A NanoBRET-Based H3R Conformational Biosensor to Study Real-Time H3 Receptor Pharmacology in Cell Membranes and Living Cells
Abstract
:1. Introduction
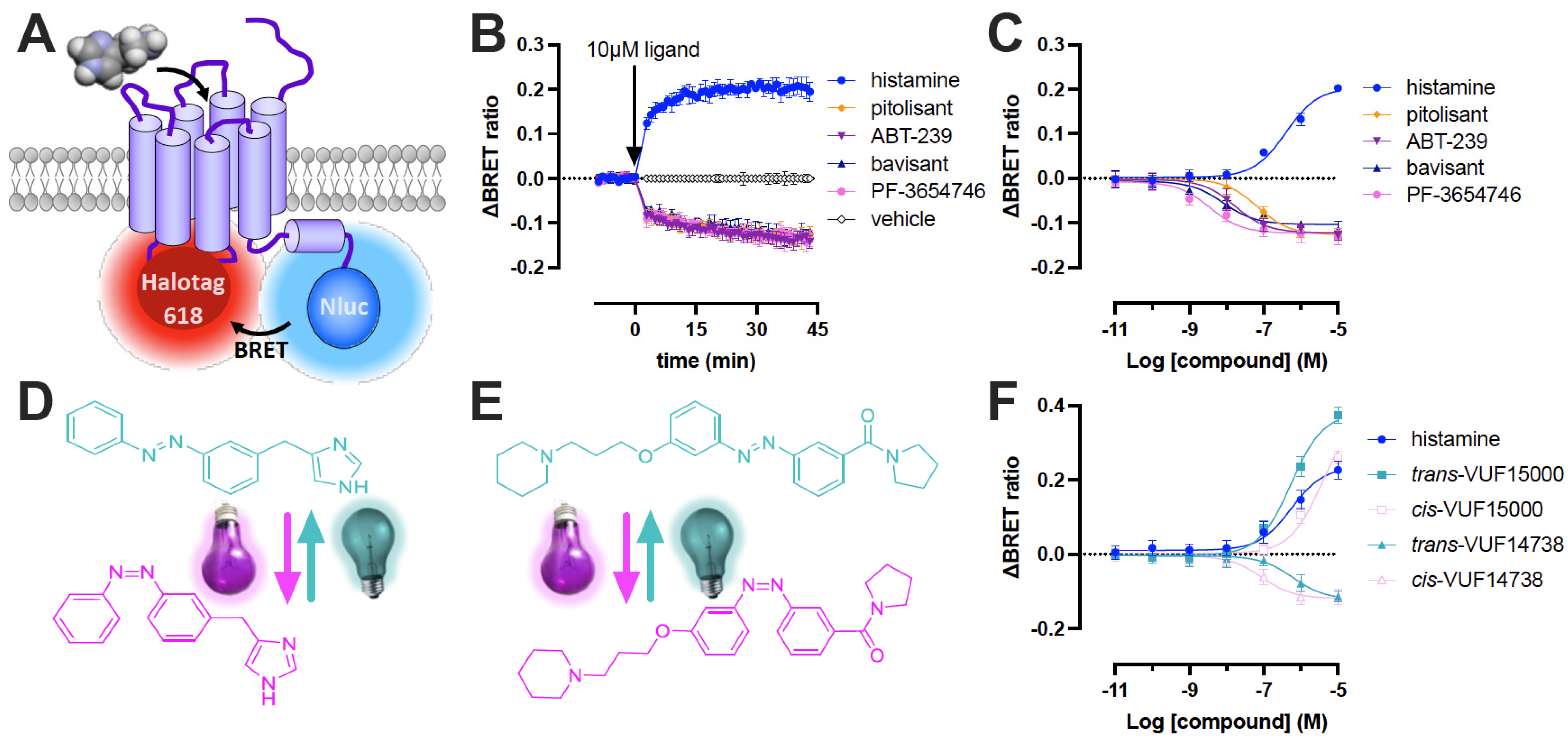
2. Results
2.1. Efficacy of (Pre) Clinical and Photoswitchable H3R Ligands on the H3R Biosensor in Living Cells
2.2. Dynamics of H3R Biosensor in Intact Cells
2.3. Behavior of the H3R Conformational Biosensor in Membrane Preparations
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Photochemistry
4.3. Cell Culture
4.4. BRET-Based H3R Biosensor Detection on Intact Cells
4.5. Membrane Preparation
4.6. [3H]NAMH Competition Binding Assay on Membranes
4.7. BRET-Based H3R Biosensor Detection on Membranes
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Nafría, J.; Tate, C.G. Cryo-Electron Microscopy: Moving Beyond X-ray Crystal Structures for Drug Receptors and Drug Development. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 51–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingler, L.M.; Lefkowitz, R.J. Conformational Basis of G Protein-Coupled Receptor Signaling Versatility. Trends Cell Biol. 2020, 30, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Seyedabadi, M.; Gharghabi, M.; Gurevich, E.V.; Gurevich, V.V. Structural basis of GPCR coupling to distinct signal transducers: Implications for biased signaling. Trends Biochem. Sci. 2022, 47, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Masureel, M.; Zou, Y.; Picard, L.-P.; Van Der Westhuizen, E.; Mahoney, J.P.; Rodrigues, J.P.G.L.M.; Mildorf, T.J.; Dror, R.O.; Shaw, D.E.; Bouvier, M.; et al. Structural insights into binding specificity, efficacy and bias of a β2AR partial agonist. Nat. Chem. Biol. 2018, 14, 1059–1066. [Google Scholar] [CrossRef]
- Shukla, A.K. Structural Basis of Partial Agonism at the β2-Adrenergic Receptor. Biochemistry 2019, 58, 137–139. [Google Scholar] [CrossRef] [Green Version]
- Gregorio, G.G.; Masureel, M.; Hilger, D.; Terry, D.S.; Juette, M.; Zhao, H.; Zhou, Z.; Perez-Aguilar, J.M.; Hauge, M.; Mathiasen, S.; et al. Single-molecule analysis of ligand efficacy in β2AR-G-protein activation. Nature 2017, 547, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Haider, R.S.; Godbole, A.; Hoffmann, C. To sense or not to sense-new insights from GPCR-based and arrestin-based biosensors. Curr. Opin. Cell Biol. 2019, 57, 16–24. [Google Scholar] [CrossRef]
- Kauk, M.; Hoffmann, C. Intramolecular and Intermolecular FRET Sensors for GPCRs—Monitoring Conformational Changes and Beyond. Trends Pharmacol. Sci. 2018, 39, 123–135. [Google Scholar] [CrossRef]
- Fernández-Dueñas, V.; Gómez-Soler, M.; López-Cano, M.; Taura, J.J.; Ledent, C.; Watanabe, M.; Jacobson, K.A.; Vilardaga, J.-P.; Ciruela, F. Uncovering caffeine's adenosine A2A receptor inverse agonism in experimental parkinsonism. ACS Chem. Biol. 2014, 9, 2496–2501. [Google Scholar] [CrossRef] [Green Version]
- Picard, L.-P.; Schönegge, A.-M.; Lohse, M.J.; Bouvier, M. Bioluminescence resonance energy transfer-based biosensors allow monitoring of ligand- and transducer-mediated GPCR conformational changes. Commun. Biol. 2018, 1, 106. [Google Scholar] [CrossRef]
- Schihada, H.; Vandenabeele, S.; Zabel, U.; Frank, M.; Lohse, M.J.; Maiellaro, I. A universal bioluminescence resonance energy transfer sensor design enables high-sensitivity screening of GPCR activation dynamics. Commun. Biol. 2018, 1, 105. [Google Scholar] [CrossRef] [PubMed]
- Schihada, H.; Ma, X.; Zabel, U.; Vischer, H.F.; Schulte, G.; Leurs, R.; Pockes, S.; Lohse, M.J. Development of a Conformational Histamine H3 Receptor Biosensor for the Synchronous Screening of Agonists and Inverse Agonists. ACS Sens. 2020, 5, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Vilardaga, J.-P.; Bünemann, M.; Krasel, C.; Castro, M.; Lohse, M.J. Measurement of the Millisecond Activation Switch of G Protein-coupled Receptors in Living Cells. Nat Biotechnol. 2003, 21, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Zürn, A.; Zabel, U.; Vilardaga, J.-P.; Schindelin, H.; Lohse, M.J.; Hoffmann, C. Fluorescence resonance energy transfer analysis of alpha 2a-adrenergic receptor activation reveals distinct agonist-specific conformational changes. Mol. Pharmacol. 2009, 75, 534–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, C.; Gaietta, G.; Bünemann, M.; Adams, S.R.; Oberdorff-Maass, S.; Behr, B.; Vilardaga, J.-P.; Tsien, R.Y.; Ellisman, M.H.; Lohse, M.J. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat. Methods 2005, 2, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, N.; Bätz, J.; Zabel, U.; Lohse, M.J.; Hoffmann, C. FRET-based sensors for the human M1-, M3-, and M5-acetylcholine receptors. Bioorg. Med. Chem. 2011, 19, 1048–1054. [Google Scholar] [CrossRef]
- Bourque, K.; Pétrin, D.; Sleno, R.; Devost, D.; Zhang, A.; Hébert, T.E. Distinct Conformational Dynamics of Three G Protein-Coupled Receptors Measured Using FlAsH-BRET Biosensors. Front. Endocrinol. 2017, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Devost, D.; Sleno, R.; Pé Trin, D.; Zhang, A.; Shinjo, Y.; Okde, R.; Aoki, J.; Inoue, A.; Hébert, T.E. Conformational profiling of the AT1 angiotensin II receptor reflects biased agonism, G protein coupling, and cellular context. J. Biol. Chem. 2017, 292, 5443–5456. [Google Scholar] [CrossRef] [Green Version]
- Sleno, R.; Pétrin, D.; Devost, D.; Goupil, E.; Zhang, A.; Hébert, T.E. Designing BRET-based conformational biosensors for G protein-coupled receptors. Methods 2016, 92, 11–18. [Google Scholar] [CrossRef]
- Szalai, B.; Barkai, L.; Turu, G.; Szidonya, L.; Várnai, P.; Hunyady, L. Allosteric interactions within the AT1 angiotensin receptor homodimer: Role of the conserved DRY motif. Biochem. Pharmacol. 2012, 84, 477–485. [Google Scholar] [CrossRef]
- Schihada, H.; Nemec, K.; Lohse, M.J.; Maiellaro, I. Bioluminescence in G Protein-Coupled Receptors Drug Screening Using Nanoluciferase and Halo-Tag Technology. Methods Mol. Biol. 2021, 2268, 137–147. [Google Scholar] [PubMed]
- Liu, Y.; Zeng, H.; Pediani, J.D.; Ward, R.J.; Chen, L.Y.; Wu, N.; Ma, L.; Tang, M.; Yang, Y.; An, S.; et al. Visualization of the activation of the histamine H3 receptor (H3R) using novel fluorescence resonance energy transfer biosensors and their potential application to the study of H3R pharmacology. FEBS J. 2018, 285, 2319–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.S.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouleau, A.; Ligneau, X.; Tardivel-Lacombe, J.; Morisset, S.; Gbahou, F.; Schwartz, J.C.; Arrang, J.M. Histamine H3-receptor-mediated [35S]GTP gamma[S] binding: Evidence for constitutive activity of the recombinant and native rat and human H3 receptors. Br. J. Pharmacol. 2002, 135, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Wieland, K.; Bongers, G.; Yamamoto, Y.; Hashimoto, T.; Yamatodani, A.; Menge, W.M.; Timmerman, H.; Lovenberg, T.W.; Leurs, R. Constitutive activity of histamine h(3) receptors stably expressed in SK-N-MC cells: Display of agonism and inverse agonism by H(3) antagonists. J. Pharmacol. Exp. Ther. 2001, 299, 908–914. [Google Scholar]
- Morisset, S.; Rouleau, A.; Ligneau, X.; Gbahou, F.; Tardivel-Lacombe, J.; Stark, H.; Schunack, W.; Ganellin, C.R.; Schwartz, J.C.; Arrang, J.M. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature 2000, 408, 860–864. [Google Scholar] [CrossRef]
- Alhusaini, M.; Eissa, N.; Saad, A.K.; Beiram, R.; Sadek, B. Revisiting Preclinical Observations of Several Histamine H3 Receptor Antagonists/Inverse Agonists in Cognitive Impairment, Anxiety, Depression, and Sleep-Wake Cycle Disorder. Front. Pharmacol. 2022, 13, 861094. [Google Scholar] [CrossRef]
- Ghamari, N.; Zarei, O.; Arias-Montaño, J.-A.; Reiner, D.; Dastmalchi, S.; Stark, H.; Hamzeh-Mivehroud, M. Histamine H3 receptor antagonists/inverse agonists: Where do they go? Pharmacol. Ther. 2019, 200, 69–84. [Google Scholar] [CrossRef]
- Urquhart, L. FDA new drug approvals in Q3 2019. Nat. Rev. Drug Discov. 2019, 18, 816. [Google Scholar] [CrossRef]
- Kollb-Sielecka, M.; Demolis, P.; Emmerich, J.; Markey, G.; Salmonson, T.; Haas, M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: Summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017, 33, 125–129. [Google Scholar] [CrossRef]
- Hauwert, N.J.; Mocking, T.A.M.; Da Costa Pereira, D.; Lion, K.; Huppelschoten, Y.; Vischer, H.F.; de Esch, I.J.P.; Wijtmans, M.; Leurs, R. A Photoswitchable Agonist for the Histamine H3 Receptor, a Prototypic Family A G-Protein-Coupled Receptor. Angew. Chem. Int. Ed. Engl. 2019, 58, 4531–4535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauwert, N.J.; Mocking, T.A.M.; Da Costa Pereira, D.; Kooistra, A.J.; Wijnen, L.M.; Vreeker, G.C.M.; Verweij, E.W.E.; De Boer, A.H.; Smit, M.J.; de Graaf, C.; et al. Synthesis and Characterization of a Bidirectional Photoswitchable Antagonist Toolbox for Real-Time GPCR Photopharmacology. J. Am. Chem. Soc. 2018, 140, 4232–4243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Shakhmin, A.; Hall, M.P.; Machleidt, T.; Walker, J.R.; Wood, K.V.; Kirkland, T.A. Coelenterazine analogues emit red-shifted bioluminescence with NanoLuc. Org. Biomol. Chem. 2017, 15, 8559–8567. [Google Scholar] [CrossRef]
- Zhang, J.; Chung, T.; Oldenburg, K. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Clark, E.A.; Hill, S.J. Differential effect of sodium ions and guanine nucleotides on the binding of thioperamide and clobenpropit to histamine H3-receptors in rat cerebral cortical membranes. Br. J. Pharmacol. 1995, 114, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Mocking, T.A.M.; Verweij, E.W.E.; Vischer, H.F.; Leurs, R. Homogeneous, Real-Time NanoBRET Binding Assays for the Histamine H3 and H4 Receptors on Living Cells. Mol. Pharmacol. 2018, 94, 1371–1381. [Google Scholar] [CrossRef] [Green Version]
- Schnell, D.; Seifert, R. Modulation of histamine H(3) receptor function by monovalent ions. Neurosci. Lett. 2010, 472, 114–118. [Google Scholar] [CrossRef]
- Kenakin, T. Biased Receptor Signaling in Drug Discovery. Pharmacol. Rev. 2019, 71, 267–315. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.C. The power issue: Determination of KB or Ki from IC50. A closer look at the Cheng-Prusoff equation, the Schild plot and related power equations. J. Pharmacol. Toxicol. Methods 2001, 46, 61–71. [Google Scholar] [CrossRef]



| Ligand | Intact Cells | Membrane Preparations | |||
|---|---|---|---|---|---|
| pEC50 | IA 1 | pEC50 | IA 1 | pKi | |
| histamine | 6.4 ± 0.1 (3) * | (+)1.00 ± 0.02 | 7.6 ± 0.1 (6) * | (+)1.00 ± 0.09 | 7.9 ± 0.1 (3) |
| pitolisant | 7.2 ± 0.2 (4) * | (−)1.00 ± 0.03 | 7.9 ± 0.3 (3) * | (−)1.00 ± 0.08 | 7.7 ± 0.3 (3) |
| ABT-239 | 7.8 ± 0.3 (4) * | (−)0.96 ± 0.05 | 8.8 ± 0.1 (3) * | (−)0.99 ± 0.04 | 9.1 ± 0.2 (3) |
| bavisant | 8.1 ± 0.2 (4) * | (−)0.77 ± 0.07 | 7.4 ± 0.1 (4) * | (−)0.88 ± 0.06 | 8.2 ± 0.2 (3) |
| PF-3654746 | 8.5 ± 0.1 (4) * | (−)0.92 ± 0.11 | 8.1 ± 0.2 (4) * | (−)1.05 ± 0.06 | 9.1 ± 0.2 (3) |
| trans-VUF15000 | 6.3 ± 0.1 (3) 2 | (+)1.36 ± 0.05 2 | n.d. | n.d. | 8.0 ± 0.2 (3) |
| cis-VUF15000 | 5.4 ± 0.2 (3) 2 | (+)1.35 ± 0.04 2 | n.d. | n.d. | 7.2 ± 0.0 (3) |
| trans-VUF14738 | 6.2 ± 0.2 (3) | n.d. 4 | n.d. | n.d. | 6.2 ± 0.1 (3) |
| cis-VUF14738 | 7.1 ± 0.2 (3) | n.d. 4 | n.d. | n.d. | 7.7 ± 0.3 (3) |
| imetit | 8.2 ± 0.1 3,* | (+)0.61 ± 0.01 | 8.8 ± 0.1 (3) * | (+)0.86 ± 0.14 | 9.1 ± 0.1 3 |
| clobenpropit | 7.4 ± 0.0 3,* | (−)0.38 ± 0.01 | 8.9 ± 0.1 (3) * | (−)0.65 ± 0.09 | 9.3 ± 0.1 3 |
| thioperamide | 7.1 ± 0.1 3 | (−)0.30 ± 0.01 | 7.2 ± 0.2 (3) | (−)0.60 ± 0.13 | 7.2 ± 0.1 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Gao, M.; Vischer, H.F.; Leurs, R. A NanoBRET-Based H3R Conformational Biosensor to Study Real-Time H3 Receptor Pharmacology in Cell Membranes and Living Cells. Int. J. Mol. Sci. 2022, 23, 8211. https://doi.org/10.3390/ijms23158211
Ma X, Gao M, Vischer HF, Leurs R. A NanoBRET-Based H3R Conformational Biosensor to Study Real-Time H3 Receptor Pharmacology in Cell Membranes and Living Cells. International Journal of Molecular Sciences. 2022; 23(15):8211. https://doi.org/10.3390/ijms23158211
Chicago/Turabian StyleMa, Xiaoyuan, Meichun Gao, Henry F. Vischer, and Rob Leurs. 2022. "A NanoBRET-Based H3R Conformational Biosensor to Study Real-Time H3 Receptor Pharmacology in Cell Membranes and Living Cells" International Journal of Molecular Sciences 23, no. 15: 8211. https://doi.org/10.3390/ijms23158211






