New Hyaluronic Acid from Plant Origin to Improve Joint Protection—An In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. Characterization of GreenIuronic®
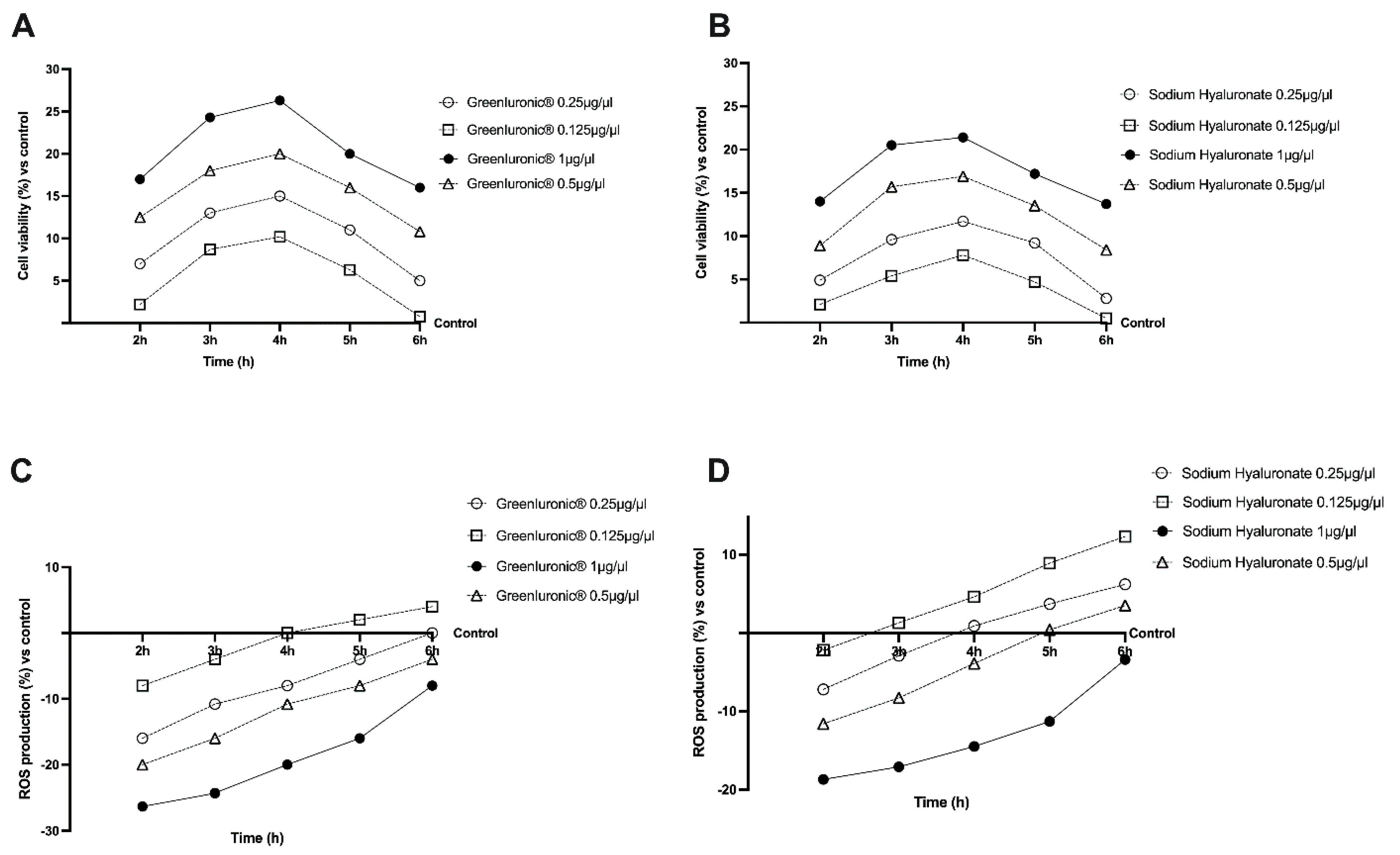
2.2. Dose–Response and Time-Course Study of GreenIuronic® on CaCo-2 Cells
2.3. Permeability Analysis of GreenIuronic® Using an In Vitro Model of Intestinal Barrier
2.4. Effects of GreenIuronic® Crossed Intestinal Barrier on Chondrocytes
2.5. Effects of HA Crossed Intestinal Barrier on Chondrocytes under OA Condition
3. Discussion
4. Materials and Methods
4.1. Agents Preparation
4.2. HPLC Analysis
4.3. Colorimetric Determination of Hyaluronic Acid
4.4. Molecular Weight Determination
4.5. Cell Culture
4.6. Experimental Protocol
4.7. Cell Viability
4.8. In Vitro Intestinal Barrier Model
4.9. Occludin Quantification Assay
4.10. Claudin 1 Detection
4.11. ZO-1 Detection
4.12. Crystal Violet Staining
4.13. ROS Production
4.14. Quantification of Hyaluronic Acid in Cell Culture
4.15. ERK/MAPK Activity
4.16. OPG Activity
4.17. NFKB Analysis
4.18. BAX Assay
4.19. Caspase 9 Assay
4.20. Western-Blot Analysis
4.21. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS | disintegrin and metalloproteinase with thrombospondin motifs |
| ANOVA | one-way analysis of variance |
| CaCo-2 | the human immortalized colorectal adenocarcinoma cell line |
| CD44 | differentiation cluster 44 |
| COX-2 | cyclooxygenase 2 |
| DMEM/F12 | Dulbecco’s modified Eagle’s medium/Nutrient F-12 Ham |
| EFSA | European Food Safety Authority |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMA | European Medicines Agency |
| ERK | extracellular signal-regulated kinases |
| ERK/MAPK | mitogen-activated protein kinases/extracellular signal-regulated kinase |
| FBS | fetal bovine serum |
| FBS | fetal bovine serum |
| FDA | US Food and Drug Administration |
| GAGs | glycosaminoglycan heteropolysaccharides family |
| HA | hyaluronic acid |
| HMWHA | high-molecular weight HA |
| HPLC | High-Performance Liquid Chromatography |
| HRMS | high-resolution mass spectrometry |
| IBD | inflammatory bowel disease |
| IL-1β | interleukin (IL)-1β |
| LMWHA | low molecular weight HA |
| LPS | lipopolysaccharide |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MMPs | matrix metalloproteinases |
| MPK-1 | mitogen-activated protein kinase phosphatase-1 |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| Na3VO4 | sodium orthovanadate |
| NFkB | nuclear factor kappa B |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| NO | nitric oxide (NO) |
| OA | Osteoarthritis |
| OPG | osteoprotegerin |
| Papp | apparent permeability coefficient |
| PBS | phosphate-buffered saline |
| PGE2 | prostaglandin E2 |
| PMSF | phenylmethanesulfonyl fluoride |
| PVDF | polyvinylidene difluoride |
| RHAMM | hyaluronan-mediated motility receptors |
| ROS | reactive oxygen species |
| TEER | transepithelial electrical resistance |
| T/C-28a2 | human chondrocyte cells |
| TJ | tight junction |
| ZO-1 | zonula occludens-1 |
Appendix A
Appendix A.1. HPLC-UV Method
- Column: Phenomenex Synergi Polar 4 µm 150 × 4.6 mm preceded by a Security guard Polar and kept at room temperature
- Mobile phase A: 340 mg of tetrabutylammonium bisulfate dissolved in 1000 mL of water HPLC grade.
- Mobile phase B: 340 mg of tetrabutylammonium bisulfate dissolved in 330 mL of water HPLC grade, then after the solution is at room temperature, brought to 1000 mL with acetonitrile.
- Wavelength: 240 nm
- Volume of injection: 30 µL
- Flow rate: 1.1 mL/min
- Gradient elution program:
Time (min) Mobile Phase B% 0.00 20 7.00 65 12.00 65 12.50 20 22.50 20
Appendix A.2. HPLC-HRMS Method
- Thermo Scientific Q-Exactive plus
- Column: Phenomenex Synergi Polar 4 µm 150 × 2.0 mm preceded by a Security guard Polar and kept at room temperature
- Mobile phase A: 0.1% formic acid in water
- Mobile phase B: 0.1% formic acid in acetonitrile
- Volume of injection: 5 µL
- Flow rate: 0.200 mL/min
- Gradient elution program:
Time (min) Mobile Phase B% 0.00 15 4.00 50 9.50 50 10.00 15 15 15 - Positive full scan.
References
- Cheng, O.T.; Souzdalnitski, D.; Vrooman, B.; Cheng, J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012, 13, 740–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belluzzi, E.; Macchi, V.; Fontanella, C.G.; Carniel, E.L.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6016. [Google Scholar] [CrossRef] [PubMed]
- Colen, S.; van den Bekerom, M.P.; Mulier, M.; Haverkamp, D. Hyaluronic acid in the treatment of knee osteoarthritis: A systematic re-view and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Niculescu-Morzsa, E.; Jeyakumar, V.; Kern, D.; Späth, S.S.; Nehrer, S. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. J. Inflamm. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmento, B.; Andrade, F.; da Silva, S.B.; Rodrigues, F.; das Neves, J.; Ferreira, D. Cell-based in vitro models for predicting drug permeability. Expert. Opin. Drug Metab. Toxicol. 2012, 8, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, M.; García-Hevia, L.; Amado, I.R.; Pastrana, L.; Gonçalves, C. In Vitro Intestinal Uptake and Permeability Of Fluorescently-Labelled Hyaluronic Acid Nanogels. Int. J. Nanomed. 2019, 14, 9077–9088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotla, N.G.; Bonam, S.R.; Rasala, S.; Wankar, J.; Bohara, R.A.; Bayry, J.; Rochev, Y.; Pandit, A. Recent advances and prospects of hyaluronan as a multi-functional therapeutic system. J. Control. Release 2021, 336, 598–620. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Cowman, M.K.; Matsuoka, S. Experimental approaches to hyaluronan structure. Carbohydr. Res. 2005, 340, 791–809. [Google Scholar] [CrossRef]
- Tømmeraas, K.; Melander, C. Kinetics of hyaluronan hydrolysis in acidic solution at various pH values. Biomacromolecules 2008, 9, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Karbownik, M.S.; Nowak, J.Z. Hyaluronan: Towards novel anti-cancer therapeutics. Pharmacol. Rep. 2013, 65, 1056–1074. [Google Scholar] [CrossRef]
- Urdiales-Gálvez, F.; Delgado, N.E.; Figueiredo, V.; Lajo-Plaza, J.V.; Mira, M.; Moreno, A.; Ortíz-Martí, F.; Del Rio-Reyes, R.; Romero-Álvarez, N.; Del Cueto, S.R.; et al. Treatment of Soft Tissue Filler Complications: Expert Consensus Recommendations. Aesthetic Plast. Surg. 2018, 42, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [Green Version]
- Akatsuka, M.; Yamamoto, Y.; Tobetto, K.; Yasui, T.; Ando, T. In vitro effects of hyaluronan on prostaglandin E2 induction by interleukin-1 in rabbit articular chondrocytes. Agents Actions 1993, 38, 122–125. [Google Scholar] [CrossRef]
- Galluccio, F.; Barskova, T.; Cerinic, M.M. Short-term effect of the combination of hyaluronic acid, chondroitin sulfate, and keratin matrix on early symptomatic knee osteoarthritis. Eur. J. Rheumatol. 2015, 2, 106–108. [Google Scholar] [CrossRef]
- Euppayo, T.; Punyapornwithaya, V.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. Effects of hyaluronic acid combined with anti-inflammatory drugs compared with hyaluronic acid alone, in clinical trials and experiments in osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 387. [Google Scholar] [CrossRef] [Green Version]
- Tarricone, E.; Elia, R.; Mattiuzzo, E.; Faggian, A.; Pozzuoli, A.; Ruggieri, P.; Brun, P. The Viability and Anti-Inflammatory Effects of Hyaluronic Acid-Chitlac-Tracimolone Acetonide- β-Cyclodextrin Complex on Human Chondrocytes. Cartilage 2021, 13, 920S–924S. [Google Scholar] [CrossRef]
- Tarricone, E.; Mattiuzzo, E.; Belluzzi, E.; Elia, R.; Benetti, A.; Venerando, R.; Vindigni, V.; Ruggieri, P.; Brun, P. Anti-Inflammatory Performance of Lactose-Modified Chitosan and Hyaluronic Acid Mixtures in an In Vitro Macrophage-Mediated Inflammation Osteoarthritis Model. Cells 2020, 9, 1328. [Google Scholar] [CrossRef]
- Wang, C.T.; Lin, Y.T.; Chiang, B.L.; Lin, Y.H.; Hou, S.M. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthr. Cartil. 2006, 14, 1237–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campo, G.M.; Avenoso, A.; Nastasi, G.; Micali, A.; Prestipino, V.; Vaccaro, M.; D’Ascola, A.; Calatroni, A.; Campo, S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1170–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julovi, S.M.; Yasuda, T.; Shimizu, M.; Hiramitsu, T.; Nakamura, T. Inhibition of interleu-kin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 2004, 50, 516–525. [Google Scholar] [CrossRef]
- Chang, C.C.; Hsieh, M.S.; Liao, S.T.; Chen, Y.H.; Cheng, C.W.; Huang, P.T.; Lin, Y.F.; Chen, C.H. Hyaluronan regulates PPARγ and inflammatory responses in IL-1β-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr. Polym. 2012, 90, 1168–1175. [Google Scholar] [CrossRef]
- Mihara, M.; Hashizume, M. The effect of high molecular hyaluronic acid on the induction of matrix degradation enzymes by IL-6, IL-1β and TNF-α. Osteoarthr. Cartil. 2012, 20, S134–S135. [Google Scholar] [CrossRef] [Green Version]
- Waddell, D.D.; Kolomytkin, O.V.; Dunn, S.; Marino, A.A. Hyaluronan suppresses IL-1beta-induced metalloproteinase activity from synovial tissue. Clin. Orthop. Relat. Res. 2007, 465, 241–248. [Google Scholar] [CrossRef]
- Kalaci, A.; Yilmaz, H.R.; Aslan, B.; Söğüt, S.; Yanat, A.N.; Uz, E. Effects of hyaluronan on nitric oxide levels and superoxide dismutase activities in synovial fluid in knee osteoarthritis. Clin. Rheumatol. 2007, 26, 1306–1311. [Google Scholar] [CrossRef]
- Karna, E.; Miltyk, W.; Surazynski, A.; Palka, J.A. Protective effect of hyaluronic acid on interleukin-1-induced deregulation of beta1-integrin and insulin-like growth factor-I receptor signaling and collagen biosynthesis in cultured human chondrocytes. Mol. Cell. Biochem. 2008, 308, 57–64. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa de Souza, A.; Vinícius Chaud, M.; Francine Alves, T.; Ferreira de Souza, J.; Andrade Santana, M.H. Hyaluronic Acid in the Intestinal Tract: Influence of Structure, Rheology, and Mucoadhesion on the Intestinal Uptake in Rats. Biomolecules 2020, 10, 1422. [Google Scholar] [CrossRef]
- Ewald, C.Y. Drug Screening Implicates Chondroitin Sulfate as a Potential Longevity Pill. Front. Aging 2021, 2, 741843. [Google Scholar] [CrossRef] [PubMed]
- Magni, A.; Agostoni, P.; Bonezzi, C.; Massazza, G.; Menè, P.; Savarino, V.; Fornasari, D. Management of Osteoarthritis: Expert Opinion on NSAIDs. Pain Ther. 2021, 10, 783–808. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Alluri, K.V.; Satish, A.R.; Mishra, S.; Golakoti, T.; Sarma, K.V.; Dey, D.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008, 10, R85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colletti, A.; Cicero, A.F.G. Nutraceutical Approach to Chronic Osteoarthritis: From Molecular Research to Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 12920. [Google Scholar] [CrossRef]
- Migliore, A.; Procopio, S. Effectiveness and utility of hyaluronic acid in osteoarthritis. Clin. Cases Miner. Bone Metab. 2015, 12, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Jevsevar, D.; Donnelly, P.; Brown, G.A.; Cummins, D.S. Viscosupplementation for Osteoarthritis of the Knee: A Systematic Review of the Evidence. J. Bone Jt. Surg. Am. 2015, 97, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, T.; Seino, S.; Sato, T.; Matsuoka, R.; Masuda, Y.; Fukui, N. Oral administration of polymer hyaluronic acid alleviates symptoms of knee osteoarthritis: A double-blind, placebo-controlled study over a 12-month period. Sci. World J. 2012, 2012, 167928. [Google Scholar] [CrossRef] [Green Version]
- Santilli, V.; Paoloni, M.; Mangone, M.; Alviti, F.; Bernetti, A. Hyaluronic acid in the management of osteoarthritis: Injection therapies innovations. Clin. Cases Miner. Bone Metab. 2016, 13, 131–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Tong, Y.; Luo, J.; Bi, Q. Development and Prospect of Intra-Articular Injection in the Treatment of Osteoarthritis: A Review. J. Pain Res. 2020, 4, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Micheloni, G.M.; Berti, M.; Perusi, F.; Sambugaro, E.; Vecchini, E.; Magnan, B. Clinical comparison of oral administration and viscosupplementation of hyaluronic acid (HA) in early knee osteoarthritis. Musculoskelet. Surg. 2017, 101, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Fredericson, M.; Altman, R.D. Hyaluronic Acid Injections or Oral Nonsteroidal Anti-inflammatory Drugs for Knee Osteoarthritis: Systematic Review and Meta-analysis of Randomized Trials. Orthop. J. Sports Med. 2020, 8, 2325967119897909. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Bedi, A.; Karlsson, J.; Sancheti, P.; Schemitsch, E. Product Differences in Intra-articular Hyaluronic Acids for Osteoarthritis of the Knee. Am. J. Sports Med. 2016, 44, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Iwaso, H.; Sato, T. Examination of the efficacy and safety of oral administration of Hyabest J, highly pure hyaluronic acid, for knee joint pain. J. Jpn. Soc. Clin. Sports Med. 2009, 17, 566–572. [Google Scholar]
- Nagaoka, I.; Nabeshima, K.; Murakami, S.; Yamamoto, T.; Watanabe, K.; Tomonaga, A.; Yamaguchi, H. Evaluation of the effects of a supplementary diet containing chicken comb extract on symptoms and cartilage metabolism in patients with knee osteoarthritis. Exp. Ther. Med. 2010, 1, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Andor, B.C.; Cerbu, S. Oral hyaluronic acid in patients with knee osteoarthritis. Progr. Nutr. 2019, 21, 243–245. [Google Scholar] [CrossRef]
- Guadagna, S.; Barattini, D.F.; Pricop, M.; Rosu, S. Oral hyaluronan for the treatment of knee osteoarthritis: A systematic review. Progr. Nutr. 2018, 20, 537–544. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guido, G.; Ausenda, G.; Iascone, V.; Chisari, E. Gut permeability and osteoarthritis, towards a mechanistic understanding of the pathogenesis: A systematic review. Ann. Med. 2021, 53, 2380–2390. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Li, B.; Zeng, B.; Chou, C.H.; Zheng, X.; Xie, J.; Li, H.; Hao, Y.; Chen, G.; et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann. Rheum. Dis. 2020, 79, 646–656. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Isa, I.L.M.; Rasala, S.; Demir, S.; Singh, R.; Baby, B.V.; Swamy, S.K.; Dockery, P.; Jala, V.R.; Rochev, Y.; et al. Modulation of Gut Barrier Functions in Ulcerative Colitis by Hyaluronic Acid System. Adv. Sci. 2022, 9, e2103189. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.W.; Zheng, H.Z.; Wang, R.; Chen, H.; Xiao, J.X.; Zheng, B.; Liu, S.L.; Ding, Y.T. Ameliorative Effects of Peptides Derived from Oyster (Crassostrea gigas) on Immunomodulatory Function and Gut Microbiota Structure in Cyclophosphamide-Treated Mice. Mar. Drugs 2021, 19, 456. [Google Scholar] [CrossRef]
- Jimbo, S.; Terashima, Y.; Teramoto, A.; Takebayashi, T.; Ogon, I.; Watanabe, K.; Sato, T.; Ichise, N.; Tohse, N.; Yamashita, T. Antinociceptive effects of hyaluronic acid on monoiodoacetate-induced ankle osteoarthritis in rats. J. Pain. Res. 2019, 12, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ge, J.; Lu, X. CircFADS2 is downregulated in osteoarthritis and suppresses LPS-induced apoptosis of chondrocytes by regulating miR-195-5p methylation. Arch. Gerontol. Geriatr. 2021, 96, 104477. [Google Scholar] [CrossRef] [PubMed]
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M.; Eto, S. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J. Bone Miner. Res. 1997, 12, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.Q.; Wang, A.T.; Yu, C.Y.; Luo, Y.; Liu, R.M.; Zhao, Y.J.; Xiao, J.H. Effect of CD44 on differentiation of human amniotic mesenchymal stem cells into chondrocytes via Smad and ERK signaling pathways. Mol. Med. Rep. 2020, 21, 2357–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Liu, Y.; Zhang, L. Tremella polysaccharide: The molecular mechanisms of its drug action. Prog. Mol. Biol. Transl. Sci. 2019, 163, 383–421. [Google Scholar] [CrossRef]
- Vivatis Pharma GMBH, 8 June 2020. Plant Producing Hyaluronic Acid. PCT/IB2021/055031. Available online: https://patents.google.com/patent/WO2021250566A1/en?oq=WO2021250566+(A1)++-++PRO-CESS+FOR+EXTRACTING+A+HYALURONIC+ACID+FROM+A+FUNGUS%2c+A+HYALURONIC+ACID+OF+PLANT+ORIGIN+AND+USE+THEREOF (accessed on 1 June 2022).
- Italian Patent. Identification and Selection of a Plant Starting Material of a Plant Chondroitin Sulfate and Hyaluronic Acid, and Transformation of such Plant Starting Material to Obtain Ingredients for Use in Foods, Supple-Ments, Medical Devices or Drugs. 102019000008409. 8 June 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020245809 (accessed on 1 June 2022).
- Ji, D.; Roman, M.; Zhou, J.; Hildreth, J. Determination of chondroitin sulfate content in raw materials and dietary supplements by high-performance liquid chromatography with ultraviolet detection after enzymatic hydrolysis: Single-laboratory validation. J. AOAC Int. 2007, 90, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Vojvodić Cebin, A.; Komes, D.; Ralet, M.C. Development and Vali-dation of HPLC-DAD Method with Pre-Column PMP Derivatization for Monomeric Profile Analysis of Polysaccharides from Agro-Industrial Wastes. Polymers 2022, 14, 544. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Cowman, M.K. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal. Biochem. 1994, 219, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Morsanuto, V.; Ruga, S.; Galla, R.; Farghali, M.; Notte, F.; Bozzo, C.; Magnani, C.; Nardone, A.; Molinari, C. Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation. Nutrients 2020, 12, 573. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, L.; Meloni, M. La valutazione dell’assorbimento intestinale in vitro. L’integratore Nutr. 2014, 17, 62–65. [Google Scholar]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, S. In Vitro permeability across CaCo-2 cells (colonic) can predict in vivo (small intestinal) absorption in man--fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in CaCo-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Christides, T.; Wray, D.; McBride, R.; Fairweather, R.; Sharp, P. Iron bioavailability from commercially available iron supplements. Eur. J. Nutr. 2015, 54, 1345–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fda.Gov. Available online: https://www.fda.gov/media/117974/download (accessed on 12 May 2021).
- Ema.Eu. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-2b-first-version_en.pdf (accessed on 6 August 2018).
- Santoro, A.; Conde, J.; Scotece, M.; Abella, V.; López, V.; Pino, J.; Gómez, R.; Gómez-Reino, J.J.; Gualillo, O. Choosing the right chondrocyte cell line: Focus on nitric oxide. J. Orthop. Res. 2015, 33, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.L.; Chow, Y.Y.; Leong, L.M.; Law, J.X.; Ghafar, N.A.; Soelaiman, I.N.; Chin, K.Y. Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis. Life 2021, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Claassen, H.; Schicht, M.; Brandt, J.; Reuse, K.; Schädlich, R.; Goldring, M.B.; Guddat, S.S.; Thate, A.; Paulsen, F. C-28/I2 and T/C-28a2 chondrocytes as well as human primary articular chondrocytes express sex hormone and insulin receptors--Useful cells in study of cartilage metabolism. Ann. Anat. 2011, 193, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Aslan, M.; Simsek, G.; Dayi, E. The effect of hyaluronic acid-supplemented bone graft in bone healing: Experimental study in rabbits. J. Biomater. Appl. 2006, 20, 209–220. [Google Scholar] [CrossRef]
- López-Senra, E.; Casal-Beiroa, P.; López-Álvarez, M.; Serra, J.; González, P.; Valcarcel, J.; Vázquez, J.A.; Burguera, E.F.; Blanco, F.J.; Magalhães, J. Impact of Prevalence Ratios of Chondroitin Sulfate (CS)-4 and -6 Isomers Derived from Marine Sources in Cell Proliferation and Chondrogenic Differentiation Processes. Mar. Drugs 2020, 18, 94. [Google Scholar] [CrossRef] [Green Version]
- Sui, C.; Zhang, L.; Hu, Y. MicroRNA-let-7a inhibition inhibits LPS-induced inflammatory injury of chondrocytes by targeting IL6R. Mol. Med. Rep. 2019, 20, 2633–2640. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Ghirlanda, S.; Ruga, S.; Notte, F.; Gaetano, L.; Uberti, F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxid. Med. Cell. Longev. 2019, 2019, 2843121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Xu, X.X.; Liu, Y.; Xi, E.Z.; An, J.J.; Tabys, D.; Liu, N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules 2019, 24, 148. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Bardelli, C.; Morsanuto, V.; Ghirlanda, S.; Cochis, A.; Molinari, C. Stimulation of the Nonneuronal Cholinergic System by Highly Diluted Acetylcholine in Keratinocytes. Cells Tissues Organs 2017, 203, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Aborehab, N.M.; El Bishbishy, M.H.; Refaiy, A.; Waly, N.E. A putative Chondroprotective role for IL-1β and MPO in herbal treatment of experimental osteoarthritis. BMC Complement. Altern. Med. 2017, 17, 495. [Google Scholar] [CrossRef] [Green Version]
- Roberts, H.M.; Moore, J.P.; Thom, J.M. The effect of aerobic walking and lower body resistance exercise on serum COMP and hyaluronan, in both males and females. Eur. J. Appl. Physiol. 2018, 118, 1095–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, C.; Morsanuto, V.; Ruga, S.; Notte, F.; Farghali, M.; Galla, R.; Uberti, F. The Role of BDNF on Aging-Modulation Markers. Brain Sci. 2020, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Vorkapic, E.; Kunath, A.; Wågsäter, D. Effects of osteoprotegerin/TNFRSF11B in two models of abdominal aortic aneurysms. Mol. Med. Rep. 2018, 18, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Morsanuto, V.; Galla, R.; Molinari, C.; Uberti, F. A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging. Brain Sci. 2020, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, I.; Hafez, H.; AlZaim, I.; Tannous, C.; Ragab, H.; Hazzaa, A.; Ketat, S.; Ghoneim, A.; Katary, M.; Abd-Alhaseeb, M.M.; et al. Transforming iodoquinol into broad spectrum anti-tumor leads: Repurposing to modulate redox homeostasis. Bioorg. Chem. 2021, 113, 105035. [Google Scholar] [CrossRef] [PubMed]
- Jieensinue, S.; Zhu, H.; Li, G.; Dong, K.; Liang, M.; Li, Y. Tanshinone IIA reduces SW837 col-orectal cancer cell viability via the promotion of mitochondrial fission by activating JNK-Mff signaling pathways. BMC Cell Biol. 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed]
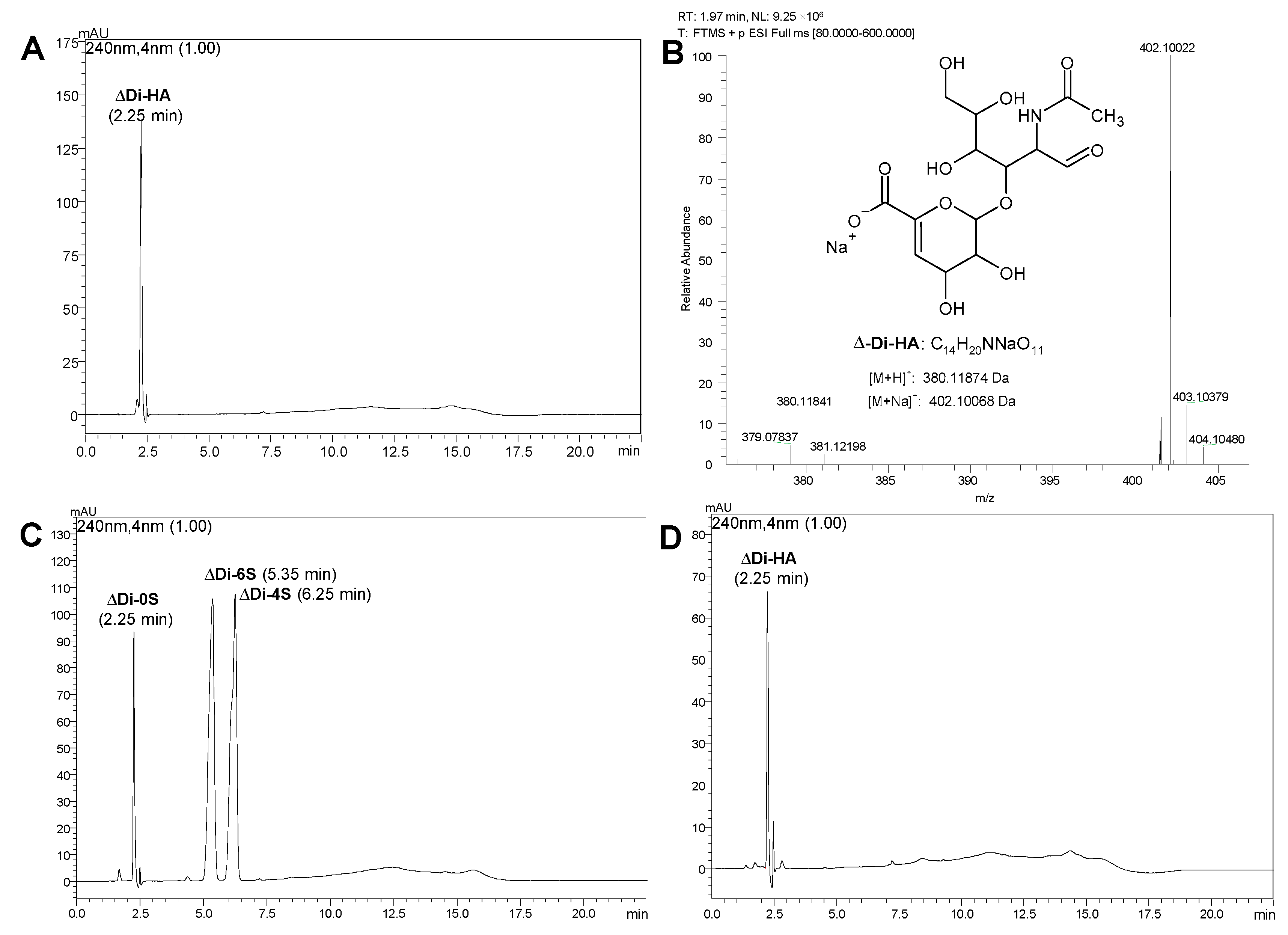






| Raw Material | Mean (%w/w) ± SD |
|---|---|
| Sodium Hyaluronate | 62.5 ± 2.121 |
| GreenIuronic® | 90.5 ± 6.364 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galla, R.; Ruga, S.; Aprile, S.; Ferrari, S.; Brovero, A.; Grosa, G.; Molinari, C.; Uberti, F. New Hyaluronic Acid from Plant Origin to Improve Joint Protection—An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8114. https://doi.org/10.3390/ijms23158114
Galla R, Ruga S, Aprile S, Ferrari S, Brovero A, Grosa G, Molinari C, Uberti F. New Hyaluronic Acid from Plant Origin to Improve Joint Protection—An In Vitro Study. International Journal of Molecular Sciences. 2022; 23(15):8114. https://doi.org/10.3390/ijms23158114
Chicago/Turabian StyleGalla, Rebecca, Sara Ruga, Silvio Aprile, Sara Ferrari, Arianna Brovero, Giorgio Grosa, Claudio Molinari, and Francesca Uberti. 2022. "New Hyaluronic Acid from Plant Origin to Improve Joint Protection—An In Vitro Study" International Journal of Molecular Sciences 23, no. 15: 8114. https://doi.org/10.3390/ijms23158114






