Prenatal Exposure to Delta-9-tetrahydrocannabinol (THC) Alters the Expression of miR-122-5p and Its Target Igf1r in the Adult Rat Ovary
Abstract
:1. Introduction
2. Results
2.1. Gestational Exposure to THC Alters miRNA Profile in Adult Rat Ovaries
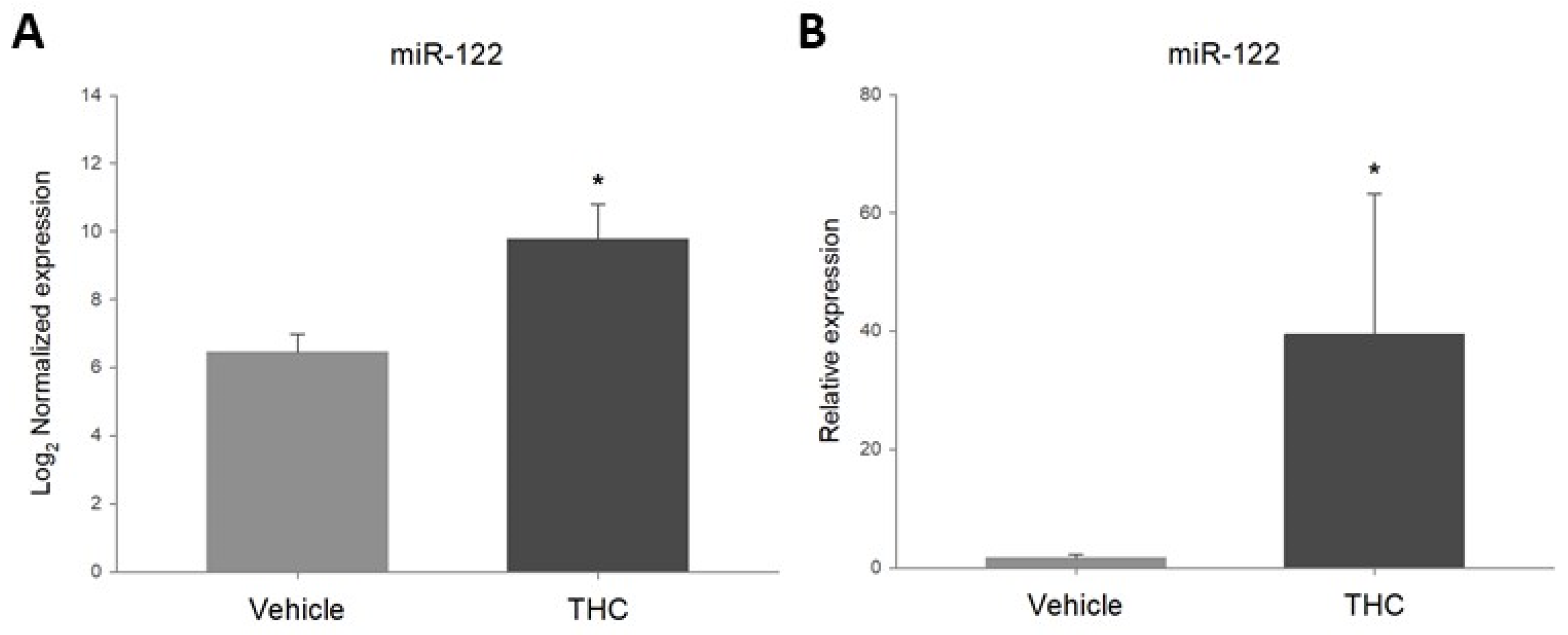
2.2. Prenatal THC-Exposure Increases Ovarian miR-122-5p Expression in Adult Offspring
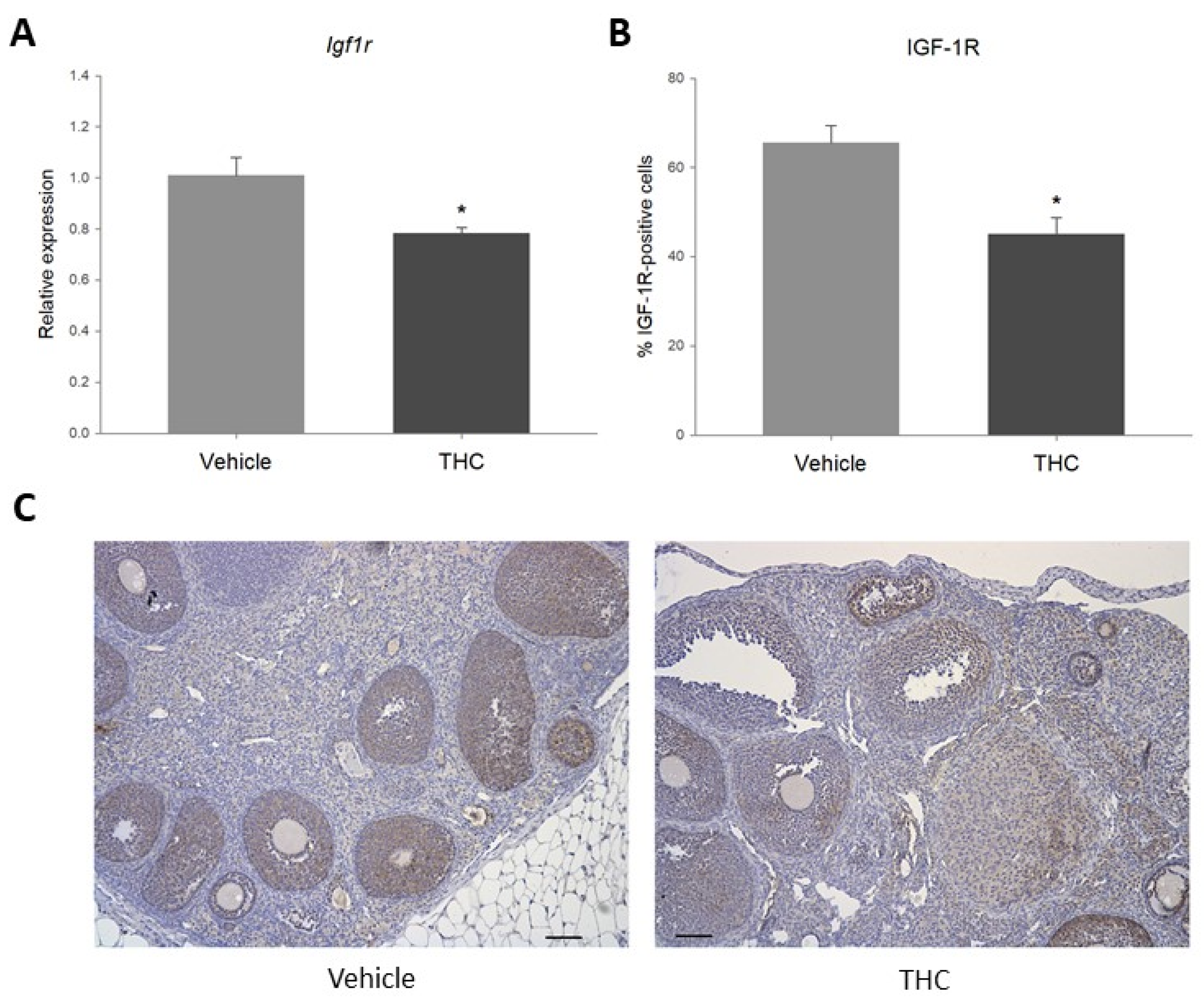
2.3. Gestational Exposure to THC Decreases Igf1r Expression in the Adult Ovary
2.4. Prenatal Exposure to THC Increases Follicular Apoptosis in the Adult Ovary
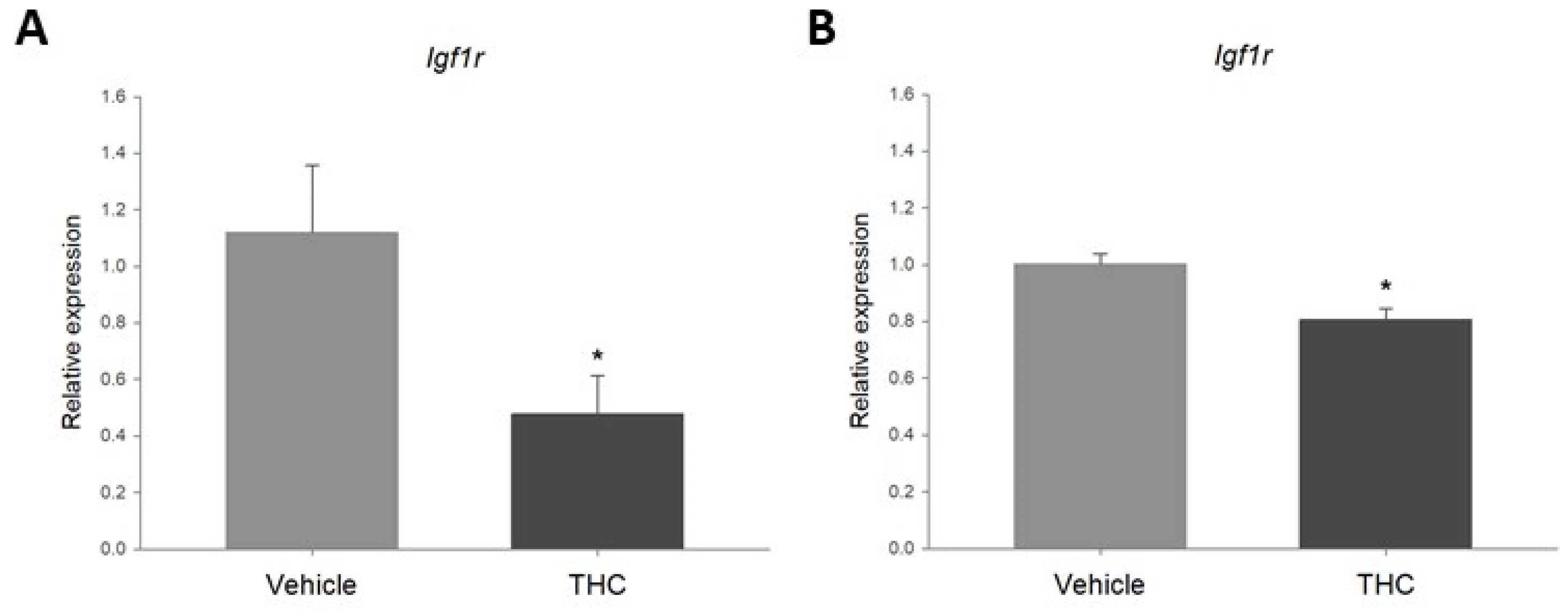
2.5. Acute Exposure to THC Decreases Expression of Igf1r in the Ovary
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Nanostring Analysis
4.3. miRNA Real-Time Quantitative PCR
4.4. Immunohistochemistry
4.5. Ovarian Explant Culture
4.6. Cell Culture
4.7. RNA Isolation and RT-qPCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Labor Unooda. World Drug Report 2021 (Set of 5 Booklets); S.l.: United Nations: 2022. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html (accessed on 16 July 2022).
- Corsi, D.J.; Hsu, H.; Weiss, D.; Fell, D.B.; Walker, M. Trends and correlates of cannabis use in pregnancy: A population-based study in Ontario, Canada from 2012 to 2017. Can. J. Public Health. 2018, 110, 76–84. [Google Scholar] [CrossRef]
- 2019 National Survey on Drug Use and Health: Women|CBHSQ Data n.d. Available online: https://www.samhsa.gov/data/report/2019-nsduh-women (accessed on 4 June 2022).
- Leemaqz, S.Y.; Dekker, G.A.; McCowan, L.M.; Kenny, L.C.; Myers, J.E.; Simpson, N.A.B.; Poston, L.; Roberts, C.T.; SCOPE Consortium. Maternal marijuana use has independent effects on risk for spontaneous preterm birth but not other common late pregnancy complications. Reprod. Toxicol. 2016, 62, 77–86. [Google Scholar] [CrossRef]
- Ko, J.Y.; Farr, S.L.; Tong, V.T.; Creanga, A.A.; Callaghan, W.M. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am. J. Obstet. Gynecol. 2015, 213, 201.e1–201.e10. [Google Scholar] [CrossRef]
- Moore, D.G.; Turner, J.D.; Parrott, A.C.; Goodwin, J.E.; Fulton, S.E.; Min, M.O.; Fox, H.C.; Braddick, F.M.B.; Axelsson, E.L.; Lynch, S.; et al. During pregnancy, recreational drug-using women stop taking ecstasy (3,4-methylenedioxy-N-methylamphetamine) and reduce alcohol consumption, but continue to smoke tobacco and cannabis: Initial findings from the Development and Infancy Study. J. Psychopharmacol. 2010, 24, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- PregnancyInfo. Available online: https://www.pregnancyinfo.ca/learn-more/ (accessed on 16 July 2022).
- Volkow, N.D.; Han, B.; Compton, W.M.; McCance-Katz, E.F. Self-reported Medical and Nonmedical Cannabis Use among Pregnant Women in the United States. JAMA 2019, 322, 167–169. [Google Scholar] [CrossRef]
- Westfall, R.E.; Janssen, P.A.; Lucas, P.; Capler, R. Survey of medicinal cannabis use among childbearing women: Patterns of its use in pregnancy and retroactive self-assessment of its efficacy against ‘morning sickness’. Complement. Ther. Clin. Pract. 2006, 12, 27–33. [Google Scholar] [CrossRef]
- Roberson, E.K.; Patrick, W.K.; Hurwitz, E.L. Marijuana Use and Maternal Experiences of Severe Nausea During Pregnancy in Hawai’i. Hawaii J. Med. Public Health 2014, 73, 283–287. [Google Scholar]
- Bayrampour, H.; Asim, A. Cannabis Use During the Pre-Conception Period and Pregnancy After Legalization. J. Obstet. Gynaecol. Can. 2021, 43, 740–745. [Google Scholar] [CrossRef]
- Singh, S.; Filion, K.B.; Abenhaim, H.A.; Eisenberg, M.J. Prevalence and outcomes of prenatal recreational cannabis use in high-income countries: A scoping review. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 8–16. [Google Scholar] [CrossRef]
- Lozano, J.; García-Algar, O.; Marchei, E.; Vall, O.; Monleon, T.; Giovannandrea, R.D.; Pichini, S. Prevalence of gestational exposure to cannabis in a Mediterranean city by meconium analysis. Acta Paediatr. 2007, 96, 1734–1737. [Google Scholar] [CrossRef]
- Metz, T.D.; Silver, R.M.; McMillin, G.A.; Allshouse, A.A.; Jensen, T.L.; Mansfield, C.; Heard, K.; Kinney, G.L.; Wymore, E.; Binswanger, I.A. Prenatal Marijuana Use by Self-Report and Umbilical Cord Sampling in a State with Marijuana Legalization. Obstet. Gynecol. 2019, 133, 98–104. [Google Scholar] [CrossRef]
- Luke, S.; Hutcheon, J.; Kendall, T. Cannabis Use in Pregnancy in British Columbia and Selected Birth Outcomes. J. Obstet. Gynaecol. Can. 2019, 41, 1311–1317. [Google Scholar] [CrossRef]
- Varner, M.W.; Silver, R.M.; Rowland Hogue, C.J.; Willinger, M.; Parker, C.B.; Thorsten, V.R.; Goldenberg, R.L.; Saade, G.R.; Dudley, D.J.; Coustan, D.; et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet. Gynecol. 2014, 123, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhu, B.; Liang, D. The associations between prenatal cannabis use disorder and neonatal outcomes. Addict. Abingdon Engl. 2021, 116, 3069–3079. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.R.; Flenady, V.J.; Gibbons, K.S.; Kingsbury, A.M.; Hurrion, E.; Mamun, A.A.; Najmna, J.M. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr. Res. 2012, 71, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Hurd, Y.L.; Wang, X.; Anderson, V.; Beck, O.; Minkoff, H.; Dow-Edwards, D. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol. 2005, 27, 221–229. [Google Scholar] [CrossRef]
- Warshak, C.R.; Regan, J.; Moore, B.; Magner, K.; Kritzer, S.; Van Hook, J. Association between marijuana use and adverse obstetrical and neonatal outcomes. J. Perinatol. 2015, 35, 991–995. [Google Scholar] [CrossRef]
- Campbell, E.E.; Gilliland, J.; Dworatzek, P.D.N.; Vrijer, B.D.; Penava, D.; Seabrook, J.A. Socioeconomic Status and Adverse Birth Outcomes: A Population-Based Canadian Sample. J. Biosoc. Sci. 2018, 50, 102–113. [Google Scholar] [CrossRef] [Green Version]
- El Marroun, H.; Tiemeier, H.; Steegers, E.A.P.; Jaddoe, V.W.V.; Hofman, A.; Verhulst, F.C.; van den Brink, W.; Huizink, A.C. Intrauterine Cannabis Exposure Affects Fetal Growth Trajectories: The Generation R Study. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 1173–1181. [Google Scholar] [CrossRef] [Green Version]
- Gabrhelík, R.; Mahic, M.; Lund, I.O.; Bramness, J.; Selmer, R.; Skovlund, E.; Handal, M.; Skurtveit, S. Cannabis Use during Pregnancy and Risk of Adverse Birth Outcomes: A Longitudinal Cohort Study. Eur. Addict. Res. 2021, 27, 131–141. [Google Scholar] [CrossRef]
- Smith, A.; Kaufman, F.; Sandy, M.S.; Cardenas, A. Cannabis Exposure during Critical Windows of Development: Epigenetic and Molecular Pathways Implicated in Neuropsychiatric Disease. Curr. Environ. Health Rep. 2020, 7, 325–342. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. A Total Synthesis of Dl-Delta-1-Tetrahydrocannabinol, the Active Constituent of Hashish. J. Am. Chem. Soc. 1965, 87, 3273–3275. [Google Scholar] [CrossRef]
- Carlier, J.; Huestis, M.A.; Zaami, S.; Pichini, S.; Busardò, F.P. Monitoring Perinatal Exposure to Cannabis and Synthetic Cannabinoids. Ther. Drug Monit. 2020, 42, 194–204. [Google Scholar] [CrossRef]
- Banu, S.K.; Stanley, J.A.; Sivakumar, K.K.; Arosh, J.A.; Barhoumi, R.; Burghardt, R.C. Identifying a novel role for X-prolyl aminopeptidase (Xpnpep) 2 in CrVI-induced adverse effects on germ cell nest breakdown and follicle development in rats. Biol. Reprod. 2015, 92, 67. [Google Scholar] [CrossRef]
- Pepe, G.J.; Lynch, T.J.; Albrecht, E.D. Regulation of baboon fetal ovarian development by placental estrogen: Onset of puberty is delayed in offspring deprived of estrogen in utero. Biol. Reprod. 2013, 89, 132. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.P.; Eriksson, J.G.; Forsén, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; Wilhelm, D.; Rodgers, R.J. Development of mammalian ovary. J. Endocrinol. 2014, 221, R145–R161. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.F.; Sauder, K.A.; Shapiro, A.L.B.; Crume, T.; Kinney, G.L.; Dabelea, D. Fetal Exposure to Cannabis and Childhood Metabolic Outcomes: The Healthy Start Study. J. Clin. Endocrinol. Metab. 2022, 107, e2862–e2869. [Google Scholar] [CrossRef]
- Campolongo, P.; Trezza, V.; Ratano, P.; Palmery, M.; Cuomo, V. Developmental consequences of perinatal cannabis exposure: Behavioral and neuroendocrine effects in adult rodents. Psychopharmacology 2011, 214, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Fried, P.A.; Hogan, M.J.; Cameron, I. Effects of prenatal marijuana on visuospatial working memory: An fMRI study in young adults. Neurotoxicol. Teratol. 2006, 28, 286–295. [Google Scholar] [CrossRef]
- Gillies, R.; Lee, K.; Vanin, S.; Laviolette, S.R.; Holloway, A.C.; Arany, E.; Hardy, D.B. Maternal exposure to Δ9-tetrahydrocannabinol impairs female offspring glucose homeostasis and endocrine pancreatic development in the rat. Reprod. Toxicol. 2020, 94, 84–91. [Google Scholar] [CrossRef]
- De Domenico, E.; Todaro, F.; Rossi, G.; Dolci, S.; Geremia, R.; Rossi, P.; Grimaldi, P. Overactive type 2 cannabinoid receptor induces meiosis in fetal gonads and impairs ovarian reserve. Cell Death Dis. 2017, 8, e3085. [Google Scholar] [CrossRef]
- Castel, P.; Barbier, M.; Poumerol, E.; Mandon-Pépin, B.; Tassistro, V.; Lepidi, H.; Pelissier-Alicot, A.L.; Manzoni, O.J.; Courbiere, B. Prenatal cannabinoid exposure alters the ovarian reserve in adult offspring of rats. Arch Toxicol. 2020, 94, 4131–4141. [Google Scholar] [CrossRef]
- Oke, S.L.; Lee, K.; Papp, R.; Laviolette, S.R.; Hardy, D.B. In Utero Exposure to Δ9-Tetrahydrocannabinol Leads to Postnatal Catch-Up Growth and Dysmetabolism in the Adult Rat Liver. Int. J. Mol. Sci. 2021, 22, 7502. [Google Scholar] [CrossRef]
- Lee, K.; Hardy, D.B. Metabolic Consequences of Gestational Cannabinoid Exposure. Int. J. Mol. Sci. 2021, 22, 9528. [Google Scholar] [CrossRef]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 2011, 70, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.K.; Itchon-Ramos, N.; Visco, Z.; Huang, Z.; Grenier, C.; Schrott, R.; Acharya, K.; Boudreau, M.H.; Price, T.M.; Raburn, D.J.; et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 2018, 13, 1208–1221. [Google Scholar] [CrossRef] [Green Version]
- Prini, P.; Penna, F.; Sciuccati, E.; Alberio, T.; Rubino, T. Chronic Δ8-THC Exposure Differently Affects Histone Modifications in the Adolescent and Adult Rat Brain. Int. J. Mol. Sci. 2017, 18, 2094. [Google Scholar] [CrossRef]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Todd, S.M.; Zhou, C.; Clarke, D.J.; Chohan, T.W.; Bahceci, D.; Arnold, J.C. Interactions between cannabidiol and Δ9-THC following acute and repeated dosing: Rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. Eur. Neuropsychopharmacol. 2017, 27, 132–145. [Google Scholar] [CrossRef]
- Yang, X.; Hegde, V.L.; Rao, R.; Zhang, J.; Nagarkatti, P.S.; Nagarkatti, M. Histone modifications are associated with Δ9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J. Biol. Chem. 2014, 289, 18707–18718. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.T.; Szutorisz, H.; Garg, P.; Martin, Q.; Landry, J.A.; Sharp, A.J.; Hurd, Y.L. Genome-Wide DNA Methylation Profiling Reveals Epigenetic Changes in the Rat Nucleus Accumbens Associated with Cross-Generational Effects of Adolescent THC Exposure. Neuropsychopharmacology. 2015, 40, 2993–3005. [Google Scholar] [CrossRef] [Green Version]
- Sido, J.M.; Yang, X.; Nagarkatti, P.S.; Nagarkatti, M. Δ9-Tetrahydrocannabinol-mediated epigenetic modifications elicit myeloid-derived suppressor cell activation via STAT3/S100A8. J. Leukoc. Biol. 2015, 97, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Juknat, A.; Gao, F.; Coppola, G.; Vogel, Z.; Kozela, E. miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia-Effect of cannabinoids. PLoS ONE 2019, 14, e0212039. [Google Scholar] [CrossRef]
- Kim, V.N. Small RNAs: Classification, biogenesis, and function. Mol. Cells 2005, 19, 1–15. [Google Scholar]
- Kim, J.; You, S. Comprehensive analysis of miRNA-mRNA interactions in ovaries of aged mice. Anim. Sci. J. 2022, 93, e13721. [Google Scholar] [CrossRef]
- Tufarelli, C.; Stanley, J.A.S.; Garrick, D.; Sharpe, J.A.; Ayyub, H.; Wood, W.G.; Higgs, D.R. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat. Genet. 2003, 34, 157–165. [Google Scholar] [CrossRef]
- Brennecke, J.; Stark, A.; Russell, R.B.; Cohen, S.M. Principles of microRNA-target recognition. PLoS Biol. 2005, 3, e85. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fang, Y.; Liu, Y.; Yang, X. MicroRNAs in ovarian function and disorders. J. Ovarian Res. 2015, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Gebremedhn, S.; Salilew-Wondim, D.; Ahmad, I.; Sahadevan, S.; Hossain, M.M.; Hoelker, M.; Rings, F.; Neuhoff, C.; Tholen, E.; Looft, C.; et al. MicroRNA Expression Profile in Bovine Granulosa Cells of Preovulatory Dominant and Subordinate Follicles during the Late Follicular Phase of the Estrous Cycle. PLoS ONE 2015, 10, e0125912. [Google Scholar] [CrossRef]
- Martínez-Peña, A.A.; Lee, K.; Petrik, J.J.; Hardy, D.B.; Holloway, A.C. Gestational exposure to Δ9-THC impacts ovarian follicular dynamics and angiogenesis in adulthood in Wistar rats. J. Dev. Orig. Health Dis. 2021, 12, 865–869. [Google Scholar] [CrossRef]
- Baumgarten, S.C.; Armouti, M.; Ko, C.; Stocco, C. IGF1R Expression in Ovarian Granulosa Cells Is Essential for Steroidogenesis, Follicle Survival, and Fertility in Female Mice. Endocrinology 2017, 158, 2309–2318. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Taverne, M.; van der Weijden, G.; Bevers, M.; Hurk, R.V.D. Immunohistochemical localisation of growth hormone (GH), GH receptor (GHR), insulin-like growth factor I (IGF-I) and type I IGF-I receptor, and gene expression of GH and GHR in rat pre-antral follicles. Zygote. 2002, 10, 85–94. [Google Scholar] [CrossRef]
- Mohammed, A.; Alghetaa, H.; Sultan, M.; Singh, N.P.; Nagarkatti, P.; Nagarkatti, M. Administration of Δ9-Tetrahydrocannabinol (THC) Post-Staphylococcal Enterotoxin B Exposure Protects Mice from Acute Respiratory Distress Syndrome and Toxicity. Front. Pharmacol. 2020, 11, 893. [Google Scholar] [CrossRef]
- Chandra, L.C.; Kumar, V.; Torben, W.; Vande Stouwe, C.; Winsauer, P.; Amedee, A.; Molina, P.E.; Mohan, M. Chronic administration of Δ9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques. J. Virol. 2015, 89, 1168–1181. [Google Scholar] [CrossRef] [Green Version]
- Hegde, V.L.; Tomar, S.; Jackson, A.; Rao, R.; Yang, X.; Singh, U.P.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Δ9-tetrahydrocannabinol in vivo: Regulation of CCAAT/enhancer-binding protein α by microRNA-690. J. Biol. Chem. 2013, 288, 36810–36826. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Cui, Z.; Li, J.; Zhang, D.; Li, Z.; Lin, Z.; Yin, H.; Ran, J.; Wang, Y.; Liu, Y. miR-122-5p regulates proliferation and apoptosis of chicken granulosa cells of hierarchal follicles by targeting MAPK3. Gene 2022, 824, 146397. [Google Scholar] [CrossRef]
- Pei, Z.J.; Zhang, Z.G.; Hu, A.X.; Yang, F.; Gai, Y. miR-122-5p inhibits tumor cell proliferation and induces apoptosis by targeting MYC in gastric cancer cells. Die Pharm.-Int. J. Pharm. Sci. 2017, 72, 344–347. [Google Scholar] [CrossRef]
- Ma, J.; Li, T.; Han, X.; Yuan, H. Knockdown of LncRNA ANRIL suppresses cell proliferation, metastasis, and invasion via regulating miR-122-5p expression in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 205–214. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Hao, J.; Huang, X.; Liu, M.; Lv, M.; Su, C.; Mu, Y.L. miRNA-122-5p in POI ovarian-derived exosomes promotes granulosa cell apoptosis by regulating BCL9. Cancer Med. 2022, 11, 2414–2426. [Google Scholar] [CrossRef]
- Menon, B.; Gulappa, T.; Menon, K.M.J. Molecular regulation of LHCGR expression by miR-122 during follicle growth in the rat ovary. Mol. Cell Endocrinol. 2017, 442, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Menon, K.M.J.; Menon, B.; Gulappa, T. Regulation of Luteinizing Hormone Receptor mRNA Expression in the Ovary: The Role of miR-122. Vitam. Horm. 2018, 107, 67–87. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, X.; Qu, Q.; Chen, J.; Fan, Z.; Zhu, D.; Miao, Y.; Hu, Z. Overexpression of miR-122 promotes apoptosis of dermal papilla cells by directly targeting IGF1R in androgenetic alopecia. Cell Biol. Int. 2022, 46, 185–191. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, J.; Ma, L.; Shan, J.; Shen, J.; Yang, Z.; Liu, L.; Luo, Y.; Yao, C.; Qian, C. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016, 371, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, H.; Yang, Z. MiR-122 Inhibits Cell Proliferation and Tumorigenesis of Breast Cancer by Targeting IGF1R. PLoS ONE 2012, 7, e47053. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Baumgarten, S.C.; Wu, Y.; Bennett, J.; Winston, N.; Hirshfeld-Cytron, J.; Stocco, C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol. Endocrinol. 2013, 27, 511–523. [Google Scholar] [CrossRef]
- Singh, R.; Chaudhary, P.; Arya, R. Role of IGF-1R in ameliorating apoptosis of GNE deficient cells. Sci. Rep. 2018, 8, 7323. [Google Scholar] [CrossRef] [Green Version]
- Mueller, B.A.; Daling, J.R.; Weiss, N.S.; Moore, D.E. Recreational drug use and the risk of primary infertility. Epidemiology. 1990, 1, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Klonoff-Cohen, H.S.; Natarajan, L.; Chen, R.V. A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. Am. J. Obstet. Gynecol. 2006, 194, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Fan, L.; Yu, Q.; Luo, S.; Wu, X.; Tang, J.; Kang, G.; Tang, L. Abnormality of Klotho Signaling Is Involved in Polycystic Ovary Syndrome. Reprod. Sci. 2018, 25, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Goto, Y.; Matsuda-Minehata, F.; Inoue, N.; Maeda, A.; Sakamaki, K.; Miyano, T. Regulation mechanism of selective atresia in porcine follicles: Regulation of granulosa cell apoptosis during atresia. J. Reprod. Dev. 2004, 50, 493–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uniyal, S.; Panda, R.P.; Chouhan, V.S.; Yadav, V.P.; Hyder, I.; Dangi, S.S.; Gupta, M.; Khan, F.A.; Sharma, G.T.; Bag, S.; et al. Expression and localization of insulin-like growth factor system in corpus luteum during different stages of estrous cycle in water buffaloes (Bubalus bubalis) and the effect of insulin-like growth factor I on production of vascular endothelial growth factor and progesterone in luteal cells cultured in vitro. Theriogenology 2015, 83, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, J.; Lawler, J.; Moorehead, R.; Bornstein, P.; Lamarre, J.; Petrik, J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J. Cell Physiol. 2007, 210, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Greenaway, J.; Connor, K.; Pedersen, H.G.; Coomber, B.L.; LaMarre, J.; Petrik, J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology 2004, 145, 2896–2905. [Google Scholar] [CrossRef] [Green Version]
- Sargent, K.M.; Lu, N.; Clopton, D.T.; Pohlmeier, W.E.; Brauer, V.M.; Ferrara, N.; Silversides, D.W.; Cupp, A.S. Loss of Vascular Endothelial Growth Factor A (VEGFA) Isoforms in Granulosa Cells Using pDmrt-1-Cre or Amhr2-Cre Reduces Fertility by Arresting Follicular Development and by Reducing Litter Size in Female Mice. PLoS ONE 2015, 10, e0116332. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.; Karanges, E.; Spiro, A.; Wong, A.; Spencer, J.; Huynh, T.; Gunasekaran, N.; Karl, T.; Long, L.E.; Huang, X.F.; et al. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 2011, 218, 443–457. [Google Scholar] [CrossRef]
- Schwope, D.M.; Karschner, E.L.; Gorelick, D.A.; Huestis, M.A. Identification of recent cannabis use: Whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin. Chem. 2011, 57, 1406–1414. [Google Scholar] [CrossRef] [Green Version]
- Falcon, M.; Pichini, S.; Joya, J.; Pujadas, M.; Sanchez, A.; Vall, O.; García Algar, O.; Luna, A.; de la Torre, R.; Rotolo, M.C.; et al. Maternal hair testing for the assessment of fetal exposure to drug of abuse during early pregnancy: Comparison with testing in placental and fetal remains. Forensic Sci. Int. 2012, 218, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Natale, B.V.; Gustin, K.N.; Lee, K.; Holloway, A.C.; Laviolette, S.R.; Natale, D.R.C.; Hardy, D.B. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 2020, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fereshteh, Z.; Schmidt, S.A.; Al-Dossary, A.A.; Accerbi, M.; Arighi, C.; Cowart, J.; Song, J.L.; Green, P.J.; Choi, K.; Yoo, S.; et al. Murine Oviductosomes (OVS) microRNA profiling during the estrous cycle: Delivery of OVS-borne microRNAs to sperm where miR-34c-5p localizes at the centrosome. Sci. Rep. 2018, 8, 16094. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, D.; Gebremedhn, S.; Salilew-Wondim, D.; Hailay, T.; Hoelker, M.; Grosse-Brinkhaus, C.; Schellander, K. MicroRNAs: Tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction 2018, 155, R121–R135. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Barnett, G.; Chiang, C.W.; Perez-Reyes, M.; Owens, S.M. Kinetic study of smoking marijuana. J. Pharmacokinet. Biopharm. 1982, 10, 495–506. [Google Scholar] [CrossRef]
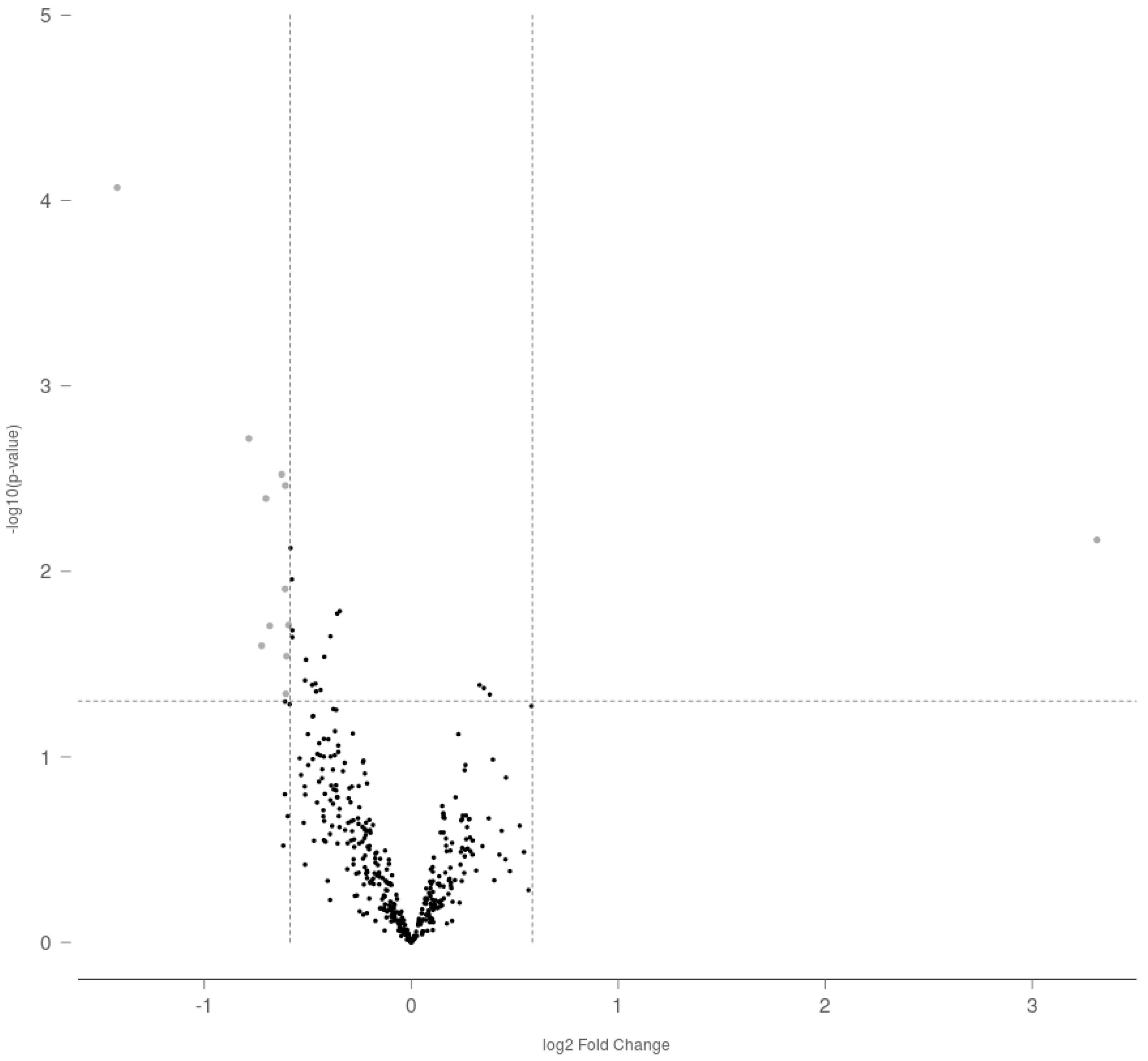

| Accession Number | Target | Effect | Fold Change | p Value |
|---|---|---|---|---|
| MIMAT0000827 | rno-miR-122-5p | Upregulated | 9.93741 | 0.00676 |
| MIMAT0000856 | rno-miR-154-5p | Downregulated | −2.67658 | 8.50 × 10−5 |
| MIMAT0000885 | rno-miR-214-3p | Downregulated | −1.72175 | 0.00192 |
| MIMAT0017813 | rno-miR-3552 | Downregulated | −1.65014 | 0.02515 |
| MIMAT0000787 | rno-miR-18a-5p | Downregulated | −1.62661 | 0.00404 |
| MIMAT0004742 | rno-miR-296-3p | Downregulated | −1.60593 | 0.01964 |
| MIMAT0005284 | rno-miR-874-3p | Downregulated | −1.54335 | 0.00299 |
| MIMAT0000882 | rno-miR-211-5p | Downregulated | −1.52523 | 0.01243 |
| MIMAT0003212 | rno-miR-20b-3p | Downregulated | −1.52416 | 0.00344 |
| MIMAT0005325 | rno-miR-598-3p | Downregulated | −1.52143 | 0.04569 |
| MIMAT0004641 | rno-miR-330-5p | Downregulated | −1.51789 | 0.02862 |
| MIMAT0005302 | rno-miR-190b-5p | Downregulated | −1.50745 | 0.01945 |
| Accession Number | RNA | Sequence (5′-) |
|---|---|---|
| MIMAT0000421 | hsa-miR-122-5p | UGGAGUGUGACAAUGGUGUUUG |
| NR_002852 | RNU5G snRNA | AUACUCUGGUUUCUCUUCAGAUCGCAUAAAUCUUUCGCCUUUUACUAAAGAUUUCCGUGGAGAGGAACAACUCUGAGUCUUAACCCAAUUUUUUGAGCCUUGCUCCGACAAGGCUA |
| MIMAT0000440 | hsa-miR-191-5p | CAACGGAAUCCCAAAAGCAGCUG |
| Accession Number | Gene Name | Symbol | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|---|
| NM_052807.2 | Insulin-like growth factor 1 receptor | Igf1r | GGAATGGGTCGTGGACAGAT | ACAATCAGCAGGATGGCAAC |
| NM_012922.2 | Caspase 3 | Casp3 | GAGCTTGGAACGCGAAGAAAA | AGAGTCCATCGACTTGCTTCC |
| NM_012512.2 | Beta-2-microglobulin | B2m | AATTCACACCCACCGAGACC | GCTCCTTCAGAGTGACGTGT |
| NM_012583.2 | Hypoxanthine phosphoribosyltransferase 1 | Hprt1 | GCAGTACAGCCCCAAAATGG | GGTCCTTTTCACCAGCAAGCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Peña, A.A.; Lee, K.; Pereira, M.; Ayyash, A.; Petrik, J.J.; Hardy, D.B.; Holloway, A.C. Prenatal Exposure to Delta-9-tetrahydrocannabinol (THC) Alters the Expression of miR-122-5p and Its Target Igf1r in the Adult Rat Ovary. Int. J. Mol. Sci. 2022, 23, 8000. https://doi.org/10.3390/ijms23148000
Martínez-Peña AA, Lee K, Pereira M, Ayyash A, Petrik JJ, Hardy DB, Holloway AC. Prenatal Exposure to Delta-9-tetrahydrocannabinol (THC) Alters the Expression of miR-122-5p and Its Target Igf1r in the Adult Rat Ovary. International Journal of Molecular Sciences. 2022; 23(14):8000. https://doi.org/10.3390/ijms23148000
Chicago/Turabian StyleMartínez-Peña, Annia A., Kendrick Lee, Madison Pereira, Ahmed Ayyash, James J. Petrik, Daniel B. Hardy, and Alison C. Holloway. 2022. "Prenatal Exposure to Delta-9-tetrahydrocannabinol (THC) Alters the Expression of miR-122-5p and Its Target Igf1r in the Adult Rat Ovary" International Journal of Molecular Sciences 23, no. 14: 8000. https://doi.org/10.3390/ijms23148000
APA StyleMartínez-Peña, A. A., Lee, K., Pereira, M., Ayyash, A., Petrik, J. J., Hardy, D. B., & Holloway, A. C. (2022). Prenatal Exposure to Delta-9-tetrahydrocannabinol (THC) Alters the Expression of miR-122-5p and Its Target Igf1r in the Adult Rat Ovary. International Journal of Molecular Sciences, 23(14), 8000. https://doi.org/10.3390/ijms23148000








