone regeneration in an equine defect model. Biomaterials 2020, 261, 120302. [Google Scholar] [CrossRef] [PubMed]
- Puiu, R.A.; Balaure, P.C.; Constantinescu, E.; Grumezescu, A.M.; Andronescu, E.; Oprea, O.-C.; Vasile, B.S.; Grumezescu, V.; Negut, I.; Nica, I.C.; et al. Anti-Cancer Nanopowders and MAPLE-Fabricated Thin Films Based on SPIONs Surface Modified with Paclitaxel Loaded β-Cyclodextrin. Pharmaceutics 2021, 13, 1356. [Google Scholar] [CrossRef] [PubMed]
- Touny, A.H.; Sikder, P.; Saleh, M.M.; Bhaduri, S.B. Facile synthesis and characterization of biphasic magnesium phosphate bioceramic nanosheets by a reflux approach. Mater. Res. Express 2019, 6, 095007. [Google Scholar] [CrossRef]
- Yu, X.; Qian, C.; Sun, L. Chemosynthesis Of Nano-Magnesium Phosphates And Its Characterization. Dig. J. Nanomater. Biostructures (DJNB) 2016, 11, 1099–1103. [Google Scholar]
- Niculescu, A.G.; Chircov, C.; Birca, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Xie, H.; Wang, P.; Wu, J. Effect of exposure of osteoblast-like cells to low-dose silver nanoparticles: Uptake, retention and osteogenic activity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Locatelli, L.; Maier, J.A.M. Silver Nanoparticles in Orthopedic Applications: New Insights on Their Effects on Osteogenic Cells. Nanomaterials 2017, 7, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, W.C.; Cinat, M.E.; Nastanski, F.; Gornick, W.B.; Wilson, S.E. New epidemiology for post-operative nosocomial infections. Am. Surg. 2000, 66, 874–878. [Google Scholar] [PubMed]
- Razavi, M.; Shepard, D.S.; Suaya, J.A.; Stason, W.B. Postoperative Staphylococcus aureus infections in Medicare beneficiaries. PLoS ONE 2014, 9, e110133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardtstock, F.; Heinrich, K.; Wilke, T.; Mueller, S.; Yu, H. Burden of Staphylococcus aureus infections after orthopedic surgery in Germany. BMC Infect. Dis. 2020, 20, 233. [Google Scholar] [CrossRef] [Green Version]
- del Pozo, J.L.; Patel, R. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, J.G.; Yu, H.; Begier, E.; Gayle, J.; Olsen, M.A. Incidence and burden of Staphylococcus aureus infection after orthopedic surgeries. Infect. Control. Hosp. Epidemiol. 2022, 43, 64–71. [Google Scholar] [CrossRef]
- Cerioli, M.; Batailler, C.; Conrad, A.; Roux, S.; Perpoint, T.; Becker, A.; Triffault-Fillit, C.; Lustig, S.; Fessy, M.H.; Laurent, F.; et al. Pseudomonas aeruginosa Implant-Associated Bone and Joint Infections: Experience in a Regional Reference Center in France. Front. Med. 2020, 7, 513242. [Google Scholar] [CrossRef]
- Pestrak, M.J.; Chaney, S.B.; Eggleston, H.C.; Dellos-Nolan, S.; Dixit, S.; Mathew-Steiner, S.S.; Roy, S.; Parsek, M.R.; Sen, C.K.; Wozniak, D.J. Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathog. 2018, 14, e1006842. [Google Scholar] [CrossRef]
- Siritongsuk, P.; Hongsing, N.; Thammawithan, S.; Daduang, S.; Klaynongsruang, S.; Tuanyok, A.; Patramanon, R. Two-Phase Bactericidal Mechanism of Silver Nanoparticles against Burkholderia pseudomallei. PLoS ONE 2016, 11, e0168098. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef] [Green Version]
- Caciandone, M.; Niculescu, A.-G.; Roșu, A.R.; Grumezescu, V.; Negut, I.; Holban, A.M.; Oprea, O.; Vasile, B.Ș.; Bîrcă, A.C.; Grumezescu, A.M.; et al. PEG-Functionalized Magnetite Nanoparticles for Modulation of Microbial Biofilms on Voice Prosthesis. Antibiotics 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, D.A.; Grumezescu, V.; Bîrcă, A.C.; Vasile, B.Ș.; Mușat, M.; Chircov, C.; Stan, M.S.; Grumezescu, A.M.; Andronescu, E.; Chifiriuc, M.C. Design, Characterization, and Antibacterial Performance of MAPLE-Deposited Coatings of Magnesium Phosphate-Containing Silver Nanoparticles in Biocompatible Concentrations. Int. J. Mol. Sci. 2022, 23, 7910. https://doi.org/10.3390/ijms23147910
Florea DA, Grumezescu V, Bîrcă AC, Vasile BȘ, Mușat M, Chircov C, Stan MS, Grumezescu AM, Andronescu E, Chifiriuc MC. Design, Characterization, and Antibacterial Performance of MAPLE-Deposited Coatings of Magnesium Phosphate-Containing Silver Nanoparticles in Biocompatible Concentrations. International Journal of Molecular Sciences. 2022; 23(14):7910. https://doi.org/10.3390/ijms23147910
Chicago/Turabian StyleFlorea, Denisa Alexandra, Valentina Grumezescu, Alexandra Cătălina Bîrcă, Bogdan Ștefan Vasile, Mihaela Mușat, Cristina Chircov, Miruna S. Stan, Alexandru Mihai Grumezescu, Ecaterina Andronescu, and Mariana Carmen Chifiriuc. 2022. "Design, Characterization, and Antibacterial Performance of MAPLE-Deposited Coatings of Magnesium Phosphate-Containing Silver Nanoparticles in Biocompatible Concentrations" International Journal of Molecular Sciences 23, no. 14: 7910. https://doi.org/10.3390/ijms23147910
APA StyleFlorea, D. A., Grumezescu, V., Bîrcă, A. C., Vasile, B. Ș., Mușat, M., Chircov, C., Stan, M. S., Grumezescu, A. M., Andronescu, E., & Chifiriuc, M. C. (2022). Design, Characterization, and Antibacterial Performance of MAPLE-Deposited Coatings of Magnesium Phosphate-Containing Silver Nanoparticles in Biocompatible Concentrations. International Journal of Molecular Sciences, 23(14), 7910. https://doi.org/10.3390/ijms23147910










