Reprogramming Metabolism of Macrophages as a Target for Kidney Dysfunction Treatment in Autoimmune Diseases
Abstract
1. Introduction
2. The Key Point of Macrophage Multifunction: Heterogeneity and Plasticity
2.1. Macrophage Plasticity
2.2. Macrophage Diversity
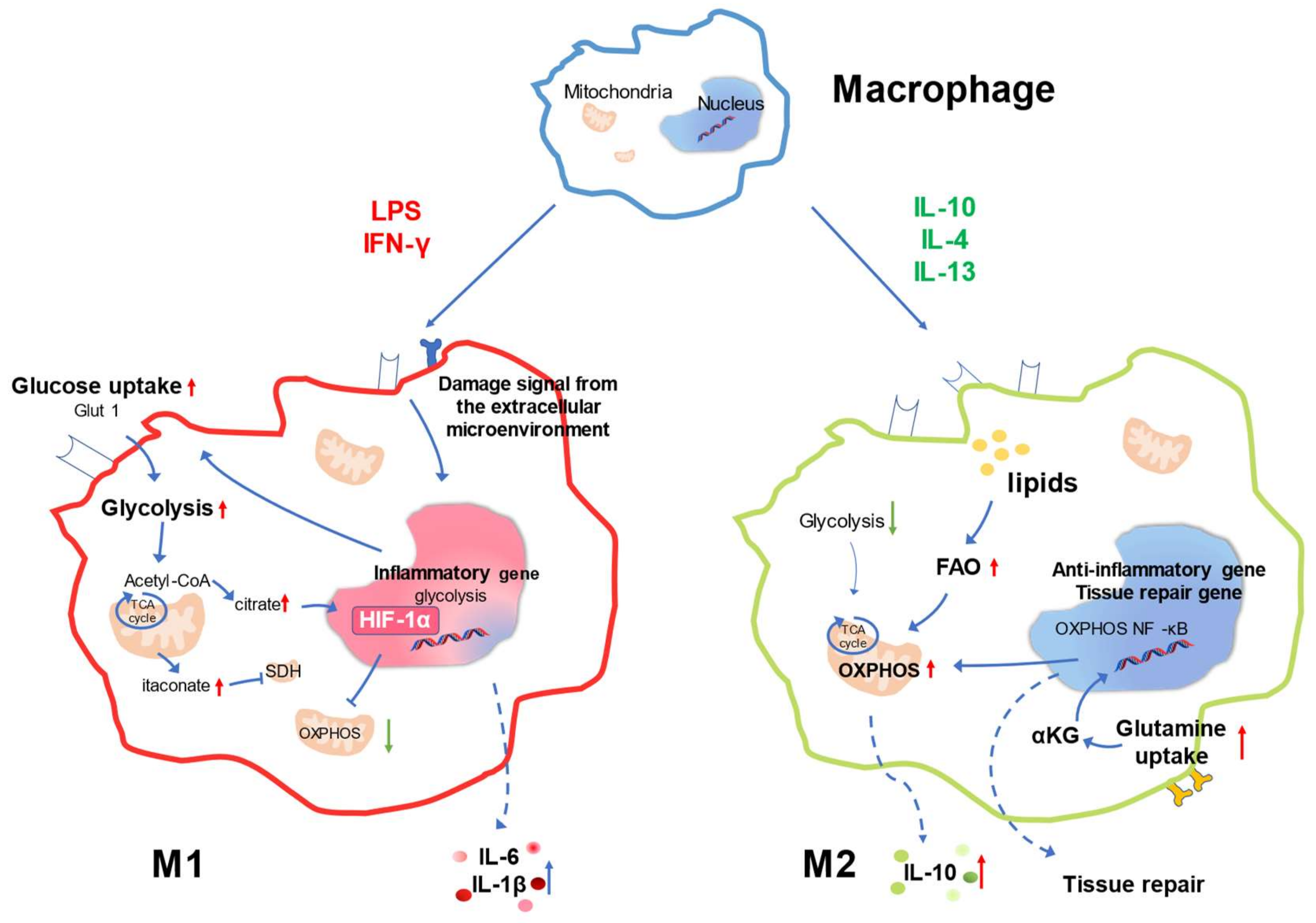
3. Metabolic Signature of Macrophage Responses
4. Changes in Macrophage Metabolic Pathways in the Injured Kidney of Autoimmune Disease
4.1. Regulation of Glucose Metabolism
4.2. Regulation of Glutamine Metabolism
4.3. Regulation of Lipid Metabolism
4.4. Changes in Macrophage Mitochondria in the Injured Kidney of Autoimmune Disease
4.5. Other Factors Contributing to Altered Macrophage Metabolism in the Injured Kidney of Autoimmune Disease
5. Macrophages in Autoimmune Disease Kidney Damage and Repair
5.1. Lupus Nephritis and Macrophages
5.2. Diabetic Nephropathy and Macrophages
5.3. Systemic Sclerosis and Macrophages
5.4. Renal Injury Induced by Other Autoimmune Diseases and Macrophages
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Tummalapalli, S.L.; Boulware, L.E.; Grams, M.E.; Ix, J.H.; Jha, V.; Kengne, A.; Madero, M.; Mihaylova, B.; Tangri, N.; et al. The case for early identification and intervention of chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021, 99, 34–47. [Google Scholar] [CrossRef]
- Jha, V.P.; Garcia-Garcia, G.P.; Iseki, K.P.; Li, Z.M.; Naicker, S.P.; Plattner, B.M.; Saran, R.P.; Wang, A.Y.P.; Yang, C.P. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Drawz, P.; Rahman, M. Chronic kidney disease. Ann. Intern. Med. 2015, 162, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Kangevari, M.; Abd-Allah, F.; Adekanmbi, V.; Adetokunboh, O.O.; Al-Mekhlafi, H.M.; Ancuceanu, R.; Appiah, S.C.Y.; Atnafu, D.D.; Ausloos, M.; Ayano, G.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Anders, H.; Saxena, R.; Zhao, M.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Huen, S.C.; Cantley, L.G. Macrophages in renal injury and repair. Annu. Rev. Physiol. 2017, 79, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Aspects Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Tang, T.; Lv, L.; Wang, B.; Cao, J.; Feng, Y.; Li, Z.; Wu, M.; Wang, F.; Wen, Y.; Zhou, L.; et al. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: An efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics 2019, 9, 4740–4755. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Xu, J.; Xie, J.; Harris, D.C.H.; Zheng, G. The role of macrophages in kidney fibrosis. Front. Physiol. 2021, 12, 705838. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Tang, P.M.; Nikolic-Paterson, D.J.; Lan, H. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Tauber, A.I. Immunity: How Elie Metchnikoff changed the course of modern medicine by Luba Vikhanski (review). B. Hist. Med. 2017, 91, 140–142. [Google Scholar] [CrossRef]
- Ezepchuk, Y.V.; Kolybo, D.V. Nobel laureate Ilya I. Metchnikoff (1845–1916). Life story and scientific heritage. Ukr. Biochem. J. 2016, 88, 98–109. [Google Scholar] [CrossRef][Green Version]
- Quaglia, M.; Dellepiane, S.; Guglielmetti, G.; Merlotti, G.; Castellano, G.; Cantaluppi, V. Extracellular vesicles as mediators of cellular crosstalk between immune system and kidney graft. Front. Immunol. 2020, 11, 74. [Google Scholar] [CrossRef]
- Wen, Y.; Yan, H.; Wang, B.; Liu, B. Macrophage heterogeneity in kidney injury and fibrosis. Front. Immunol. 2021, 12, 681748. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ren, J.; Gui, Y.; Wei, W.; Shu, B.; Lu, Q.; Xue, X.; Sun, X.; He, W.; Yang, J.; et al. Wnt/β -catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J. Am. Soc. Nephrol. 2018, 29, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Feng, Y.; Wu, M.; Wang, B.; Li, Z.; Zhong, X.; Wu, W.; Chen, J.; Ni, H.; Tang, T.; et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020, 27, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dai, S.; Feng, D.; Qin, Z.; Peng, X.; Sakamuri, S.S.V.P.; Ren, M.; Huang, L.; Cheng, M.; Mohammad, K.E.; et al. Distinct fate, dynamics and niches of renal macrophages of bone marrow or embryonic origins. Nat. Commun. 2020, 11, 2280. [Google Scholar] [CrossRef]
- Chen, T.; Cao, Q.; Wang, Y.; Harris, D.C.H. M2 macrophages in kidney disease: Biology, therapies, and perspectives. Kidney Int. 2019, 95, 760–773. [Google Scholar] [CrossRef]
- Shapouri Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Muñoz, J.; Akhavan, N.S.; Mullins, A.P.; Arjmandi, B.H. Macrophage polarization and osteoporosis: A review. Nutrients 2020, 12, 2999. [Google Scholar] [CrossRef]
- Gentek, R.; Molawi, K.; Sieweke, M.H. Tissue macrophage identity and self-renewal. Immunol. Rev. 2014, 262, 56–73. [Google Scholar] [CrossRef]
- Emile, J.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdel-Wahab, O.; Allen, C.E.; et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 127, 2672–2681. [Google Scholar] [CrossRef]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of resident tissue macrophage identity and function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Chen, Q.; Jiao, F.; Shi, C.; Pei, M.; Lv, J.; Zhang, H.; Wang, L.; Gong, Z. TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Proliferat. 2020, 53, e12829. [Google Scholar] [CrossRef]
- Wen, Y.; Lu, X.; Ren, J.; Privratsky, J.R.; Yang, B.; Rudemiller, N.P.; Zhang, J.; Griffiths, R.; Jain, M.K.; Nedospasov, S.A.; et al. KLF4 in macrophages attenuates TNF-α-mediated kidney injury and fibrosis. J. Am. Soc. Nephrol. 2019, 30, 1925–1938. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-kB and MAPK pathways. BMC Immunol. 2020, 21, 1–32. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, P.; Song, X.; Zhang, H.; Ma, S.; Wang, J.; Li, W.; Lv, R.; Liu, X.; Ma, S.; et al. Salmonella Typhimurium reprograms macrophage metabolism via T3SS effector SopE2 to promote intracellular replication and virulence. Nat. Commun. 2021, 12, 879. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, C.; Nandakumar, K.S.; Ronald, G.; Gladue, R. Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediat. Inflamm. 2020, 2020, 3830212–3830220. [Google Scholar] [CrossRef]
- Díaz-Bulnes, P.; Saiz, M.L.; López-Larrea, C.; Rodríguez, R.M. Crosstalk between hypoxia and ER stress response: A key regulator of macrophage polarization. Front. Immunol. 2019, 10, 2951. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Jeon, R.; Vuckovic, I.; Jiang, X.; Lerman, A.; Folmes, C.D.; Dzeja, P.D.; Herrmann, J. Interferon gamma induces reversible metabolic reprogramming of M1 macrophages to sustain cell viability and pro-inflammatory activity. EBioMedicine 2018, 30, 303–316. [Google Scholar] [CrossRef]
- Juhas, U.; Ryba-Stanisławowska, M.; Szargiej, P.; Myśliwska, J. Different pathways of macrophage activation and polarization. Postȩpy Hig. Med. Doświadczalnej 2015, 69, 496–502. [Google Scholar] [CrossRef]
- Beyer, M.; Mallmann, M.R.; Xue, J.; Staratschek-Jox, A.; Vorholt, D.; Krebs, W.; Sommer, D.; Sander, J.; Mertens, C.; Nino-Castro, A.; et al. High-resolution transcriptome of human macrophages. PLoS ONE 2012, 7, e45466. [Google Scholar] [CrossRef]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.; Ruhrberg, C.; Cantley, L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fu, M.; Xin, H. Polarizing Macrophages In Vitro; Springer: New York, NY, USA, 2018; Volume 1784, pp. 119–126. [Google Scholar]
- Yang, R.; Liao, Y.; Wang, L.; He, P.; Hu, Y.; Yuan, D.; Wu, Z.; Sun, X. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front. Immunol. 2019, 10, 2346. [Google Scholar] [CrossRef]
- Li, L.; Wei, K.; Ding, Y.; Ahati, P.; Xu, H.; Fang, H.; Wang, H. M2a macrophage-secreted CHI3L1 promotes extracellular matrix metabolic imbalances via activation of IL-13Rα2/MAPK pathway in rat intervertebral disc degeneration. Front. Immunol. 2021, 12, 666361. [Google Scholar] [CrossRef]
- Liu, D.; Wei, Y.; Liu, Y.; Wu, T.; Hu, J.; Lu, H. The long non-coding RNA NEAT1/miR-224-5p/IL-33 axis modulates macrophage M2a polarization and A1 astrocyte activation. Mol. Neurobiol. 2021, 58, 4506–4519. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liu, X.; Wang, H.; Guo, S. The M2a macrophage subset may be critically involved in the fibrogenesis of endometriosis in mice. Reprod. Biomed. Online 2018, 37, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukocyte Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Philipp, D.; Suhr, L.; Wahlers, T.; Choi, Y.; Paunel-Görgülü, A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res. Ther. 2018, 9, 286. [Google Scholar] [CrossRef]
- Yue, Y.; Huang, S.; Wang, L.; Wu, Z.; Liang, M.; Li, H.; Lv, L.; Li, W.; Wu, Z. M2b macrophages regulate cardiac fibroblast activation and alleviate cardiac fibrosis after reperfusion injury. Circ. J. Off. J. Jpn. Circ. Soc. 2020, 84, 626–635. [Google Scholar] [CrossRef]
- Tian, L.; Yu, Q.; Liu, D.; Chen, Z.; Zhang, Y.; Lu, J.; Ma, X.; Huang, F.; Han, J.; Wei, L.; et al. Epithelial-mesenchymal transition of peritoneal mesothelial cells is enhanced by M2c macrophage polarization. Immunol. Investig. 2022, 51, 301–315. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, H.; Lee, H.; Hong, M.; Park, S.; Bae, H. Magnolol attenuates cisplatin-induced muscle wasting by M2c macrophage activation. Front. Immunol. 2020, 11, 77. [Google Scholar] [CrossRef]
- Miki, S.; Suzuki, J.; Takashima, M.; Ishida, M.; Kokubo, H.; Yoshizumi, M. S-1-Propenylcysteine promotes IL-10-induced M2c macrophage polarization through prolonged activation of IL-10R/STAT3 signaling. Sci. Rep. UK 2021, 11, 22469. [Google Scholar] [CrossRef]
- Stockinger, B.; Meglio, P.D.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Yue, T.; Sun, F.; Yang, C.; Wang, F.; Luo, J.; Yang, P.; Xiong, F.; Zhang, S.; Yu, Q.; Wang, C. The AHR signaling attenuates autoimmune responses during the development of type 1 diabetes. Front. Immunol. 2020, 11, 1510. [Google Scholar] [CrossRef]
- Zhang, T.; Li, R.; Li, H.; Xiang, N.; Tan, Z.; Wang, G.; Li, X. The contribution of genetic variation and aberrant methylation of aryl hydrocarbon receptor signaling pathway genes to rheumatoid arthritis. Front. Immunol. 2022, 13, 823863. [Google Scholar] [CrossRef]
- Yang, X.X.; Liu, H.H.; Ye, T.T.; Duan, C.C.; Lv, P.P.; Wu, X.X.; Liu, J.J.; Jiang, K.K.; Lu, H.H.; Yang, H.H.; et al. AhR activation attenuates calcium oxalate nephrocalcinosis by diminishing M1 macrophage polarization and promoting M2 macrophage polarization. Theranostics 2020, 10, 12011–12025. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Mylonas, K.J.; Anderson, J.; Sheldrake, T.A.; Hesketh, E.E.; Richards, J.A.; Ferenbach, D.A.; Kluth, D.C.; Savill, J.; Hughes, J. Granulocyte macrophage-colony stimulating factor: A key modulator of renal mononuclear phagocyte plasticity. Immunobiology 2019, 224, 60–74. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Y.; Zheng, D.; Sun, Y.; Wang, C.; Wang, X.M.; Lee, V.W.S.; Wang, Y.; Zheng, G.; Tan, T.K.; et al. Failed renoprotection by alternatively activated bone marrow macrophages is due to a proliferation-dependent phenotype switch in vivo. Kidney Int. 2014, 85, 794–806. [Google Scholar] [CrossRef]
- Perdiguero, E.G.; Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 2016, 17, 2–8. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Kenigsberg, E.; Keren-Shaul, H.; Elefant, N.; Paul, F.; Zaretsky, I.; Mildner, A.; Cohen, N.; Jung, S.; Tanay, A.; et al. Massively parallel single-cell RNA-Seq for marker-free decomposition of tissues into cell types. Science 2014, 343, 776–779. [Google Scholar] [CrossRef]
- Specht, H.; Emmott, E.; Petelski, A.A.; Huffman, R.G.; Perlman, D.H.; Serra, M.; Kharchenko, P.; Koller, A.; Slavov, N. Single-cell proteomic and transcriptomic analysis of macrophage heterogeneity using SCoPE2. Genome Biol. 2021, 22, 50. [Google Scholar] [CrossRef]
- Conway, B.R.; O’Sullivan, E.D.; Cairns, C.; O’Sullivan, J.; Simpson, D.J.; Salzano, A.; Connor, K.; Ding, P.; Humphries, D.; Stewart, K.; et al. Kidney single-cell atlas reveals myeloid heterogeneity in progression and regression of kidney disease. J. Am. Soc. Nephrol. 2020, 31, 2833–2854. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The metabolic signature of macrophage responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Lian, G.; Zhang, S.; Wang, X.; Jiang, C.; Wang, H. HIF1α-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediat. Inflamm. 2017, 2017, 9029310–9029327. [Google Scholar] [CrossRef]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. How metabolism generates signals during innate immunity and inflammation. J. Biol. Chem. 2013, 288, 22893–22898. [Google Scholar] [CrossRef]
- Kieler, M.; Hofmann, M.; Schabbauer, G. More than just protein building blocks: How amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021, 288, 3694–3714. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Humphries, F.; Shmuel-Galia, L.; Ketelut-Carneiro, N.; Li, S.; Wang, B.; Nemmara, V.V.; Wilson, R.; Jiang, Z.; Khalighinejad, F.; Muneeruddin, K.; et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 2020, 369, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Dong, L.; Song, Y.; Zhang, Y.; Zhao, W.; Wang, C.; Lin, H.; Al Ani, M.K.; Liu, W.; Xue, R.; Yang, L. Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J. Cell. Physiol. 2021, 236, 6376–6390. [Google Scholar] [CrossRef]
- EL Kasmi, K.C.; Stenmark, K.R. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin. Immunol. 2015, 27, 267–275. [Google Scholar] [CrossRef]
- Luan, H.; Horng, T. Dynamic changes in macrophage metabolism modulate induction and suppression of Type I inflammatory responses. Curr. Opin. Immunol. 2021, 73, 9–15. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Rayees, S.; Rochford, I.; Joshi, J.C.; Joshi, B.; Banerjee, S.; Mehta, D. Macrophage TLR4 and PAR2 signaling: Role in regulating vascular inflammatory injury and repair. Front. Immunol. 2020, 11, 2091. [Google Scholar] [CrossRef]
- Lauterbach, M.A.; Hanke, J.E.; Serefidou, M.; Mangan, M.S.J.; Kolbe, C.; Hess, T.; Rothe, M.; Kaiser, R.; Hoss, F.; Gehlen, J.; et al. Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-citrate lyase. Immunity 2019, 51, 997–1011. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.; Chou, C.; Vavakova, M.; et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [CrossRef]
- Zanoni, I.; Tan, Y.; Di Gioia, M.; Broggi, A.; Ruan, J.; Shi, J.; Donado, C.A.; Shao, F.; Wu, H.; Springstead, J.R.; et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016, 352, 1232–1236. [Google Scholar] [CrossRef]
- Di Gioia, M.; Spreafico, R.; Springstead, J.R.; Mendelson, M.M.; Joehanes, R.; Levy, D.; Zanoni, I. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat. Immunol. 2020, 21, 42–53. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef]
- Díaz-Gerevini, G.T.; Daín, A.; Pasqualini, M.E.; López, C.B.; Eynard, A.R.; Repossi, G. Diabetic encephalopathy: Beneficial effects of supplementation with fatty acids ω3 and nordihydroguaiaretic acid in a spontaneous diabetes rat model. Lipids Health Dis. 2019, 18, 43. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, Y.; Mu, Y.; Zhang, X.; Liu, L.; Hou, X.; Zhang, L.; Xu, X.; Ji, A.; Cao, R.; et al. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr. Res. 2013, 33, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Ariyoshi, W.; Yoshioka, Y.; Hikiji, H.; Nishihara, T.; Okinaga, T. Docosahexaenoic acid enhances M2 macrophage polarization via the p38 signaling pathway and autophagy. J. Cell. Biochem. 2019, 120, 12604–12617. [Google Scholar] [CrossRef]
- Eslick, S.; Williams, E.J.; Berthon, B.S.; Wright, T.; Karihaloo, C.; Gately, M.; Wood, L.G. Weight loss and short-chain fatty acids reduce systemic inflammation in monocytes and adipose tissue macrophages from obese subjects. Nutrients 2022, 14, 765. [Google Scholar] [CrossRef]
- Benmoussa, K.; Garaude, J.; Acín-Pérez, R. How mitochondrial metabolism contributes to macrophage phenotype and functions. J. Mol. Biol. 2018, 430, 3906–3921. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Bhatia, D.; Chung, K.; Nakahira, K.; Patino, E.; Rice, M.C.; Torres, L.K.; Muthukumar, T.; Choi, A.M.; Akchurin, O.M.; Choi, M.E. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. JCI Insight 2019, 4, e132826. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Niegowska, M.; Eames, H.L.; Almuttaqi, H.; Arru, G.; Erre, G.L.; Passiu, G.; Khoyratty, T.E.; van Grinsven, E.; Udalova, I.A.; et al. Antibody response to homologous epitopes of Epstein-Barr virus, Mycobacterium avium subsp. paratuberculosis and IRF5 in patients with different connective tissue diseases and in mouse model of antigen-induced arthritis. J. Transl. Autoimmun. 2020, 3, 100048. [Google Scholar] [CrossRef]
- Bo, M.; Niegowska, M.; Erre, G.L.; Piras, M.; Longu, M.G.; Manchia, P.; Manca, M.; Passiu, G.; Sechi, L.A. Rheumatoid arthritis patient antibodies highly recognize IL-2 in the immune response pathway involving IRF5 and EBV antigens. Sci. Rep. UK 2018, 8, 1788–1789. [Google Scholar] [CrossRef]
- Xu, W.; Pan, H.; Xu, Y.; Ye, D. Interferon regulatory factor 5 and autoimmune lupus. Expert Rev. Mol. Med. 2013, 15, e6. [Google Scholar] [CrossRef]
- Stein, T.; Wollschlegel, A.; Te, H.; Weiss, J.; Joshi, K.; Kinzel, B.; Billich, A.; Guntermann, C.; Lehmann, J.C.U. Interferon regulatory factor 5 and nuclear factor kappa-B exhibit cooperating but also divergent roles in the regulation of pro-inflammatory cytokines important for the development of TH1 and TH17 responses. FEBS J. 2018, 285, 3097–3113. [Google Scholar] [CrossRef]
- Jing, C.; Castro-Dopico, T.; Richoz, N.; Tuong, Z.K.; Ferdinand, J.R.; Lok, L.S.C.; Loudon, K.W.; Banham, G.D.; Mathews, R.J.; Cader, Z.; et al. Macrophage metabolic reprogramming presents a therapeutic target in lupus nephritis. Proc. Natl. Acad. Sci. USA 2020, 117, 15160–15171. [Google Scholar] [CrossRef]
- Zubair, K.; You, C.; Kwon, G.; Kang, K. Two faces of macrophages: Training and tolerance. Biomedicines 2021, 9, 1596. [Google Scholar] [CrossRef]
- Zoshima, T.; Baba, T.; Tanabe, Y.; Ishida, Y.; Nakatani, K.; Nagata, M.; Mukaida, N.; Kawano, M. CCR2- and CCR5-mediated macrophage infiltration contributes to glomerular endocapillary hypercellularity in antibody-induced lupus nephritis. Rheumatology 2021, 61, 3033–3048. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhao, Y.; Jandeleit-Dahm, K. Macrophage phenotype and its relationship with renal function in human diabetic nephropathy. PLoS ONE 2019, 14, e221991. [Google Scholar] [CrossRef]
- Zhu, Q.J.; Zhu, M.; Xu, X.X.; Meng, X.M.; Wu, Y.G. Exosomes from high glucose–treated macrophages activate glomerular mesangial cells via TGF-β1/Smad3 pathway in vivo and in vitro. FASEB J. 2019, 33, 9279–9290. [Google Scholar] [CrossRef]
- Narváez, J. Systemic lupus erythematosus 2020. Med. Clin. Barc. 2020, 155, 494. [Google Scholar] [CrossRef]
- Wafa, A.; Hicham, H.; Naoufal, R.; Hajar, K.; Rachid, R.; Souad, B.; Mouna, M.; Zoubida, M.T.; Mohamed, A. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome: A study of 20 Moroccan adult patients. Clin. Rheumatol. 2022, 16, 743–749. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Ching, C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, C81–C96. [Google Scholar] [CrossRef]
- Burbano, C.; Villar-Vesga, J.; Vásquez, G.; Muñoz-Vahos, C.; Rojas, M.; Castaño, D. Proinflammatory differentiation of macrophages through microparticles that form immune complexes leads to T- and B-cell activation in systemic autoimmune diseases. Front. Immunol. 2019, 10, 2058. [Google Scholar] [CrossRef]
- Kamal, M.; Gabr, H.; Anwar, S.; Bastawy, S.; Salah, L. The relation of CD4+CD25+Foxp3+ regulatory T cells concentration with disease activity and damage index in systemic lupus erythematosus. Lupus 2022, 31, 463–471. [Google Scholar] [CrossRef]
- Venkatadri, R.; Sabapathy, V.; Dogan, M.; Sharma, R. Targeting regulatory T cells for therapy of Lupus Nephritis. Front. Pharmacol. 2021, 12, 806612. [Google Scholar] [CrossRef]
- Udompornpitak, K.; Bhunyakarnjanarat, T.; Charoensappakit, A.; Dang, C.P.; Saisorn, W.; Leelahavanichkul, A. Lipopolysaccharide-enhanced responses against aryl hydrocarbon receptor in FcgRIIb-deficient macrophages, a profound impact of an environmental toxin on a Lupus-like mouse model. Int. J. Mol. Sci. 2021, 22, 4199. [Google Scholar] [CrossRef]
- Varghese, B.; Haase, N.; Low, P.S. Depletion of folate-receptor-positive macrophages leads to alleviation of symptoms and prolonged survival in two murine models of systemic Lupus erythematosus. Mol. Pharmaceut. 2007, 4, 679–685. [Google Scholar] [CrossRef]
- Dufour, A.; Bellac, C.L.; Eckhard, U.; Solis, N.; Klein, T.; Kappelhoff, R.; Fortelny, N.; Jobin, P.; Rozmus, J.; Mark, J.; et al. C-terminal truncation of IFN-γ inhibits proinflammatory macrophage responses and is deficient in autoimmune disease. Nat. Commun. 2018, 9, 2416–2418. [Google Scholar] [CrossRef]
- Chalmers, S.A.; Chitu, V.; Herlitz, L.C.; Sahu, R.; Stanley, E.R.; Putterman, C. Macrophage depletion ameliorates nephritis induced by pathogenic antibodies. J. Autoimmun. 2014, 57, 42–52. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Montserrat-de La Paz, S.; Sanchez-Hidalgo, M.; Cardeno, A.; Bermudez, B.; Muriana, F.J.G.; Alarcon-de-la-Lastra, C. Virgin olive oil and its phenol fraction modulate monocyte/macrophage functionality: A potential therapeutic strategy in the treatment of systemic lupus erythematosus. Brit. J. Nutr. 2018, 120, 681–692. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; Villegas, I.; Cárdeno, A.; Talero, E.; Sánchez-Hidalgo, M.; Motilva, V.; Alarcón De La Lastra, C. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clin. Nutr. 2010, 29, 663–673. [Google Scholar] [CrossRef]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Alarcón-de-la-Lastra, C. Extra virgin olive oil polyphenolic extracts downregulate inflammatory responses in LPS-activated murine peritoneal macrophages suppressing NFκB and MAPK signalling pathways. Food Funct. 2014, 5, 127–1277. [Google Scholar] [CrossRef]
- Voloshyna, I.; Teboul, I.; Kasselman, L.J.; Salama, M.; Carsons, S.E.; DeLeon, J.; Mattana, J.; Miyawaki, N.; Reiss, A.B. Macrophage lipid accumulation in the presence of immunosuppressive drugs mycophenolate mofetil and cyclosporin A. Inflamm. Res. 2019, 68, 787–799. [Google Scholar] [CrossRef]
- Tesch, G.H. Diabetic nephropathy—Is this an immune disorder? Clin. Sci. 2017, 131, 2183–2199. [Google Scholar] [CrossRef]
- Zorena, K.; Michalska, M.; Kurpas, M.; Jaskulak, M.; Murawska, A.; Rostami, S. Environmental factors and the risk of developing type 1 diabetes-old disease and new data. Biology 2022, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Morse, Z.J.; Horwitz, M.S. Innate viral receptor signaling determines type 1 diabetes onset. Front. Endocrinol. 2017, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Calle, P.; Hotter, G. Macrophage phenotype and fibrosis in diabetic nephropathy. Int. J. Mol. Sci. 2020, 21, 2806. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.V.; Tan, S.H.; Candasamy, M.; Bhattamisra, S.K. Diabetic nephropathy: An update on pathogenesis and drug development. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 754–762. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Nephro. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.; Deng, T.; Ge, H.; Xiao, X. Identification of C3 as a therapeutic target for diabetic nephropathy by bioinformatics analysis. Sci. Rep. UK 2020, 10, 13468. [Google Scholar] [CrossRef]
- Pini, A.; Verta, R.; Grange, C.; Gurrieri, M.; Rosa, A.C. Histamine and diabetic nephropathy: An up-to-date overview. Clin. Sci. 2019, 133, 41–54. [Google Scholar] [CrossRef]
- Opazo-Ríos, L.; Mas, S.; Marín-Royo, G.; Mezzano, S.; Gómez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and diabetic nephropathy: Novel mechanistic insights and therapeutic opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Zhu, X.; Liu, Y.; Wu, B.; Guo, Y.; Liu, B.; Zhang, X. Effects of autophagy on macrophage adhesion and migration in diabetic nephropathy. Ren. Fail. 2019, 41, 682–690. [Google Scholar] [CrossRef]
- Landis, R.C.; Quimby, K.R.; Greenidge, A.R. M1/M2 macrophages in diabetic nephropathy: Nrf2/HO-1 as therapeutic targets. Curr. Pharm. Des. 2018, 24, 2241–2249. [Google Scholar] [CrossRef]
- Xie, F.; Lei, J.; Ran, M.; Li, Y.; Deng, L.; Feng, J.; Zhong, Y.; Li, J.; Antonio, B.; Brunetti, A. Attenuation of diabetic nephropathy in diabetic mice by Fasudil through regulation of macrophage polarization. J. Diabetes Res. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Jiandong, L.; Yang, Y.; Peng, J.; Xiang, M.; Wang, D.; Xiong, G.; Li, S. Trichosanthes kirilowii lectin ameliorates streptozocin-induced kidney injury via modulation of the balance between M1/M2 phenotype macrophage. Biomed. Pharmacother. 2019, 109, 93–102. [Google Scholar] [CrossRef]
- Hatanaka, T.; Ogawa, D.; Tachibana, H.; Eguchi, J.; Inoue, T.; Yamada, H.; Takei, K.; Makino, H.; Wada, J. Inhibition of SGLT2 alleviates diabetic nephropathy by suppressing high glucose-induced oxidative stress in type 1 diabetic mice. Pharmacol. Res. Perspect. 2016, 4, e239. [Google Scholar] [CrossRef]
- Wang, X.; Yao, B.; Wang, Y.; Fan, X.; Wang, S.; Niu, A.; Yang, H.; Fogo, A.; Zhang, M.; Harris, R.C. Macrophage cyclooxygenase-2 protects against development of diabetic nephropathy. Diabetes 2017, 66, 494–504. [Google Scholar] [CrossRef]
- Lee, S.E.; Jang, J.E.; Kim, H.S.; Jung, M.K.; Ko, M.S.; Kim, M.; Park, H.S.; Oh, W.; Choi, S.J.; Jin, H.J.; et al. Mesenchymal stem cells prevent the progression of diabetic nephropathy by improving mitochondrial function in tubular epithelial cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Rackham, C.L.; Hubber, E.L.; Czajka, A.; Malik, A.N.; King, A.J.F.; Jones, P.M. Optimizing beta cell function through mesenchymal stromal cell-mediated mitochondria transfer. Stem Cells 2020, 38, 574–584. [Google Scholar] [CrossRef]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Krasnodembskaya, A.D. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef]
- Zeng, H.; Qi, X.; Xu, X.; Wu, Y. TAB1 regulates glycolysis and activation of macrophages in diabetic nephropathy. Inflamm. Res. 2020, 69, 1215–1234. [Google Scholar] [CrossRef]
- Wetzel, M.D.; Stanley, K.; Maity, S.; Madesh, M.; Bopassa, J.C.; Awad, A.S. Homoarginine ameliorates diabetic nephropathy independent of nitric oxide synthase-3. Physiol. Rep. 2021, 9, e14766. [Google Scholar] [CrossRef]
- Torres, Á.; Muñoz, K.; Nahuelpán, Y.; Saez, A.R.; Mendoza, P.; Jara, C.; Cappelli, C.; Suarez, R.; Oyarzún, C.; Quezada, C.; et al. Intraglomerular monocyte/macrophage infiltration and macrophage–myofibroblast transition during diabetic nephropathy is regulated by the A2B adenosine receptor. Cells 2020, 9, 1051. [Google Scholar] [CrossRef]
- Wetzel, M.D.; Gao, T.; Venkatachalam, M.; Morris, S.M.; Awad, A.S. l-Homoarginine supplementation prevents diabetic kidney damage. Physiol. Rep. 2019, 7, e14235. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhu, X.; Guo, Y.; Yang, Y.; Jiang, Y.; Liu, B. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy. J. Cell. Physiol. 2019, 234, 6917–6926. [Google Scholar] [CrossRef]
- Tang, C.; Kanter, J.E.; Bornfeldt, K.E.; Leboeuf, R.C.; Oram, J.F. Diabetes reduces the cholesterol exporter ABCA1 in mouse macrophages and kidneys. J. Lipid Res. 2010, 51, 1719–1728. [Google Scholar] [CrossRef]
- Abdolmaleki, F.; Kovanen, P.T.; Mardani, R.; Gheibi-hayat, S.M.; Bo, S.; Sahebkar, A. Resolvins: Emerging players in autoimmune and inflammatory diseases. Clin. Rev. Allergy Immunol. 2019, 58, 82–91. [Google Scholar] [CrossRef]
- Brown, M.; O’Reilly, S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin. Exp. Immunol. 2019, 195, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Prescott, R.J.; Freemont, A.J.; Jones, C.J.P.; Hoyland, J.; Fielding, P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J. Pathol. 1992, 166, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Christmann, R.B.; Sampaio Barros, P.; Stifano, G.; Borges, C.L.; Carvalho, C.R.; Kairalla, R.; Parra, E.R.; Spira, A.; Simms, R.; Capellozzi, V.L.; et al. Association of interferon- and transforming growth factor β–regulated genes and macrophage activation with systemic sclerosis–related progressive lung fibrosis. Arthritis Rheumatol. 2014, 66, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Pap, T.; Kowal Bielecka, O.; Meyringer, R.; Guiducci, S.; Landthaler, M.; Schölmerich, J.; Michel, B.A.; Gay, R.E.; Matucci Cerinic, M.; et al. Overexpression of monocyte chemoattractant protein 1 in systemic sclerosis: Role of platelet-derived growth factor and effects on monocyte chemotaxis and collagen synthesis. Arthritis Rheum. 2001, 44, 2665–2678. [Google Scholar] [CrossRef]
- Iliopoulos, G.; Daoussis, D. Renal dysfunction in systemic sclerosis beyond scleroderma renal crisis. Rheumatol. Int. 2021, 41, 1203–1208. [Google Scholar] [CrossRef]
- Trostle, D.C.; Bedetti, C.D.; Steen, V.D.; Al Sabbagh, M.R.; Zee, B.; Medsger, T.A. Renal vascular histology and morphometry in systemic sclerosis. a case–control autopsy study. Arthritis Rheum. 1988, 31, 393–400. [Google Scholar] [CrossRef]
- Chrabaszcz, M.; Małyszko, J.; Sikora, M.; Alda-Malicka, R.; Stochmal, A.; Matuszkiewicz-Rowinska, J.; Rudnicka, L. Renal Involvement in Systemic Sclerosis: An Update. Kidney Blood Press. R. 2020, 45, 532–548. [Google Scholar] [CrossRef]
- Lescoat, A.; Lelong, M.; Jeljeli, M.; Piquet-Pellorce, C.; Morzadec, C.; Ballerie, A.; Jouneau, S.; Jego, P.; Vernhet, L.; Batteux, F.; et al. Combined anti-fibrotic and anti-inflammatory properties of JAK-inhibitors on macrophages in vitro and in vivo: Perspectives for scleroderma-associated interstitial lung disease. Biochem. Pharmacol. 2020, 178, 114103. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wu, G.; Zhou, Q.; Yue, H.; Rao, L.; Yuan, T.; Mo, B.; Wang, F.; Chen, L.; et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci. Adv. 2021, 7, eabb6075. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preyers, F.W.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; Loenhout, J.V.; de Jong, D.J.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Raphael, I.; Joern, R.R.; Forsthuber, T.G. Memory CD4 + T cells in immunity and autoimmune diseases. Cells 2020, 9, 531. [Google Scholar] [CrossRef]
- Kieffer, T.E.C.; Laskewitz, A.; Scherjon, S.A.; Faas, M.M.; Prins, J.R. Memory T cells in pregnancy. Front. Immunol. 2019, 10, 625. [Google Scholar] [CrossRef]
- Jeljeli, M.; Riccio, L.G.C.; Doridot, L.; Chêne, C.; Nicco, C.; Chouzenoux, S.; Deletang, Q.; Allanore, Y.; Kavian, N.; Batteux, F. Trained immunity modulates inflammation-induced fibrosis. Nat. Commun. 2019, 10, 5615–5670. [Google Scholar] [CrossRef]
- Atzeni, F.; Muto, P.; Rodríguez-Carrio, J.; Masala, I.F. Frequency of renal function parameter abnormalities in patients with psoriatic arthritis and rheumatoid arthritis: Real-world evidence from clinical practice. J. Clin. Med. 2022, 11, 1029. [Google Scholar] [CrossRef]
- Möller, B.; Pruijm, M.; Adler, S.; Scherer, A.; Villiger, P.M.; Finckh, A.; Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) Foundation. Chronic NSAID use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann. Rheum. Dis. 2015, 74, 718–723. [Google Scholar] [CrossRef]
- Bo, M.; Jasemi, S.; Uras, G.; Erre, G.L.; Passiu, G.; Sechi, L.A. Role of infections in the pathogenesis of rheumatoid arthritis: Focus on Mycobacteria. Microorganisms 2020, 8, 1459. [Google Scholar] [CrossRef]
- Lin, Y.; Nhieu, J.; Liu, P.; Le, G.; Lee, D.J.; Wei, C.; Lin, Y.; Oh, S.; Lowe, D.; Wei, L. CRABP1-CaMKII-Agrn regulates the maintenance of neuromuscular junction in spinal motor neuron. Cell Death Differ. 2022. [Google Scholar] [CrossRef] [PubMed]
- Carlos, C.P.; Carvalho, E.P.; Angeli Junior, E.V.; Garcia Filho, G.F.; Doná, J.P.L.; Batanero, R.P.D.O.; Guena, R.D.O.; Agren, C.; Baptista, M.A.S.F.; Bizotto, T.S.G.; et al. Angiotensin involvement in kidney injury induced by rheumatoid arthritis in rat. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1271–1279. [Google Scholar] [CrossRef]
- Li, Z.; Hao, H.; Gao, Y.; Wang, Z.; Lu, W.; Liu, J. Expression and localization analyses of the cholinergic anti-inflammatory pathway and α7nAchR in different tissues of rats with rheumatoid arthritis. Acta Histochem. 2019, 121, 742–749. [Google Scholar] [CrossRef]
- Simón-Vázquez, R.; Tsapis, N.; Lorscheider, M.; Rodríguez, A.; Calleja, P.; Mousnier, L.; de Miguel Villegas, E.; González-Fernández, Á.; Fattal, E. Improving dexamethasone drug loading and efficacy in treating arthritis through a lipophilic prodrug entrapped into PLGA-PEG nanoparticles. Drug Deliv. Transl. Res. 2022, 12, 1270–1284. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Chen, H.; Zhang, J.; He, W. Reprogramming Metabolism of Macrophages as a Target for Kidney Dysfunction Treatment in Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 8024. https://doi.org/10.3390/ijms23148024
Tian F, Chen H, Zhang J, He W. Reprogramming Metabolism of Macrophages as a Target for Kidney Dysfunction Treatment in Autoimmune Diseases. International Journal of Molecular Sciences. 2022; 23(14):8024. https://doi.org/10.3390/ijms23148024
Chicago/Turabian StyleTian, Feng, Hui Chen, Jianmin Zhang, and Wei He. 2022. "Reprogramming Metabolism of Macrophages as a Target for Kidney Dysfunction Treatment in Autoimmune Diseases" International Journal of Molecular Sciences 23, no. 14: 8024. https://doi.org/10.3390/ijms23148024
APA StyleTian, F., Chen, H., Zhang, J., & He, W. (2022). Reprogramming Metabolism of Macrophages as a Target for Kidney Dysfunction Treatment in Autoimmune Diseases. International Journal of Molecular Sciences, 23(14), 8024. https://doi.org/10.3390/ijms23148024





