The Effect of N-Acetylation on the Anti-Inflammatory Activity of Chitooligosaccharides and Its Potential for Relieving Endotoxemia
Abstract
:1. Introduction
2. Results
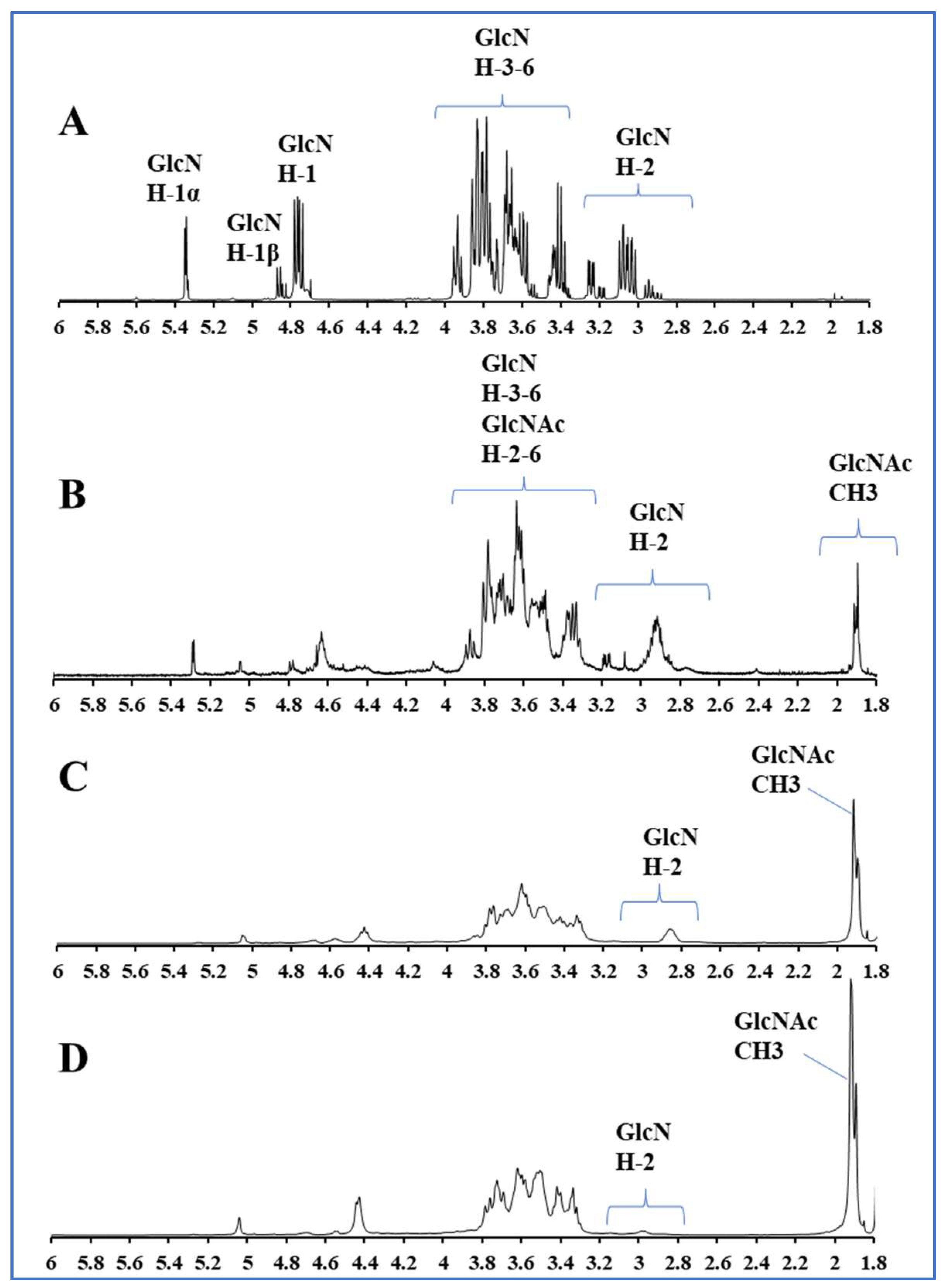
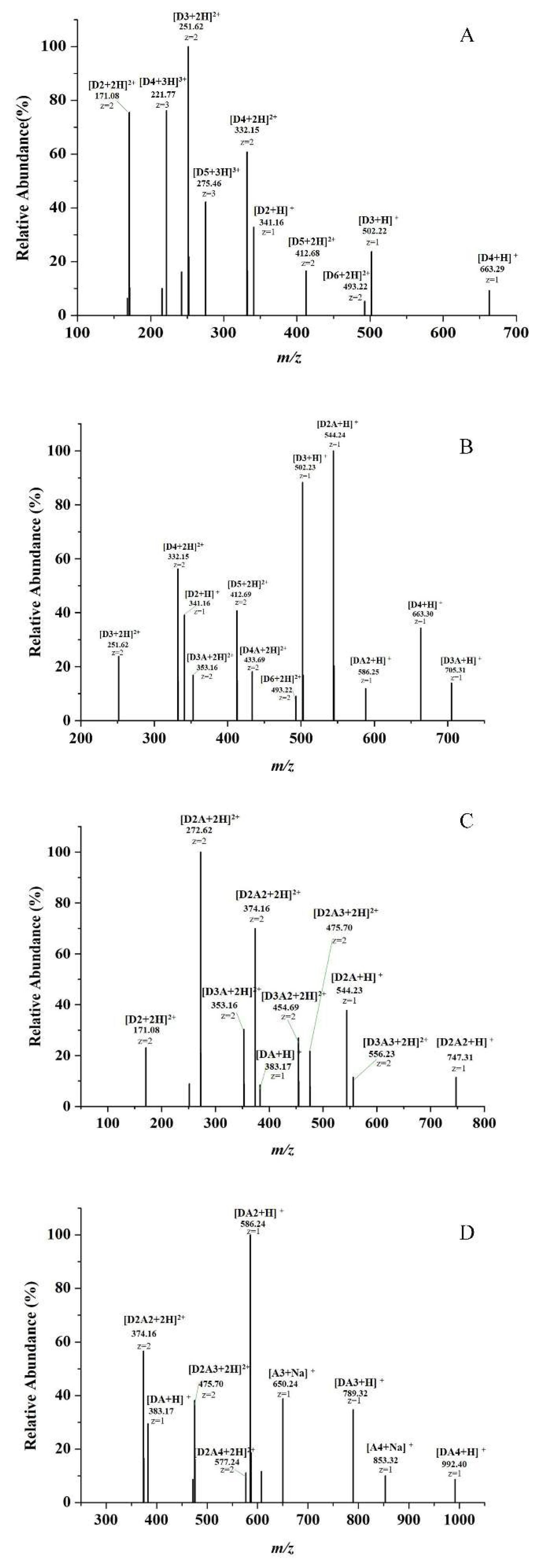
2.1. Preparation and Characterization of COSs with Different DAs
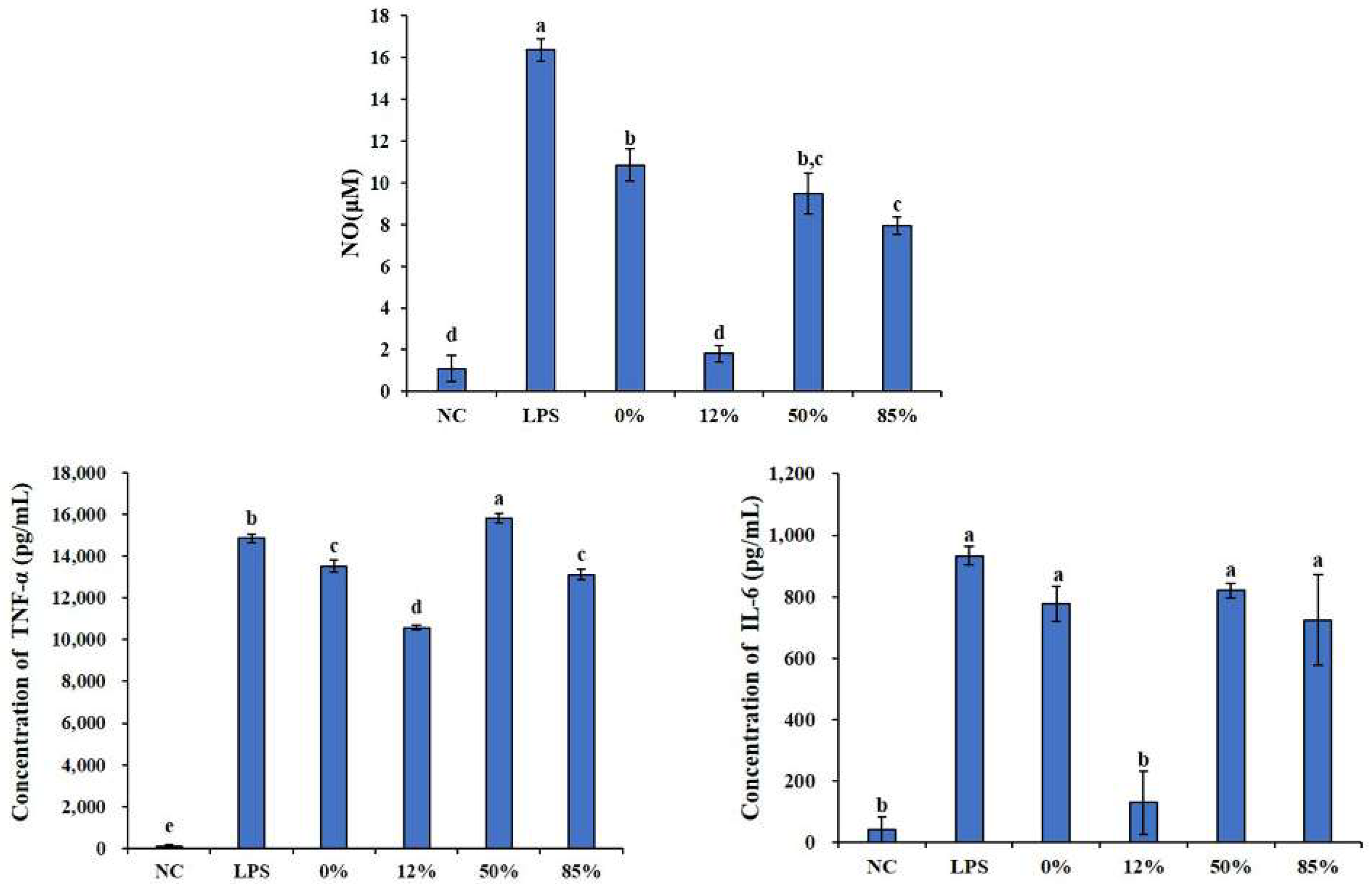
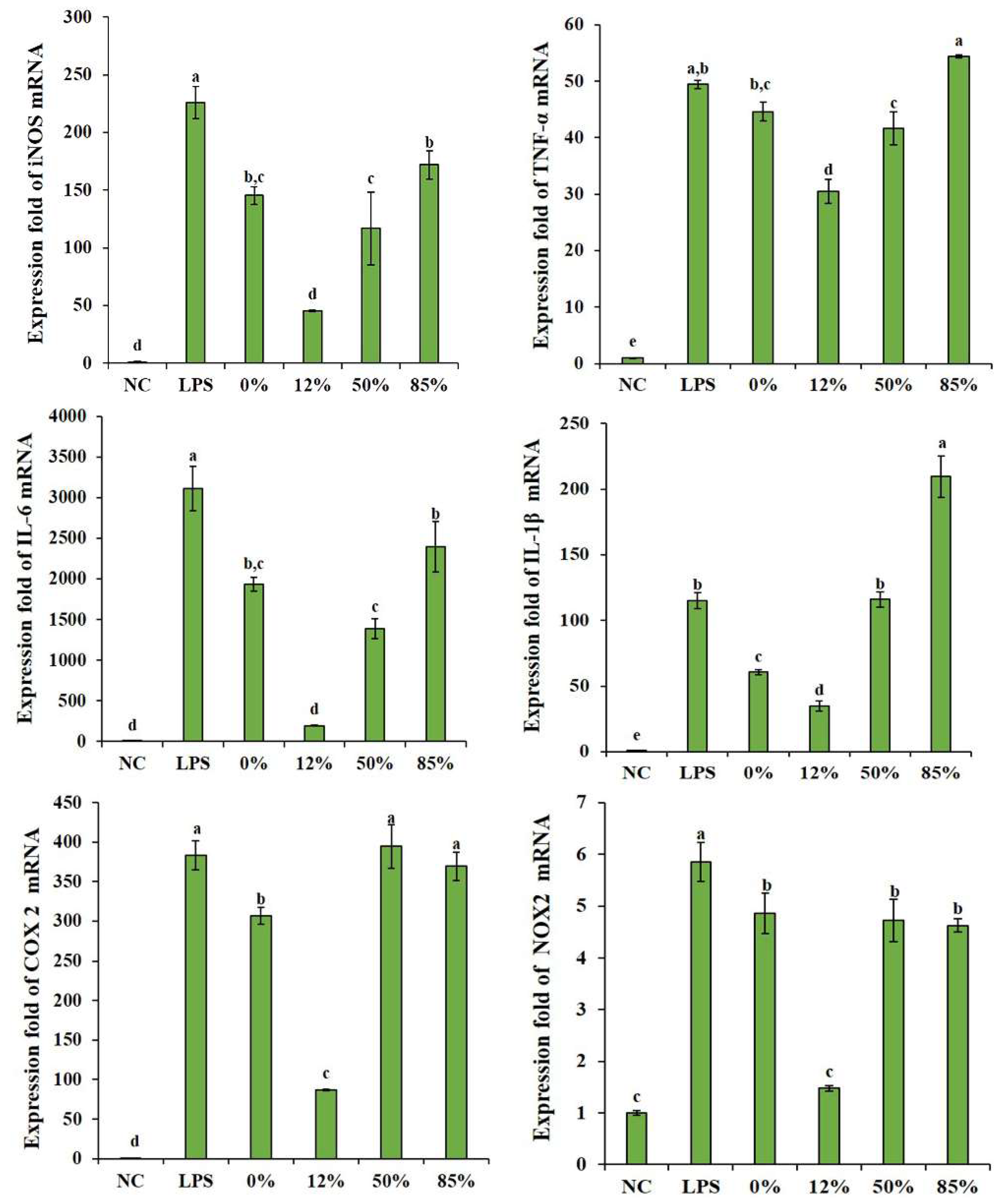
2.2. Effects of COSs with Different DAs on Inflammatory Cytokines
2.3. Effects of COSs with Different DAs on NF-κB Signaling Pathway
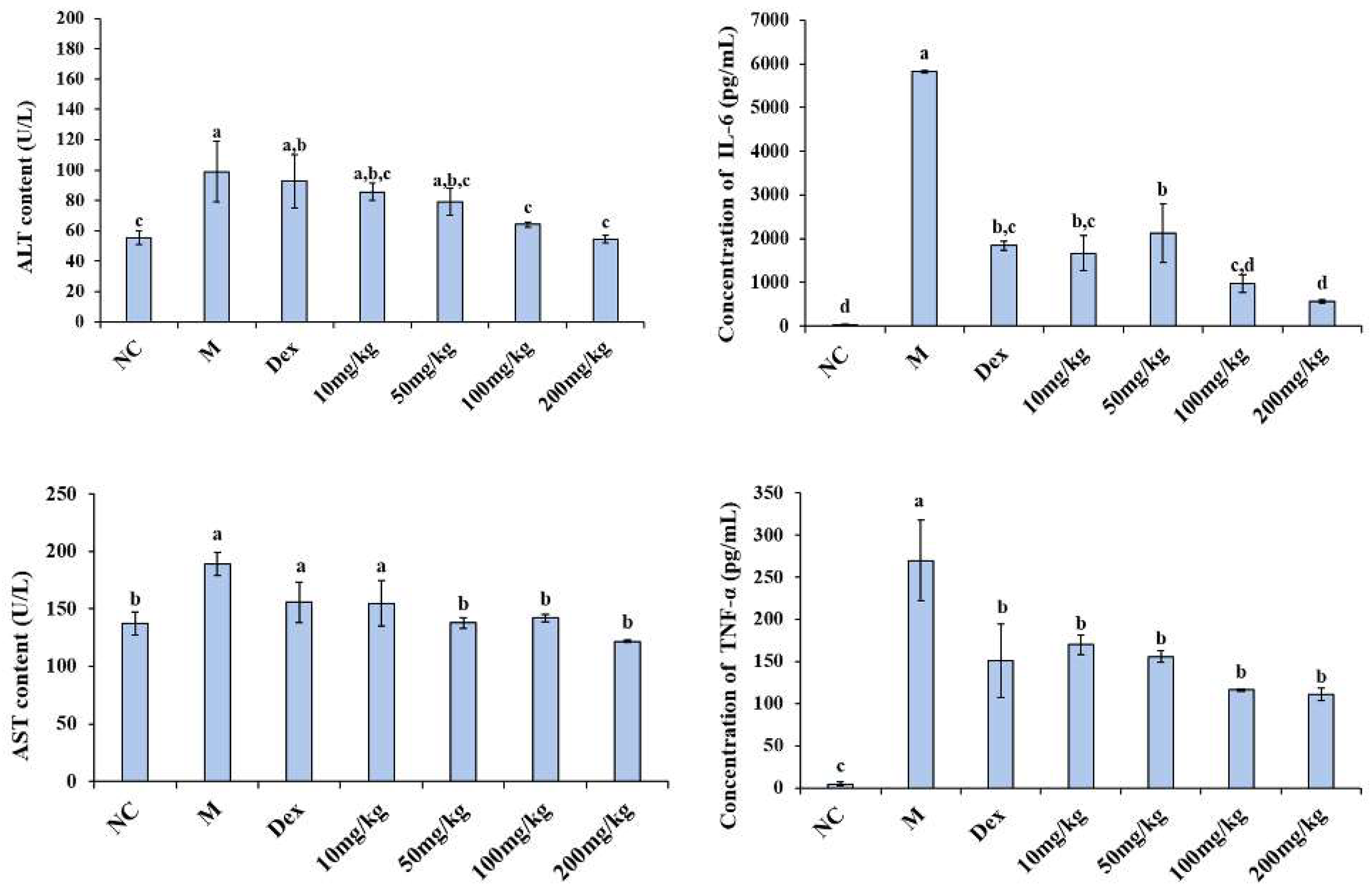
2.4. Alleviating Effect of 12% COS on Endotoxemia in Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation and Characterization of COSs with Different DAs
4.3. Cell Culture and Mouse Model
4.4. Experimental Design of Cell and Animal
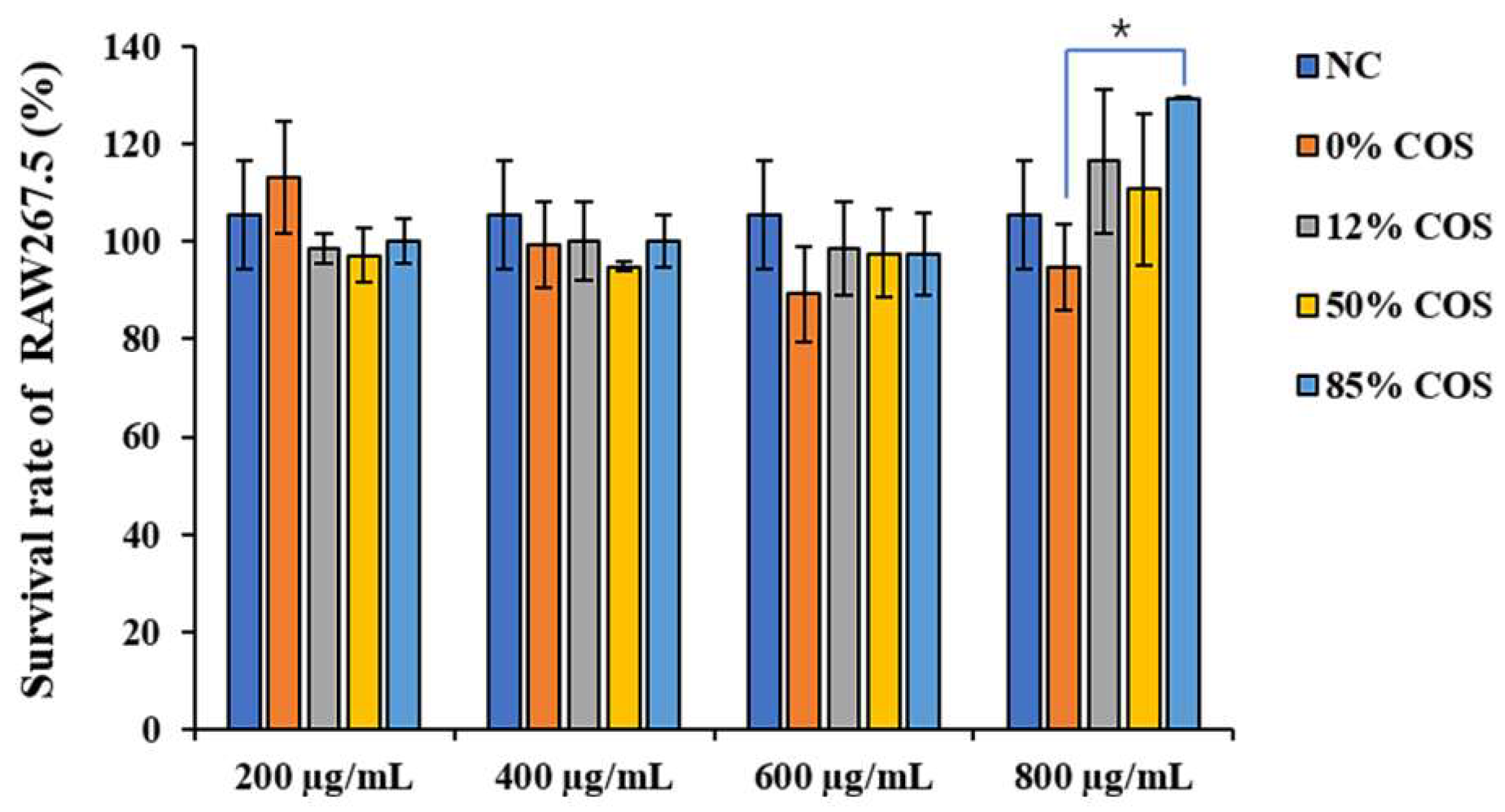
4.5. Determination of Cell Viability
4.6. Biochemical Index Determination and Enzyme-Linked Immunosorbent Assay
4.7. RT-qPCR and WB Analysis
4.8. Observing p65 Nuclear Translocation by IF
4.9. Histological Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H. Lipopolysaccharide: Basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest. Res. 2014, 12, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.Y.; Shen, H.H.; Kung, C.W.; Chen, S.Y.; Lu, C.H.; Lee, Y.M. The Role of HSP70 in the Protective Effects of NVP-AUY922 on Multiple Organ Dysfunction Syndrome in Endotoxemic Rats. Front. Pharmacol. 2021, 12, 724515. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Hsieh, Y.J.; Lu, C.W.; Lee, C.J.; Hsu, B.G. Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species. Int. J. Mol. Sci. 2021, 22, 11190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yin, L.; Zhang, L.; Jiang, D.; Liu, L.; Ji, H. Chitoheptaose Promotes Heart Rehabilitation in a Rat Myocarditis Model by Improving Antioxidant, Anti-Inflammatory, and Antiapoptotic Properties. Oxidative Med. Cell. Longev. 2020, 2020, 2394704. [Google Scholar] [CrossRef] [Green Version]
- Zweifach, B.W.; Janoff, A. Bacterial endotoxemia. Annu. Rev. Med. 1965, 16, 201–220. [Google Scholar] [CrossRef]
- Esmon, C.T. Regulation of blood coagulation. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 349–360. [Google Scholar] [CrossRef]
- Bernard, G.R.; Vincent, J.L.; Laterre, P.; LaRosa, S.P.; Dhainaut, J.F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [Green Version]
- Sozinov, A.S. Systemic endotoxemia during chronic viral hepatitis. Bull. Exp. Biol. Med. 2002, 133, 153–155. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse Effects of Nonsteroidal Antiinflammatory Drugs: An Update of Gastrointestinal, Cardiovascular and Renal Complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, W.M. Adverse reactions to nonsteroidal anti-inflammatory drugs diclofenac compared with other nonsteroidal anti-inflammatory drugs. Am. J. Med. 1986, 80, 70–80. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [Green Version]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Hao, W.; Li, K.; Li, P. Review: Advances in preparation of chitooligosaccharides with heterogeneous sequences and their bioactivity. Carbohydr. Polym. 2021, 252, 117206. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, Y.N.; Ognyanov, M.H.; Kiyohara, H.; Batsalova, T.G.; Dzhambazov, B.M.; Ciz, M.; Denev, P.N.; Yamada, H.; Paulsen, B.S.; Vasicek, O.; et al. Acidic polysaccharide complexes from purslane, silver linden and lavender stimulate Peyer’s patch immune cells through innate and adaptive mechanisms. Int. J. Biol. Macromol. 2017, 105, 730–740. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.H.; Vasicek, O.; Rise, F.; Denev, P.N.; Yamada, H.; Lojek, A.; et al. The common lavender (Lavandula angustifolia Mill.) pectic polysaccharides modulate phagocytic leukocytes and intestinal Peyer’s patch cells. Carbohydr. Polym. 2017, 174, 948–959. [Google Scholar] [CrossRef]
- Jitprasertwong, P.; Khamphio, M.; Petsrichuang, P.; Eijsink, V.G.H.; Poolsri, W.; Muanprasat, C.; Rangnoi, K.; Yamabhai, M. Anti-inflammatory activity of soluble chito-oligosaccharides (CHOS) on VitD3-induced human THP-1 monocytes. PLoS ONE 2021, 16, e0246381. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xu, Q.S.; Du, Y.G.; Xu, J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-kappaB and endothelial inflammatory response. Carbohydr. Polym. 2014, 99, 568–578. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, A.; Wang, H.; Su, J.; Liu, X. Mannose Receptor Mediates the Activation of Chitooligosaccharides on Blunt Snout Bream (Megalobrama amblycephala) Macrophages. Front. Immunol. 2021, 12, 686846. [Google Scholar] [CrossRef]
- Guo, J.; Liao, M.; Zhu, Y.; Hu, X.; Wang, J. The protective role of Chitooligosaccharides against chronic ulcerative colitis induced by dextran sulfate sodium in mice. J. Funct. Foods 2021, 87, 104809. [Google Scholar] [CrossRef]
- Gu, M.; Pan, S.; Li, Q.; Qi, Z.; Deng, W.; Bai, N. Chitosan and chitooligosaccharides attenuate soyabean meal-induced intestinal inflammation of turbot (Scophthalmus maximus): Possible involvement of NF-small ka, CyrillicB, activator protein-1 and mitogen-activated protein kinases pathways. Br. J. Nutr. 2021, 126, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Panda, S.K.; Agarwal, B.; Behera, S.; Ali, S.M.; Pulse, M.E.; Solomkin, J.S.; Opal, S.M.; Bhandari, V.; Acharya, S. Novel Chitohexaose Analog Protects Young and Aged mice from CLP Induced Polymicrobial Sepsis. Sci. Rep. 2019, 9, 2904. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Kidibule, P.; Míguez, N.; Fernández-Arrojo, L.; Ballesteros, A.O.; Fernández-Lobato, M.; Plou, F.J. Tailored Enzymatic Synthesis of Chitooligosaccharides with Different Deacetylation Degrees and Their Anti-Inflammatory Activity. Catalysts 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A.J.B. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef]
- Mills, C.D.; Thomas, A.C.; Lenz, L.L.; Munder, M. Macrophage: SHIP of Immunity. Front. Immunol. 2014, 5, 620. [Google Scholar] [CrossRef] [Green Version]
- Eberhart, C.E.; Coffey, R.J.; Radhika, A.; Giardiello, F.M.; Ferrenbach, S.; Dubois, R.N. Up-regulation of cyclooxygenase-2 gene-expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994, 107, 1183–1188. [Google Scholar] [CrossRef]
- Singel, K.L.; Segal, B.H. NOX2-dependent regulation of inflammation. Clin. Sci. 2016, 130, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.; Grailer, J.J.; Wang, N.; Wang, M.; Yao, J.; Zhong, R.; Gao, G.F.; Ward, P.A.; Tan, D.X.; et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J. Pineal Res. 2016, 60, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Li, Z.Q.; Mo, Z.Q.; Xu, S.; Mao, H.H.; Shi, D.; Li, Z.W.; Dan, X.M.; Luo, X.C. Immunomodulatory Effects of N-Acetyl Chitooligosaccharides on RAW264.7 Macrophages. Mar. Drugs 2020, 18, 421. [Google Scholar] [CrossRef] [PubMed]
- Allan, R. Vaccinia tricks Toll. Genome Biol. 2000, 1, reports0079. [Google Scholar] [CrossRef]
- Wroblewski, F.; Ladue, J.S. Serum glutamic pyruvic transaminase in cardiac and hepatic disease. Proc. Soc. Exp. Biol. Med. 1956, 91, 569–571. [Google Scholar] [CrossRef]
- Ma, P.; Liu, H.-T.; Wei, P.; Xu, Q.-S.; Bai, X.-F.; Du, Y.-G.; Yu, C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011, 84, 1391–1398. [Google Scholar] [CrossRef]
- Barman, P.K.; Mukherjee, R.; Prusty, B.K.; Suklabaidya, S.; Senapati, S.; Ravindran, B. Chitohexaose protects against acetaminophen-induced hepatotoxicity in mice. Cell Death Dis. 2016, 7, e2224. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Senevirathne, M.; Ahn, C.B.; Kim, S.K.; Je, J.Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorg. Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef]
- Basa, S.; Nampally, M.; Honorato, T.; Das, S.N.; Podile, A.R.; El Gueddari, N.E.; Moerschbacher, B.M. The Pattern of Acetylation Defines the Priming Activity of Chitosan Tetramers. J. Am. Chem. Soc. 2020, 142, 1975–1986. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Xing, R.; Qin, Y.; Li, P. Preparation, characterization and antioxidant activity of two partially N-acetylated chitotrioses. Carbohydr. Polym. 2013, 92, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, S.; Xing, R.; Yu, H.; Qin, Y.; Li, R.; Li, P. High-resolution separation of homogeneous chitooligomers series from 2-mers to 7-mers by ion-exchange chromatography. J. Sep. Sci. 2013, 36, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Li, K.; Ma, Y.; Li, R.; Xing, R.; Yu, H.; Li, P. Preparation and Antioxidant Activity of Chitosan Dimers with Different Sequences. Mar. Drugs 2021, 19, 366. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan Oligosaccharide Attenuates Nonalcoholic Fatty Liver Disease Induced by High Fat Diet through Reducing Lipid Accumulation, Inflammation and Oxidative Stress in C57BL/6 Mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival-application to proliferation and cyto-toxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-kappaB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef]
- Flameng, W.; Borgers, M.; Daenen, W.; Stalpaert, G. Ultrastructural and cytochemical correlates of myocardial protection by cardiac hypothermia in man. J. Thorac. Cardiovasc. Surg. 1980, 79, 413–424. [Google Scholar] [CrossRef]



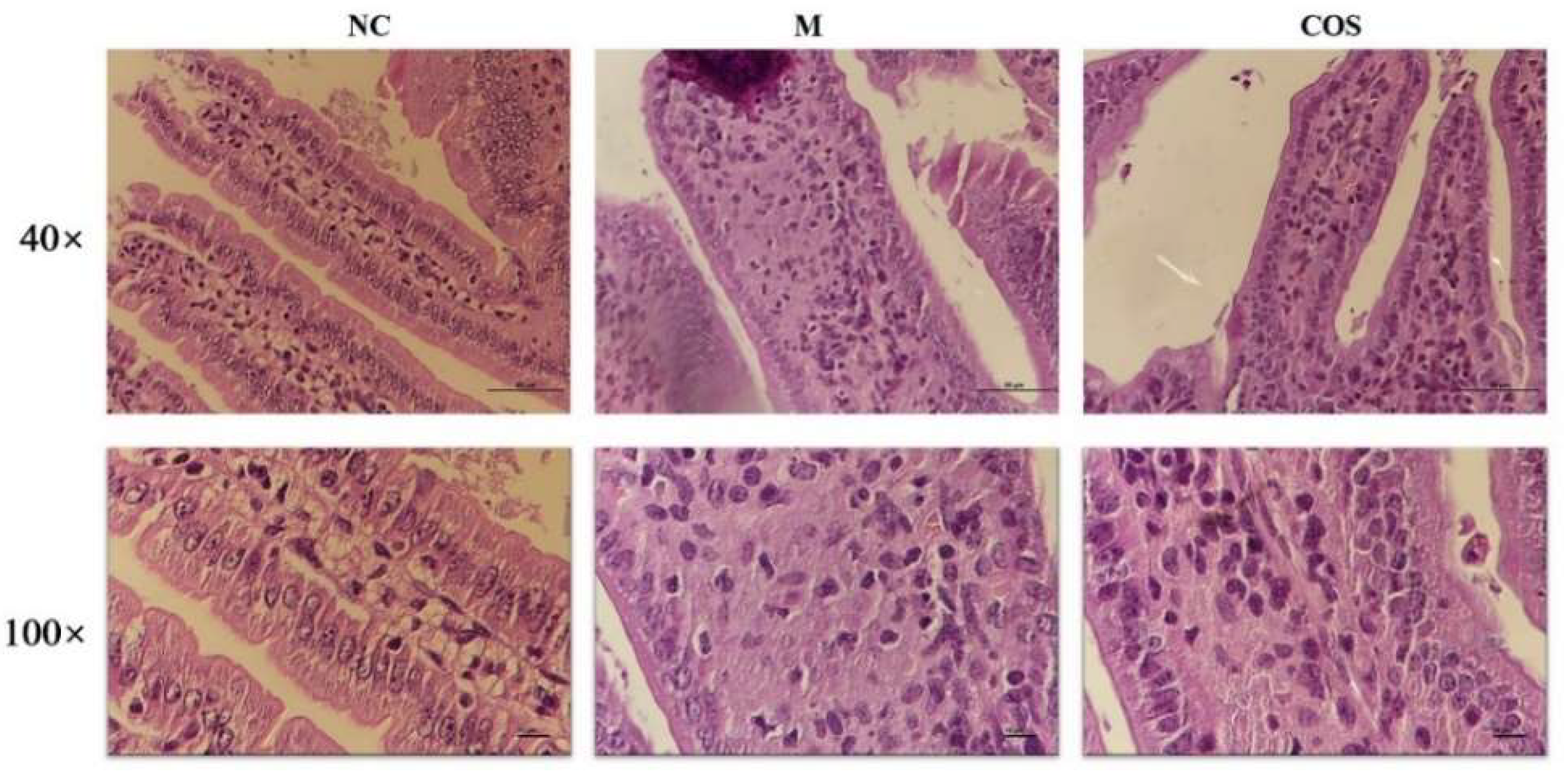
| Group | NC | M | COS |
|---|---|---|---|
| Score | 1.0125 ± 0.15519 a | 3.5625 ± 0.14752 c | 1.4571 ± 0.11722 b |
| Group | NC | M | COS |
|---|---|---|---|
| Height/crypt depth | 3.49 ± 0.10179 a | 1.85 ± 0.089 c | 2.21 ± 0.090 b |
| Primer. | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| GAPDH | ACTCACGGCAAATTCAACGGCA | GACTCCACGACATACTCAGCAC |
| iNOS | GCCCAGGAGGAGAGAGAT | GCAAAGAGGACTGCGGCT |
| IL-6 | AGACTTCCATCCAGTTGCCTTCTTG | CATGTGTAATTAAGCCTCCGACTTGTG |
| TNF-α | TGCCTATGTCTCAGCCTCTTC | GAGGCCATTTGGGAACTTCT |
| IL-1β | GCAGAGCACAAGCCTGTCTTCC | ACCTGTCTTGGCCGAGGACTAAG |
| COX-2 | AAATGCTGGTGTGGAAGGT | TTGTTGCTCTAGGCTTTGCT |
| NOX2 | CCTTACTGGCTGGGATGA | GCAATGGTCTTGAACTCGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.; Li, K.; Ge, X.; Yang, H.; Xu, C.; Liu, S.; Yu, H.; Li, P.; Xing, R. The Effect of N-Acetylation on the Anti-Inflammatory Activity of Chitooligosaccharides and Its Potential for Relieving Endotoxemia. Int. J. Mol. Sci. 2022, 23, 8205. https://doi.org/10.3390/ijms23158205
Hao W, Li K, Ge X, Yang H, Xu C, Liu S, Yu H, Li P, Xing R. The Effect of N-Acetylation on the Anti-Inflammatory Activity of Chitooligosaccharides and Its Potential for Relieving Endotoxemia. International Journal of Molecular Sciences. 2022; 23(15):8205. https://doi.org/10.3390/ijms23158205
Chicago/Turabian StyleHao, Wentong, Kecheng Li, Xiangyun Ge, Haoyue Yang, Chaojie Xu, Song Liu, Huahua Yu, Pengcheng Li, and Ronge Xing. 2022. "The Effect of N-Acetylation on the Anti-Inflammatory Activity of Chitooligosaccharides and Its Potential for Relieving Endotoxemia" International Journal of Molecular Sciences 23, no. 15: 8205. https://doi.org/10.3390/ijms23158205






