High-Density Lipoproteins: A Role in Inflammation in COPD
Abstract
:1. Introduction
2. Clinical Links between HDL and COPD
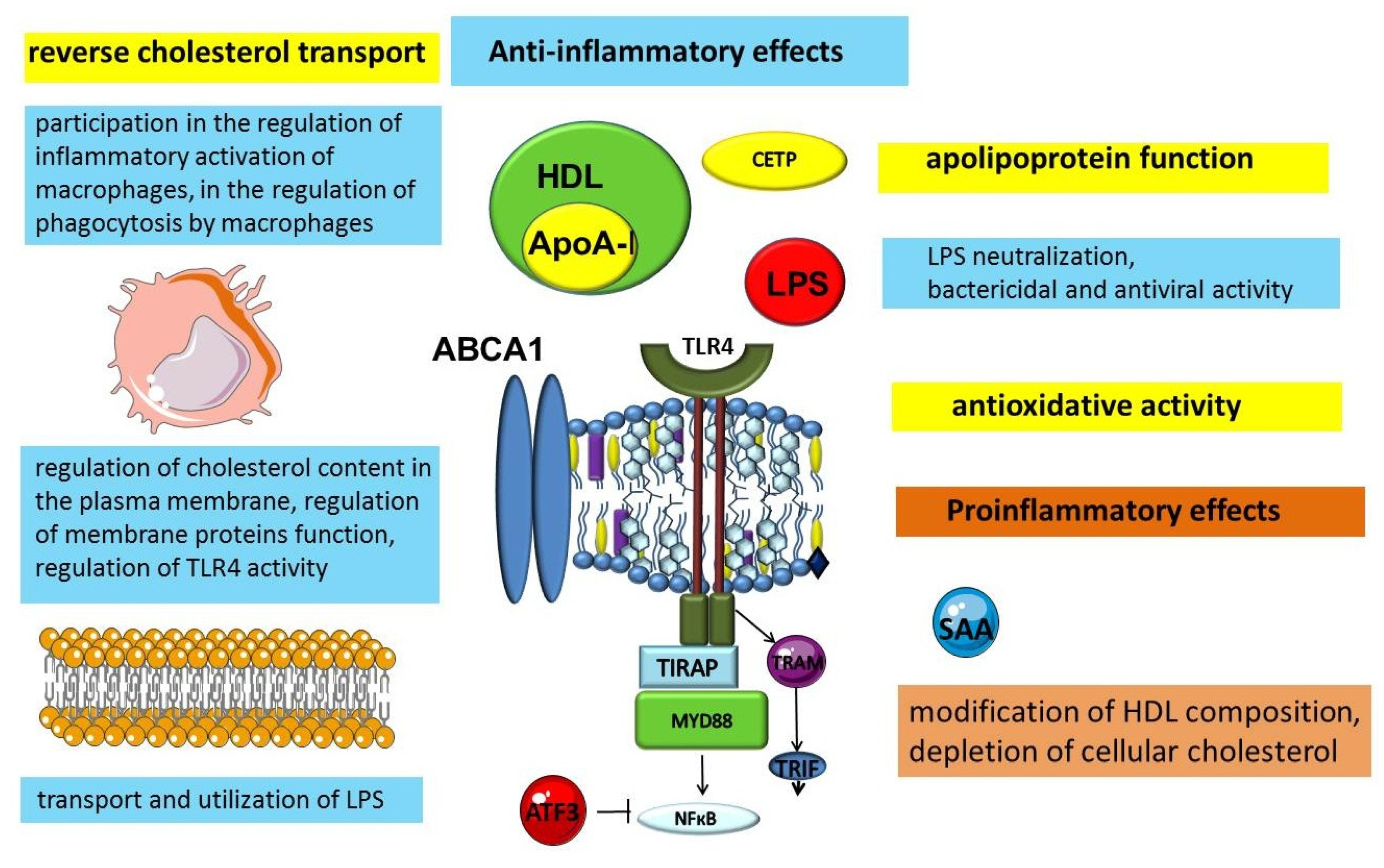
3. Anti-Inflammatory Function of HDL
3.1. The Significance of Apolipoproteins in the Anti-Inflammatory Function of HDLs
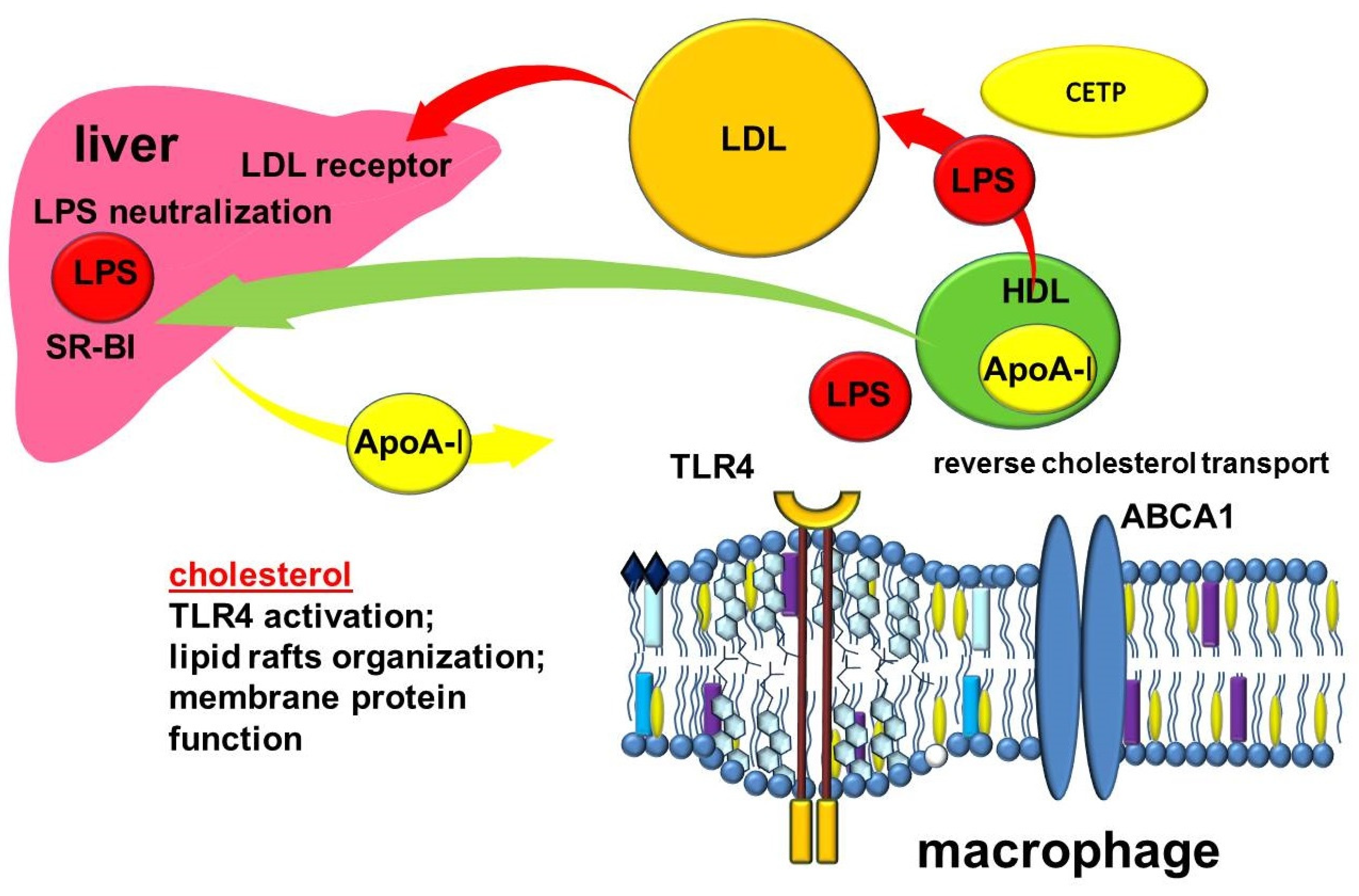
3.2. The Importance of ABC-Mediated Lipid Transport in the Regulation of Inflammation
3.3. Other Anti-Inflammatory Mechanisms of HDL
4. Proinflammatory Function of HDL
5. Other Transport Functions of HDL
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- Blanco, I.; Diego, I.; Bueno, P.; Casas-Maldonado, F.; Miravitlles, M. Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur. Respir. J. 2019, 54, 1900610. [Google Scholar] [CrossRef]
- Søgaard, M.; Madsen, M.; Løkke, A.; Hilberg, O.; Sørensen, H.T.; Thomsen, R.W. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Westbroek, L.F.; Klijnsma, M.; Salome, P.; Sekhuis, L.M.; Rolink, E.; Korsmit, E.; Kerstjens, H.A.M.; Group, L.C.C.P.S. Reducing the Number of Hospitalization Days for COPD: Setting up a Transmural-Care Pathway. Int. J. Chronic Obstruct. Pulm. Dis. 2020, 15, 2367–2377. [Google Scholar] [CrossRef]
- Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 20 July 2022).
- García Castillo, E.; Alonso Pérez, T.; Ancochea, J.; Pastor Sanz, M.T.; Almagro, P.; Martínez-Camblor, P.; Miravitlles, M.; Rodríguez-Carballeira, M.; Navarro, A.; Lamprecht, B.; et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2015 and GOLD 2019 staging: A pooled analysis of individual patient data. ERJ Open Res. 2020, 6, 00253–02020. [Google Scholar] [CrossRef]
- Hogg, J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004, 364, 709–721. [Google Scholar] [CrossRef]
- Global Strategy for Prevention, Diagnosis and Management of COPD: 2022 Report. 2022, p. 177. Available online: https://goldcopd.org/2022-gold-reports-2/ (accessed on 20 July 2022).
- Marciniuk, D.D.; Goodridge, D.; Hernandez, P.; Rocker, G.; Balter, M.; Bailey, P.; Ford, G.; Bourbeau, J.; O’Donnell, D.E.; Maltais, F.; et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: A Canadian Thoracic Society clinical practice guideline. Can. Respir. J. 2011, 18, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Miravitlles, M.; Menezes, A.; López Varela, M.V.; Casas, A.; Ugalde, L.; Ramirez-Venegas, A.; Mendoza, L.; López, A.; Wehrmeister, F.C.; Surmont, F.; et al. Prevalence and impact of respiratory symptoms in a population of patients with COPD in Latin America: The LASSYC observational study. Respir. Med. 2018, 134, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Gephine, S.; Mucci, P.; Grosbois, J.-M.; Maltais, F.; Saey, D. Physical Frailty in COPD Patients with Chronic Respiratory Failure. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1381–1392. [Google Scholar] [CrossRef]
- Bernard, S.; LeBlanc, P.; Whittom, F.; Carrier, G.; Jobin, J.; Belleau, R.; Maltais, F. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 629–634. [Google Scholar] [CrossRef]
- Laniado-Laborín, R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int. J. Environ. Res. Public Health 2009, 6, 209–224. [Google Scholar] [CrossRef] [Green Version]
- Marsh, S.; Aldington, S.; Shirtcliffe, P.; Weatherall, M.; Beasley, R. Smoking and COPD: What really are the risks? Eur. Respir. J. 2006, 28, 883–884. [Google Scholar] [CrossRef]
- Franciosi, L.; Postma, D.S.; van den Berge, M.; Govorukhina, N.; Horvatovich, P.L.; Fusetti, F.; Poolman, B.; Lodewijk, M.E.; Timens, W.; Bischoff, R.; et al. Susceptibility to COPD: Differential proteomic profiling after acute smoking. PLoS ONE 2014, 9, e102037. [Google Scholar] [CrossRef]
- Leem, A.Y.; Park, B.; Kim, Y.S.; Chang, J.; Won, S.; Jung, J.Y. Longitudinal decline in lung function: A community-based cohort study in Korea. Sci. Rep. 2019, 9, 13614. [Google Scholar] [CrossRef] [Green Version]
- Safitri, W.; Martini, S.; Artanti, K.D.; Li, C.-Y. Smoking from a Younger Age Is the Dominant Factor in the Incidence of Chronic Obstructive Pulmonary Disease: Case-Control Study. Int. J. Environ. Res. Public Health 2021, 18, 6047. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Gao, X.; Lv, Y.; Zhou, L.; Shi, J.; Wei, W.; Huang, J.; Deng, L.; Wang, Z.; et al. Epidemiological evidence relating risk factors to chronic obstructive pulmonary disease in China: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0261692. [Google Scholar] [CrossRef]
- Jiang, X.-Q.; Mei, X.-D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar] [CrossRef]
- Duan, R.-R.; Hao, K.; Yang, T. Air pollution and chronic obstructive pulmonary disease. Chronic Dis. Transl. Med. 2020, 6, 260–269. [Google Scholar] [CrossRef]
- Denden, S.; Khelil, A.H.; Knani, J.; Lakhdar, R.; Perrin, P.; Lefranc, G.; Chibani, J.B. Alpha-1 antitrypsin gene polymorphism in Chronic Obstructive Pulmonary Disease (COPD). Genet. Mol. Biol. 2010, 33, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Angelis, N.; Porpodis, K.; Zarogoulidis, P.; Spyratos, D.; Kioumis, I.; Papaiwannou, A.; Pitsiou, G.; Tsakiridis, K.; Mpakas, A.; Arikas, S.; et al. Airway inflammation in chronic obstructive pulmonary disease. J. Thorac. Dis. 2014, 6 (Suppl. S1), S167–S172. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Martin, R.J. Airway inflammation in chronic obstructive pulmonary disease: Comparisons with asthma. J. Allergy Clin. Immunol. 2003, 112, 819–827; quiz 828. [Google Scholar] [CrossRef]
- Kotlyarov, S. Involvement of the Innate Immune System in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2022, 23, 985. [Google Scholar] [CrossRef]
- Vlahos, R.; Bozinovski, S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front. Immunol. 2014, 5, 435. [Google Scholar] [CrossRef] [Green Version]
- Jasper, A.E.; McIver, W.J.; Sapey, E.; Walton, G.M. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Research 2019, 8, 557. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Crystal, R.G. Innate immunity and chronic obstructive pulmonary disease: A mini-review. Gerontology 2013, 59, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Monsó, E. Microbiome in chronic obstructive pulmonary disease. Ann. Transl. Med. 2017, 5, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Bafadhel, M.; Haldar, K.; Spivak, A.; Mayhew, D.; Miller, B.E.; Tal-Singer, R.; Johnston, S.L.; Ramsheh, M.Y.; Barer, M.R.; et al. Lung microbiome dynamics in COPD exacerbations. Eur. Respir. J. 2016, 47, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- Kerkhof, M.; Voorham, J.; Dorinsky, P.; Cabrera, C.; Darken, P.; Kocks, J.W.; Sadatsafavi, M.; Sin, D.D.; Carter, V.; Price, D.B. Association between COPD exacerbations and lung function decline during maintenance therapy. Thorax 2020, 75, 744–753. [Google Scholar] [CrossRef]
- Makris, D.; Moschandreas, J.; Damianaki, A.; Ntaoukakis, E.; Siafakas, N.M.; Milic Emili, J.; Tzanakis, N. Exacerbations and lung function decline in COPD: New insights in current and ex-smokers. Respir. Med. 2007, 101, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Groenewegen, K.H.; Postma, D.S.; Hop, W.C.; Wielders, P.L.; Schlösser, N.J.; Wouters, E.F. Increased systemic inflammation is a risk factor for COPD exacerbations. Chest 2008, 133, 350–357. [Google Scholar] [CrossRef]
- Oudijk, E.J.D.; Lammers, J.W.J.; Koenderman, L. Systemic inflammation in chronic obstructive pulmonary disease. Eur. Respir. J. 2003, 22, 5s–13s. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, Z.; Dong, L.; Wu, Y.; Shen, H.; Chen, Z. Lipid metabolism in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Kotlyarov, S.; Kotlyarova, A. The Role of ABC Transporters in Lipid Metabolism and the Comorbid Course of Chronic Obstructive Pulmonary Disease and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 6711. [Google Scholar] [CrossRef]
- Naik, D.; Joshi, A.; Paul, T.V.; Thomas, N. Chronic obstructive pulmonary disease and the metabolic syndrome: Consequences of a dual threat. Indian J. Endocrinol. Metab. 2014, 18, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Nordestgaard, B.G. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: A contemporary primary prevention cohort. Lancet 2020, 396, 1644–1652. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef]
- Atar, D.; Jukema, J.W.; Molemans, B.; Taub, P.R.; Goto, S.; Mach, F.; CerezoOlmos, C.; Underberg, J.; Keech, A.; Tokgözoğlu, L.; et al. New cardiovascular prevention guidelines: How to optimally manage dyslipidaemia and cardiovascular risk in 2021 in patients needing secondary prevention? Atherosclerosis 2021, 319, 51–61. [Google Scholar] [CrossRef]
- Zafirova-Ivanovska, B.; Stojkovikj, J.; Dokikj, D.; Anastasova, S.; Debresliovska, A.; Zejnel, S.; Stojkovikj, D. The Level of Cholesterol in COPD Patients with Severe and Very Severe Stage of the Disease. Open Access Maced. J. Med. Sci. 2016, 4, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Jungck, D.; Zickfeld, M.I.; Albuscheit, T.; Brandenburger, E.; Knobloch, J.; Koch, A. HDL serum levels are significantly lower in patients with COPD than in never-smokers. Eur. Respir. J. 2017, 50, PA1204. [Google Scholar] [CrossRef]
- Rafie, S.; Moitra, S.; Brashier, B.B. Association between the Serum Metabolic Profile and Lung Function in Chronic Obstructive Pulmonary Disease. Turk. Thorac. J. 2018, 19, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkart, K.M.; Manichaikul, A.; Wilk, J.B.; Ahmed, F.S.; Burke, G.L.; Enright, P.; Hansel, N.N.; Haynes, D.; Heckbert, S.R.; Hoffman, E.A.; et al. APOM and high-density lipoprotein cholesterol are associated with lung function and per cent emphysema. Eur. Respir. J. 2014, 43, 1003–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zhang, J.; Huang, Y. Prediction of cardiovascular risk in patients with chronic obstructive pulmonary disease: A study of the National Health and Nutrition Examination Survey database. BMC Cardiovasc. Disord. 2021, 21, 417. [Google Scholar] [CrossRef] [PubMed]
- Tantucci, C.; Modina, D. Lung function decline in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Marott, J.L.; Ingebrigtsen, T.S.; Çolak, Y.; Vestbo, J.; Lange, P. Lung Function Trajectories Leading to Chronic Obstructive Pulmonary Disease as Predictors of Exacerbations and Mortality. Am. J. Respir. Crit. Care Med. 2020, 202, 210–218. [Google Scholar] [CrossRef]
- Oelsner, E.; Balte, P.; Schwartz, J.E.; Burkart, K.M.; Cassano, P.; Jacobs, D.R.; Kalhan, R.; Kronmal, R.; Loehr, L.R.; O’Connor, G.T.; et al. LATE-BREAKING ABSTRACT: High density lipoprotein cholesterol (HDL-C) and longitudinal lung function in six United States (US) cohorts. Eur. Respir. J. 2016, 48, OA2001. [Google Scholar] [CrossRef]
- Park, J.H.; Mun, S.; Choi, D.P.; Lee, J.Y.; Kim, H.C. Association between high-density lipoprotein cholesterol level and pulmonary function in healthy Korean adolescents: The JS high school study. BMC Pulm. Med. 2017, 17, 190. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Ramírez, S.; Paniagua-Pérez, A.; Castro-Serna, D.; Ledesma-Velázquez, A.; Rubio-Guerra, A.; Vargas-Ayala, G. Effect of the components of the metabolic syndrome on pulmonary function. The unexpected role of high-density lipoprotein cholesterol. Cir. Cir. 2018, 86, 175–181. [Google Scholar] [CrossRef]
- Reed, R.M.; Hashmi, S.; Eberlein, M.; Iacono, A.; Netzer, G.; DeFilippis, A.; Girgis, R.E.; Toth, P.P.; Scharf, S.; Jones, S. Impact of lung transplantation on serum lipids in COPD. Respir. Med. 2011, 105, 1961–1968. [Google Scholar] [CrossRef] [Green Version]
- Reed, R.M.; Iacono, A.; DeFilippis, A.; Eberlein, M.; Girgis, R.E.; Jones, S. Advanced chronic obstructive pulmonary disease is associated with high levels of high-density lipoprotein cholesterol. J. Heart Lung Transplant. 2011, 30, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, B.; Miao, X.; Ma, J.; Wang, J.; Ding, K.; Chen, X.; Hu, Q.; Fu, F.; Zeng, T.; et al. The Relationship of Lymphocyte to High-Density Lipoprotein Ratio with Pulmonary Function in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Wang, L.; Shen, T.; Du, W.; Liu, Z.; Chen, R.; Hu, M. High apolipoprotein M serum levels correlate with chronic obstructive pulmonary disease. Lipids Health Dis. 2016, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Can, U.; Yerlikaya, F.H.; Yosunkaya, S. Role of oxidative stress and serum lipid levels in stable chronic obstructive pulmonary disease. J. Chin. Med. Assoc. 2015, 78, 702–708. [Google Scholar] [CrossRef] [Green Version]
- Xuan, L.; Han, F.; Gong, L.; Lv, Y.; Wan, Z.; Liu, H.; Zhang, D.; Jia, Y.; Yang, S.; Ren, L.; et al. Association between chronic obstructive pulmonary disease and serum lipid levels: A meta-analysis. Lipids Health Dis. 2018, 17, 263. [Google Scholar] [CrossRef] [Green Version]
- Burkart, K.M.; Ahmed, F.S.; Watson, K.; Hoffman, E.A.; Burke, G.L.; Barr, R.G. Association between high density lipoproteins (hdl) cholesterol and ct percent emphysema. The mesa lung study. In B37. Chronic Obstructive Pulmonary Disease Pathogenesis I; American Thoracic Society: New York, NY, USA, 2010; p. A2878. [Google Scholar]
- Ogawa, E.; Nakano, Y.; Ohara, T.; Muro, S.; Hirai, T.; Sato, S.; Sakai, H.; Tsukino, M.; Kinose, D.; Nishioka, M.; et al. Body mass index in male patients with COPD: Correlation with low attenuation areas on CT. Thorax 2009, 64, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Coxson, H.O.; Chan, I.H.; Mayo, J.R.; Hlynsky, J.; Nakano, Y.; Birmingham, C.L. Early emphysema in patients with anorexia nervosa. Am. J. Respir. Crit. Care Med. 2004, 170, 748–752. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Betsuyaku, T. The chronic obstructive pulmonary disease comorbidity spectrum in Japan differs from that in western countries. Respir. Investig. 2015, 53, 259–270. [Google Scholar] [CrossRef]
- Viniol, C.; Vogelmeier, C.F. Exacerbations of COPD. Eur. Respir. Rev. 2018, 27, 170103. [Google Scholar] [CrossRef] [Green Version]
- Oelsner, E.; Burkart, K.; Jacobs, D.; Loehr, L.; Kalhan, R.; O’Connor, G.; Tsai, M.; White, W.; Kronmal, R.; Folsom, A.; et al. LATE-BREAKING ABSTRACT: High density lipoprotein cholesterol (HDL) and severe chronic lung disease (CLD) exacerbations in the general population. Eur. Respir. J. 2015, 46, PA615. [Google Scholar] [CrossRef]
- Madsen, C.M.; Varbo, A.; Tybjærg-Hansen, A.; Frikke-Schmidt, R.; Nordestgaard, B.G. U-shaped relationship of HDL and risk of infectious disease: Two prospective population-based cohort studies. Eur. Heart J. 2017, 39, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- García-Olmos, L.; Alberquilla, Á.; Ayala, V.; García-Sagredo, P.; Morales, L.; Carmona, M.; de Tena-Dávila, M.J.; Pascual, M.; Muñoz, A.; Salvador, C.H.; et al. Comorbidity in patients with chronic obstructive pulmonary disease in family practice: A cross sectional study. BMC Fam. Pract. 2013, 14, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragoso, E.; André, S.; Boleo-Tomé, J.P.; Areias, V.; Munhá, J.; Cardoso, J. Understanding COPD: A vision on phenotypes, comorbidities and treatment approach. Rev. Port. Pneumol. 2016, 22, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzikhan, N.; Lahousse, L.; Verhamme, K.M.C.; Franco, O.H.; Ikram, A.M.; Stricker, B.H.; Brusselle, G.G. COPD is associated with an increased risk of peripheral artery disease and mortality. ERJ Open Res. 2018, 4, 00086–02018. [Google Scholar] [CrossRef]
- Aisanov, Z.; Khaltaev, N. Management of cardiovascular comorbidities in chronic obstructive pulmonary disease patients. J. Thorac. Dis. 2020, 12, 2791–2802. [Google Scholar] [CrossRef]
- Cavaillès, A.; Brinchault-Rabin, G.; Dixmier, A.; Goupil, F.; Gut-Gobert, C.; Marchand-Adam, S.; Meurice, J.-C.; Morel, H.; Person-Tacnet, C.; Leroyer, C.; et al. Comorbidities of COPD. Eur. Respir. Rev. 2013, 22, 454–475. [Google Scholar] [CrossRef]
- Enriquez, J.R.; Parikh, S.V.; Selzer, F.; Jacobs, A.K.; Marroquin, O.; Mulukutla, S.; Srinivas, V.; Holper, E.M. Increased adverse events after percutaneous coronary intervention in patients with COPD: Insights from the National Heart, Lung, and Blood Institute dynamic registry. Chest 2011, 140, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Li, C.-L.; Cao, J.; Feng, J. Metabolic syndrome prevalence in patients with obstructive sleep apnea syndrome and chronic obstructive pulmonary disease: Relationship with systemic inflammation. Clin. Respir. J. 2020, 14, 1159–1165. [Google Scholar] [CrossRef]
- Kuklisova, Z.; Tkacova, R.; Joppa, P.; Wouters, E.; Sastry, M. Severity of nocturnal hypoxia and daytime hypercapnia predicts CPAP failure in patients with COPD and obstructive sleep apnea overlap syndrome. Sleep Med. 2017, 30, 139–145. [Google Scholar] [CrossRef]
- Khatri, S.B.; Ioachimescu, O.C. The intersection of obstructive lung disease and sleep apnea. Cleve. Clin. J. Med. 2016, 83, 127–140. [Google Scholar] [CrossRef]
- Choi, H.S.; Rhee, C.K.; Park, Y.B.; Yoo, K.H.; Lim, S.Y. Metabolic Syndrome in Early Chronic Obstructive Pulmonary Disease: Gender Differences and Impact on Exacerbation and Medical Costs. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2873–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, K.C.; Subhankar, S.; Mohanta, P.C.; Jagaty, S.K.; Dutta, P.; Pothal, S. Prevalence of metabolic syndrome in chronic obstructive pulmonary disease and its correlation with severity of disease. J. Fam. Med. Prim. Care 2022, 11, 2094–2098. [Google Scholar] [CrossRef]
- Geslain-Biquez, C.; Vol, S.; Tichet, J.; Caradec, A.; D’Hour, A.; Balkau, B. The metabolic syndrome in smokers. The D.E.S.I.R. study. Diabetes Metab. 2003, 29, 226–234. [Google Scholar] [CrossRef]
- Sun, Y.; Milne, S.; Jaw, J.E.; Yang, C.X.; Xu, F.; Li, X.; Obeidat, M.e.; Sin, D.D. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: A meta-analysis of clinical trials. Respir. Res. 2019, 20, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divo, M.J.; Cabrera, C.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.M.; de-Torres, J.P.; Zulueta, J.; Zagaceta, J.; Sanchez-Salcedo, P.; Berto, J.; et al. Comorbidity Distribution, Clinical Expression and Survival in COPD Patients with Different Body Mass Index. Chronic Obstr. Pulm. Dis. J. COPD Found. 2014, 1, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigsby, M.R.; Siddharthan, T.; Pollard, S.L.; Chowdhury, M.; Rubinstein, A.; Miranda, J.J.; Bernabe-Ortiz, A.; Alam, D.; Kirenga, B.; Jones, R.; et al. Low Body Mass Index Is Associated with Higher Odds of COPD and Lower Lung Function in Low- and Middle-Income Countries. COPD J. Chronic Obstr. Pulm. Dis. 2019, 16, 58–65. [Google Scholar] [CrossRef]
- Kim, E.K.; Singh, D.; Park, J.H.; Park, Y.B.; Kim, S.I.; Park, B.; Park, J.; Kim, J.H.; Kim, M.A.; Lee, J.H.; et al. Impact of Body Mass Index Change on the Prognosis of Chronic Obstructive Pulmonary Disease. Respiration 2020, 99, 943–953. [Google Scholar] [CrossRef]
- Wada, H.; Ikeda, A.; Maruyama, K.; Yamagishi, K.; Barnes, P.J.; Tanigawa, T.; Tamakoshi, A.; Iso, H. Low BMI and weight loss aggravate COPD mortality in men, findings from a large prospective cohort: The JACC study. Sci. Rep. 2021, 11, 1531. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.H.; Kim, H.J.; Park, J.-Y.; Lee, H.S.; Byun, M.K. The effect of low body mass index on the development of chronic obstructive pulmonary disease and mortality. J. Intern. Med. 2019, 286, 573–582. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Wang, Z.; Yu, F.; Xu, Q.; Guo, W.; Wu, C.; He, J. Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine 2016, 95, e4225. [Google Scholar] [CrossRef]
- Iyer, A.S.; Dransfield, M.T. The “Obesity Paradox” in Chronic Obstructive Pulmonary Disease: Can It Be Resolved? Ann. Am. Thorac. Soc. 2018, 15, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Ejike, C.O.; Wise, R.A.; McCormack, M.C.; Brigham, E.P. Investigation of the Obesity Paradox in Chronic Obstructive Pulmonary Disease, According to Smoking Status, in the United States. Am. J. Epidemiol. 2019, 188, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.F.; Stratton, R.J.; Kurukulaaratchy, R.; Elia, M. S163 The ‘Obesity Paradox’ in chronic obstructive pulmonary disease. Thorax 2010, 65, A73–A74. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Aguilar, E.; Orbe, J.; Fernández-Montero, A.; Fernández-Alonso, S.; Rodríguez, J.A.; Fernández-Alonso, L.; Páramo, J.A.; Roncal, C. Reduced high-density lipoprotein cholesterol: A valuable, independent prognostic marker in peripheral arterial disease. J. Vasc. Surg. 2017, 66, 1527–1533.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanbay, M.; Solak, Y.; Unal, H.U.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Oguz, Y.; Eyileten, T.; Vural, A.; et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int. Urol. Nephrol. 2014, 46, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Kundi, H.; Kiziltunc, E.; Cetin, M.; Cicekcioglu, H.; Cetin, Z.G.; Cicek, G.; Ornek, E. Association of monocyte/HDL-C ratio with SYNTAX scores in patients with stable coronary artery disease. Herz 2016, 41, 523–529. [Google Scholar] [CrossRef]
- Bolayir, A.; Gokce, S.F.; Cigdem, B.; Bolayir, H.A.; Yildiz, O.K.; Bolayir, E.; Topaktas, S.A. Monocyte/high-density lipoprotein ratio predicts the mortality in ischemic stroke patients. Neurol. Neurochir. Pol. 2018, 52, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Yakar, H.I.; Kanbay, A. Could monocyte level/HDL cholesterol ratio predict cardiovascular diseases in patients with COPD? Niger. J. Clin. Pract. 2020, 23, 450–455. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, J.; Zou, H.; Li, M.; Sun, W.; Kong, X. Monocyte-to-high-density lipoprotein-cholesterol ratio (MHR) and the risk of all-cause and cardiovascular mortality: A nationwide cohort study in the United States. Lipids Health Dis. 2022, 21, 30. [Google Scholar] [CrossRef]
- Acikgoz, N.; Kurtoğlu, E.; Yagmur, J.; Kapicioglu, Y.; Cansel, M.; Ermis, N. Elevated Monocyte to High-Density Lipoprotein Cholesterol Ratio and Endothelial Dysfunction in Behçet Disease. Angiology 2018, 69, 65–70. [Google Scholar] [CrossRef]
- Yılmaz, M.; Kayançiçek, H. A New Inflammatory Marker: Elevated Monocyte to HDL Cholesterol Ratio Associated with Smoking. J. Clin. Med. 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.P.; Hopkins, R.J. Update on the potential role of statins in chronic obstructive pulmonary disease and its co-morbidities. Expert Rev. Respir. Med. 2013, 7, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Hopkins, R.; Eaton, T. Pharmacological actions of statins: Potential utility in COPD. Eur. Respir. Rev. 2009, 18, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.; Perrem, L.; Khashan, A.S.; Henry, M.T.; Ni Chroinin, M. Statins versus placebo for people with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019, 7, Cd011959. [Google Scholar] [CrossRef] [PubMed]
- Rezk, N.A.; Elewa, A. Anti inflammatory effects of statin in COPD. Egypt. J. Chest Dis. Tuberc. 2013, 62, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Damkjær, M.; Håkansson, K.; Kallemose, T.; Ulrik, C.S.; Godtfredsen, N. Statins in High-Risk Chronic Obstructive Pulmonary Disease Outpatients: No Impact on Time to First Exacerbation and All-Cause Mortality—The STATUETTE Cohort Study. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 579–589. [Google Scholar] [CrossRef]
- Raymakers, A.J.; Sadatsafavi, M.; Sin, D.D.; De Vera, M.A.; Lynd, L.D. The impact of statin drug use on all-cause mortality in patients with COPD: A population-based cohort study. Chest 2017, 152, 486–493. [Google Scholar] [CrossRef]
- Cao, C.; Wu, Y.; Xu, Z.; Lv, D.; Zhang, C.; Lai, T.; Li, W.; Shen, H. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: A systematic review and meta-analysis of observational research. Sci. Rep. 2015, 5, 16461. [Google Scholar] [CrossRef] [Green Version]
- Ingebrigtsen, T.S.; Marott, J.L.; Nordestgaard, B.G.; Lange, P.; Hallas, J.; Vestbo, J. Statin use and exacerbations in individuals with chronic obstructive pulmonary disease. Thorax 2015, 70, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Yayan, J.; Bald, M.; Franke, K.-J. No Independent Influence of Statins on the Chronic Obstructive Pulmonary Disease Exacerbation Rate: A Cohort Observation Study Over 10 Years. Int. J. Gen. Med. 2021, 14, 2883–2892. [Google Scholar] [CrossRef]
- Smith, M.C.; Ashdown, H.F.; Sheppard, J.P.; Butler, C.C.; Bankhead, C. Statin prescription in patients with chronic obstructive pulmonary disease and risk of exacerbations: A retrospective cohort study in the Clinical Practice Research Datalink. BMJ Open 2021, 11, e050757. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-J.; Lin, C.-L.; Hsu, C.Y.; Shae, Z.; Kao, C.-H. Associations between statins and coronary artery disease and stroke risks in patients with asthma–chronic obstructive pulmonary disease overlap syndrome: A time-dependent regression study. Atherosclerosis 2019, 283, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Jankowski, V.; Bender, G.; Zewinger, S.; Rye, K.-A.; van der Vorst, E.P.C. Interaction between high-density lipoproteins and inflammation: Function matters more than concentration! Adv. Drug Deliv. Rev. 2020, 159, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Bandeali, S.; Farmer, J. High-density lipoprotein and atherosclerosis: The role of antioxidant activity. Curr. Atheroscler. Rep. 2012, 14, 101–107. [Google Scholar] [CrossRef]
- Sallese, A.; Suzuki, T.; McCarthy, C.; Bridges, J.; Filuta, A.; Arumugam, P.; Shima, K.; Ma, Y.; Wessendarp, M.; Black, D.; et al. Targeting cholesterol homeostasis in lung diseases. Sci. Rep. 2017, 7, 10211. [Google Scholar] [CrossRef]
- Remmerie, A.; Scott, C.L. Macrophages and lipid metabolism. Cell. Immunol. 2018, 330, 27–42. [Google Scholar] [CrossRef]
- Carey, B.; Trapnell, B.C. The molecular basis of pulmonary alveolar proteinosis. Clin. Immunol. 2010, 135, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Pian, M.S.; Dobbs, L.G. Lipoprotein-stimulated surfactant secretion in alveolar type II cells: Mediation by heterotrimeric G proteins. Am. J. Physiol. 1997, 273, L634–L639. [Google Scholar] [CrossRef] [Green Version]
- Kolleck, I.; Schlame, M.; Fechner, H.; Looman, A.C.; Wissel, H.; Rüstow, B. HDL is the major source of vitamin E for type II pneumocytes. Free Radic. Biol. Med. 1999, 27, 882–890. [Google Scholar] [CrossRef]
- Han, R. Plasma lipoproteins are important components of the immune system. Microbiol. Immunol. 2010, 54, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. High-density lipoproteins and the immune system. J. Lipids 2013, 2013, 684903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, N.; Glass, C.K. Regulation of macrophage function in inflammation and atherosclerosis. J. Lipid Res. 2009, 50, S277–S281. [Google Scholar] [CrossRef] [Green Version]
- Zannis, V.I.; Cole, F.S.; Jackson, C.L.; Kurnit, D.M.; Karathanasis, S.K. Distribution of apolipoprotein A-I, C-II, C-III, and E mRNA in fetal human tissues. Time-dependent induction of apolipoprotein E mRNA by cultures of human monocyte-macrophages. Biochemistry 1985, 24, 4450–4455. [Google Scholar] [CrossRef]
- Kingwell, B.A.; Chapman, M.J.; Kontush, A.; Miller, N.E. HDL-targeted therapies: Progress, failures and future. Nat. Rev. Drug Discov. 2014, 13, 445–464. [Google Scholar] [CrossRef]
- Zannis, V.I.; Fotakis, P.; Koukos, G.; Kardassis, D.; Ehnholm, C.; Jauhiainen, M.; Chroni, A. HDL Biogenesis, Remodeling, and Catabolism. In High Density Lipoproteins: From Biological Understanding to Clinical Exploitation; von Eckardstein, A., Kardassis, D., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 53–111. [Google Scholar]
- Zhao, G.-J.; Yin, K.; Fu, Y.-C.; Tang, C.-K. The interaction of ApoA-I and ABCA1 triggers signal transduction pathways to mediate efflux of cellular lipids. Mol. Med. 2012, 18, 149–158. [Google Scholar] [CrossRef]
- Smoak, K.A.; Aloor, J.J.; Madenspacher, J.; Merrick, B.A.; Collins, J.B.; Zhu, X.; Cavigiolio, G.; Oda, M.N.; Parks, J.S.; Fessler, M.B. Myeloid differentiation primary response protein 88 couples reverse cholesterol transport to inflammation. Cell Metab. 2010, 11, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Chen, W.; Linsel-Nitschke, P.; Martinez, L.O.; Agerholm-Larsen, B.; Silver, D.L.; Tall, A.R. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J. Clin. Investig. 2003, 111, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Cooke, A.L.; Morris, J.; Melchior, J.T.; Street, S.E.; Jerome, W.G.; Huang, R.; Herr, A.B.; Smith, L.E.; Segrest, J.P.; Remaley, A.T.; et al. A thumbwheel mechanism for APOA1 activation of LCAT activity in HDL. J. Lipid Res. 2018, 59, 1244–1255. [Google Scholar] [CrossRef] [Green Version]
- Sorci-Thomas, M.G.; Bhat, S.; Thomas, M.J. Activation of lecithin:cholesterol acyltransferase by HDL ApoA-I central helices. Clin. Lipidol. 2009, 4, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, A.; Melchior, J.T.; Morris, J.C.; Huang, R.; Jerome, W.G.; Davidson, W.S. Abstract 541: The Molecular Interaction of Apolipoprotein A-I Containing High Density Lipoproteins with Lecithin: Cholesterol Acyl Transferase. Arterioscler. Thromb. Vasc. Biol. 2016, 36, A541. [Google Scholar] [CrossRef]
- Casteleijn, M.G.; Parkkila, P.; Viitala, T.; Koivuniemi, A. Interaction of lecithin:cholesterol acyltransferase with lipid surfaces and apolipoprotein A-I-derived peptides. J. Lipid Res. 2018, 59, 670–683. [Google Scholar] [CrossRef] [Green Version]
- Sankaranarayanan, S.; Oram, J.F.; Asztalos, B.F.; Vaughan, A.M.; Lund-Katz, S.; Adorni, M.P.; Phillips, M.C.; Rothblat, G.H. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 2009, 50, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Levine, S.J. Emerging Roles of Apolipoprotein E and Apolipoprotein A-I in the Pathogenesis and Treatment of Lung Disease. Am. J. Respir. Cell Mol. Biol. 2016, 55, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Yao, X.; Levine, S.J. High-density Lipoproteins and Apolipoprotein A-I: Potential New Players in the Prevention and Treatment of Lung Disease. Front. Pharmacol. 2016, 7, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Xu, H.; Shi, Y.; Nandedkar, S.; Zhang, H.; Gao, H.; Feroah, T.; Weihrauch, D.; Schulte, M.L.; Jones, D.W.; et al. Genetic deletion of apolipoprotein A-I increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. J. Lipid Res. 2010, 51, 2560–2570. [Google Scholar] [CrossRef] [Green Version]
- Provost, P.R.; Boucher, E.; Tremblay, Y. Apolipoprotein A-I, A-II, C-II, and H expression in the developing lung and sex difference in surfactant lipids. J. Endocrinol. 2009, 200, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Lee, J.M.; Park, S.W.; Kim, K.S.; Lee, M.W.; Paik, S.; Jang, A.S.; Kim, D.J.; Uh, S.; Kim, Y.; et al. Attenuation of Cigarette Smoke-Induced Emphysema in Mice by Apolipoprotein A-1 Overexpression. Am. J. Respir. Cell Mol. Biol. 2016, 54, 91–102. [Google Scholar] [CrossRef]
- Slagter, S.N.; van Vliet-Ostaptchouk, J.V.; Vonk, J.M.; Boezen, H.M.; Dullaart, R.P.F.; Kobold, A.C.M.; Feskens, E.J.; van Beek, A.P.; van der Klauw, M.M.; Wolffenbuttel, B.H.R. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med. 2013, 11, 195. [Google Scholar] [CrossRef] [Green Version]
- Jubinville, É.; Talbot, M.; Bérubé, J.-C.; Hamel-Auger, M.; Maranda-Robitaille, M.; Beaulieu, M.-J.; Aubin, S.; Paré, M.-È.; Kallend, D.G.; Arsenault, B.; et al. Interplay between cigarette smoking and pulmonary reverse lipid transport. Eur. Respir. J. 2017, 50, 1700681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barochia, A.V.; Kaler, M.; Cuento, R.A.; Gordon, E.M.; Weir, N.A.; Sampson, M.; Fontana, J.R.; MacDonald, S.; Moss, J.; Manganiello, V.; et al. Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 990–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotlyarov, S.N.; Kotlyarova, A.A. Role of lipid metabolism and systemic inflammation in the development of atherosclerosis in animal models. IP Pavlov. Russ. Med. Biol. Her. 2021, 29, 134–146. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emancipator, K.; Csako, G.; Elin, R.J. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect. Immun. 1992, 60, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Liao, X.L.; Lou, B.; Wu, M.P. Role of apolipoprotein A-I in protecting against endotoxin toxicity. Acta Biochim. Biophys. Sin. 2004, 36, 419–424. [Google Scholar] [CrossRef]
- Jiao, Y.L.; Wu, M.P. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine 2008, 43, 83–87. [Google Scholar] [CrossRef]
- Henning, M.F.; Herlax, V.; Bakás, L. Contribution of the C-terminal end of apolipoprotein AI to neutralization of lipopolysaccharide endotoxic effect. Innate Immun. 2011, 17, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Van Linthout, S.; Spillmann, F.; Graiani, G.; Miteva, K.; Peng, J.; Van Craeyveld, E.; Meloni, M.; Tölle, M.; Escher, F.; Subasigüller, A.; et al. Down-regulation of endothelial TLR4 signalling after apo A-I gene transfer contributes to improved survival in an experimental model of lipopolysaccharide-induced inflammation. J. Mol. Med. 2011, 89, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Sharifov, O.F.; Xu, X.; Gaggar, A.; Grizzle, W.E.; Mishra, V.K.; Honavar, J.; Litovsky, S.H.; Palgunachari, M.N.; White, C.R.; Anantharamaiah, G.M.; et al. Anti-inflammatory mechanisms of apolipoprotein A-I mimetic peptide in acute respiratory distress syndrome secondary to sepsis. PLoS ONE 2013, 8, e64486. [Google Scholar] [CrossRef]
- Brandenburg, K.; Jürgens, G.; Andrä, J.; Lindner, B.; Koch, M.H.J.; Blume, A.; Garidel, P. Biophysical characterization of the interaction of high-density lipoprotein (HDL) with endotoxins. Eur. J. Biochem. 2002, 269, 5972–5981. [Google Scholar] [CrossRef] [PubMed]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11, 1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, D.M.; Parker, T.S.; Donnelly, T.M.; Walsh, A.; Rubin, A.L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 12040–12044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurfel, M.M.; Kunitake, S.T.; Lichenstein, H.; Kane, J.P.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994, 180, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, M.A.; Adamek, M.; Bilińska, B.; Hejmej, A.; Steinhagen, D.; Ciereszko, A. Characterization, expression and antibacterial properties of apolipoproteins A from carp (Cyprinus carpio L.) seminal plasma. Fish Shellfish. Immunol. 2014, 41, 389–401. [Google Scholar] [CrossRef]
- Dietrich, M.A.; Nynca, J.; Adamek, M.; Steinhagen, D.; Karol, H.; Ciereszko, A. Expression of apolipoprotein A-I and A-II in rainbow trout reproductive tract and their possible role in antibacterial defence. Fish Shellfish. Immunol. 2015, 45, 750–756. [Google Scholar] [CrossRef]
- Karan, S.; Mohapatra, A.; Sahoo, P.K.; Garg, L.C.; Dixit, A. Structural-functional characterization of recombinant Apolipoprotein A-I fromLabeo rohitademonstrates heat-resistant antimicrobial activity. Appl. Microbiol. Biotechnol. 2020, 104, 145–159. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Q.; Chen, J. Identification, expression analysis, and antibacterial activity of Apolipoprotein A-I from amphioxus (Branchiostoma belcheri). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 238, 110329. [Google Scholar] [CrossRef]
- Biedzka-Sarek, M.; Metso, J.; Kateifides, A.; Meri, T.; Jokiranta, T.S.; Muszyński, A.; Radziejewska-Lebrecht, J.; Zannis, V.; Skurnik, M.; Jauhiainen, M. Apolipoprotein A-I Exerts Bactericidal Activity against Yersinia enterocolitica Serotype O:3*. J. Biol. Chem. 2011, 286, 38211–38219. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.P.; Chopra, A.K.; Coppenhaver, D.H.; Ananatharamaiah, G.M.; Baron, S. Lipoproteins account for part of the broad non-specific antiviral activity of human serum. Antivir. Res. 1999, 42, 211–218. [Google Scholar] [CrossRef]
- Tang, C.; Houston, B.A.; Storey, C.; LeBoeuf, R.C. Both STAT3 activation and cholesterol efflux contribute to the anti-inflammatory effect of apoA-I/ABCA1 interaction in macrophages. J. Lipid Res. 2016, 57, 848–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.M.; Handa, P.; Tateya, S.; Schwartz, J.; Tang, C.; Mitra, P.; Oram, J.F.; Chait, A.; Kim, F. Apolipoprotein A-I attenuates palmitate-mediated NF-κB activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLoS ONE 2012, 7, e33917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Li, L.; Xie, W.; Wu, J.F.; Yao, F.; Tan, Y.L.; Xia, X.D.; Liu, X.Y.; Liu, D.; Lan, G.; et al. Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation. Atherosclerosis 2016, 248, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Low, H.; Mukhamedova, N.; Capettini, L.d.S.A.; Xia, Y.; Carmichael, I.; Cody, S.H.; Huynh, K.; Ditiatkovski, M.; Ohkawa, R.; Bukrinsky, M.; et al. Cholesterol Efflux-Independent Modification of Lipid Rafts by AIBP (Apolipoprotein A-I Binding Protein). Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2346–2359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, G.J.; Yin, K.; Xia, X.D.; Gong, D.; Zhao, Z.W.; Chen, L.Y.; Zheng, X.L.; Tang, X.E.; Tang, C.K. Apolipoprotein A-1 Binding Protein Inhibits Inflammatory Signaling Pathways by Binding to Apolipoprotein A-1 in THP-1 Macrophages. Circ. J. 2018, 82, 1396–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Wallace, A.M.; Schneider, D.A.; Burg, E.; Kim, J.; Alekseeva, E.; Ubags, N.D.; Cool, C.D.; Fang, L.; Suratt, B.T.; et al. AIBP augments cholesterol efflux from alveolar macrophages to surfactant and reduces acute lung inflammation. JCI Insight 2018, 3, e120519. [Google Scholar] [CrossRef] [Green Version]
- Furlaneto, C.J.; Ribeiro, F.P.; Hatanaka, E.; Souza, G.M.; Cassatella, M.A.; Campa, A. Apolipoproteins A-I and A-II downregulate neutrophil functions. Lipids 2002, 37, 925–928. [Google Scholar] [CrossRef]
- Meilhac, O.; Tanaka, S.; Couret, D. High-Density Lipoproteins Are Bug Scavengers. Biomolecules 2020, 10, 598. [Google Scholar] [CrossRef]
- Jacobo-Albavera, L.; Domínguez-Pérez, M.; Medina-Leyte, D.J.; González-Garrido, A.; Villarreal-Molina, T. The Role of the ATP-Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021, 22, 1593. [Google Scholar] [CrossRef]
- Chai, A.B.; Ammit, A.J.; Gelissen, I.C. Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Respir. Res. 2017, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Yvan-Charvet, L.; Ranalletta, M.; Wang, N.; Han, S.; Terasaka, N.; Li, R.; Welch, C.; Tall, A.R. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Investig. 2007, 117, 3900–3908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, K.; Tomioka, M.; Ueda, K. Function and regulation of ABCA1—Membrane meso-domain organization and reorganization. FEBS J. 2011, 278, 3190–3203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schwabe, R.F.; DeVries-Seimon, T.; Yao, P.M.; Gerbod-Giannone, M.C.; Tall, A.R.; Davis, R.J.; Flavell, R.; Brenner, D.A.; Tabas, I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: Model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 2005, 280, 21763–21772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerbod-Giannone, M.-C.; Li, Y.; Holleboom, A.; Han, S.; Hsu, L.-C.; Tabas, I.; Tall, A.R. TNFα induces ABCA1 through NF-κB in macrophages and in phagocytes ingesting apoptotic cells. Proc. Natl. Acad. Sci. USA 2006, 103, 3112–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, N.; Abe-Dohmae, S.; Sato, R.; Yokoyama, S. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J. Lipid Res. 2006, 47, 1915–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe-Dohmae, S.; Ikeda, Y.; Matsuo, M.; Hayashi, M.; Okuhira, K.-i.; Ueda, K.; Yokoyama, S. Human ABCA7 Supports Apolipoprotein-mediated Release of Cellular Cholesterol and Phospholipid to Generate High Density Lipoprotein*. J. Biol. Chem. 2004, 279, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Abe-Dohmae, S.; Iwamoto, N.; Fitzgerald, M.L.; Yokoyama, S. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7 [S]. J. Lipid Res. 2010, 51, 2591–2599. [Google Scholar] [CrossRef] [Green Version]
- Bates, S.R.; Tao, J.-Q.; Collins, H.L.; Francone, O.L.; Rothblat, G.H. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 289, L980–L989. [Google Scholar] [CrossRef]
- McNeish, J.; Aiello, R.J.; Guyot, D.; Turi, T.; Gabel, C.; Aldinger, C.; Hoppe, K.L.; Roach, M.L.; Royer, L.J.; de Wet, J.; et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA 2000, 97, 4245–4250. [Google Scholar] [CrossRef] [Green Version]
- Kotlyarov, S.; Kotlyarova, A. Bioinformatic Analysis of ABCA1 Gene Expression in Smoking and Chronic Obstructive Pulmonary Disease. Membranes 2021, 11, 674. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, J.; Wang, Y.; Sun, J. High density lipoprotein promoting proliferation and migration of type II alveolar epithelial cells during inflammation state. Lipids Health Dis. 2017, 16, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Out, R.; Hoekstra, M.; Habets, K.; Meurs, I.; de Waard, V.; Hildebrand, R.B.; Wang, Y.; Chimini, G.; Kuiper, J.; Van Berkel, T.J.; et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, A.M.; Oram, J.F. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006, 47, 2433–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelissen, I.C.; Harris, M.; Rye, K.A.; Quinn, C.; Brown, A.J.; Kockx, M.; Cartland, S.; Packianathan, M.; Kritharides, L.; Jessup, W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessup, W.; Gelissen, I.C.; Gaus, K.; Kritharides, L. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 2006, 17, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Vaughan, A.M. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006, 99, 1031–1043. [Google Scholar] [CrossRef]
- Seres, L.; Cserepes, J.; Elkind, N.B.; Törocsik, D.; Nagy, L.; Sarkadi, B.; Homolya, L. Functional ABCG1 expression induces apoptosis in macrophages and other cell types. Biochim. Biophys. Acta 2008, 1778, 2378–2387. [Google Scholar] [CrossRef] [Green Version]
- Wojcik, A.J.; Skaflen, M.D.; Srinivasan, S.; Hedrick, C.C. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J. Immunol. 2008, 180, 4273–4282. [Google Scholar] [CrossRef] [Green Version]
- Baldan, A.; Gonen, A.; Choung, C.; Que, X.; Marquart, T.J.; Hernandez, I.; Bjorkhem, I.; Ford, D.A.; Witztum, J.L.; Tarling, E.J. ABCG1 is required for pulmonary B-1 B cell and natural antibody homeostasis. J. Immunol. 2014, 193, 5637–5648. [Google Scholar] [CrossRef] [Green Version]
- Baldán, Á.; Gomes, A.V.; Ping, P.; Edwards, P.A. Loss of ABCG1 Results in Chronic Pulmonary Inflammation. J. Immunol. 2008, 180, 3560–3568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churg, A.; Zhou, S.; Wright, J.L. Matrix metalloproteinases in COPD. Eur. Respir. J. 2012, 39, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Sng, J.J.; Prazakova, S.; Thomas, P.S.; Herbert, C. MMP-8, MMP-9 and Neutrophil Elastase in Peripheral Blood and Exhaled Breath Condensate in COPD. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Draper, D.W.; Madenspacher, J.H.; Dixon, D.; King, D.H.; Remaley, A.T.; Fessler, M.B. ATP-binding cassette transporter G1 deficiency dysregulates host defense in the lung. Am. J. Respir. Crit. Care Med. 2010, 182, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-T.; Kreutzberger, A.J.B.; Lee, J.; Kiessling, V.; Tamm, L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids 2016, 199, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Devaux, P.F.; Morris, R. Transmembrane Asymmetry and Lateral Domains in Biological Membranes. Traffic 2004, 5, 241–246. [Google Scholar] [CrossRef]
- Song, Y.; Kenworthy, A.K.; Sanders, C.R. Cholesterol as a co-solvent and a ligand for membrane proteins. Protein Sci. 2014, 23, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Fantini, J.; Epand, R.M.; Barrantes, F.J. Cholesterol-Recognition Motifs in Membrane Proteins. In Direct Mechanisms in Cholesterol Modulation of Protein Function; Rosenhouse-Dantsker, A., Bukiya, A.N., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–25. [Google Scholar]
- Fantini, J.; Barrantes, F. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, L.J.; Rao, G.; Jones, P.M.; Glancey, E.; Aleidi, S.M.; George, A.M.; Brown, A.J.; Gelissen, I.C. Cholesterol sensing by the ABCG1 lipid transporter: Requirement of a CRAC motif in the final transmembrane domain. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 956–964. [Google Scholar] [CrossRef]
- Sidletskaya, K.; Vitkina, T.; Denisenko, Y. The Role of Toll-Like Receptors 2 and 4 in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Du, X.; Poltorak, A. Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: Genetic and evolutionary studies. J. Endotoxin Res. 2001, 7, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.W.; Kwon, M.-J.; Choi, A.M.K.; Kim, H.-P.; Nakahira, K.; Hwang, D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar] [CrossRef] [Green Version]
- Sarir, H.; Mortaz, E.; Karimi, K.; Kraneveld, A.D.; Rahman, I.; Caldenhoven, E.; Nijkamp, F.P.; Folkerts, G. Cigarette smoke regulates the expression of TLR4 and IL-8 production by human macrophages. J. Inflamm. 2009, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shan, P.; Jiang, G.; Cohn, L.; Lee, P.J. Toll-like receptor 4 deficiency causes pulmonary emphysema. J. Clin. Investig. 2006, 116, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Ruysschaert, J.M.; Lonez, C. Role of lipid microdomains in TLR-mediated signalling. Biochim. Biophys. Acta 2015, 1848, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Woollard, K.J.; Hoang, A.; Mukhamedova, N.; Stirzaker, R.A.; McCormick, S.P.; Remaley, A.T.; Sviridov, D.; Chin-Dusting, J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2071–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.; Gelissen, I.C.; Ammit, A.J. Regulation of ATP binding cassette transporter A1 (ABCA1) expression: Cholesterol-dependent and—Independent signaling pathways with relevance to inflammatory lung disease. Respir. Res. 2020, 21, 250. [Google Scholar] [CrossRef]
- Sonett, J.; Goldklang, M.; Sklepkiewicz, P.; Gerber, A.; Trischler, J.; Zelonina, T.; Westerterp, M.; Lemaître, V.; Okada, Y.; D’Armiento, J. A critical role for ABC transporters in persistent lung inflammation in the development of emphysema after smoke exposure. FASEB J. 2018, 32, fj201701381. [Google Scholar] [CrossRef]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef]
- Lewis, G.F.; Rader, D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blauw, L.L.; Wang, Y.; Willems van Dijk, K.; Rensen, P.C.N. A Novel Role for CETP as Immunological Gatekeeper: Raising HDL to Cure Sepsis? Trends Endocrinol. Metab. 2020, 31, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Azzam, K.M.; Fessler, M.B. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol. Metab. 2012, 23, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topchiy, E.; Cirstea, M.; Kong, H.J.; Boyd, J.H.; Wang, Y.; Russell, J.A.; Walley, K.R. Lipopolysaccharide Is Cleared from the Circulation by Hepatocytes via the Low Density Lipoprotein Receptor. PLoS ONE 2016, 11, e0155030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Ji, A.; de Beer, F.C.; Tannock, L.R.; van der Westhuyzen, D.R. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Investig. 2008, 118, 364–375. [Google Scholar] [CrossRef] [Green Version]
- Munford, R.S.; Weiss, J.P.; Lu, M. Biochemical transformation of bacterial lipopolysaccharides by acyloxyacyl hydrolase reduces host injury and promotes recovery. J. Biol. Chem. 2020, 295, 17842–17851. [Google Scholar] [CrossRef]
- Shrestha, S.; Wu, B.J.; Guiney, L.; Barter, P.J.; Rye, K.-A. Cholesteryl ester transfer protein and its inhibitors. J. Lipid Res. 2018, 59, 772–783. [Google Scholar] [CrossRef] [Green Version]
- Trinder, M.; Genga, K.R.; Kong, H.J.; Blauw, L.L.; Lo, C.; Li, X.; Cirstea, M.; Wang, Y.; Rensen, P.C.N.; Russell, J.A.; et al. Cholesteryl Ester Transfer Protein Influences High-Density Lipoprotein Levels and Survival in Sepsis. Am. J. Respir. Crit. Care Med. 2019, 199, 854–862. [Google Scholar] [CrossRef]
- Cazita, P.M.; Barbeiro, D.F.; Moretti, A.I.; Quintão, E.C.; Soriano, F.G. Human cholesteryl ester transfer protein expression enhances the mouse survival rate in an experimental systemic inflammation model: A novel role for CETP. Shock 2008, 30, 590–595. [Google Scholar] [CrossRef]
- Santana, K.G.; Righetti, R.F.; Breda, C.N.d.S.; Domínguez-Amorocho, O.A.; Ramalho, T.; Dantas, F.E.B.; Nunes, V.S.; Tibério, I.d.F.L.C.; Soriano, F.G.; Câmara, N.O.S.; et al. Cholesterol-Ester Transfer Protein Alters M1 and M2 Macrophage Polarization and Worsens Experimental Elastase-Induced Pulmonary Emphysema. Front. Immunol. 2021, 12, 684076. [Google Scholar] [CrossRef]
- Ma, L.; Dong, F.; Zaid, M.; Kumar, A.; Zha, X. ABCA1 protein enhances Toll-like receptor 4 (TLR4)-stimulated interleukin-10 (IL-10) secretion through protein kinase A (PKA) activation. J. Biol. Chem. 2012, 287, 40502–40512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tall, A.R. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J. Intern. Med. 2008, 263, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [Green Version]
- Di Bartolo, B.A.; Duong, M.; Nicholls, S.J. Clinical trials with cholesteryl ester transfer protein inhibitors. Curr. Opin. Lipidol 2016, 27, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, L.A.; Sugarman, E.D.; Bourassa, P.A.; Sand, T.M.; Zimetti, F.; Gao, F.; Rothblat, G.H.; Milici, A.J. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J. Lipid Res. 2007, 48, 1263–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Pritchard, D.K.; Becker, L.; Hoofnagle, A.N.; Tanimura, N.; Bammler, T.K.; Beyer, R.P.; Bumgarner, R.; Vaisar, T.; Beer, M.C.d.; et al. High-Density Lipoprotein Suppresses the Type I Interferon Response, a Family of Potent Antiviral Immunoregulators, in Macrophages Challenged With Lipopolysaccharide. Circulation 2010, 122, 1919–1927. [Google Scholar] [CrossRef]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A.; et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Rohatgi, A.; Westerterp, M.; Eckardstein, A.v.; Remaley, A.; Rye, K.-A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
- Ansell, B.J.; Fonarow, G.C.; Fogelman, A.M. High-density lipoprotein: Is it always atheroprotective? Curr. Atheroscler. Rep. 2006, 8, 405–411. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C.; Theodorou, K.; Wu, Y.; Hoeksema, M.A.; Goossens, P.; Bursill, C.A.; Aliyev, T.; Huitema, L.F.A.; Tas, S.W.; Wolfs, I.M.J.; et al. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-κB/STAT1-IRF1 Signaling. Cell Metab. 2017, 25, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotakis, P.; Kothari, V.; Thomas, D.G.; Westerterp, M.; Molusky, M.M.; Altin, E.; Abramowicz, S.; Wang, N.; He, Y.; Heinecke, J.W.; et al. Anti-Inflammatory Effects of HDL (High-Density Lipoprotein) in Macrophages Predominate Over Proinflammatory Effects in Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e253–e272. [Google Scholar] [CrossRef] [PubMed]
- Van Lenten, B.J.; Hama, S.Y.; de Beer, F.C.; Stafforini, D.M.; McIntyre, T.M.; Prescott, S.M.; La Du, B.N.; Fogelman, A.M.; Navab, M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Investig. 1995, 96, 2758–2767. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Anantharamaiah, G.M.; Fogelman, A.M. The role of dysfunctional HDL in atherosclerosis. J. Lipid Res. 2009, 50, S145–S149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- G, H.B.; Rao, V.S.; Kakkar, V.V. Friend Turns Foe: Transformation of Anti-Inflammatory HDL to Proinflammatory HDL during Acute-Phase Response. Cholesterol 2011, 2011, 274629. [Google Scholar] [CrossRef] [Green Version]
- Uhlar, C.M.; Whitehead, A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999, 265, 501–523. [Google Scholar] [CrossRef]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D.; et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016, 126, 266–281. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Abbasi, A.; Rossiter, H.B.; Su, X.; Liu, H.; Pi, Y.; Sang, L.; Zhong, W.; Yang, Q.; Guo, X.; et al. Serum Amyloid A in Stable COPD Patients is Associated with the Frequent Exacerbator Phenotype. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2379–2388. [Google Scholar] [CrossRef]
- Bozinovski, S.; Hutchinson, A.; Thompson, M.; Macgregor, L.; Black, J.; Giannakis, E.; Karlsson, A.S.; Silvestrini, R.; Smallwood, D.; Vlahos, R.; et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, K.C.; Remaley, A.T. Lipid-based carriers of microRNAs and intercellular communication. Curr. Opin. Lipidol. 2012, 23, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roffel, M.P.; Bracke, K.R.; Heijink, I.H.; Maes, T. miR-223: A Key Regulator in the Innate Immune Response in Asthma and COPD. Front. Med. 2020, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, M.; Gerlic, M.; O’Neill, L.A.J.; Masters, S.L. miR-223: Infection, inflammation and cancer. J. Intern. Med. 2013, 274, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Johnnidis, J.B.; Harris, M.H.; Wheeler, R.T.; Stehling-Sun, S.; Lam, M.H.; Kirak, O.; Brummelkamp, T.R.; Fleming, M.D.; Camargo, F.D. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008, 451, 1125–1129. [Google Scholar] [CrossRef]
- Ezzie, M.E.; Crawford, M.; Cho, J.-H.; Orellana, R.; Zhang, S.; Gelinas, R.; Batte, K.; Yu, L.; Nuovo, G.; Galas, D.; et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax 2012, 67, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Schuliga, M. NF-kappaB Signaling in Chronic Inflammatory Airway Disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef]
- Zhou, W.; Pal, A.S.; Hsu, A.Y.-H.; Gurol, T.; Zhu, X.; Wirbisky-Hershberger, S.E.; Freeman, J.L.; Kasinski, A.L.; Deng, Q. MicroRNA-223 Suppresses the Canonical NF-κB Pathway in Basal Keratinocytes to Dampen Neutrophilic Inflammation. Cell Rep. 2018, 22, 1810–1823. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wang, Y.; Ma, Y.; Yang, J. MiR-223 plays a protecting role in neutrophilic asthmatic mice through the inhibition of NLRP3 inflammasome. Respir. Res. 2020, 21, 116. [Google Scholar] [CrossRef]
- Leuenberger, C.; Schuoler, C.; Bye, H.; Mignan, C.; Rechsteiner, T.; Hillinger, S.; Opitz, I.; Marsland, B.; Faiz, A.; Hiemstra, P.S.; et al. MicroRNA-223 controls the expression of histone deacetylase 2: A novel axis in COPD. J. Mol. Med. 2016, 94, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Tabet, F.; Vickers, K.C.; Cuesta Torres, L.F.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S.; et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014, 5, 3292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, C.H.; Noronha Filho, A.J.; Marques e Silva, R.M.F.; da Cruz, T.F.; de Oliveira Monteiro, V.; Pio, M.; Rufino, R.L. Alpha 1-antitrypsin deficiency in patients with chronic obstructive pulmonary disease patients: Is systematic screening necessary? BMC Res. Notes 2019, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, J.-A.; Ortega-Gomez, A.; Rubio-Navarro, A.; Louedec, L.; Ho-Tin-Noé, B.; Caligiuri, G.; Nicoletti, A.; Levoye, A.; Plantier, L.; Meilhac, O. High-Density Lipoproteins Potentiate α1-Antitrypsin Therapy in Elastase-Induced Pulmonary Emphysema. Am. J. Respir. Cell Mol. Biol. 2014, 51, 536–549. [Google Scholar] [CrossRef]
- Gordon, S.M.; Sviridov, D.; Sakurai, T.; Freeman, L.; Remaley, A.T. Abstract 29: Alpha-1-antitrypsin Protects High Density Lipoprotein From Functional Inactivation by Elastase. Arterioscler. Thromb. Vasc. Biol. 2016, 36, A29. [Google Scholar] [CrossRef]
- Segal, L.; Lewis, E.C. The lipid ties of α1-antitrypsin: Structural and functional aspects. Cell Immunol. 2022, 375, 104528. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, R.L.; Wolfbauer, G.; Albers, J.J.; Munford, R.S. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J. Biol. Chem. 1999, 274, 34116–34122. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlyarov, S. High-Density Lipoproteins: A Role in Inflammation in COPD. Int. J. Mol. Sci. 2022, 23, 8128. https://doi.org/10.3390/ijms23158128
Kotlyarov S. High-Density Lipoproteins: A Role in Inflammation in COPD. International Journal of Molecular Sciences. 2022; 23(15):8128. https://doi.org/10.3390/ijms23158128
Chicago/Turabian StyleKotlyarov, Stanislav. 2022. "High-Density Lipoproteins: A Role in Inflammation in COPD" International Journal of Molecular Sciences 23, no. 15: 8128. https://doi.org/10.3390/ijms23158128





