miR-10 and Its Negative Correlation with Serum IL-35 Concentration and Positive Correlation with STAT5a Expression in Patients with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results
2.1. miRNA Expression in Serum
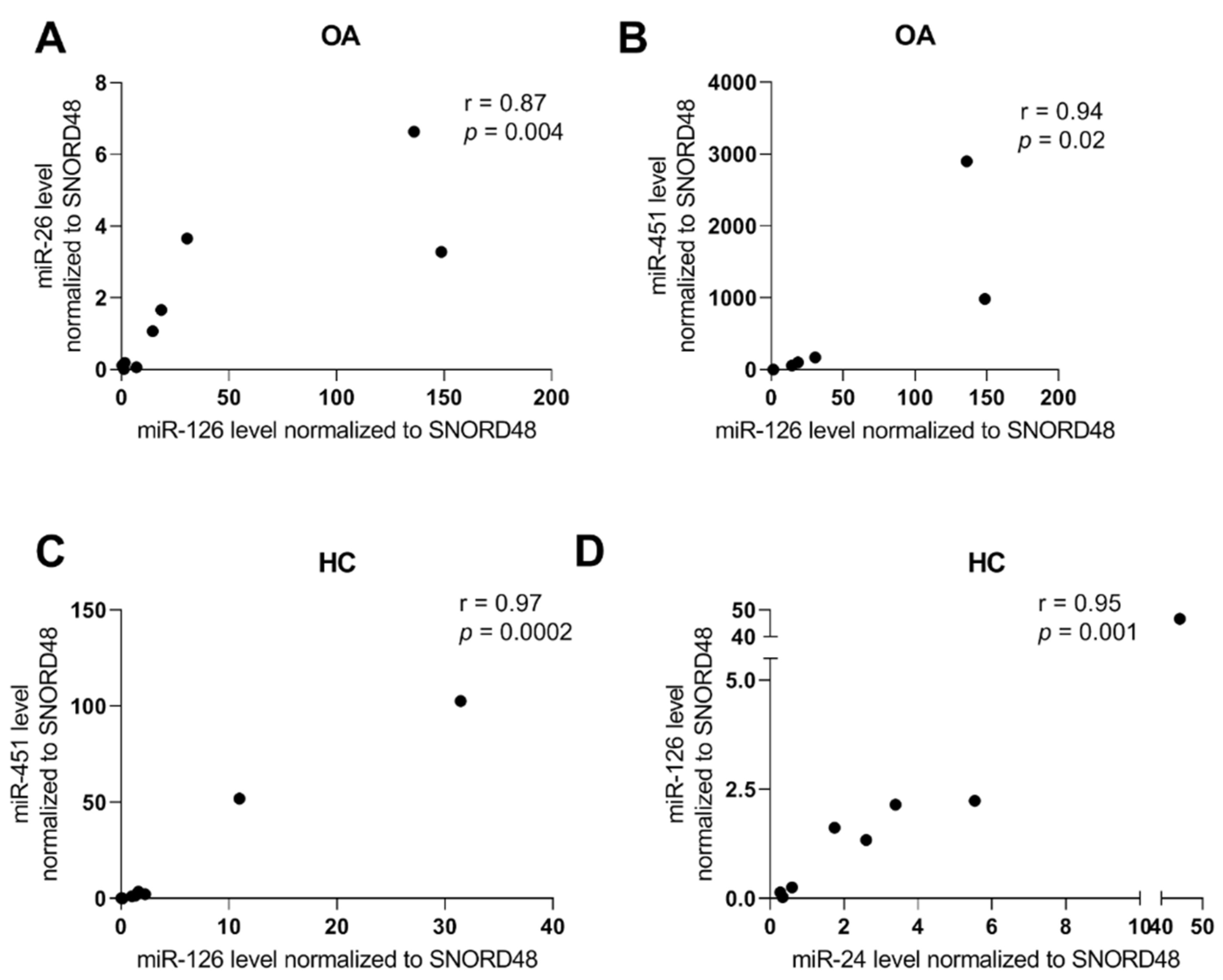
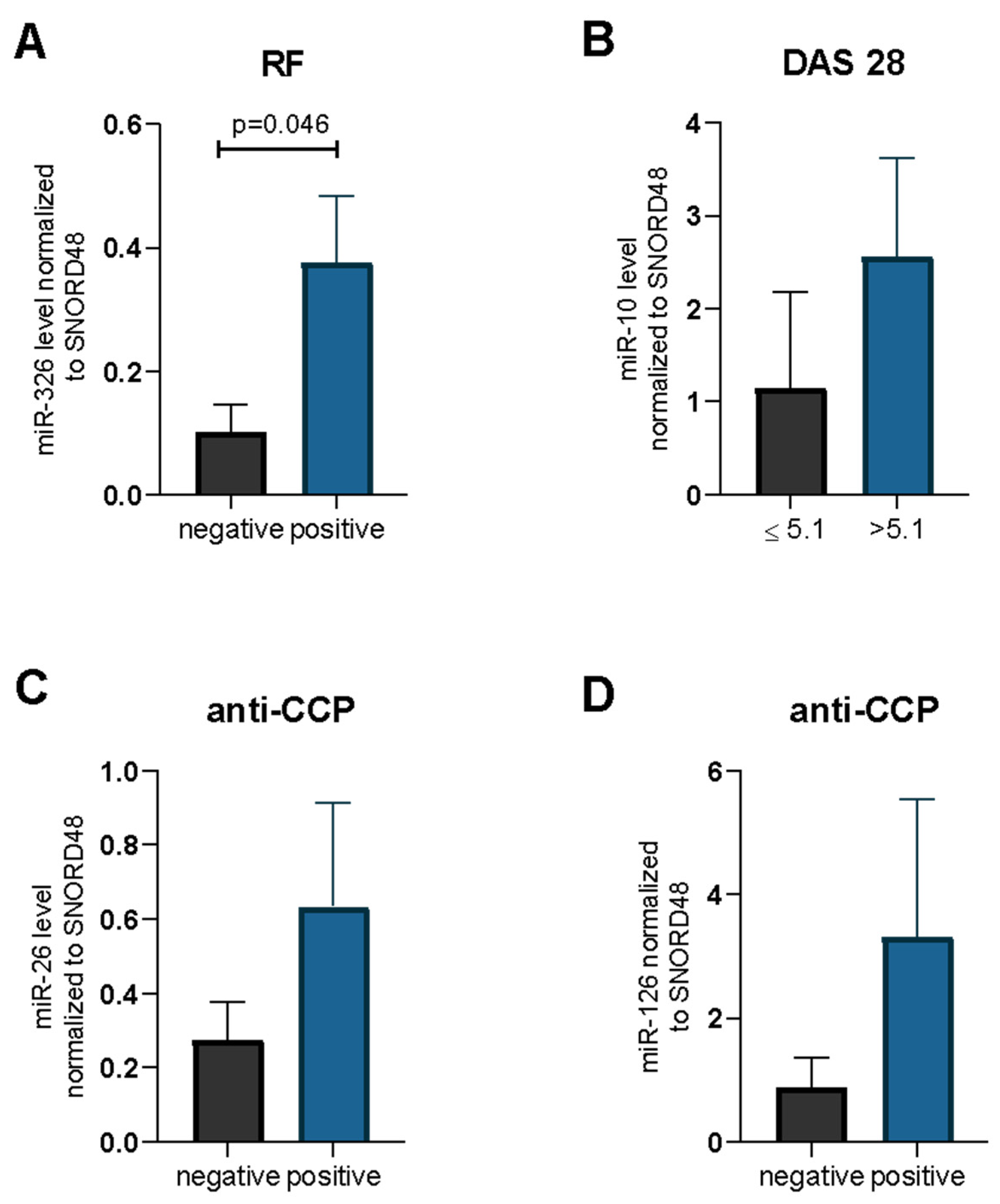
2.2. miRNA and RA/OA Phenotype
2.3. Cytokine Serum Levels
2.4. miRNA Expression Levels in Serum and Correlation between Transcriptional Factors
2.5. miRNA Expression Levels in Serum and Correlation between Cytokine Levels
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. miRNA Isolation
4.3. miRNA Expression
4.4. Cytokine Serum Levels
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houge, I.S.; Hoff, M.; Thomas, R.; Videm, V. Mortality is increased in patients with rheumatoid arthritis or diabetes compared to the general population—The Nord-Trøndelag Health Study. Sci. Rep. 2020, 10, 3593. [Google Scholar] [CrossRef]
- Veronese, N.; Cereda, E.; Maggi, S.; Luchini, C.; Solmi, M.; Smith, T.; Denkinger, M.; Hurley, M.; Thompson, T.; Manzato, E.; et al. Osteoarthritis and mortality: A prospective cohort study and systematic review with meta-analysis. Semin. Arthritis Rheum. 2016, 46, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, R.J.; Nelson, A.E.; Callahan, L.F. Knee and hip osteoarthritis as predictors of premature death: A review of the evidence. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 24–30. [Google Scholar] [PubMed]
- Vicente, R.; Noël, D.; Pers, Y.M.; Apparailly, F.; Jorgensen, C. Deregulation and therapeutic potential of microRNAs in arthritic diseases. Nat. Rev. Rheumatol. 2016, 12, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.S.; Huang, Y.M.; Cai, D.K.; Liu, J.W.; Cao, X.W. Analysis of differences in the molecular mechanism of rheumatoid arthritis and osteoarthritis based on integration of gene expression profiles. Immunol. Lett. 2015, 168, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, J.; Clanchy, F.I.L.; Taher, T.E.; Al-Bogami, M.; Ong, V.H.; Abraham, D.J.; Williams, R.O.; Mageed, R.A. Functional and phenotypic heterogeneity of Th17 cells in health and disease. Eur. J. Clin. Investig. 2019, 49, e13032. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Zhu, P. Functional niche of inflamed synovium for Th17-cell expansion and activation in rheumatoid arthritis: Implication to clinical therapeutics. Autoimmun. Rev. 2012, 11, 844–851. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Sun, C.; Liu, T.; Liang, T.; Zhan, L.; Lin, X.; Feng, X.-H. STAT3 selectively interacts with Smad3 to antagonize TGF-β signaling HHS Public Access. Oncogene 2016, 35, 4388–4398. [Google Scholar] [CrossRef] [Green Version]
- Paradowska-Gorycka, A.; Romanowska-Próchnicka, K.; Haladyj, E.; Manczak, M.; Maslinski, S.; Olesinska, M. Association of the Smad3 and NFATc2 gene polymorphisms and their serum levels with susceptibility to rheumatoid arthritis in Polish cohorts. Clin. Exp. Immunol. 2015, 179, 444–453. [Google Scholar] [CrossRef] [Green Version]
- García-Bermúdez, M.; López-Mejías, R.; Genre, F.; Castañeda, S.; González-Juanatey, C.; Llorca, J.; Corrales, A.; Miranda-Filloy, J.A.; Rueda-Gotor, J.; Gómez-Vaquero, C.; et al. SMAD3 rs17228212 Gene Polymorphism Is Associated with Reduced Risk to Cerebrovascular Accidents and Subclinical Atherosclerosis in Anti-CCP Negative Spanish Rheumatoid Arthritis Patients. PLoS ONE 2013, 8, e77695. [Google Scholar] [CrossRef]
- Farrar, M.A.; Owen, D.L. STAT5 and CD4+ T Cell Immunity. F1000Research 2017, 6, 32. [Google Scholar]
- Lamana, A.; Villares, R.; Seoane, I.V.; Andrés, N.; Lucas, P.; Emery, P.; Vital, E.M.; Triguero-Martínez, A.; Marquez, A.; Ortiz, A.M.; et al. Identification of a Human SOCS1 Polymorphism That Predicts Rheumatoid Arthritis Severity. Front. Immunol. 2020, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, W.D.; Peng, H.; Pan, H.F.; Ye, D.Q. SOCS signaling in autoimmune diseases: Molecular mechanisms and therapeutic implications. Eur. J. Immunol. 2014, 44, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.E.; Maney, N.J.; Nair, N.; Lendrem, D.W.; Skelton, A.J.; Diboll, J.; Brown, P.M.; Smith, G.R.; Carmody, R.J.; Barton, A.; et al. Expression of STAT3-regulated genes in circulating CD4+ T cells discriminates rheumatoid arthritis independently of clinical parameters in early arthritis. Rheumatology 2019, 58, 1250–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltanzadeh-Yamchi, M.; Shahbazi, M.; Aslani, S.; Mohammadnia-Afrouzi, M. MicroRNA signature of regulatory T cells in health and autoimmunity. Biomed. Pharmacother. 2018, 100, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, Y.; Guo, Q.; Zou, L.; Zhang, J.; Fang, Y.; Zhang, J.; Zhang, J.; Fu, X.; Liu, H.; et al. Altered microRNA expression profile with miR-146a upregulation in CD4+T cells from patients with rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R81. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Wu, H.; Chan, V.; Lau, C.S.; Lu, Q. Transcriptional and epigenetic regulation of follicular T-helper cells and their role in autoimmunity. Autoimmunity 2017, 50, 71–81. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Wajda, A.; Romanowska-Próchnicka, K.; Walczuk, E.; Kuca-Warnawin, E.; Kmiolek, T.; Stypinska, B.; Rzeszotarska, E.; Majewski, D.; Jagodzinski, P.P.; et al. Th17/Treg-Related Transcriptional Factor Expression and Cytokine Profile in Patients with Rheumatoid Arthritis. Front. Immunol. 2020, 11, 572858. [Google Scholar] [CrossRef]
- Kmiołek, T.; Rzeszotarska, E.; Wajda, A.; Walczuk, E.; Kuca-Warnawin, E.; Romanowska-Próchnicka, K.; Stypinska, B.; Majewski, D.; Jagodzinski, P.P.; Pawlik, A.; et al. The interplay between transcriptional factors and micrornas as an important factor for th17/treg balance in ra patients. Int. J. Mol. Sci. 2020, 21, 7169. [Google Scholar] [CrossRef]
- Chandan, K.; Gupta, M.; Sarwat, M. Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Front. Immunol. 2020, 10, 3081. [Google Scholar] [CrossRef] [Green Version]
- McAlexander, M.A.; Phillips, M.J.; Witwer, K.W. Comparison of methods for miRNA extraction from plasma and quantitative recovery of RNA from cerebrospinal fluid. Front. Genet. 2013, 4, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufourd, T.; Robil, N.; Mallet, D.; Carcenac, C.; Boulet, S.; Brishoual, S.; Rabois, E.; Houeto, J.L.; De La Grange, P.; Carnicella, S. Plasma or serum? A qualitative study on rodents and humans using high-throughput microRNA sequencing for circulating biomarkers. Biol. Methods Protoc. 2019, 4, bpz006. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churov, A.V.; Oleinik, E.K.; Knip, M. MicroRNAs in rheumatoid arthritis: Altered expression and diagnostic potential. Autoimmun. Rev. 2015, 14, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Prajzlerová, K.; Kryštůfková, O.; Hánová, P.; Horváthová, V.; Gregová, M.; Pavelka, K.; Vencovský, J.; Šenolt, L.; Filková, M. High miR-451 expression in peripheral blood mononuclear cells from subjects at risk of developing rheumatoid arthritis. Sci. Rep. 2021, 11, 4719. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.; Noerholm, M.; Ståhlberg, N.; Mouritzen, P.; Glue, C. miRCURYTM LNA research tools for microRNA. Nat. Methods 2006, 3, 1–2. [Google Scholar] [CrossRef]
- Hussain, N.; Zhu, W.; Jiang, C.; Xu, J.; Geng, M.; Wu, X.; Hussain, S.; Wang, B.; Rajoka, M.S.R.; Li, Y.; et al. Down-regulation of miR-10a-5p promotes proliferation and restricts apoptosis via targeting T-box transcription factor 5 in inflamed synoviocytes. Biosci. Rep. 2018, 38, BSR20180003. [Google Scholar] [CrossRef] [Green Version]
- Mu, N.; Gu, J.; Huang, T.; Zhang, C.; Shu, Z.; Li, M.; Hao, Q.; Li, W.; Zhang, W.; Zhao, J.; et al. A novel NF-κB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci. Rep. 2016, 6, 20059. [Google Scholar] [CrossRef]
- Fish, J.E.; Cybulsky, M.I. Taming endothelial activation with a microRNA. J. Clin. Investig. 2012, 122, 1967–1970. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Belkin, N.; Feinberg, M.W. Endothelial MicroRNAs and atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 372. [Google Scholar] [CrossRef]
- Sun, W.; Ma, Y.; Chen, P.; Wang, D. MicroRNA-10a silencing reverses cisplatin resistance in the A549/cisplatin human lung cancer cell line via the transforming growth factor-β/Smad2/STAT3/STAT5 pathway. Mol. Med. Rep. 2015, 11, 3854–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njock, M.S.; Cheng, H.S.; Dang, L.T.; Nazari-Jahantigh, M.; Lau, A.C.; Boudreau, E.; Roufaiel, M.; Cybulsky, M.I.; Schober, A.; Fish, J.E. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood 2015, 125, 3202–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, G.; Qiu, G.; Ge, M.; Meng, J.; Zhang, G.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. MiR-10a in Peripheral Blood Mononuclear Cells Is a Biomarker for Sepsis and Has Anti-Inflammatory Function. Mediat. Inflamm. 2020, 2020, 4370983. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.L.; Jie, L.F.; Cheng, Q.; Bin, D.Y.; Dan, C.W. Pathogenesis and Function of Interleukin-35 in Rheumatoid Arthritis. Front. Pharmacol. 2021, 12, 655114. [Google Scholar] [CrossRef] [PubMed]
- Filková, M.; Vernerová, Z.; Hulejová, H.; Prajzlerová, K.; Veigl, D.; Pavelka, K.; Vencovský, J.; Šenolt, L. Pro-inflammatory effects of interleukin-35 in rheumatoid arthritis. Cytokine 2015, 73, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Xu, D.; Xue, F.; Li, M.; Wang, X.; Liu, G. Interleukin-35 sensitizes monocytes from patients with asthma to glucocorticoid therapy by regulating p38 MAPK. Exp. Ther. Med. 2020, 19, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, L.; Liu, S.; Wu, J.; Xia, L.; Shen, H.; Lu, J. Elevated serum IL-35 levels in rheumatoid arthritis are associated with disease activity. J. Investig. Med. 2019, 67, 707–710. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Wajda, A.; Haładyj, E.; Romanowska-Próchnicka, K.; Felis-Giemza, A.; Nałęcz-Janik, J.; Walczyk, M.; Olesińska, M.; Tarnacka, B.; Paradowska-Gorycka, A. IL-35, TNF-α, BAFF, and VEGF serum levels in patients with different rheumatic diseases. Reumatologia 2019, 57, 145–150. [Google Scholar] [CrossRef]
- Sui, X.; Liu, H.; Zhou, Y. Expression of miR-495 and miR-326 in peripheral blood of rheumatoid arthritis patients and its significance. Exp. Ther. Med. 2020, 3766–3774. [Google Scholar] [CrossRef]
- Albrecht, K.; Zink, A. Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: A review of data from randomized clinical trials and cohort studies. Arthritis Res. Ther. 2017, 19, 68. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Xu, C.; Pan, Z.; Zhang, Y.; Xu, Z.; Chen, Y.; Li, T.; Li, X.; Liu, Y.; Huangfu, L.; et al. The antifibrotic effects and mechanisms of microRNA-26a action in idiopathic pulmonary fibrosis. Mol. Ther. 2014, 22, 1122–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, B.K.; Gagan, J.; Yan, Z.; Dutta, A. miR-26a is required for skeletal muscle differentiation and regeneration in mice. Genes Dev. 2012, 26, 2180–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Wang, P.; Wu, Y.; Zhong, J.; Chen, Q.; Wang, D.; Chen, H.; Hu, S.; Wu, Q. MiR-26a Reduces Inflammatory Responses via Inhibition of PGE2 Production by Targeting COX-2. Inflammation 2022, 45, 1484–1496. [Google Scholar] [CrossRef]
- Rasmussen, T.K.; Andersen, T.; Hvid, M.; Hetland, M.L.; Hørslev-Petersen, K.; Stengaard-Pedersen, K.; Holm, C.K.; Deleuran, B. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J. Rheumatol. 2010, 37, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Chen, Y.M.; Chen, H.H.; Hsieh, C.W.; Lin, C.C.; Lan, J.L. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res. Ther. 2011, 13, R126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, S.K.; Cho, M.L.; Park, M.K.; Oh, H.J.; Park, J.S.; Her, Y.M.; Lee, S.Y.; Youn, J.; Ju, J.H.; Park, K.S.; et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 2012, 64, 740–751. [Google Scholar] [CrossRef]
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; La Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic Diet and microRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Cannataro, R.; Perri, M.; Gallelli, L.; Caroleo, M.C.; De Sarro, G.; Cione, E. Ketogenic Diet Acts on Body Remodeling and MicroRNAs Expression Profile. MicroRNA 2019, 8, 116–126. [Google Scholar] [CrossRef]




| Parameter | RA n = 23 | OA n = 26 | HC n = 29 |
|---|---|---|---|
| age, years (median, range) | 57.5 (21–75) | 67 (28–85) | 46 (41–63) |
| female/male | 21/1 | 16/10 | 20/9 |
| ESR—mm/h (mean ± SD) | 29.86 ± 23.98 | 14.72 ± 8.369 | - |
| CRP—mg/dL (mean ± SD) | 21.68 ± 20.89 | 5.696 ± 1.396 | - |
| Disease duration, years (median, range) | 11.5 (0.5–21) | - | - |
| DAS-28 (mean ± SD) | 4.993 ± 1.425 | - | - |
| VAS (mm, mean ± SD) | 58 ± 23.44 | - | - |
| PLT (mean ± SD) | - | 226.4 ± 52.88 | - |
| vitamin D (mean ± SD) | - | 31.83 ± 24.88 | - |
| RF positivity, n (%) | 13/9 | - | - |
| anti-CCP positivity, n (%) | 17/5 | - | - |
| Medication: | - | - | |
| cDMARDS: MTX/SSA/LEF, n (%) | 7 (30%) | - | - |
| GKS + cDMARDS, n (%) | 5 (22%) | - | - |
| antimalarials + cDMARDS, n (%) | 5 (22%) | - | - |
| GKS + antimalarials, n (%) | 1 (4%) | - | - |
| BIOLOGICS + cDMARDS, n (%) | 3 (13%) | - | - |
| no drug, n (%) | 1 (4%) | - | - |
| no data, n (%) | 1 (4%) | - | - |
| Parameter | RA n = 12 | OA n = 9 | HC n = 9 |
|---|---|---|---|
| age, years (median, range) | 51 (21–75) | 73 (28–80) | 45.5 (44–60) |
| female/male | 10/4 | 5/4 | 4/5 |
| ESR—mm/h (mean ± SD) | 35 ± 21.59 | 18.11 ± 9.02 | - |
| CRP-mg/dL (mean ± SD) | 24.92 ± 19.72 | 6.111 ± 1.764 | - |
| Disease duration, years (median, range) | 6.5 (0.5–18) | - | - |
| DAS-28 (mean ± SD) | 5.018 ± 1.438 | - | - |
| VAS (mm, mean ± SD) | 64 ± 25.05 | - | - |
| PLT (mean ± SD) | - | 245 ± 52.97 | - |
| vitamin D (mean ± SD) | - | 37.07 ± 6.111 | - |
| RF positivity, n (%) | 13/9 | - | - |
| anti-CCP positivity, n (%) | 17/5 | - | - |
| Medication: | - | - | |
| cDMARDS: MTX/SSA/LEF, n (%) | 2 (9%) | - | - |
| GKS + cDMARDS, n(%) | 2 (9%) | - | - |
| antimalarials + cDMARDS, n (%) | 3 (14%) | - | - |
| GKS + immunosuppressants, n (%) | 1 (6%) | - | - |
| BIOLOGICS + cDMARDS, n (%) | 2 (9%) | - | - |
| no drug, n (%) | 1 (6%) | - | - |
| no data, n (%) | 1 (6%) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradowska-Gorycka, A.; Wajda, A.; Rzeszotarska, E.; Kmiolek, T.; Stypinska, B.; Dudek, E.; Romanowska-Prochnicka, K.; Syrowka, P. miR-10 and Its Negative Correlation with Serum IL-35 Concentration and Positive Correlation with STAT5a Expression in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 7925. https://doi.org/10.3390/ijms23147925
Paradowska-Gorycka A, Wajda A, Rzeszotarska E, Kmiolek T, Stypinska B, Dudek E, Romanowska-Prochnicka K, Syrowka P. miR-10 and Its Negative Correlation with Serum IL-35 Concentration and Positive Correlation with STAT5a Expression in Patients with Rheumatoid Arthritis. International Journal of Molecular Sciences. 2022; 23(14):7925. https://doi.org/10.3390/ijms23147925
Chicago/Turabian StyleParadowska-Gorycka, Agnieszka, Anna Wajda, Ewa Rzeszotarska, Tomasz Kmiolek, Barbara Stypinska, Ewa Dudek, Katarzyna Romanowska-Prochnicka, and Piotr Syrowka. 2022. "miR-10 and Its Negative Correlation with Serum IL-35 Concentration and Positive Correlation with STAT5a Expression in Patients with Rheumatoid Arthritis" International Journal of Molecular Sciences 23, no. 14: 7925. https://doi.org/10.3390/ijms23147925
APA StyleParadowska-Gorycka, A., Wajda, A., Rzeszotarska, E., Kmiolek, T., Stypinska, B., Dudek, E., Romanowska-Prochnicka, K., & Syrowka, P. (2022). miR-10 and Its Negative Correlation with Serum IL-35 Concentration and Positive Correlation with STAT5a Expression in Patients with Rheumatoid Arthritis. International Journal of Molecular Sciences, 23(14), 7925. https://doi.org/10.3390/ijms23147925








