Molecular Identification of the G-Protein Genes and Their Expression Profiles in Response to Nitrogen Deprivation in Brassica napus
Abstract
:1. Introduction
2. Results
2.1. Identification and Analysis of G-Protein Genes in B. napus
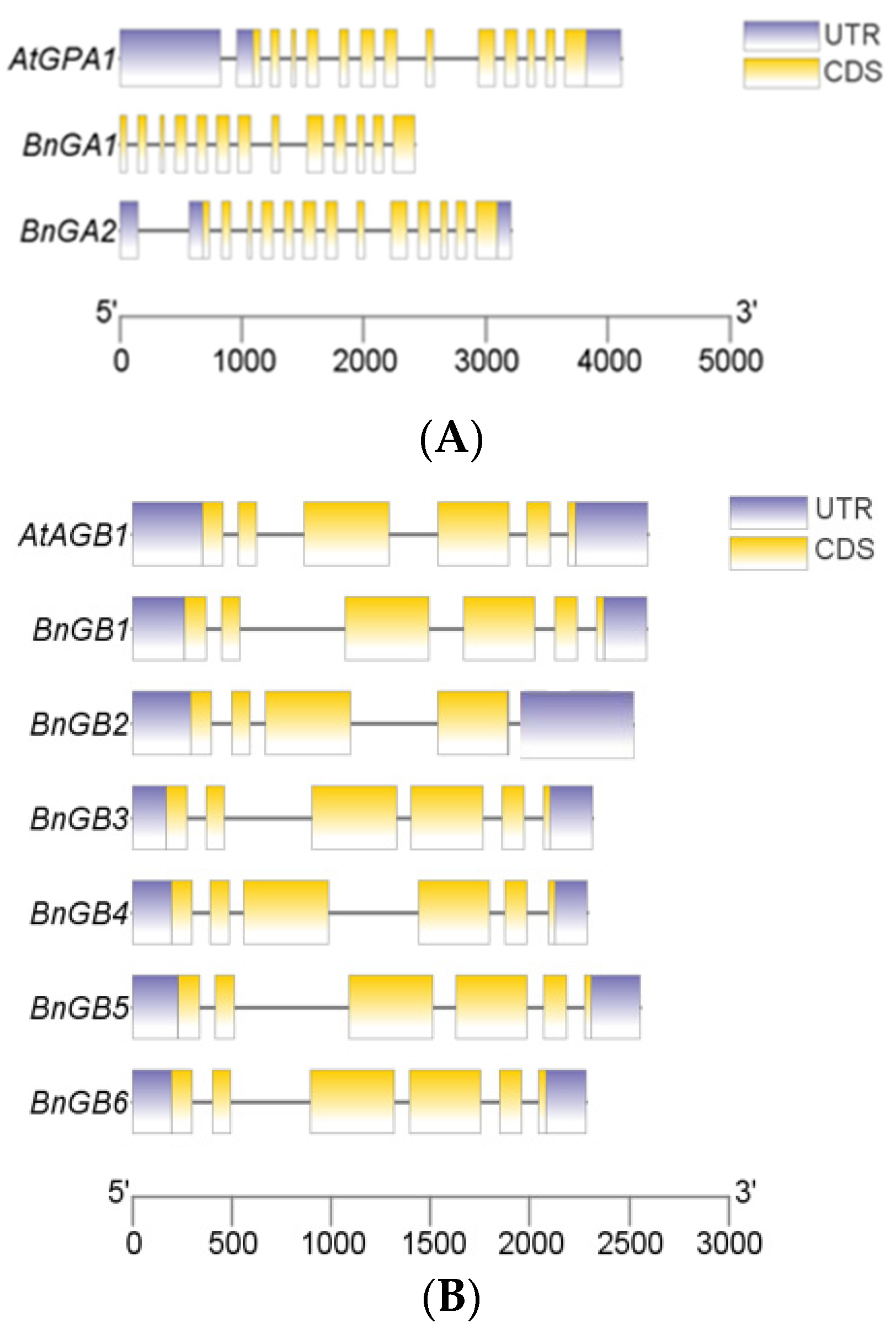
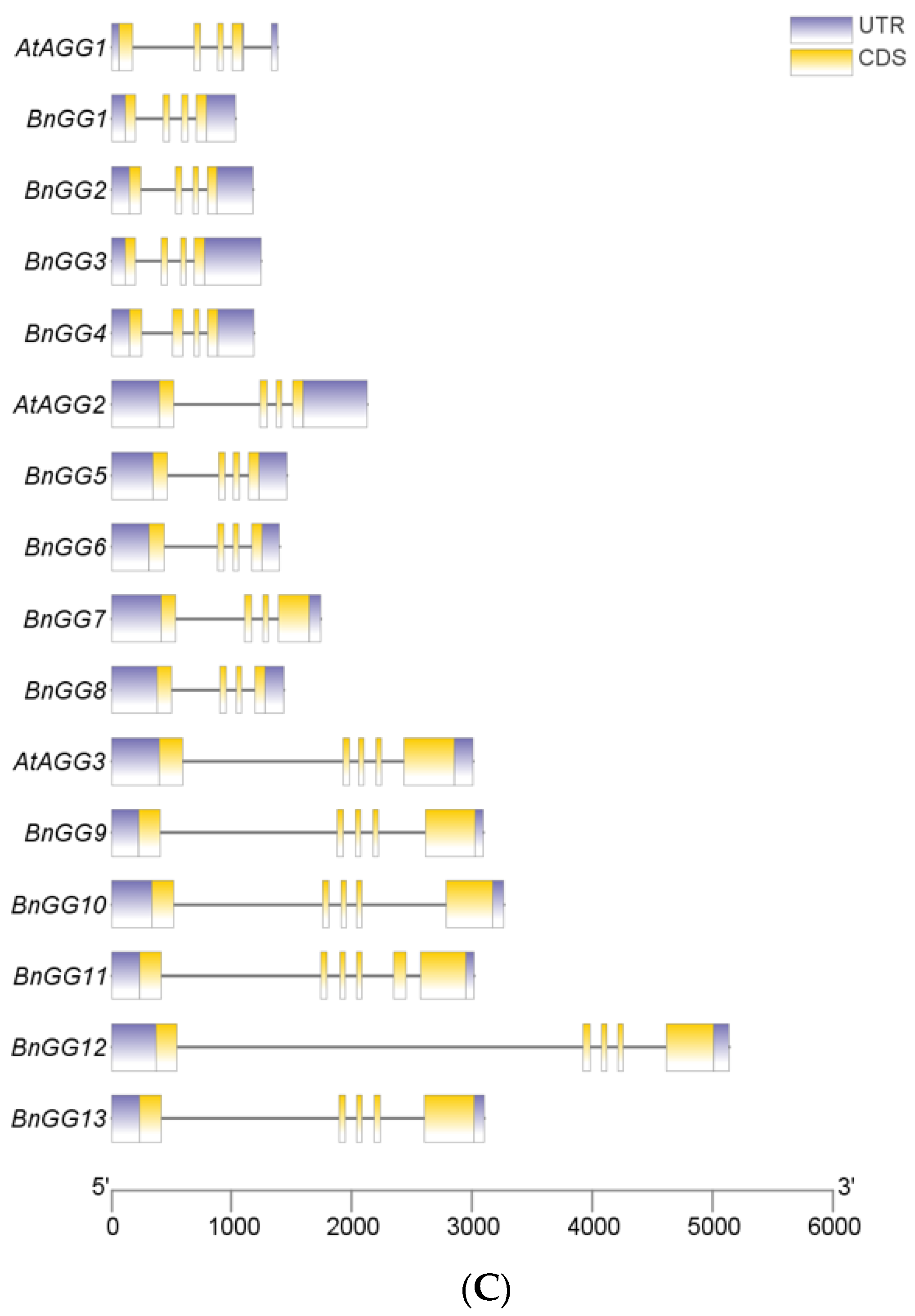
2.2. Gene Structure Analyses
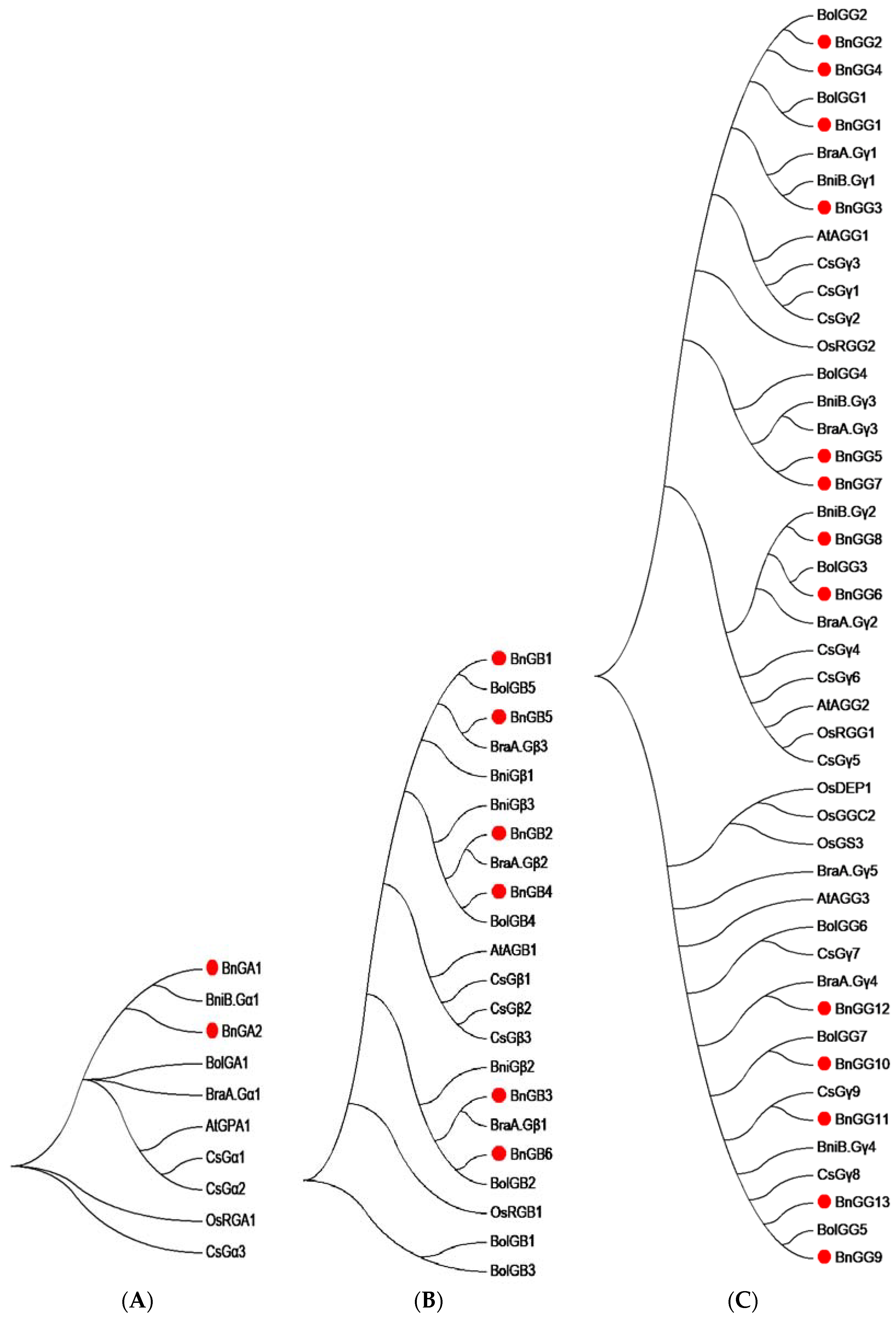
2.3. Phylogenetic Analysis of G-Protein
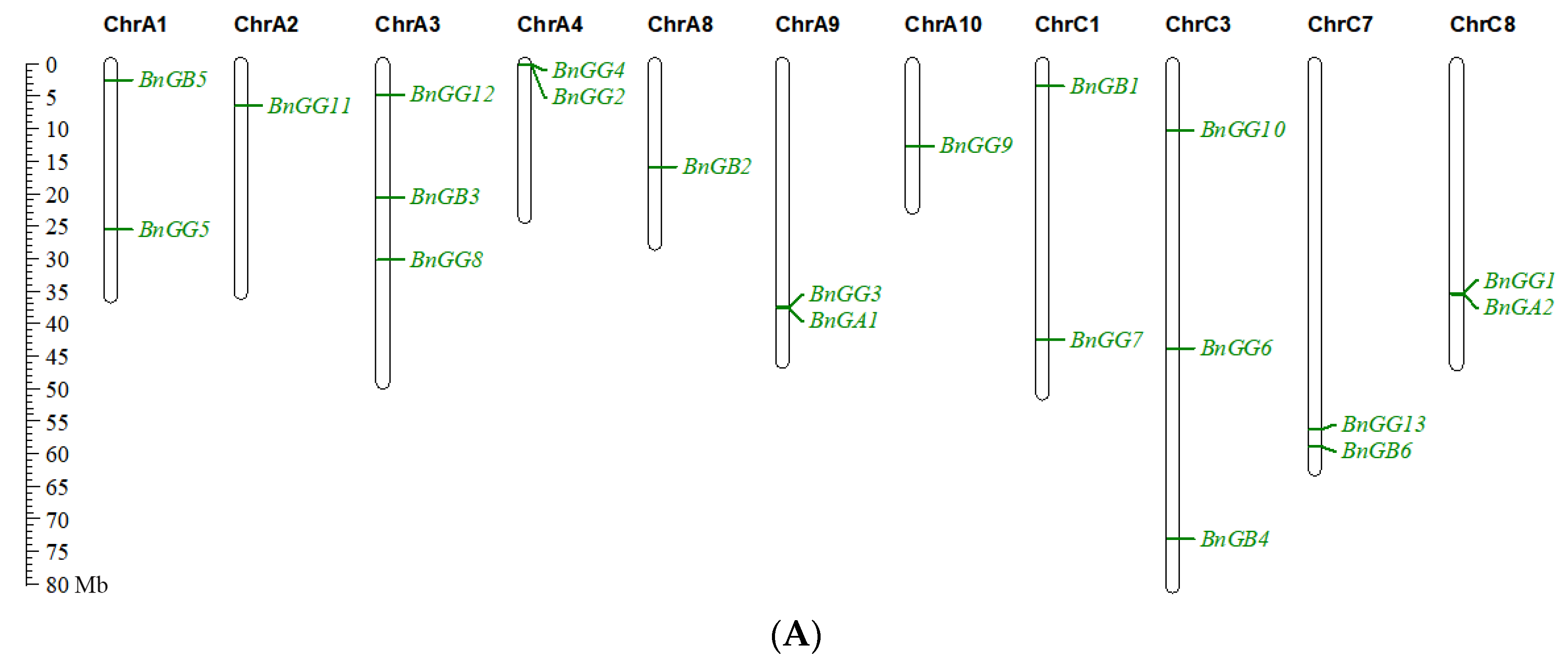
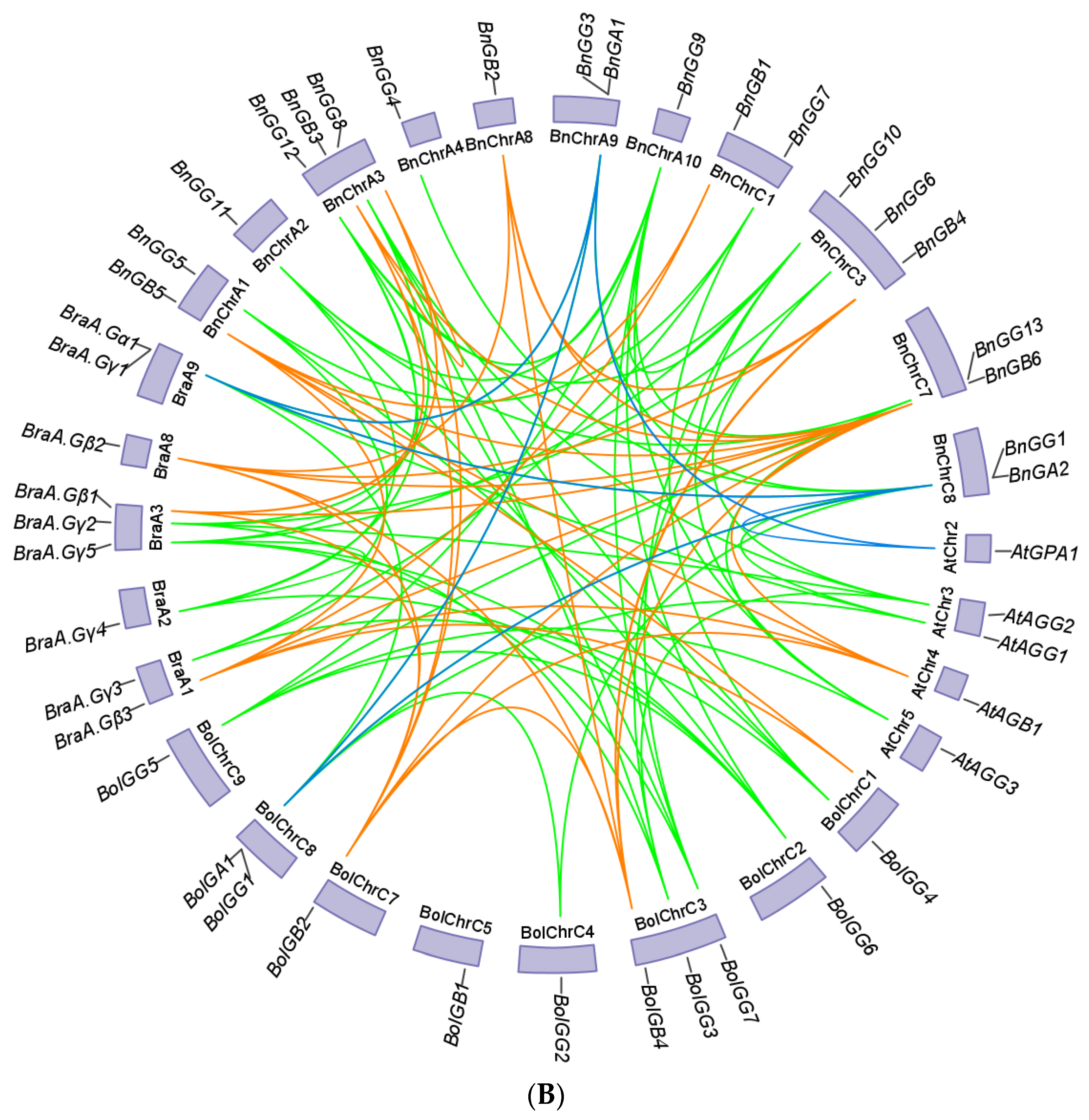
2.4. Chromosomal Location and Gene Duplication of G-Protein
2.5. Analysis of Cis-Acting Element in G-Protein Genes’ Promoters
2.6. Expression Patterns of G-Protein Genes in Different Tissues
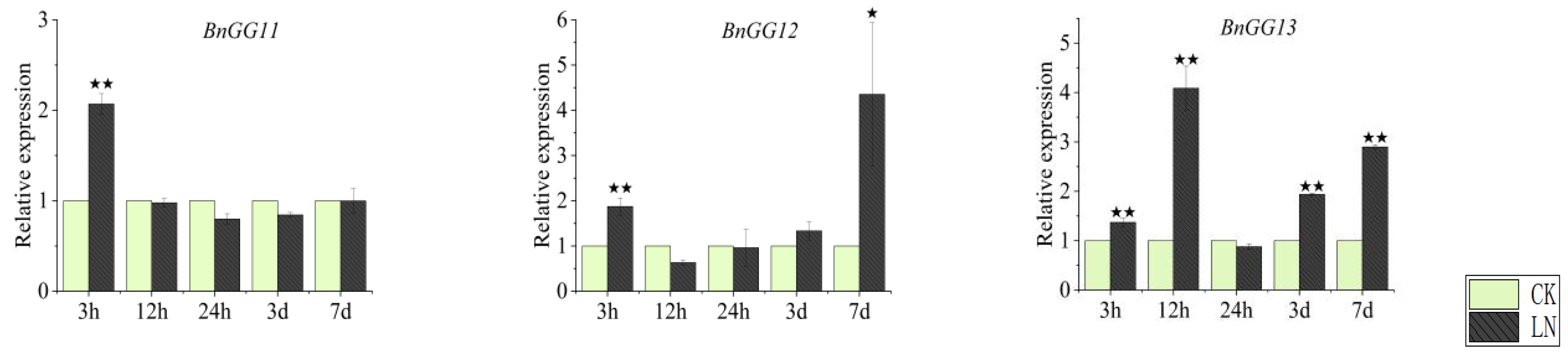
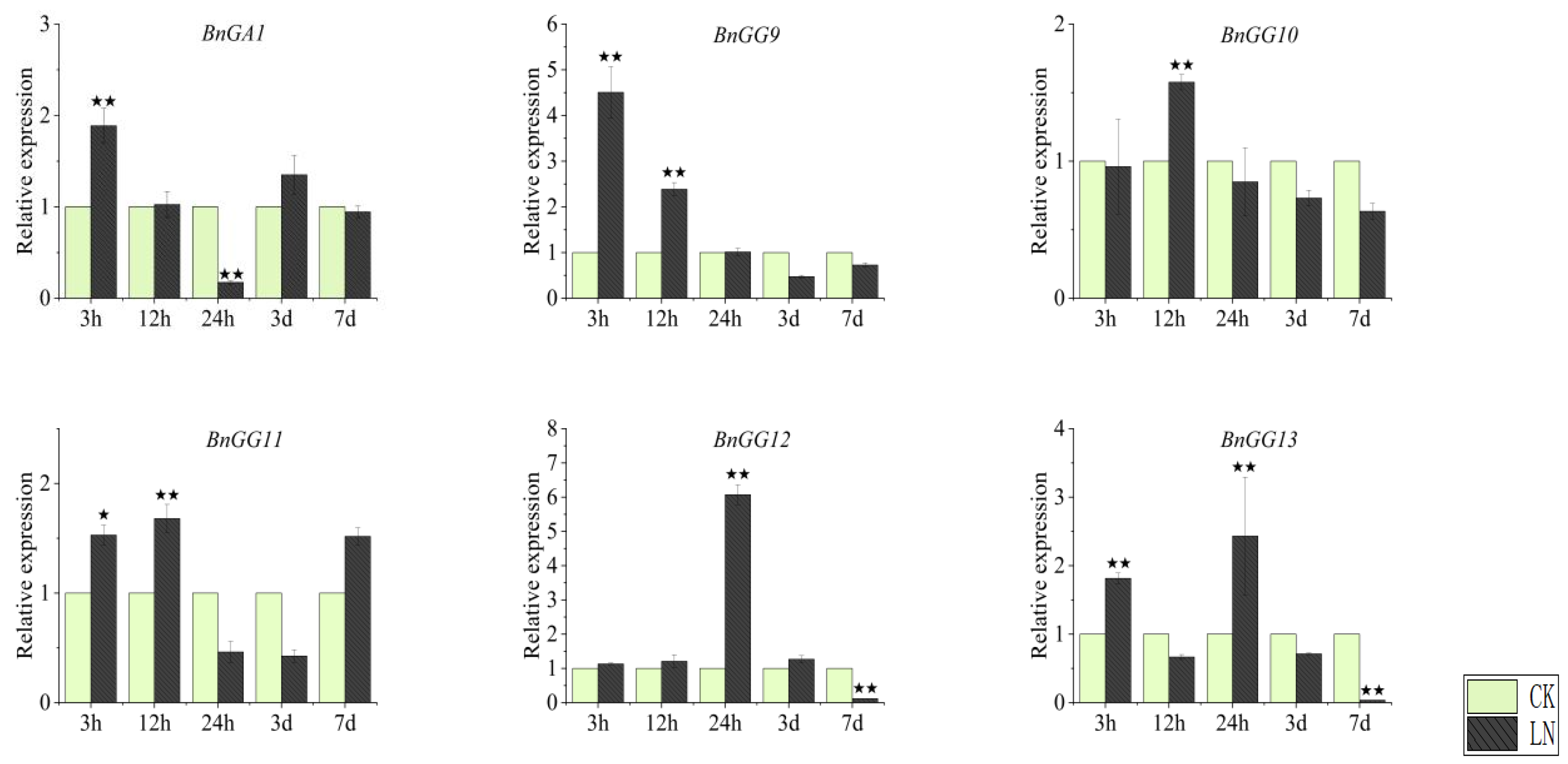
2.7. Expression Patterns of G-Protein Genes under Nitrogen Deficiency
3. Discussion
4. Materials and Methods
4.1. Identification of the G-Protein Family Members in Brassica napus Genome
4.2. Sequence Analysis and Structural Characterization
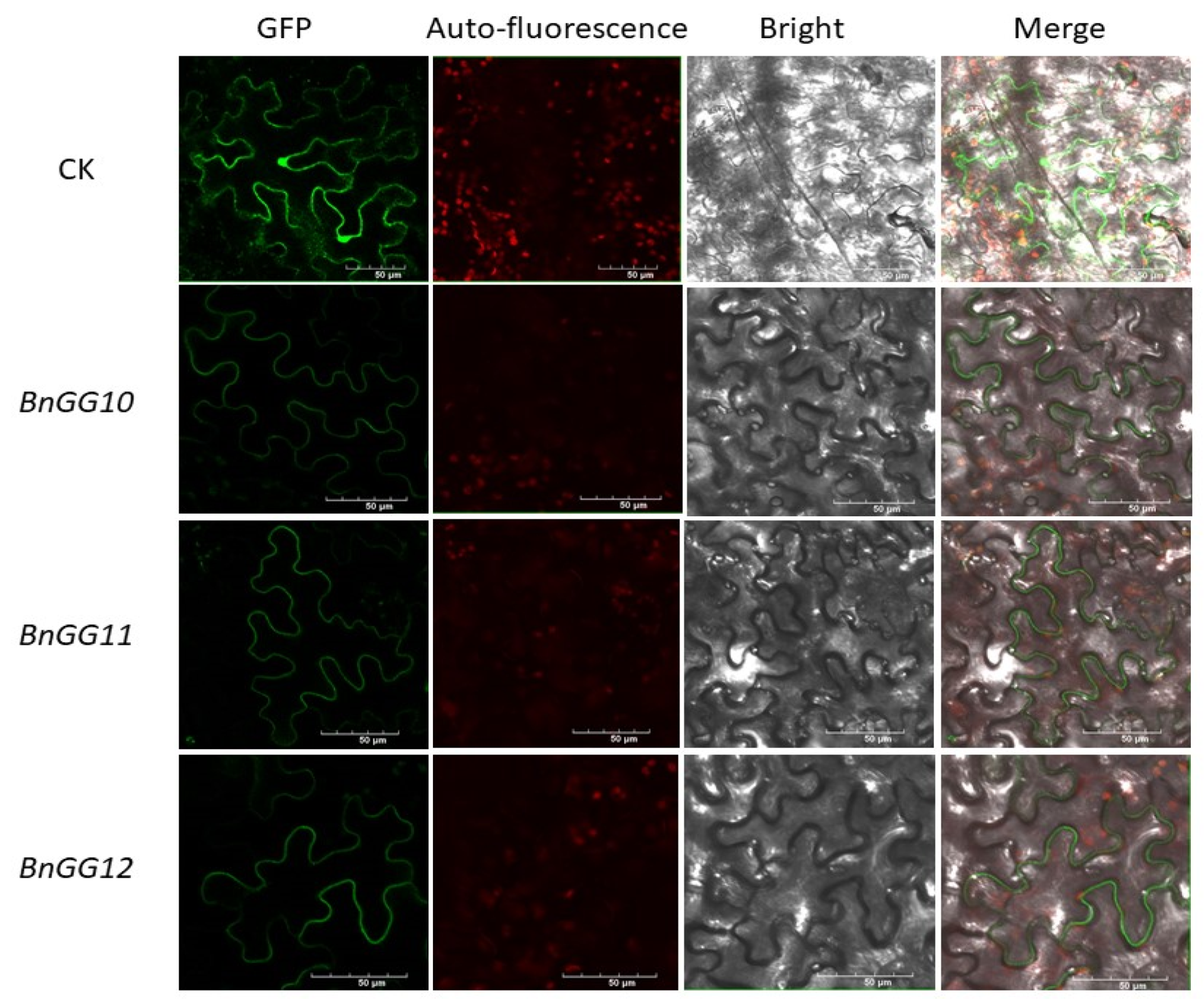
4.3. Phylogenetic Analysis and Subcellular Localization of G-Protein
4.4. Chromosomal Localization and Gene Duplication
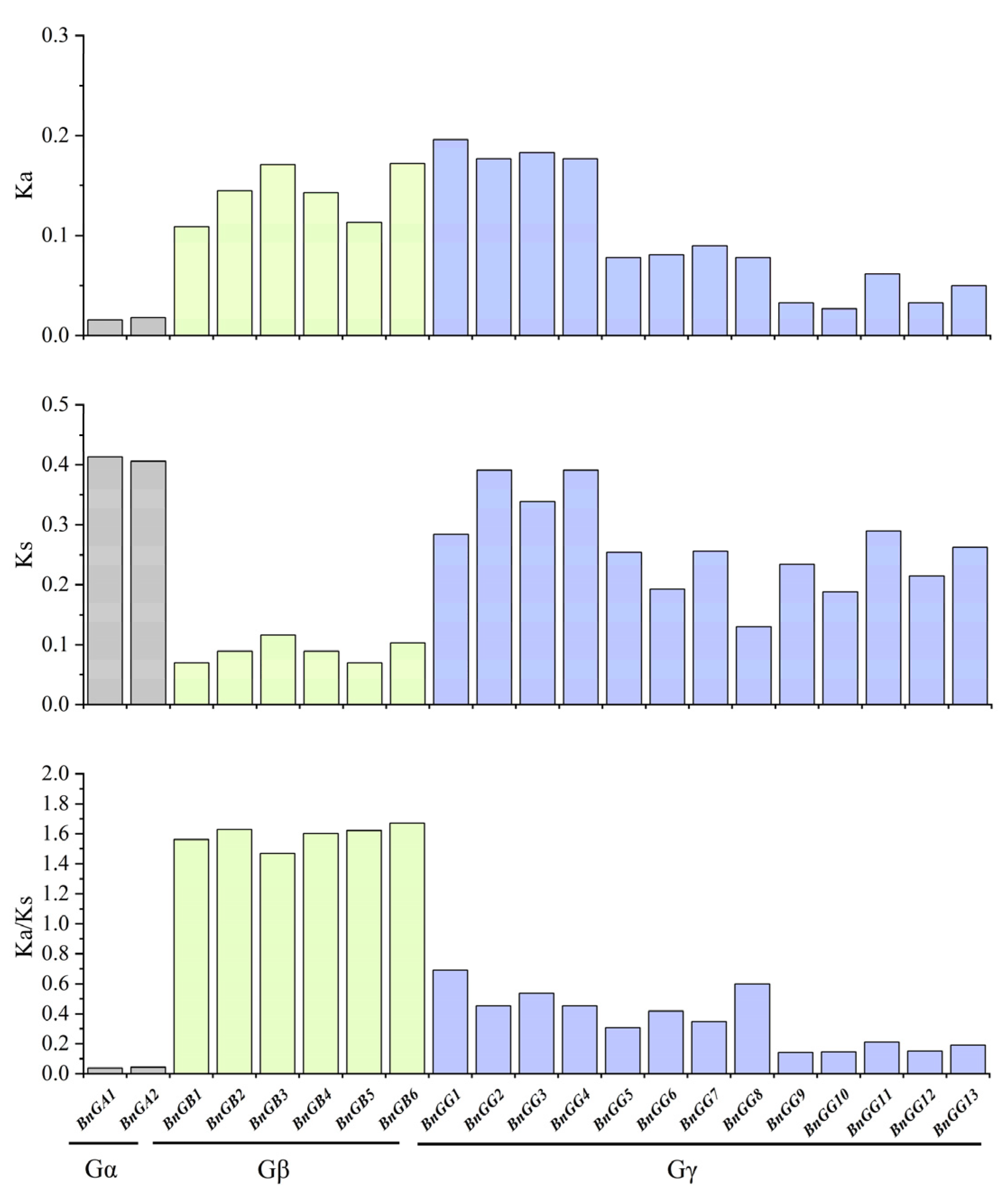
4.5. Calculation of the Ka/Ks Values of G-Protein Genes
4.6. Analysis of Cis-Acting Element in B. napus G-Protein Genes’ Promoters
4.7. Plant Materials and Nitrogen Deprivation Treatments
4.8. RNA Isolation, RNA-Sequencing, and Quantitative Reverse-Transcription PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilman, A.G. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 1987, 56, 615–649. [Google Scholar] [CrossRef]
- Klopffleisch, K.; Phan, N.; Augustin, K.; Bayne, R.S.; Booker, K.S.; Botella, J.R.; Carpita, N.C.; Carr, T.; Chen, J.G.; Cooke, T.R. Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol. Syst. Biol. 2011, 7, 532. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-Y.; Urano, D. Genetic and systematic approaches toward G protein-coupled abiotic stress signaling in plants. Front. Plant Sci. 2018, 9, 1378. [Google Scholar] [CrossRef]
- Ofoe, R. Signal transduction by plant heterotrimeric G-protein. Plant Biol. 2021, 23, 3–10. [Google Scholar] [CrossRef]
- Temple, B.R.S.; Jones, A.M. The plant heterotrimeric G-protein complex. Annu. Rev. Plant Biol. 2007, 58, 249–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, G.; Kostenis, E. Heterotrimeric G-proteins: A short history. Br. J. Pharmacol. 2006, 147, S46–S55. [Google Scholar] [CrossRef] [Green Version]
- Chakravorty, D.; Trusov, Y.; Botella, J.R. Site-directed mutagenesis of the Arabidopsis heterotrimeric G protein β subunit suggests divergent mechanisms of effector activation between plant and animal G proteins. Planta 2012, 235, 615–627. [Google Scholar] [CrossRef]
- Arya, G.C.; Kumar, R.; Bisht, N.C. Evolution, expression differentiation and interaction specificity of heterotrimeric G-protein subunit gene family in the mesohexaploid Brassica rapa. PLoS ONE 2014, 9, e105771. [Google Scholar] [CrossRef] [PubMed]
- Roy Choudhury, S.; Marlin, M.A.; Pandey, S. The Role of Gβ Protein in Controlling Cell Expansion via Potential Interaction with Lipid Metabolic Pathways. Plant Physiol. 2019, 179, 1159–1175. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.Y.; Xu, Y.Z.; Fan, W.; Long, D.P.; Cao, B.N.; Xiang, Z.H.; Zhao, A.C. Identification of the genes involved in heterotrimeric G-protein signaling in mulberry and their regulation by abiotic stresses and signal molecules. Biol. Plant. 2018, 62, 277–286. [Google Scholar] [CrossRef]
- Ullah, H.; Chen, J.-G.; Young, J.C.; Im, K.-H.; Sussman, M.R.; Jones, A.M. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 2001, 292, 2066–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, H.; Chen, J.-G.; Temple, B.; Boyes, D.C.; Alonso, J.M.; Davis, K.R.; Ecker, J.R.; Jones, A.M. The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 2003, 15, 393–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, Y.; Kato, T.; Ohki, S.; Ishikawa, A.; Kitano, H.; Sasaki, T.; Asahi, T.; Iwasaki, Y. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc. Natl. Acad. Sci. USA 1999, 96, 7575–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Liu, Y.; Zheng, L.; Chen, L.; Li, N.; Corke, F.; Lu, Y.; Fu, X.; Zhu, Z.; Bevan, M.W. The plant-specific G protein γ subunit AGG3 influences organ size and shape in Arabidopsis thaliana. New Phytol. 2012, 194, 690–703. [Google Scholar] [CrossRef]
- Kumar, R.; Bisht, N.C. Heterotrimeric Gα subunit regulates plant architecture, organ size and seed weight in the oilseed Brassica juncea. Plant Mol. Biol. 2020, 104, 549–560. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Wang, X.; Yang, M.; Guo, C.; Xiao, K. TaNBP1, a guanine nucleotide-binding subunit gene of wheat, is essential in the regulation of N starvation adaptation via modulating N acquisition and ROS homeostasis. BMC Plant Biol. 2018, 18, 167. [Google Scholar] [CrossRef]
- Sun, H.; Qian, Q.; Wu, K.; Luo, J.; Wang, S.; Zhang, C.; Ma, Y.; Liu, Q.; Huang, X.; Yuan, Q. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 2014, 46, 652–656. [Google Scholar] [CrossRef]
- Xu, X.; Wu, K.; Xu, R.; Yu, J.; Wang, J.; Zhao, Y.; Wang, Y.; Song, W.; Wang, S.; Gao, Z. Pyramiding of the dep1-1 and NAL1 NJ6 Alleles Achieves Sustainable Improvements in Nitrogen-Use Efficiency and Grain Yield in Japonica Rice Breeding. J. Genet. Genom. 2019, 46, 325–328. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, M.; Gu, S.; Sun, J.; Ma, Z.; Wang, L.; Zheng, W.; Xu, Z. DEP1 is involved in regulating the carbon–nitrogen metabolic balance to affect grain yield and quality in rice (Oriza sativa L.). PLoS ONE 2019, 14, e0213504. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, N.; Kanyuka, K.; Jaiswal, D.K.; Kumar, A.; Arora, V.; Malik, A.; Gupta, N.; Hooley, R.; Raghuram, N. GCR1 and GPA1 coupling regulates nitrate, cell wall, immunity and light responses in Arabidopsis. Sci. Rep. 2019, 9, 5838. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Jones, A.M.; Gao, Y. G proteins sculp root architecture in response to nitrogen in rice and Arabidopsis. Plant Sci. 2018, 274, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Roy Choudhury, S.; Vijayakumar, A.; Hovis, L.; Rhodes, Z.; Polzin, R.; Blumenthal, D.; Pandey, S. Arabidopsis type III Gγ Protein AGG3 is a positive regulator of yield and stress responses in the model monocot Setaria viridis. Front. Plant Sci. 2018, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell 1995, 7, 859–868. [Google Scholar] [PubMed] [Green Version]
- Liu, X.; Zhang, F. Nitrogen fertilizer induced greenhouse gas emissions in China. Curr. Opin. Environ. Sustain. 2011, 3, 407–413. [Google Scholar]
- Zou, X.Y.; Liu, B.L.; Song, L.Q.; Guan, C.Y. Effects of nitrogen application on yield and nitrogen use efficiency of rapeseed (Brassica napus). J. Appl. Ecol. 2016, 27, 1169–1176. [Google Scholar]
- Gao, Y.; Li, T.; Liu, Y.; Ren, C.; Zhao, Y.; Wang, M. Isolation and characterization of gene encoding G protein α subunit protein responsive to plant hormones and abiotic stresses in Brassica napus. Mol. Biol. Rep. 2010, 37, 3957–3965. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Li, T.; Ren, C.; Liu, Y.; Wang, M. Cloning and characterization of a G protein β subunit gene responsive to plant hormones and abiotic stresses in Brassica napus. Plant Mol. Biol. Rep. 2010, 28, 450–459. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Zhao, Y.; Ren, C.; Zhang, Y.; Wang, M. Isolation and characterization of a G protein γ subunit gene responsive to plant hormones and abiotic stresses in Brassica napus L. Acta Physiol. Plant. 2011, 33, 391–399. [Google Scholar] [CrossRef]
- Nagaharu, U.; Nagaharu, N. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- Cheng, F.; Mandáková, T.; Wu, J.; Xie, Q.; Lysak, M.A.; Wang, X. Deciphering the diploid ancestral genome of the mesohexaploid Brassica rapa. Plant Cell 2013, 25, 1541–1554. [Google Scholar] [CrossRef] [Green Version]
- Chakravorty, D.; Trusov, Y.; Zhang, W.; Acharya, B.R.; Sheahan, M.B.; McCurdy, D.W.; Assmann, S.M.; Botella, J.R. An atypical heterotrimeric G-protein γ-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J. 2011, 67, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Nekrutenko, A.; Makova, K.D.; Li, W.-H. The KA/KS ratio test for assessing the protein-coding potential of genomic regions: An empirical and simulation study. Genome Res. 2002, 12, 198–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Arya, G.C.; Bisht, N.C. Differential expression and interaction specificity of the heterotrimeric G-protein family in Brassica nigra reveal their developmental-and condition-specific roles. Plant Cell Physiol. 2014, 55, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Assmann, S.M.; Fedoroff, N.V. Characterization of the Arabidopsis heterotrimeric G protein. J. Biol. Chem. 2008, 283, 13913–13922. [Google Scholar] [CrossRef] [Green Version]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.P.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.P.; Zhao, M.; Ma, J.; Yu, J.; Huang, S. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef]
- Seo, H.-S.; Kim, H.-Y.; Jeong, J.-Y.; Lee, S.-Y.; Cho, M.-J.; Bahk, J.-D. Molecular cloning and characterization of RGA1 encoding a G protein α subunit from rice (Oryza sativa L. IR-36). Plant Mol. Biol. 1995, 27, 1119–1131. [Google Scholar] [CrossRef]
- Roy Choudhury, S.; Riesselman, A.J.; Pandey, S. Constitutive or seed-specific overexpression of A rabidopsis G-protein γ subunit 3 (AGG 3) results in increased seed and oil production and improved stress tolerance in C amelina sativa. Plant Biotechnol. J. 2014, 12, 49–59. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Zhang, X.; Liu, Y.; Li, N.; Li, Y. Roles of the Arabidopsis G protein γ subunit AGG3 and its rice homologs GS3 and DEP1 in seed and organ size control. Plant Signal. Behav. 2012, 7, 1357–1359. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated profile HMM searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [Green Version]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. In Circular. California Agricultural Experiment Station; CABI: Wallingford, UK, 1950; Volume 347. [Google Scholar]
- Qin, L.; Walk, T.C.; Han, P.; Chen, L.; Zhang, S.; Li, Y.; Hu, X.; Xie, L.; Yang, Y.; Liu, J. Adaption of roots to nitrogen deficiency revealed by 3D quantification and proteomic analysis. Plant Physiol. 2019, 179, 329–347. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Qin, L.; Li, Y.; Liao, X.; Xu, Z.; Hu, X.; Xie, L.; Yu, C.; Wu, Y.; Xing, L. Identification of suitable reference genes in leaves and roots of rapeseed (Brassica napus L.) under different nutrient deficiencies. J. Integrat. Agric. 2017, 16, 809–819. [Google Scholar] [CrossRef] [Green Version]



| Name | Gene ID | Chr. | Genomic Location | AA | MW (kDa) | pI |
|---|---|---|---|---|---|---|
| BnGA1 | 106368534 | A9 | 37616342–37619503 | 383 | 44.51 | 5.83 |
| BnGA2 | 106416165 | C8 | 35538778–35541981 | 383 | 44.52 | 5.83 |
| BnGB1 | 106355793 | C1 | 3466087–3468674 | 377 | 40.95 | 7.12 |
| BnGB2 | 106361031 | A8 | 15871124–15873524 | 327 | 35.42 | 6.81 |
| BnGB3 | 106393802 | A3 | 20605662–20607978 | 378 | 41.03 | 6.84 |
| BnGB4 | 106434467 | C3 | 73099901–73102187 | 378 | 40.98 | 6.66 |
| BnGB5 | 111200422 | A1 | 2483391–2485944 | 377 | 40.96 | 7.12 |
| BnGB6 | 111207516 | C7 | 58909449–58911731 | 378 | 40.95 | 6.66 |
| BnGG1 | 106415923 | C8 | 35416454–35417485 | 89 | 10.03 | 4.32 |
| BnGG2 | 106444922 | A4 | 160931–162114 | 93 | 10.44 | 4.87 |
| BnGG3 | 111200375 | A9 | 37488644–37489890 | 89 | 9.95 | 4.23 |
| BnGG4 | 106444926 | A4 | 148539–149721 | 102 | 11.55 | 8.96 |
| BnGG5 | 106378744 | A1 | 25494898–25496358 | 101 | 11.46 | 5.67 |
| BnGG6 | 106416661 | C3 | 43845900–43847294 | 101 | 11.22 | 4.69 |
| BnGG7 | 106453742 | C1 | 42473926–42475664 | 156 | 17.81 | 9.35 |
| BnGG8 | 106444151 | A3 | 30215792–30217228 | 101 | 11.16 | 4.69 |
| BnGG9 | 106370440 | A10 | 12691034–12694126 | 243 | 26.59 | 8.71 |
| BnGG10 | 106385330 | C3 | 10289041–10292301 | 236 | 25.67 | 8.71 |
| BnGG11 | 106425695 | A2 | 6534486–6537500 | 265 | 28.96 | 8.78 |
| BnGG12 | 106443216 | A3 | 4798356–4803485 | 237 | 25.76 | 8.71 |
| BnGG13 | 106409403 | C7 | 56275611–56278720 | 246 | 26.86 | 8.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Nan, Y.; Atif, A.; Hu, W.; Zhang, Y.; Tian, H.; Gao, Y. Molecular Identification of the G-Protein Genes and Their Expression Profiles in Response to Nitrogen Deprivation in Brassica napus. Int. J. Mol. Sci. 2022, 23, 8151. https://doi.org/10.3390/ijms23158151
Xie Y, Nan Y, Atif A, Hu W, Zhang Y, Tian H, Gao Y. Molecular Identification of the G-Protein Genes and Their Expression Profiles in Response to Nitrogen Deprivation in Brassica napus. International Journal of Molecular Sciences. 2022; 23(15):8151. https://doi.org/10.3390/ijms23158151
Chicago/Turabian StyleXie, Yuyu, Yunyou Nan, Ayub Atif, Wencong Hu, Yanfeng Zhang, Hui Tian, and Yajun Gao. 2022. "Molecular Identification of the G-Protein Genes and Their Expression Profiles in Response to Nitrogen Deprivation in Brassica napus" International Journal of Molecular Sciences 23, no. 15: 8151. https://doi.org/10.3390/ijms23158151







