Therapeutic Benefits of Selenium in Hematological Malignancies
Abstract
:1. Introduction
2. Regulation of Signaling Pathways by Selenium in Cancer
2.1. Selenoproteins Play a Role in Cancer
2.2. Role of Selenoproteins in Hematological Malignancies
2.3. Inorganic and Organic Selenium Compounds Exert Therapeutic Activities
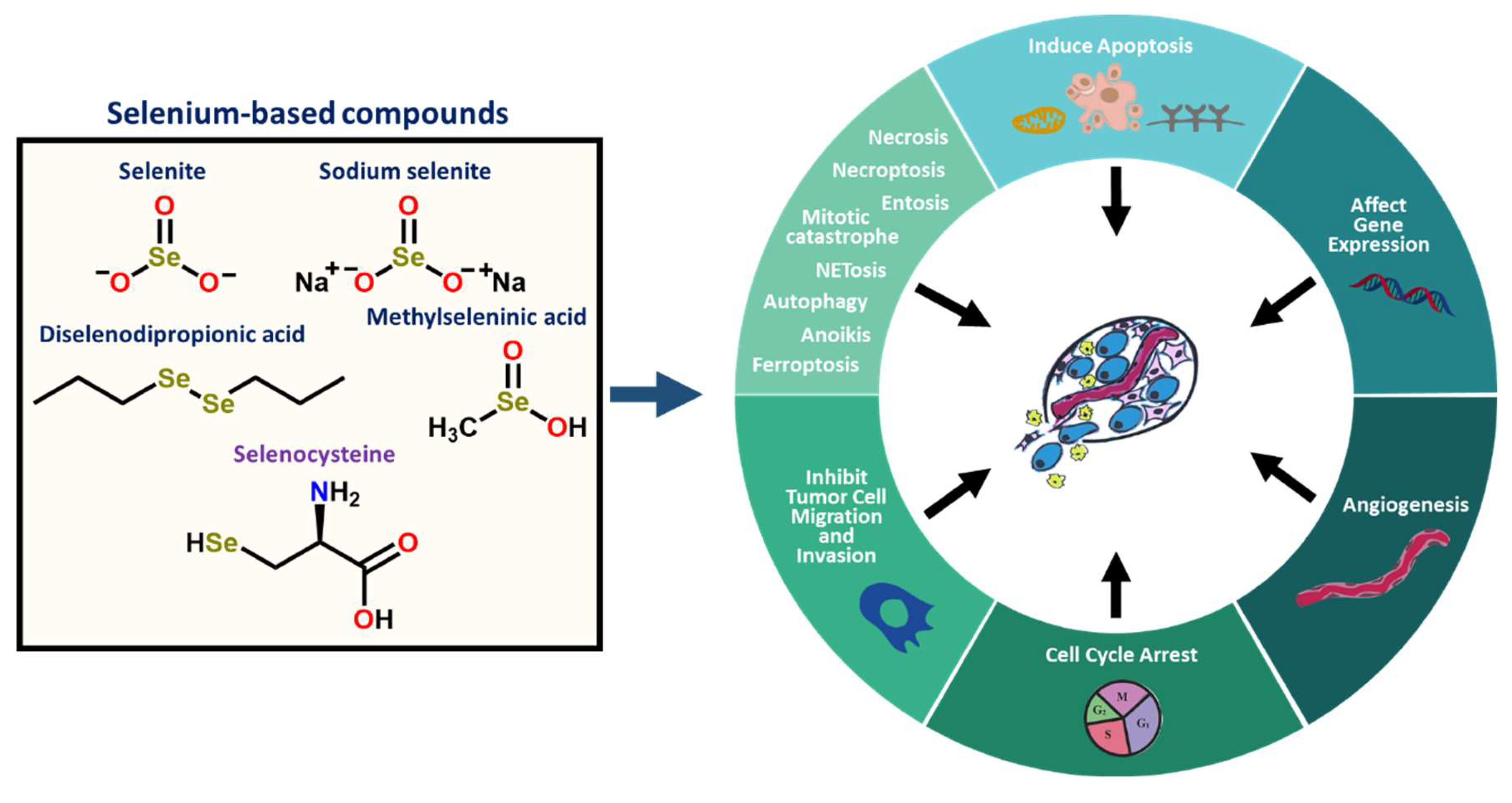
2.4. Selenium-Based Compounds or Proteins Act by Various Modes of Action
3. Role of Selenium and Its Therapeutic Advantages in Various Cancers
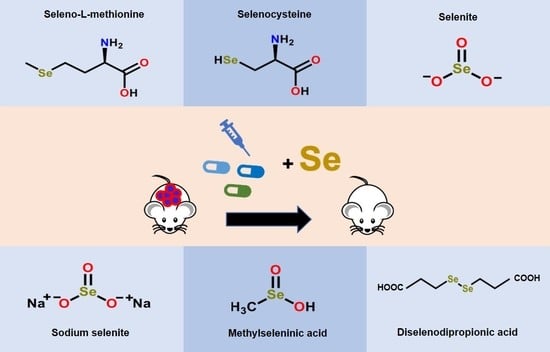
3.1. Pre-Clinical Selenium-Based Therapeutic Studies Are Promising in Disease Animal Models
3.2. Clinical Studies Support Adjuvant Selenium Supplementation
4. Selenium Reduces Disease Progression in Blood Cancers
4.1. Selenium Induces a Cytotoxic Effect in Leukemia/Lymphoma Cells
4.2. Selenium Impacts AML in In Vitro and In Vivo Models
4.3. Selenium Is Potent in Leukemia Stem Cells through In Vitro and In Vivo AML/CML Models
4.4. Selenium Is Therapeutically Valuable in Patients with Hematological Malignancies
5. Selenium Alleviates Adverse Effects Associated with Radiotherapy or Chemotherapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug Targets 2004, 4, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, M.-E.M.; Tao, L.; Medeiros, B.C.; Clarke, C.A. Improvements in the early death rate among 9380 patients with acute myeloid leukemia after initial therapy: A SEER database analysis. Cancer 2015, 121, 2004–2012. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Newcomb, P.A.; Carbone, P.P. Cancer Treatment and Age: Patient Perspectives. JNCI J. Natl. Cancer Inst. 1993, 85, 1580–1584. [Google Scholar] [CrossRef]
- Pui, C.H.; Mullighan, C.G.; Evans, W.E.; Relling, M.V. Pediatric acute lymphoblastic leukemia: Where are we going and how do we get there? Blood 2012, 120, 1165–1174. [Google Scholar] [CrossRef] [Green Version]
- Beyar-Katz, O.; Gill, S. Novel Approaches to Acute Myeloid Leukemia Immunotherapy. Clin. Cancer Res. 2018, 24, 5502–5515. [Google Scholar] [CrossRef] [Green Version]
- Koreth, J.; Schlenk, R.; Kopecky, K.J.; Honda, S.; Sierra, J.; Djulbegovic, B.J.; Wadleigh, M.; DeAngelo, D.J.; Stone, R.M.; Sakamaki, H.; et al. Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia in First Complete Remission: Systematic Review and Meta-analysis of Prospective Clinical Trials. JAMA 2009, 301, 2349–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadia, T.M.; Ravandi, F.; Cortes, J.; Kantarjian, H. New drugs in acute myeloid leukemia. Ann. Oncol. 2016, 27, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Pazirandeh, A.; Assadi Nejad, M.; Vossogh, P. Determination of selenium in blood serum of children with acute leukemia and effect of chemotherapy on serum selenium level. J. Trace Elem. Med. Biol. 1999, 13, 242–246. [Google Scholar] [CrossRef]
- Atieh, M.; Molouk, H.; AhmadReza, S.; Rocsanna, N.; Mohammad, A.; Ardeshir, G. Trace elements (Se, Zn, and Cu) levels in patients with newly diagnosed acute leukemia. Int. J. Hematol.-Oncol. Stem Cell Res. 2015, 6, 5–10. [Google Scholar]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.R.; Hajra, S.; Baral, R.; Bhattacharya, S. Use of selenium as micronutrients and for future anticancer drug: A review. Nucleus 2020, 63, 107–118. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [Green Version]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, M.A.; Sulieman, S.; Muhling, K.H. Regulation of Selenium/Sulfur Interactions to Enhance Chemopreventive Effects: Lessons to Learn from Brassicaceae. Molecules 2020, 25, 5846. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Moulis, J.M.; Gaillard, J.; Lutz, M. Replacement of Sulfur by Selenium in Iron-Sulfur Proteins. Adv. Inorg. Chem. 1992, 38, 73–115. [Google Scholar] [CrossRef]
- Ip, C.; Ganther, H.E. Comparison of selenium and sulfur analogs in cancer prevention. Carcinogenesis 1992, 13, 1167–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweizer, U.; Fradejas-Villar, N. Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 2016, 30, 3669–3681. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Li, D.; Feng, X.; Zhao, F.; Li, C.; Zheng, S.; Lyu, J. A pan-cancer study of selenoprotein genes as promising targets for cancer therapy. BMC Med. Genomics 2021, 14, 78. [Google Scholar] [CrossRef]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Jablonska, E.; Vinceti, M. Selenium and Human Health: Witnessing a Copernican Revolution? J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 328–368. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The Epidemiology of Selenium and Human Cancer. Adv. Cancer Res. 2017, 136, 1–48. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.L.; Zhang, J.L.; Zhang, Z.W.; Yao, H.D.; Sun, G.; Xu, S.W. Protective roles of selenium on nitric oxide-mediated apoptosis of immune organs induced by cadmium in chickens. Biol. Trace Elem. Res. 2014, 159, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Zamamiri-Davis, F.; Stewart, J.B.; Thompson, J.T.; Sordillo, L.M.; Reddy, C.C. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: Role of nuclear factor-kappaB in up-regulation. Biochem. J. 2002, 366, 203–209. [Google Scholar] [CrossRef] [Green Version]
- The PyMOL Molecular Graphics System, Version 2.2 Schrödinger, LLC. Available online: https://pymol.org/ (accessed on 28 June 2021).
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Allan, C.B.; Lacourciere, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium. Annu. Rev. Nutr. 1999, 19, 1–16. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [Green Version]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [Green Version]
- Handa, E.; Puspitasari, I.M.; Abdulah, R.; Yamazaki, C.; Kameo, S.; Nakano, T.; Koyama, H. Recent advances in clinical studies of selenium supplementation in radiotherapy. J. Trace Elem. Med. Biol. 2020, 62, 126653. [Google Scholar] [CrossRef]
- Muecke, R.; Schomburg, L.; Buentzel, J.; Kisters, K.; Micke, O. Selenium or no selenium--that is the question in tumor patients: A new controversy. Integr. Cancer Ther. 2010, 9, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, Y.; Aref, S.; Taalab, M.; Ayed, M.; Mabed, M. Implications of Glutathione Peroxidase 3 Expression in a Cohort of Egyptian Patients with Acute Myeloid Leukemia. Asian Pac. J. Cancer Prev. 2020, 21, 3567–3572. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ma, W.; Liu, P.; Zhou, F. Overview of thioredoxin system and targeted therapies for acute leukemia. Mitochondrion 2019, 47, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, M.; Zhang, J.; Ma, Y.; Qu, W.; Liang, J.; Wu, H.; Wen, H. Peperomin E and its orally bioavailable analog induce oxidative stress-mediated apoptosis of acute myeloid leukemia progenitor cells by targeting thioredoxin reductase. Redox Biol. 2019, 24, 101153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Pan, Y.; Wei, Y.; Zhang, R.; Bai, G.; Shen, Q.; Meng, S.; Le, X.-F.; Andreeff, M.; Claret, F.X. Jab1/Csn5–Thioredoxin Signaling in Relapsed Acute Monocytic Leukemia under Oxidative Stress. Clin. Cancer Res. 2017, 23, 4450–4461. [Google Scholar] [CrossRef] [Green Version]
- Fedirko, V.; Jenab, M.; Meplan, C.; Jones, J.S.; Zhu, W.; Schomburg, L.; Siddiq, A.; Hybsier, S.; Overvad, K.; Tjonneland, A.; et al. Association of Selenoprotein and Selenium Pathway Genotypes with Risk of Colorectal Cancer and Interaction with Selenium Status. Nutrients 2019, 11, 935. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, D.L.; Yoo, M.H.; Carlson, B.A.; Gladyshev, V.N. Selenoproteins that function in cancer prevention and promotion. Biochim. Biophys. Acta 2009, 1790, 1541–1545. [Google Scholar] [CrossRef] [Green Version]
- Kaweme, N.M.; Zhou, S.; Changwe, G.J.; Zhou, F. The significant role of redox system in myeloid leukemia: From pathogenesis to therapeutic applications. Biomark. Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F.; et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell. Biol. 2004, 24, 9414–9423. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, D.; Wu, W.; Huang, D.; Zheng, H.; Aihaiti, Y. A Pan-Cancer Analysis of the Role of Selenoprotein P mRNA in Tumorigenesis. Int. J. Gen. Med. 2021, 14, 7471–7485. [Google Scholar] [CrossRef]
- Wei, J.; Xie, Q.; Liu, X.; Wan, C.; Wu, W.; Fang, K.; Yao, Y.; Cheng, P.; Deng, D.; Liu, Z. Identification the prognostic value of glutathione peroxidases expression levels in acute myeloid leukemia. Ann. Transl. Med. 2020, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Kinowaki, Y.; Kurata, M.; Ishibashi, S.; Ikeda, M.; Tatsuzawa, A.; Yamamoto, M.; Miura, O.; Kitagawa, M.; Yamamoto, K. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab. Investig. 2018, 98, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Kurata, M.; Onishi, I.; Kinowaki, Y.; Sato, Y.; Shiono, S.; Ishibashi, S.; Ikeda, M.; Yamamoto, M.; Kitagawa, M.; et al. SECISBP2 is a novel prognostic predictor that regulates selenoproteins in diffuse large B-cell lymphoma. Lab. Investig. 2021, 101, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Eagle, K.; Jiang, Y.; Shi, X.; Li, M.; Obholzer, N.P.; Hu, T.; Perez, M.W.; Koren, J.V.; Kitano, A.; Yi, J.S.; et al. An oncogenic enhancer encodes selective selenium dependency in AML. Cell Stem Cell 2022, 29, 386–399.e387. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Benedetti, R.; Handzlik, J.; Zwergel, C.; Battistelli, C. The innovative potential of selenium-containing agents for fighting cancer and viral infections. Drug Discov. Today 2021, 26, 256–263. [Google Scholar] [CrossRef]
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule Selenium-Containing Compounds: Recent Development and Therapeutic Applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef]
- Soriano-Garcia, M. Organoselenium compounds as potential therapeutic and chemopreventive agents: A review. Curr. Med. Chem. 2004, 11, 1657–1669. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, M. The Inhibitory Efficacy of Methylseleninic Acid Against Colon Cancer Xenografts in C57BL/6 Mice. Nutr. Cancer 2015, 67, 831–838. [Google Scholar] [CrossRef]
- Powers, M.; Liu, L.; Deemer, D.; Chen, S.; Scholl, A.; Yoshinaga, M.; Liu, Z. Selenite Inhibits Notch Signaling in Cells and Mice. Int. J. Mol. Sci. 2021, 22, 2518. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Peng, J.; Guo, X.; Tan, W. Expression Profile Analysis of Selenium-Related Genes in Peripheral Blood Mononuclear Cells of Patients with Keshan Disease. Bio. Med. Res. Int. 2019, 2019, 4352905. [Google Scholar] [CrossRef] [PubMed]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium Compounds as Novel Adjuvants of Chemotherapy Drugs—A Promising Approach to Fight Cancer Drug Resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangard-Rafsanjani, Z.; Gholami, K.; Hadjibabaie, M.; Shamshiri, A.R.; Alimoghadam, K.; Sarayani, A.; Mojtahedzadeh, M.; Ostadali-Dehaghi, M.; Ghavamzadeh, A. The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: A randomized clinical trial. Bone Marrow Transplant. 2013, 48, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Muecke, R.; Schomburg, L.; Glatzel, M.; Berndt-Skorka, R.; Baaske, D.; Reichl, B.; Buentzel, J.; Kundt, G.; Prott, F.J.; Devries, A.; et al. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 828–835. [Google Scholar] [CrossRef]
- Varela-Lopez, A.; Battino, M.; Navarro-Hortal, M.D.; Giampieri, F.; Forbes-Hernandez, T.Y.; Romero-Marquez, J.M.; Collado, R.; Quiles, J.L. An update on the mechanisms related to cell death and toxicity of doxorubicin and the protective role of nutrients. Food Chem. Toxicol. 2019, 134, 110834. [Google Scholar] [CrossRef]
- Cao, S.; Durrani, F.A.; Tóth, K.; Rustum, Y.M. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br. J. Cancer 2014, 110, 1733–1743. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising Selenium for Modulation of Cancer Treatments. Anticancer Res. 2017, 37, 6497–6509. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.O.; Jacobson, G.M.; Goodman, H.J.B.; Bird, S.; Jameson, M.B. Comparative Safety and Pharmacokinetic Evaluation of Three Oral Selenium Compounds in Cancer Patients. Biol. Trace Elem. Res. 2019, 189, 395–404. [Google Scholar] [CrossRef]
- Ip, C.; Hayes, C.; Budnick, R.M.; Ganther, H.E. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991, 51, 595–600. [Google Scholar]
- Pinto, J.T.; Krasnikov, B.F.; Alcutt, S.; Jones, M.E.; Dorai, T.; Villar, M.T.; Artigues, A.; Li, J.; Cooper, A.J. Kynurenine aminotransferase III and glutamine transaminase L are identical enzymes that have cysteine S-conjugate beta-lyase activity and can transaminate L-selenomethionine. J. Biol. Chem. 2014, 289, 30950–30961. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdiglesias, V.; Pásaro, E.; Méndez, J.; Laffon, B. In Vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: A review. Arch. Toxicol. 2010, 84, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Soukupová, K.; Rudolf, E. Suppression of proliferation and activation of cell death by sodium selenite involves mitochondria and lysosomes in chemoresistant bladder cancer cells. J. Trace Elem. Med. Biol. 2019, 52, 58–67. [Google Scholar] [CrossRef]
- Goel, A.; Fuerst, F.; Hotchkiss, E.; Boland, C.R. Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol. Ther. 2006, 5, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Wang, Z.; Ganther, H.; Lu, J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol. Cancer Ther. 2002, 1, 1059–1066. [Google Scholar]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium Compounds, Apoptosis and Other Types of Cell Death: An Overview for Cancer Therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Subburayan, K.; Thayyullathil, F.; Pallichankandy, S.; Cheratta, A.R.; Galadari, S. Superoxide-mediated ferroptosis in human cancer cells induced by sodium selenite. Transl. Oncol. 2020, 13, 100843. [Google Scholar] [CrossRef]
- Ingold, I.; Conrad, M. Selenium and iron, two elemental rivals in the ferroptotic death process. Oncotarget 2018, 9, 22241–22242. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Prabhu, K.S.; Mastro, A.M. Is Selenium a Potential Treatment for Cancer Metastasis? Nutrients 2013, 5, 1149–1168. [Google Scholar] [CrossRef] [Green Version]
- Plano, D.; Baquedano, Y.; Ibáñez, E.; Jiménez, I.; Palop, J.A.; Spallholz, J.E.; Sanmartín, C. Antioxidant-Prooxidant Properties of a New Organoselenium Compound Library. Molecules 2010, 15, 7292–7312. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Yoon, S.I.; Shin, S.H.; Koh, Y.A.; Lee, S.-J.; Lee, Y.-S.; Bae, S. p53-mediated enhancement of radiosensitivity by selenophosphate synthetase 1 overexpression. J. Cell. Phys. 2006, 209, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Lancia, J.K.; Mercer, T.I.; Ip, C. Selenium compounds regulate p53 by common and distinctive mechanisms. Anticancer Res. 2004, 24, 1401–1408. [Google Scholar] [PubMed]
- Fischer, J.L.; Mihelc, E.M.; Pollok, K.E.; Smith, M.L. Chemotherapeutic selectivity conferred by selenium: A role for p53-dependent DNA repair. Mol. Cancer Ther. 2007, 6, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.R.; Sweeney, C.; Smith, M.L. Selenomethionine induction of DNA repair response in human fibroblasts. Oncogene 2002, 21, 3663–3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.R.; Kelley, M.R.; Smith, M.L. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc. Natl. Acad. Sci. USA 2002, 99, 14548–14553. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, L.; Murthy, K.G.; Zhu, C.; Curran, T.; Xanthoudakis, S.; Prives, C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997, 11, 558–570. [Google Scholar] [CrossRef] [Green Version]
- Seemann, S.; Hainaut, P. Roles of thioredoxin reductase 1 and APE/Ref-1 in the control of basal p53 stability and activity. Oncogene 2005, 24, 3853–3863. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Wang, J.; Wu, X.; Yang, C.S.; Zhang, J. Selenium nanoparticles act as an intestinal p53 inhibitor mitigating chemotherapy-induced diarrhea in mice. Pharmacol. Res. 2019, 149, 104475. [Google Scholar] [CrossRef]
- Cao, S.; Durrani, F.A.; Rustum, Y.M. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin. Cancer Res. 2004, 10, 2561–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakih, M.; Cao, S.; Durrani, F.A.; Rustum, Y.M. Selenium protects against toxicity induced by anticancer drugs and augments antitumor activity: A highly selective, new, and novel approach for the treatment of solid tumors. Clin. Colorectal Cancer 2005, 5, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.E.; Stratton, M.S.; Lillico, A.J.; Fakih, M.; Natarajan, R.; Clark, L.C.; Marshall, J.R. A report of high-dose selenium supplementation: Response and toxicities. J. Trace Elem. Med. Biol. 2004, 18, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Hama, S.; Kogure, K.; Tsuchiya, H. Selenate induces epithelial-mesenchymal transition in a colorectal carcinoma cell line by AKT activation. Exp. Cell Res. 2013, 319, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wei, W.; Hua, F.; Cao, T.; Dong, H.; Yang, T.; Yang, Y.; Pan, H.; Xu, C. Requirement for ERK activity in sodium selenite-induced apoptosis of acute promyelocytic leukemia-derived NB4 cells. J. Biochem. Mol. Biol. 2007, 40, 196–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Huang, F.; Liu, Y.; Yang, Y.; Jiang, Q.; Xu, C. Autophagy inhibition through PI3K/Akt increases apoptosis by sodium selenite in NB4 cells. BMB Rep. 2009, 42, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.D.; Fu, X.Y.; Zhang, Z.Y.; Cao, M.Z.; Sun, J.Y.; Yang, M.F.; Fu, X.T.; Zhao, S.J.; Shao, L.R.; Zhang, H.F.; et al. Selenocysteine induces apoptosis in human glioma cells: Evidence for TrxR1-targeted inhibition and signaling crosstalk. Sci. Rep. 2017, 7, 6465. [Google Scholar] [CrossRef] [Green Version]
- Wallenberg, M.; Misra, S.; Wasik, A.M.; Marzano, C.; Bjornstedt, M.; Gandin, V.; Fernandes, A.P. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell. Mol. Med. 2014, 18, 671–684. [Google Scholar] [CrossRef]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother. Pharmacol. 2010, 66, 475–484. [Google Scholar] [CrossRef]
- Li, G.X.; Lee, H.J.; Wang, Z.; Hu, H.; Liao, J.D.; Watts, J.C.; Combs, G.F., Jr.; Lu, J. Superior In Vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis 2008, 29, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Tobe, T.; Ueda, K.; Ando, M.; Okamoto, Y.; Kojima, N. Thiol-mediated multiple mechanisms centered on selenodiglutathione determine selenium cytotoxicity against MCF-7 cancer cells. J. Biol. Inorg. Chem. 2015, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wu, M.; Botnen, J.H. Methylselenol, a selenium metabolite, induces cell cycle arrest in G1 phase and apoptosis via the extracellular-regulated kinase 1/2 pathway and other cancer signaling genes. J. Nutr. 2009, 139, 1613–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhou, L.; Du, J.; Li, M.; Qian, C.; Cheng, Y.; Peng, Y.; Xie, J.; Wang, D. Induction of apoptosis in human multiple myeloma cell lines by ebselen via enhancing the endogenous reactive oxygen species production. Biomed. Res. Int. 2014, 2014, 696107. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Li, J.; Yin, H.W.; Wang, L.H.; Tang, W.C.; Zhao, F.; Liu, X.M.; Zeng, H.H. Augmented antitumor effects of combination therapy of cisplatin with ethaselen as a novel thioredoxin reductase inhibitor on human A549 cell In Vivo. Investig. New Drugs 2010, 28, 205–215. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Fu, J.; Yin, H.; Xiong, K.; Tan, Q.; Jin, H.; Li, J.; Wang, T.; Tang, W.; et al. Ethaselen: A potent mammalian thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radic. Biol. Med. 2012, 52, 898–908. [Google Scholar] [CrossRef]
- Alvarez-Perez, M.; Ali, W.; Marc, M.A.; Handzlik, J.; Dominguez-Alvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef] [Green Version]
- De Souza, D.; Mariano, D.O.; Nedel, F.; Schultze, E.; Campos, V.F.; Seixas, F.; da Silva, R.S.; Munchen, T.S.; Ilha, V.; Dornelles, L.; et al. New organochalcogen multitarget drug: Synthesis and antioxidant and antitumoral activities of chalcogenozidovudine derivatives. J. Med. Chem. 2015, 58, 3329–3339. [Google Scholar] [CrossRef]
- Zhang, S.; An, B.; Li, J.; Hu, J.; Huang, L.; Li, X.; Chan, A.S.C. Synthesis and evaluation of selenium-containing indole chalcone and diarylketone derivatives as tubulin polymerization inhibition agents. Org. Biomol. Chem. 2017, 15, 7404–7410. [Google Scholar] [CrossRef]
- Dos Santos Edos, A.; Hamel, E.; Bai, R.; Burnett, J.C.; Tozatti, C.S.; Bogo, D.; Perdomo, R.T.; Antunes, A.M.; Marques, M.M.; Matos Mde, F.; et al. Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorg. Med. Chem. Lett. 2013, 23, 4669–4673. [Google Scholar] [CrossRef] [Green Version]
- Savegnago, L.; Jesse, C.R.; Nogueira, C.W. Structural modifications into diphenyl diselenide molecule do not cause toxicity in mice. Environ. Toxicol. Pharmacol. 2009, 27, 271–276. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Azambuja, J.H.; da Silveira, E.F.; Marcondes Sari, M.H.; da Cruz Weber Fulco, B.; Costa Prado, V.; Gelsleichter, N.E.; Beckenkamp, L.R.; da Cruz Fernandes, M.; Spanevello, R.M.; et al. Antitumor action of diphenyl diselenide nanocapsules: In Vitro assessments and preclinical evidence in an animal model of glioblastoma multiforme. J. Trace Elem. Med. Biol. 2019, 55, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Suchocki, P.; Misiewicz-Krzeminska, I.; Skupinska, K.; Niedzwiecka, K.; Lubelska, K.; Fijalek, Z.; Kasprzycka-Guttman, T. Selenitetriglicerydes affect CYP1A1 and QR activity by involvement of reactive oxygen species and Nrf2 transcription factor. Pharmacol. Rep. 2010, 62, 352–361. [Google Scholar] [CrossRef]
- Wu, J.C.; Wang, F.Z.; Tsai, M.L.; Lo, C.Y.; Badmaev, V.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Se-Allylselenocysteine induces autophagy by modulating the AMPK/mTOR signaling pathway and epigenetic regulation of PCDH17 in human colorectal adenocarcinoma cells. Mol. Nutr. Food Res. 2015, 59, 2511–2522. [Google Scholar] [CrossRef]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiolog. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef] [Green Version]
- Shamberger, R.J.; Frost, D.V. Possible protective effect of selenium against human cancer. Can. Med. Assoc. J. 1969, 100, 682. [Google Scholar] [PubMed]
- Hadjibabaie, M.; Iravani, M.; Shamshiri, A.R.; Zaker, Z.; Mousavi, A.; Alimoghaddam, K.; Bahar, B.; Kalantar, E.; Ghavamzadeh, A. The prevalence of low selenium levels in adult patients undergoing bone marrow transplantation: A brief communication. Nutr. Cancer 2008, 60, 837–839. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zhu, Y.J.; Li, W.G. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol. Trace Elem. Res. 1997, 56, 117–124. [Google Scholar] [CrossRef]
- Sandsveden, M.; Nilsson, E.; Borgquist, S.; Rosendahl, A.H.; Manjer, J. Prediagnostic serum selenium levels in relation to breast cancer survival and tumor characteristics. Int. J. Cancer 2020, 147, 2424–2436. [Google Scholar] [CrossRef]
- Pakmanesh, F.; Moslemi, D.; Mahjoub, S. Pre and post chemotherapy evaluation of breast cancer patients: Biochemical approach of serum selenium and antioxidant enzymes. Casp. J. Intern. Med. 2020, 11, 403–409. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species-A Critical Review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef] [PubMed]
- Prokopczyk, B.; Rosa, J.G.; Desai, D.; Amin, S.; Sohn, O.S.; Fiala, E.S.; El-Bayoumy, K. Chemoprevention of lung tumorigenesis induced by a mixture of benzo (a) pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by the organoselenium compound 1,4-phenylenebis (methylene) selenocyanate. Cancer Lett. 2000, 161, 35–46. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xu, T. Elemental Selenium at Nano Size (Nano-Se) as a Potential Chemopreventive Agent with Reduced Risk of Selenium Toxicity: Comparison with Se-Methylselenocysteine in Mice. Toxicol. Sci. 2007, 101, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, C. Factors influencing the anticarcinogenic efficacy of selenium in dimethylbenz[a]anthracene-induced mammary tumorigenesis in rats. Cancer Res. 1981, 41, 2683–2686. [Google Scholar]
- Clayton, C.C.; Baumann, C.A. Diet and azo dye tumors; effect of diet during a period when the dye is not fed. Cancer Res. 1949, 9, 575–582. [Google Scholar]
- Medina, D.; Shepherd, F.S. Selenium inhibition of the neoplastic transformation in preneoplastic mammary cell populations. Cancer Lett. 1984, 24, 227–234. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, W.; Bartolo, P. A concise review on the role of selenium for bone cancer applications. Bone 2021, 149, 115974. [Google Scholar] [CrossRef]
- Jiang, Z.; Chi, J.; Li, H.; Wang, Y.; Liu, W.; Han, B. Effect of chitosan oligosaccharide-conjugated selenium on improving immune function and blocking gastric cancer growth. Eur. J. Pharmacol. 2021, 891, 173673. [Google Scholar] [CrossRef]
- Karelia, D.N.; Kim, S.; Pandey, M.K.; Plano, D.; Amin, S.; Lu, J.; Sharma, A.K. Novel Seleno-Aspirinyl Compound AS-10 Induces Apoptosis, G1 Arrest of Pancreatic Ductal Adenocarcinoma Cells, Inhibits Their NF-κB Signaling, and Synergizes with Gemcitabine Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 4966. [Google Scholar] [CrossRef]
- Ju Han, H.; Sub Byun, W.; Ho Lee, G.; Kyung Kim, W.; Jang, K.; Yang, S.; Yang, J.; Woo Ha, M.; Hong, S.; Lee, J.; et al. Synthesis and biological activity of selenopsammaplin A and its analogues as antitumor agents with DOT1L inhibitory activity. Bioorganic Med. Chem. 2021, 35, 116072. [Google Scholar] [CrossRef]
- Csonka, A.; Kincses, A.; Nové, M.; Vadas, Z.; Sanmartín, C.; Domínguez-Álvarez, E.; Spengler, G. Selenoesters and Selenoanhydrides as Novel Agents Against Resistant Breast Cancer. Anticancer Res. 2019, 39, 3777–3783. [Google Scholar] [CrossRef]
- Almeida, G.M.; Rafique, J.; Saba, S.; Siminski, T.; Mota, N.S.R.S.; Filho, D.W.; Braga, A.L.; Pedrosa, R.C.; Ourique, F. Novel selenylated imidazo [1, 2-a] pyridines for breast cancer chemotherapy: Inhibition of cell proliferation by Akt-mediated regulation, DNA cleavage and apoptosis. Biochem. Biophys. Res. Commun. 2018, 503, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.M.; Kaul, A.; Dong, Y.; Ip, C.; Rowan, B.G. Attenuation of Estrogen Receptor α (ERα) Signaling by Selenium in Breast Cancer Cells via Downregulation of ERα Gene Expression. Breast Cancer Res. Treat. 2005, 92, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Sorolla, A.; Fromont, J.; Blancafort, P.; Flematti, G.R. Aurantoside C Targets and Induces Apoptosis in Triple Negative Breast Cancer Cells. Mar. Drugs 2018, 16, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Ahn, J.H.; Kim, S.B. How shall we treat early triple-negative breast cancer (TNBC): From the current standard to upcoming immuno-molecular strategies. ESMO Open 2018, 3, e000357. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.H.; Hsia, S.; Chung, C.H.; Lin, Y.C.; Shih, M.Y.; Chen, P.C.; Hsu, G.W.; Fan, C.T.; Peng, C.L. Combination of Fish Oil and Selenium Enhances Anticancer Efficacy and Targets Multiple Signaling Pathways in Anti-VEGF Agent Treated-TNBC Tumor-Bearing Mice. Mar. Drugs 2021, 19, 193. [Google Scholar] [CrossRef] [PubMed]
- Linderholm, B.K.; Hellborg, H.; Johansson, U.; Elmberger, G.; Skoog, L.; Lehtio, J.; Lewensohn, R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann. Oncol. 2009, 20, 1639–1646. [Google Scholar] [CrossRef]
- Montero, A.J.; Escobar, M.; Lopes, G.; Gluck, S.; Vogel, C. Bevacizumab in the treatment of metastatic breast cancer: Friend or foe? Curr. Oncol. Rep. 2012, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mohsena, M.A.; Abdel-Malakb, C.A.; Nassef, S.M. Anti-angiogenic targeted therapy in triple negative breast cancer HCC-1806 cell line. Int. J. Sci. Eng. Res. 2018, 9, 93. [Google Scholar]
- Yin, T.; He, S.; Ye, T.; Shen, G.; Wan, Y.; Wang, Y. Antiangiogenic therapy using sunitinib combined with rapamycin retards tumor growth but promotes metastasis. Transl. Oncol. 2014, 7, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.H.; Tzeng, Y.T.; Lai, G.M.; Chang, C.L.; Hu, M.H.; Tsai, W.L.; Liu, Y.R.; Hsia, S.; Chuang, S.E.; Chiou, T.J.; et al. Omega-3 Fatty Acid-Enriched Fish Oil and Selenium Combination Modulates Endoplasmic Reticulum Stress Response Elements and Reverses Acquired Gefitinib Resistance in HCC827 Lung Adenocarcinoma Cells. Mar. Drugs 2020, 18, 399. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, D.W. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients 2014, 6, 5184–5223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, R.; Kim, M.J.; Sohn, I.; Kim, S.; Kim, I.; Ryu, J.M.; Choi, H.J.; Kim, J.M.; Lee, S.K.; Yu, J.; et al. Serum Trace Elements and Their Associations with Breast Cancer Subgroups in Korean Breast Cancer Patients. Nutrients 2018, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.H.; Hsia, S.; Chung, C.H.; Lin, Y.C.; Shih, M.Y.; Chen, P.C.; Peng, C.L.; Henning, S.M.; Hsu, G.W.; Li, Z. Nutritional supplements in combination with chemotherapy or targeted therapy reduces tumor progression in mice bearing triple-negative breast cancer. J. Nutr. Biochem. 2021, 87, 108504. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Carrier, L.; Belame, A.; Thiyagarajah, A.; Salvo, V.A.; Burow, M.E.; Rowan, B.G. Combination of methylselenocysteine with tamoxifen inhibits MCF-7 breast cancer xenografts in nude mice through elevated apoptosis and reduced angiogenesis. Breast Cancer Res. Treat. 2009, 118, 33–43. [Google Scholar] [CrossRef]
- Duffield-Lillico, A.J.; Dalkin, B.L.; Reid, M.E.; Turnbull, B.W.; Slate, E.H.; Jacobs, E.T.; Marshall, J.R.; Clark, L.C.; Nutritional Prevention of Cancer Study Group. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003, 91, 608–612. [Google Scholar] [CrossRef]
- Della Rovere, F.; Granata, A.; Familiari, D.; Zirilli, A.; Cimino, F.; Tomaino, A. Histamine and selenium in lung cancer. Anticancer Res. 2006, 26, 2937–2942. [Google Scholar]
- Duffield-Lillico, A.J.; Reid, M.E.; Turnbull, B.W.; Combs, G.F., Jr.; Slate, E.H.; Fischbach, L.A.; Marshall, J.R.; Clark, L.C. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: A summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 630–639. [Google Scholar]
- Li, W.Q.; Zhang, J.Y.; Ma, J.L.; Li, Z.X.; Zhang, L.; Zhang, Y.; Guo, Y.; Zhou, T.; Li, J.Y.; Shen, L.; et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: Follow-up of a randomized intervention trial. BMJ 2019, 366, l5016. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-J.; Chen, Y.; Zhang, Y.-Q.; Zhou, M.-Z.; Song, X.-M.; Zhang, B.-Z.; Luo, L.; Xu, P.-M.; Zhao, Y.-N.; Zhao, Y.-B.; et al. The protective role of selenium on the toxicity of cisplatin-contained chemotherapy regimen in cancer patients. Biol. Trace Elem. Res. 1997, 56, 331–341. [Google Scholar] [CrossRef]
- Sieja, K.; Talerczyk, M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol. Oncol. 2004, 93, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.A.; Endermann, T.; Stephan, C.; Stoedter, M.; Behrends, T.; Wolff, I.; Jung, K.; Schomburg, L. Selenoprotein P status correlates to cancer-specific mortality in renal cancer patients. PLoS ONE 2012, 7, e46644. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Hoffmann, P.R. Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin. Cell Dev. Biol. 2021, 115, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N.; White, D.A.; Schneider, C.J. Cancer mortality correlation studies-IV: Associations with dietary intakes and blood levels of certain trace elements, notably Se-antagonists. Bioinorg. Chem. 1977, 7, 35–56. [Google Scholar] [CrossRef]
- Thomson, C.D. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef] [Green Version]
- McConnell, K.P.; Cooper, B.J. Distribution of selenium in serum proteins and red blood cells after subcutaneous injection of sodium selenite containing radioselenium. J. Biol. Chem. 1950, 183, 459–466. [Google Scholar] [CrossRef]
- Asfour, I.A.; El-Tehewi, M.M.; Ahmed, M.H.; Abdel-Sattar, M.A.; Moustafa, N.N.; Hegab, H.M.; Fathey, O.M. High-dose sodium selenite can induce apoptosis of lymphoma cells in adult patients with non-Hodgkin’s lymphoma. Biol. Trace Elem. Res. 2009, 127, 200–210. [Google Scholar] [CrossRef]
- Kim, T.; Jung, U.; Cho, D.Y.; Chung, A.S. Se-methylselenocysteine induces apoptosis through caspase activation in HL-60 cells. Carcinogenesis 2001, 22, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Jüliger, S.; Goenaga-Infante, H.; Lister, T.A.; Fitzgibbon, J.; Joel, S.P. Chemosensitization of B-cell lymphomas by methylseleninic acid involves nuclear factor-kappaB inhibition and the rapid generation of other selenium species. Cancer Res. 2007, 67, 10984–10992. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Bao, Y.; Ma, D.; Zi, Y.; Yang, C.; Yang, M.; Xing, M.; Yang, W. Sodium selenite inhibits leukemia HL-60 cell proliferation and induces cell apoptosis by enhancing the phosphorylation of JNK1 and increasing the expression of p21 and p27. Int. J. Mol. Med. 2014, 34, 1175–1179. [Google Scholar] [CrossRef]
- Last, K.; Maharaj, L.; Perry, J.; Strauss, S.; Fitzgibbon, J.; Lister, T.A.; Joel, S. The activity of methylated and non-methylated selenium species in lymphoma cell lines and primary tumours. Ann. Oncol. 2006, 17, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Cao, W.; Xu, H.; Zhu, M.; Wang, J.; Ke, X. Treatment with a selenium-platinum compound induced T-cell acute lymphoblastic leukemia/lymphoma cells apoptosis through the mitochondrial signaling pathway. Oncol. Lett. 2017, 13, 1702–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqa, A.; Munir, R.; Faisal, M. Antitumor effects of sodium selenite on acute lymphocytic leukemia. J. Cancer Res. Ther. 2021, 17, 266–268. [Google Scholar] [CrossRef]
- Lu, J.; Kaeck, M.; Jiang, C.; Wilson, A.C.; Thompson, H.J. Selenite induction of DNA strand breaks and apoptosis in mouse leukemic L1210 cells. Biochem. Pharmacol. 1994, 47, 1531–1535. [Google Scholar] [CrossRef]
- Jiang, X.R.; Macey, M.G.; Lin, H.X.; Newland, A.C. The anti-leukaemic effects and the mechanism of sodium selenite. Leuk. Res. 1992, 16, 347–352. [Google Scholar] [CrossRef]
- Misra, S.; Selvam, A.K.; Wallenberg, M.; Ambati, A.; Matolcsy, A.; Magalhaes, I.; Lauter, G.; Björnstedt, M. Selenite promotes all-trans retinoic acid-induced maturation of acute promyelocytic leukemia cells. Oncotarget 2016, 7, 74686–74700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Shi, X.; Eagle, K.; Lin, C.Y.; Nakada, D. Integrating Enhancer Profiling and CRISPR Dropout Screen Revealed Selenoprotein Synthesis Pathway as a New Vulnerability in AML. Blood 2019, 134, 639. [Google Scholar] [CrossRef]
- Khalkar, P.; Ali, H.A.; Codó, P.; Argelich, N.D.; Martikainen, A.; Arzenani, M.K.; Lehmann, S.; Walfridsson, J.; Ungerstedt, J.; Fernandes, A.P. Selenite and methylseleninic acid epigenetically affects distinct gene sets in myeloid leukemia: A genome wide epigenetic analysis. Free Radic. Biol. Med. 2018, 117, 247–257. [Google Scholar] [CrossRef]
- Jin, Y.; Cai, L.; Yang, Q.; Luo, Z.; Liang, L.; Liang, Y.; Wu, B.; Ding, L.; Zhang, D.; Xu, X.; et al. Anti-leukemia activities of selenium nanoparticles embedded in nanotube consisted of triple-helix β-d-glucan. Carbohydr. Polym. 2020, 240, 116329. [Google Scholar] [CrossRef]
- Doro, N.C.; Lal, D.; Rustum, Y.; Wang, E.S. Selenium Enhances the Anti-Angiogenic Effects of Bevacuzimab (Anti-VEGF Antibody) in Human Acute Myeloid Leukemia Xenograft Models. Blood 2007, 110, 4216. [Google Scholar] [CrossRef]
- Annageldiyev, C.; Tan, S.F.; Thakur, S.; Dhanyamraju, P.K.; Ramisetti, S.R.; Bhadauria, P.; Schick, J.; Zeng, Z.; Sharma, V.; Dunton, W.; et al. The PI3K/AKT Pathway Inhibitor ISC-4 Induces Apoptosis and Inhibits Growth of Leukemia in Preclinical Models of Acute Myeloid Leukemia. Front. Oncol. 2020, 10, 393. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Sharma, A.; Desai, D.; Madhunapantula, S.V.; Huh, S.J.; Robertson, G.P.; Amin, S. Synthesis and Anticancer Activity Comparison of Phenylalkyl Isoselenocyanates with Corresponding Naturally Occurring and Synthetic Isothiocyanates. J. Med. Chem. 2008, 51, 7820–7826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Sharma, A.K.; Madhunapantula, S.V.; Desai, D.; Huh, S.J.; Mosca, P.; Amin, S.; Robertson, G.P. Targeting Akt3 signaling in malignant melanoma using isoselenocyanates. Clin. Cancer Res. 2009, 15, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.E.; Gallant, J.-N.; Dicker, D.T.; Amin, S.; Irby, R.B.; Sharma, A.K.; El-Deiry, W.S. Correction: The Akt Inhibitor ISC-4 Synergizes with Cetuximab in 5-FU-Resistant Colon Cancer. PLoS ONE 2013, 8, e59380. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Kline, C.L.; Berg, A.; Amin, S.; Irby, R.B. The Akt inhibitor ISC-4 activates prostate apoptosis response protein-4 and reduces colon tumor growth in a nude mouse model. Clin. Cancer Res. 2011, 17, 4474–4483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, U.H.; Kaushal, N.; Hegde, S.; Finch, E.R.; Kudva, A.K.; Kennett, M.J.; Jordan, C.T.; Paulson, R.F.; Prabhu, K.S. Selenium suppresses leukemia through the action of endogenous eicosanoids. Cancer Res. 2014, 74, 3890–3901. [Google Scholar] [CrossRef] [Green Version]
- Savona, M.; Talpaz, M. Getting to the stem of chronic myeloid leukaemia. Nat. Rev. Cancer 2008, 8, 341–350. [Google Scholar] [CrossRef]
- Crews, L.A.; Jamieson, C.H. Selective elimination of leukemia stem cells: Hitting a moving target. Cancer Lett. 2013, 338, 15–22. [Google Scholar] [CrossRef]
- Pear, W.S.; Miller, J.P.; Xu, L.; Pui, J.C.; Soffer, B.; Quackenbush, R.C.; Pendergast, A.M.; Bronson, R.; Aster, J.C.; Scott, M.L.; et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 1998, 92, 3780–3792. [Google Scholar] [CrossRef]
- Zuo, L.; Li, J.; Yang, Y.; Wang, X.; Shen, T.; Xu, C.M.; Zhang, Z.N. Sodium selenite induces apoptosis in acute promyelocytic leukemia-derived NB4 cells by a caspase-3-dependent mechanism and a redox pathway different from that of arsenic trioxide. Ann. Hematol. 2004, 83, 751–758. [Google Scholar] [CrossRef]
- Guan, L.; Han, B.; Li, J.; Li, Z.; Huang, F.; Yang, Y.; Xu, C. Exposure of human leukemia NB4 cells to increasing concentrations of selenite switches the signaling from pro-survival to pro-apoptosis. Ann. Hematol. 2009, 88, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Weisberger, A.S.; Suhrland, L.G. Studies on analogues of L-cysteine and L-cystine. III. The effect of selenium cystine on leukemia. Blood 1956, 11, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch, E.R.; Tukaramrao, D.B.; Goodfield, L.L.; Quickel, M.D.; Paulson, R.F.; Prabhu, K.S. Activation of PPARγ by endogenous prostaglandin J(2) mediates the antileukemic effect of selenium in murine leukemia. Blood 2017, 129, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.W.; Horng, I.S.; Hsu, K.H.; Chiang, Y.C.; Liaw, Y.F.; Chen, C.J. Plasma selenium levels and risk of hepatocellular carcinoma among men with chronic hepatitis virus infection. Am. J. Epidemiol. 1999, 150, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Asfour, I.A.; El-kholy, N.M.; Ayoub, M.S.; Ahmed, M.B.; Bakarman, A.A. Selenium and glutathione peroxidase status in adult Egyptian patients with acute myeloid leukemia. Biol. Trace Elem. Res. 2009, 132, 85–92. [Google Scholar] [CrossRef]
- Zuo, X.L.; Chen, J.M.; Zhou, X.; Li, X.Z.; Mei, G.Y. Levels of selenium, zinc, copper, and antioxidant enzyme activity in patients with leukemia. Biol. Trace Elem. Res. 2006, 114, 41–53. [Google Scholar] [CrossRef]
- Stevens, J.; Waters, R.; Sieniawska, C.; Kassam, S.; Montoto, S.; Fitzgibbon, J.; Rohatiner, A.; Lister, A.; Joel, S. Serum selenium concentration at diagnosis and outcome in patients with haematological malignancies. Br. J. Haematol. 2011, 154, 448–456. [Google Scholar] [CrossRef]
- Last, K.W.; Cornelius, V.; Delves, T.; Sieniawska, C.; Fitzgibbon, J.; Norton, A.; Amess, J.; Wilson, A.; Rohatiner, A.Z.; Lister, T.A. Presentation serum selenium predicts for overall survival, dose delivery, and first treatment response in aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 2003, 21, 2335–2341. [Google Scholar] [CrossRef]
- Jameson, M.B.; Evans, S.O. Adding Selenium to Chemotherapy and Radiation in Haematological Malignancies: Clinical Trials Are Justified. Acta Haematol. 2014, 132, 254–255. [Google Scholar] [CrossRef]
- Velicer, C.M.; Ulrich, C.M. Vitamin and Mineral Supplement Use among US Adults after Cancer Diagnosis: A Systematic Review. J. Clin. Oncol. 2008, 26, 665–673. [Google Scholar] [CrossRef]
- Morel, S.; Amre, D.; Teasdale, E.; Caru, M.; Laverdiere, C.; Krajinovic, M.; Sinnett, D.; Curnier, D.; Levy, E.; Marcil, V. Dietary Intakes Are Associated with HDL-Cholesterol in Survivors of Childhood Acute Lymphoblastic Leukaemia. Nutrients 2019, 11, 2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, K.C.; Vieira, M.L.; Beltrame, R.L.; Cartum, J.; Alves, S.I.; Azzalis, L.A.; Junqueira, V.B.; Pereira, E.C.; Fonseca, F.L. Impact of Selenium Supplementation in Neutropenia and Immunoglobulin Production in Childhood Cancer Patients. J. Med. Food 2016, 19, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Wong, Y.-S.; Chen, T.; Zhang, H.; Liu, C.; Zheng, W. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials 2011, 32, 9068–9076. [Google Scholar] [CrossRef] [PubMed]
- Schueller, P.; Puettmann, S.; Micke, O.; Senner, V.; Schaefer, U.; Willich, N. Selenium Influences the Radiation Sensitivity of C6 Rat Glioma Cells. Anticancer Res. 2004, 24, 2913–2918. [Google Scholar]
- Asfour, I.A.; Fayek, M.; Raouf, S.; Soliman, M.; Hegab, H.M.; El-Desoky, H.; Saleh, R.; Moussa, M.A. The impact of high-dose sodium selenite therapy on Bcl-2 expression in adult non-Hodgkin’s lymphoma patients: Correlation with response and survival. Biol. Trace Elem. Res. 2007, 120, 1–10. [Google Scholar] [CrossRef]
- Asfour, I.A.; El Shazly, S.; Fayek, M.H.; Hegab, H.M.; Raouf, S.; Moussa, M.A. Effect of high-dose sodium selenite therapy on polymorphonuclear leukocyte apoptosis in non-Hodgkin’s lymphoma patients. Biol. Trace Elem. Res. 2006, 110, 19–32. [Google Scholar] [CrossRef]
- Barton, M.B.; Jacob, S.; Shafiq, J.; Wong, K.; Thompson, S.R.; Hanna, T.P.; Delaney, G.P. Estimating the demand for radiotherapy from the evidence: A review of changes from 2003 to 2012. Radiother. Oncol. 2014, 112, 140–144. [Google Scholar] [CrossRef]
- Citrin, D.E.; Mitchell, J.B. Mechanisms of Normal Tissue Injury From Irradiation. Semin. Radiat. Oncol. 2017, 27, 316–324. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J. The protective effects of trace elements against side effects induced by ionizing radiation. Radiat. Oncol. J. 2015, 33, 66–74. [Google Scholar] [CrossRef]
- Lobb, R.J.; Jacobson, G.M.; Cursons, R.T.; Jameson, M.B. The Interaction of Selenium with Chemotherapy and Radiation on Normal and Malignant Human Mononuclear Blood Cells. Int. J. Mol. Sci. 2018, 19, 3167. [Google Scholar] [CrossRef] [Green Version]
- Dennert, G.; Horneber, M. Selenium for alleviating the side effects of chemotherapy, radiotherapy and surgery in cancer patients. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- Muecke, R.; Micke, O.; Schomburg, L.; Buentzel, J.; Adamietz, I.A.; Huebner, J. Serum selenium deficiency in patients with hematological malignancies: Is a supplementation study mandatory? Acta Haematol. 2014, 132, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.L.; Fonseca, F.L.; Costa, L.G.; Beltrame, R.L.; Chaves, C.M.; Cartum, J.; Alves, S.I.; Azzalis, L.A.; Junqueira, V.B.; Pereria, E.C.; et al. Supplementation with selenium can influence nausea, fatigue, physical, renal, and liver function of children and adolescents with cancer. J. Med. Food 2015, 18, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Komarov, P.G.; Komarova, E.A.; Kondratov, R.V.; Christov-Tselkov, K.; Coon, J.S.; Chernov, M.V.; Gudkov, A.V. A Chemical Inhibitor of P53 That Protects Mice from the Side Effects of Cancer Therapy. Science 1999, 285, 1733. [Google Scholar] [CrossRef] [Green Version]
- Lammers, T.; Subr, V.; Ulbrich, K.; Peschke, P.; Huber, P.E.; Hennink, W.E.; Storm, G.; Kiessling, F. Hpma-Based Polymer Therapeutics Improve the Efficacy of Surgery, of Radiotherapy and of Chemotherapy Combinations. Nanomedicine 2010, 5, 1501. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Xu, J.; Li, Y.; He, J.; Yang, Y.; Huynh, T.; Ni, P.; Duan, G.; Yang, Z.; et al. Low-Dose X-ray-Responsive Diselenide Nanocarriers for Effective Delivery of Anticancer Agents. ACS Appl. Mater. Interfaces 2020, 12, 43398–43407. [Google Scholar] [CrossRef]
- Krishnan, S.; Chadha, A.S.; Suh, Y.; Chen, H.C.; Rao, A.; Das, P.; Minsky, B.D.; Mahmood, U.; Delclos, M.E.; Sawakuchi, G.O.; et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 755. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Storer, B.E.; Iyer, J.G.; Moshiri, A.; Parvathaneni, U.; Byrd, D.; Sober, A.J.; Sondak, V.K.; Gershenwald, J.E.; Nghiem, P. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases from the National Cancer Data Base. J. Natl. Cancer Inst. 2016, 108, djw042. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Y.; Chou, H.Y.; Yuh, C.H.; Mekuria, S.L.; Kao, Y.C.; Tsai, H.C. Radiation-Sensitive Dendrimer-Based Drug Delivery System. Adv. Sci. 2018, 5, 1700339. [Google Scholar] [CrossRef]
- Liu, T.I.; Yang, Y.C.; Chiang, W.H.; Hung, C.K.; Tsai, Y.C.; Chiang, C.S.; Lo, C.L.; Chiu, H.C. Radiotherapy-Controllable Chemotherapy from Reactive Oxygen Species-Responsive Polymeric Nanoparticles for Effective Local Dual Modality Treatment of Malignant Tumors. Biomacromolecules 2018, 19, 3825. [Google Scholar] [CrossRef]
- Baskar, R.; Yap, S.P.; Chua, K.L.M.; Itahana, K. The Diverse and Complex Roles of Radiation on Cancer Treatment: Therapeutic Target and Genome Maintenance. Am. J. Cancer Res. 2012, 2, 372. [Google Scholar] [PubMed]
- Deepagan, V.G.; Kwon, S.; You, D.G.; Nguyen, V.Q.; Um, W.; Ko, H.; Lee, H.; Jo, D.G.; Kang, Y.M.; Park, J.H. In Situ Diselenide-Crosslinked Polymeric Micelles for Ros-Mediated Anticancer Drug Delivery. Biomaterials 2016, 103, 56. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jin, Y.; Qi, R.; Peng, S.; Fan, B. Oxidation Responsive Mono-Cleavable Amphiphilic Di-Block Polymer Micelles Labeled with a Single Diselenide. Polym. Chem. 2013, 4, 4017. [Google Scholar] [CrossRef]
- Li, T.Y.; Pan, S.J.; Gao, S.Q.; Xiang, W.T.; Sun, C.X.; Cao, W.; Xu, H.P. Diselenide-Pemetrexed Assemblies for Combined Cancer Immuno-, Radio-, and Chemotherapies. Angew. Chem. Int. Ed. 2020, 59, 2700. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive Oxygen Species in Cancer Cells: Live by the Sword, Die by the Sword. Cancer Cell 2006, 10, 175. [Google Scholar] [CrossRef] [Green Version]
- De Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible Polymeric Nanoparticles Degrade and Release Cargo in Response to Biologically Relevant Levels of Hydrogen Peroxide. J. Am. Chem. Soc. 2012, 134, 15758. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J. Am. Chem. Soc. 2010, 132, 442. [Google Scholar] [CrossRef]
- Guleria, A.; Baby, C.M.; Tomy, A.; Maurya, D.K.; Neogy, S.; Debnath, A.K.; Adhikari, S. Size Tuning, Phase Stabilization, and Anticancer Efficacy of Amorphous Selenium Nanoparticles: Effect of Ion-Pair Interaction, −OH Functionalization, and Reuse of RTILs as Host Matrix. J. Phys. Chem. 2021, 125, 13933–13945. [Google Scholar] [CrossRef]
- Wan, X.; Wu, T.; Song, L.; Pan, W.; Li, N.; Tang, B. Selenium-engineered covalent organic frameworks for high-efficiency and long-acting cancer therapy. Chem. Commun. 2021, 57, 6145–6148. [Google Scholar] [CrossRef]
- Barbanente, A.; Palazzo, B.; Esposti, L.D.; Adamiano, A.; Iafisco, M.; Ditaranto, N.; Migoni, D.; Gervaso, F.; Nadar, R.; Ivanchenko, P.; et al. Selenium-doped hydroxyapatite nanoparticles for potential application in bone tumor therapy. J. Inorg. Biochem. 2021, 215, 111334. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-Containing Nanoparticles Combine the Nk Cells Mediated Immunotherapy with Radiotherapy and Chemotherapy. Adv. Mater. 2020, 32, 1907568. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, X.; Chen, P.; Zhang, L.; Yang, C.S.; Zhang, J. Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free Radic. Biol. Med. 2018, 126, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Toubhans, B.; Gazze, S.A.; Bissardon, C.; Bohic, S.; Gourlan, A.T.; Gonzalez, D.; Charlet, L.; Conlan, R.S.; Francis, L.W. Selenium nanoparticles trigger alterations in ovarian cancer cell biomechanics. Nanomedicine Nanotechnol. Biol. Med. 2020, 29, 102258. [Google Scholar] [CrossRef] [PubMed]
- Sonkusre, P. Specificity of Biogenic Selenium Nanoparticles for Prostate Cancer Therapy with Reduced Risk of Toxicity: An In Vitro and In Vivo Study. Front. Oncol. 2020, 9, 1541. [Google Scholar] [CrossRef] [Green Version]





| S. No. | Compound | Redox Property | Cytotoxicity Mechanism | References |
|---|---|---|---|---|
| 1 | Selenate | Proapoptotic, genotoxic | Activates protein phosphatase 2A, which inhibits various signaling cascades such as phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Induces apoptosis, but at a relatively high concentration. | [95] |
| 2 | Selenite/Sodium selenite (SS) | Proapoptotic, prooxidative, genotoxic, inhibits cell proliferation | Activation of extracellular signal-regulated protein kinase (ERK) pathway. Inhibition of autophagy through PI3K/Akt pathway | [96,97] |
| 3 | Selenocysteine (SeCys) | Antioxidant | Induces apoptosis through the cell cycle arrest, and oxidative damage. Paraptotic-like effect mediated by ER stress. | [98,99] |
| 4 | Selenomethionine (SeMet) | Proapoptotic, proliferation inhibition | Pro-apoptotic effects in several cancer cell lines. Activation of p53-dependent proteins. Non-toxic and non-genotoxic. | [1,85] |
| 5 | Methylselenocysteine (MSC) | Proapoptotic, anti-angiogenic, proliferation inhibition | Anti-cancer effects in various cell lines, including promyelocytic leukemia. ER stress and mitochondrial dysfunction/signaling | [83,100] |
| 6 | Methylselenic acid (MSA) | Pro-apoptotic, anti-inflammatory, pro-oxidant, anti-angiogenic | Induces cytotoxicity through DNA damage. Regulation of PI3k/Akt, ERK1/2, and p38 pathways | [77,101] |
| 7 | Selenodiglutathione (SDG) | Antioxidant, pro-apoptotic | Induction of apoptosis through ROS and oxidative damage. | [102] |
| 8 | Methylselenol | Pro-apoptotic; inhibits cell growth | Inhibition of the ERK1/2 pathway activation and c-Myc expression. Induces cell cycle arrest. | [103] |
| 9 | Ebselen | Anti-inflammatory, antioxidant, protects against oxidative stress as well as DNA damage | As an antioxidant, Ebselen induces apoptosis through many pathways. Induces ROS generation and oxidative damage | [104] |
| 10 | Ethaselen | Proliferation suppression, synergistically effective with cisplatin against resistant leukemic cells | Induces ROS and apoptosis by TrxR inhibition | [105,106] |
| 11 | Dimethyl diselenide | Antioxidant | Induces NADPH quinone oxidoreductase | [107] |
| 12 | Zidovudine derivatives | Pro-apoptotic | Induced apoptosis through the mitochondrial pathway | [108] |
| 13 | Phenylindolyl Ketone Derivative | Pro-apoptotic | Induced apoptosis through cell cycle arrest and inhibition of tubulin polymerization. | [109] |
| 14 | Combretastatin 4-A analog | Inhibit tubulin polymerization | Inhibition of cell growth. | [110] |
| 15 | Diphenyl diselenide | Antioxidant, inhibitor of nociception | Protective against genotoxic substances. Induces apoptosis through oxidative damage. | [111,112] |
| 16 | Selol | Cytotoxic effects, inhibits proliferation, apoptotic | Induced apoptosis in resistant cancer cell lines including leukemia through oxidative damage. | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehudin, M.A.; Golla, U.; Trivedi, D.; Potlakayala, S.D.; Rudrabhatla, S.V.; Desai, D.; Dovat, S.; Claxton, D.; Sharma, A. Therapeutic Benefits of Selenium in Hematological Malignancies. Int. J. Mol. Sci. 2022, 23, 7972. https://doi.org/10.3390/ijms23147972
Ehudin MA, Golla U, Trivedi D, Potlakayala SD, Rudrabhatla SV, Desai D, Dovat S, Claxton D, Sharma A. Therapeutic Benefits of Selenium in Hematological Malignancies. International Journal of Molecular Sciences. 2022; 23(14):7972. https://doi.org/10.3390/ijms23147972
Chicago/Turabian StyleEhudin, Melanie A., Upendarrao Golla, Devnah Trivedi, Shobha D. Potlakayala, Sairam V. Rudrabhatla, Dhimant Desai, Sinisa Dovat, David Claxton, and Arati Sharma. 2022. "Therapeutic Benefits of Selenium in Hematological Malignancies" International Journal of Molecular Sciences 23, no. 14: 7972. https://doi.org/10.3390/ijms23147972