Sweet Taste Signaling: The Core Pathways and Regulatory Mechanisms
Abstract
1. Introduction
2. Organization of the Taste System
3. Pathways Mediating Sweet Taste Signaling
3.1. Sweet Taste Stimuli Are Structurally Diverse
3.2. The G-Protein-Coupled Receptor Pathway for Sweet Taste Signaling
3.2.1. The Primary Sweet Taste Receptor Is a Heterodimer of Taste 1 Receptor Members 2 and 3 (TAS1R2 + TAS1R3)
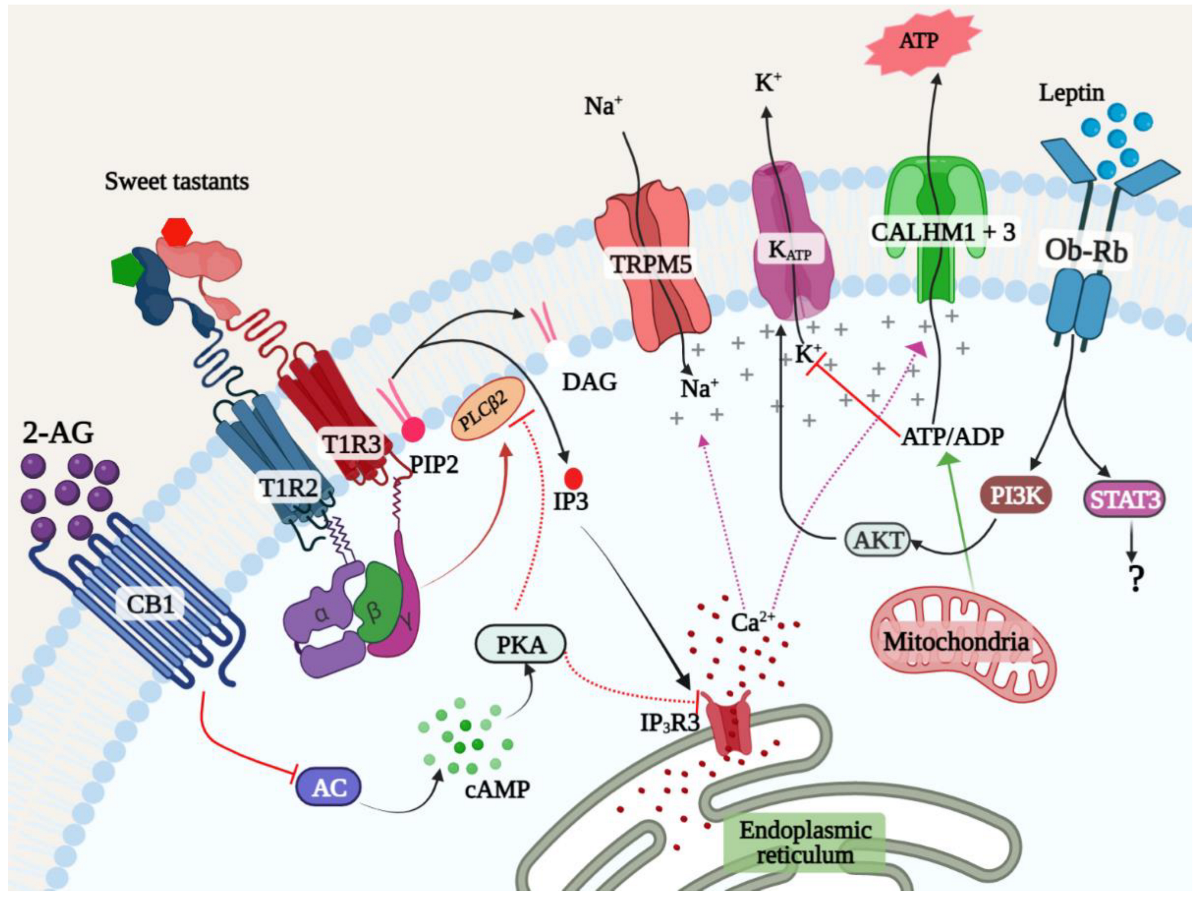
3.2.2. STR-Mediated Signal Transduction Pathways
3.2.3. STR-Independent Sweet Taste Signaling Pathways
3.3. Physiological and Behavioral Significance of Sweet Taste Signaling through Multiple Pathways
4. Regulation of Sweet Taste Signaling
4.1. Regulation of STR Anterograde Transport
4.2. STR Endocytosis and Adaptation
4.3. Promiscuous G-Protein Coupling Affect Signaling Pathways Downstream of the STR
4.4. Roles of G-Protein Signaling Regulators
4.5. Interaction of G-Proteins with PDZ Domain-Containing Proteins May Regulate Their Microvillar Localization
4.6. Hormonal Regulation of STR Signaling
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral coding of taste. Neuron 2014, 81, 984–1000. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K. Why do we like sweet taste: A bitter tale? Physiol. Behav. 2016, 164 Pt B, 432–437. [Google Scholar] [CrossRef]
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J.P. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef]
- Derby, C.D.; Kozma, M.T.; Senatore, A.; Schmidt, M. Molecular Mechanisms of Reception and Perireception in Crustacean Chemoreception: A Comparative Review. Chem. Senses 2016, 41, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Jordano, P.; Forget, P.M.; Lambert, J.E.; Bohning-Gaese, K.; Traveset, A.; Wright, S.J. Frugivores and seed dispersal: Mechanisms and consequences for biodiversity of a key ecological interaction. Biol. Lett. 2011, 7, 321–323. [Google Scholar] [CrossRef]
- Sussman, R.W. Primate origins and the evolution of angiosperms. Am. J. Primatol. 1991, 23, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Abbott, E. Sugar: A Bittersweet History; Penguin: Toronto, ON, Canada, 2008; 453p. [Google Scholar]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.L.; Martin, A.A.; Clark, K.; Swithers, S.E. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: Implications for the learned control of energy and body weight regulation. Q. J. Exp. Psychol. 2011, 64, 1430–1441. [Google Scholar] [CrossRef]
- Wise, P.M.; Nattress, L.; Flammer, L.J.; Beauchamp, G.K. Reduced dietary intake of simple sugars alters perceived sweet taste intensity but not perceived pleasantness. Am. J. Clin. Nutr. 2016, 103, 50–60. [Google Scholar] [CrossRef]
- Kapsimali, M.; Barlow, L.A. Developing a sense of taste. Semin. Cell Dev. Biol. 2013, 24, 200–209. [Google Scholar] [CrossRef]
- Barlow, L.A. Progress and renewal in gustation: New insights into taste bud development. Development 2015, 142, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, S.C.; Finger, T.E. Recent advances in taste transduction and signaling. F1000Research 2019, 8, 2117. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, S.C.; Finger, T.E. A taste for ATP: Neurotransmission in taste buds. Front. Cell. Neurosci. 2013, 7, 264. [Google Scholar] [CrossRef]
- Dando, R.; Roper, S.D. Acetylcholine is released from taste cells, enhancing taste signalling. J. Physiol. 2012, 590, 3009–3017. [Google Scholar] [CrossRef]
- Huang, Y.A.; Pereira, E.; Roper, S.D. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS ONE 2011, 6, e25471. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D. Chemical and electrical synaptic interactions among taste bud cells. Curr. Opin. Physiol. 2021, 20, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.A.; Stone, L.M.; Pereira, E.; Yang, R.; Kinnamon, J.C.; Dvoryanchikov, G.; Chaudhari, N.; Finger, T.E.; Kinnamon, S.C.; Roper, S.D. Knocking out P2X receptors reduces transmitter secretion in taste buds. J. Neurosci. 2011, 31, 13654–13661. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Maruyama, Y.; Lu, K.S.; Pereira, E.; Plonsky, I.; Baur, J.E.; Wu, D.; Roper, S.D. Mouse taste buds use serotonin as a neurotransmitter. J. Neurosci. 2005, 25, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, B.C.; Sukumaran, S.K.; Margolskee, R.F.; Bachmanov, A.A. Amiloride-Insensitive Salt Taste Is Mediated by Two Populations of Type III Taste Cells with Distinct Transduction Mechanisms. J. Neurosci. 2016, 36, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Perea-Martinez, I.; Nagai, T.; Chaudhari, N. Functional Cell Types in Taste Buds Have Distinct Longevities. PLoS ONE 2013, 8, e53399. [Google Scholar] [CrossRef] [PubMed]
- Barlow, L.A.; Klein, O.D. Developing and regenerating a sense of taste. Curr. Top. Dev. Biol. 2015, 111, 401–419. [Google Scholar]
- Miura, H.; Scott, J.K.; Harada, S.; Barlow, L.A. Sonic hedgehog-Expressing Basal Cells Are General Post-Mitotic Precursors of Functional Taste Receptor Cells. Dev. Dyn. 2014, 243, 1286–1297. [Google Scholar] [CrossRef]
- Ahmed, J.; Preissner, S.; Dunkel, M.; Worth, C.L.; Eckert, A.; Preissner, R. SuperSweet—A resource on natural and artificial sweetening agents. Nucleic Acids Res. 2011, 39, D377–D382. [Google Scholar] [CrossRef]
- Picone, D.; Temussi, P.A. Dissimilar sweet proteins from plants: Oddities or normal components? Plant Sci. 2012, 195, 135–142. [Google Scholar] [CrossRef]
- Wintjens, R.; Viet, T.M.; Mbosso, E.; Huet, J. Hypothesis/review: The structural basis of sweetness perception of sweet-tasting plant proteins can be deduced from sequence analysis. Plant Sci. 2011, 181, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ceunen, S.; Geuns, J.M. Steviol glycosides: Chemical diversity, metabolism, and function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Cui, M.; Zhao, B.; Liu, Z.; Snyder, L.A.; Benard, L.M.; Osman, R.; Margolskee, R.F.; Max, M. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J. Biol. Chem. 2005, 280, 15238–15246. [Google Scholar] [CrossRef] [PubMed]
- Pothuraju, R.; Sharma, R.K.; Chagalamarri, J.; Jangra, S.; Kumar Kavadi, P. A systematic review of Gymnema sylvestre in obesity and diabetes management. J. Sci. Food Agric. 2014, 94, 834–840. [Google Scholar] [CrossRef]
- McLaughlin, S.K.; McKinnon, P.J.; Margolskee, R.F. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 1992, 357, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.T.; Gannon, K.S.; Margolskee, R.F. Transduction of bitter and sweet taste by gustducin. Nature 1996, 381, 796–800. [Google Scholar] [CrossRef]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef]
- Max, M.; Shanker, Y.G.; Huang, L.; Rong, M.; Liu, Z.; Campagne, F.; Weinstein, H.; Damak, S.; Margolskee, R.F. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat. Genet. 2001, 28, 58–63. [Google Scholar] [CrossRef]
- Montmayeur, J.P.; Liberles, S.D.; Matsunami, H.; Buck, L.B. A candidate taste receptor gene near a sweet taste locus. Nat. Neurosci. 2001, 4, 492–498. [Google Scholar] [CrossRef]
- Cui, M.; Jiang, P.; Maillet, E.; Max, M.; Margolskee, R.F.; Osman, R. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr. Pharm. Des. 2006, 12, 4591–4600. [Google Scholar] [CrossRef]
- Kurihara, Y. Characteristics of antisweet substances, sweet proteins, and sweetness-inducing proteins. Crit. Rev. Food Sci. Nutr. 1992, 32, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Ellaithy, A.; Gonzalez-Maeso, J.; Logothetis, D.A.; Levitz, J. Structural and Biophysical Mechanisms of Class C G Protein-Coupled Receptor Function. Trends Biochem. Sci. 2020, 45, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- DuBois, G.E. Molecular mechanism of sweetness sensation. Physiol. Behav. 2016, 164 Pt B, 453–463. [Google Scholar] [CrossRef]
- Jiang, P.; Cui, M.; Zhao, B.; Snyder, L.A.; Benard, L.M.; Osman, R.; Max, M.; Margolskee, R.F. Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J. Biol. Chem. 2005, 280, 34296–34305. [Google Scholar] [CrossRef]
- Jiang, P.; Ji, Q.; Liu, Z.; Snyder, L.A.; Benard, L.M.; Margolskee, R.F.; Max, M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J. Biol. Chem. 2004, 279, 45068–45075. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Koyama, N. High activity of adenyl cyclase in olfactory and gustatory organs. Biochem. Biophys. Res. Commun. 1972, 48, 30–34. [Google Scholar] [CrossRef]
- Abaffy, T.; Trubey, K.R.; Chaudhari, N. Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am. J. Physiol.-Cell Physiol. 2003, 284, C1420–C1428. [Google Scholar] [CrossRef]
- Avenet, P.; Lindemann, B. Patch-clamp study of isolated taste receptor cells of the frog. J. Membr. Biol. 1987, 97, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Striem, B.J.; Pace, U.; Zehavi, U.; Naim, M.; Lancet, D. Sweet tastants stimulate adenylate cyclase coupled to GTP-binding protein in rat tongue membranes. Biochem. J. 1989, 260, 121–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell 2003, 112, 293–301. [Google Scholar] [CrossRef]
- Huang, L.; Shanker, Y.G.; Dubauskaite, J.; Zheng, J.Z.; Yan, W.; Rosenzweig, S.; Spielman, A.I.; Max, M.; Margolskee, R.F. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat. Neurosci. 1999, 2, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Clapp, T.R.; Stone, L.M.; Margolskee, R.F.; Kinnamon, S.C. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Huang, L.; Rong, M.; Kozak, J.A.; Preuss, A.K.; Zhang, H.; Max, M.; Margolskee, R.F. A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 2002, 5, 1169–1176. [Google Scholar] [CrossRef]
- Liu, D.; Liman, E.R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA 2003, 100, 15160–15165. [Google Scholar] [CrossRef] [PubMed]
- Taruno, A.; Vingtdeux, V.; Ohmoto, M.; Ma, Z.; Dvoryanchikov, G.; Li, A.; Adrien, L.; Zhao, H.; Leung, S.; Abernethy, M.; et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 2013, 495, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Taruno, A.; Ohmoto, M.; Jyotaki, M.; Lim, J.C.; Miyazaki, H.; Niisato, N.; Marunaka, Y.; Lee, R.J.; Hoff, H.; et al. CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes. Neuron 2018, 98, 547–561.e10. [Google Scholar] [CrossRef]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S.; Jiang, P.; Ninomiya, Y.; Margolskee, R.F. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Perez, C.A.; Shigemura, N.; Yoshida, R.; Mosinger, B., Jr.; Glendinning, J.I.; Ninomiya, Y.; et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem. Senses 2006, 31, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Eddy, M.C.; Eschle, B.K.; Peterson, D.; Lauras, N.; Margolskee, R.F.; Delay, E.R. A conditioned aversion study of sucrose and SC45647 taste in TRPM5 knockout mice. Chem. Senses 2012, 37, 391–401. [Google Scholar] [CrossRef]
- Ohkuri, T.; Yasumatsu, K.; Horio, N.; Jyotaki, M.; Margolskee, R.F.; Ninomiya, Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am. J. Physiol.-Regul. Integr] Comp. Physiol. 2009, 296, R960–R971. [Google Scholar] [CrossRef] [PubMed]
- Armour, S.L.; Frueh, A.; Knudsen, J.G. Sodium, Glucose and Dysregulated Glucagon Secretion: The Potential of Sodium Glucose Transporters. Front. Pharmacol. 2022, 13, 837664. [Google Scholar] [CrossRef] [PubMed]
- Hjorne, A.P.; Modvig, I.M.; Holst, J.J. The Sensory Mechanisms of Nutrient-Induced GLP-1 Secretion. Metabolites 2022, 12, 420. [Google Scholar] [CrossRef]
- Ishihara, H. Metabolism-secretion coupling in glucose-stimulated insulin secretion. Diabetol. Int. 2022, 13, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.; Satin, L.S. Beta-Cell Ion Channels and Their Role in Regulating Insulin Secretion. Compr. Physiol. 2021, 11, 1–21. [Google Scholar] [PubMed]
- Sukumaran, S.K.; Yee, K.K.; Iwata, S.; Kotha, R.; Quezada-Calvillo, R.; Nichols, B.L.; Mohan, S.; Pinto, B.M.; Shigemura, N.; Ninomiya, Y.; et al. Taste cell-expressed alpha-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc. Natl. Acad. Sci. USA 2016, 113, 6035–6040. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Ohkuri, T.; Yoshida, R.; Iwata, S.; Margolskee, R.F.; Ninomiya, Y. Sodium-glucose cotransporter 1 as a sugar taste sensor in mouse tongue. Acta Physiol. 2020, 230, e13529. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.K.; Sukumaran, S.K.; Kotha, R.; Gilbertson, T.A.; Margolskee, R.F. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc. Natl. Acad. Sci. USA 2011, 108, 5431–5436. [Google Scholar] [CrossRef]
- Toyono, T.; Seta, Y.; Kataoka, S.; Oda, M.; Toyoshima, K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res. 2011, 345, 243–252. [Google Scholar] [CrossRef]
- Merigo, F.; Benati, D.; Cristofoletti, M.; Osculati, F.; Sbarbati, A. Glucose transporters are expressed in taste receptor cells. J. Anat. 2011, 219, 243–252. [Google Scholar] [CrossRef]
- Mandel, A.L.; Peyrot des Gachons, C.; Plank, K.L.; Alarcon, S.; Breslin, P.A. Individual differences in AMY1 gene copy number, salivary alpha-amylase levels, and the perception of oral starch. PLoS ONE 2010, 5, e13352. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.L.; Breslin, P.A. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J. Nutr. 2012, 142, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Breslin, P.A.S.; Izumi, A.; Tharp, A.; Ohkuri, T.; Yokoo, Y.; Flammer, L.J.; Rawson, N.E.; Margolskee, R.F. Evidence that human oral glucose detection involves a sweet taste pathway and a glucose transporter pathway. PLoS ONE 2021, 16, e0256989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Klebansky, B.; Fine, R.M.; Liu, H.; Xu, H.; Servant, G.; Zoller, M.; Tachdjian, C.; Li, X. Molecular mechanism of the sweet taste enhancers. Proc. Natl. Acad. Sci. USA 2010, 107, 4752–4757. [Google Scholar] [CrossRef]
- Servant, G.; Tachdjian, C.; Tang, X.Q.; Werner, S.; Zhang, F.; Li, X.; Kamdar, P.; Petrovic, G.; Ditschun, T.; Java, A.; et al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc. Natl. Acad. Sci. USA 2010, 107, 4746–4751. [Google Scholar] [CrossRef]
- Beloto-Silva, O.; Machado, U.F.; Oliveira-Souza, M. Glucose-induced regulation of NHEs activity and SGLTs expression involves the PKA signaling pathway. J. Membr. Biol. 2011, 239, 157–165. [Google Scholar] [CrossRef]
- Yuan, Y.; Kong, F.; Xu, H.; Zhu, A.; Yan, N.; Yan, C. Cryo-EM structure of human glucose transporter GLUT4. Nat. Commun. 2022, 13, 2671. [Google Scholar] [CrossRef]
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.S.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Lubitz, G.S.; Shelling, S. Taste of glucose elicits cephalic-phase insulin release in mice. Physiol. Behav. 2018, 192, 200–205. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Frim, Y.G.; Hochman, A.; Lubitz, G.S.; Basile, A.J.; Sclafani, A. Glucose elicits cephalic-phase insulin release in mice by activating KATP channels in taste cells. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 312, R597–R610. [Google Scholar] [CrossRef]
- Gant, N.; Stinear, C.M.; Byblow, W.D. Carbohydrate in the mouth immediately facilitates motor output. Brain Res. 2010, 1350, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I.; Gillman, J.; Zamer, H.; Margolskee, R.F.; Sclafani, A. The role of T1r3 and Trpm5 in carbohydrate-induced obesity in mice. Physiol. Behav. 2012, 107, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Murovets, V.O.; Bachmanov, A.A.; Travnikov, S.V.; Churikova, A.A.; Zolotarev, V.A. The Involvement of the T1R3 Receptor Protein in the Control of Glucose Metabolism in Mice at Different Levels of Glycemia. J. Evol. Biochem. Physiol. 2014, 50, 334–344. [Google Scholar] [CrossRef][Green Version]
- Siderovski, D.P.; Willard, F.S. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int. J. Biol. Sci. 2005, 1, 51–66. [Google Scholar] [CrossRef]
- Cherfils, J.; Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef]
- Ishii, H.; Sato, Y.; Takei, M.; Nishio, S.; Komatsu, M. Glucose-incretin interaction revisited. Endocr. J. 2011, 58, 519–525. [Google Scholar] [CrossRef]
- Young, B.; Wertman, J.; Dupre, D.J. Regulation of GPCR Anterograde Trafficking by Molecular Chaperones and Motifs. Prog. Mol. Biol. Transl. Sci. 2015, 132, 289–305. [Google Scholar] [PubMed]
- Arvan, P.; Zhao, X.; Ramos-Castaneda, J.; Chang, A. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic 2002, 3, 771–780. [Google Scholar] [CrossRef]
- Ilegems, E.; Iwatsuki, K.; Kokrashvili, Z.; Benard, O.; Ninomiya, Y.; Margolskee, R.F. REEP2 enhances sweet receptor function by recruitment to lipid rafts. J. Neurosci. 2010, 30, 13774–13783. [Google Scholar] [CrossRef]
- Saito, H.; Kubota, M.; Roberts, R.W.; Chi, Q.; Matsunami, H. RTP family members induce functional expression of mammalian odorant receptors. Cell 2004, 119, 679–691. [Google Scholar] [CrossRef]
- Benovic, J.L.; DeBlasi, A.; Stone, W.C.; Caron, M.G.; Lefkowitz, R.J. Beta-adrenergic receptor kinase: Primary structure delineates a multigene family. Science 1989, 246, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, R.J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998, 273, 18677–18680. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, V.V.; Gurevich, E.V. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 2006, 110, 465–502. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, V.V.; Gurevich, E.V. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front. Pharmacol. 2019, 10, 125. [Google Scholar] [CrossRef]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Kusakabe, Y.; Miura, H.; Shindo, Y.; Ninomiya, Y.; Hino, A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem. Biophys. Res. Commun. 2003, 312, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, Y.; Yamaguchi, E.; Tanemura, K.; Kameyama, K.; Chiba, N.; Arai, S.; Emori, Y.; Abe, K. Identification of two alpha-subunit species of GTP-binding proteins, Galpha15 and Galphaq, expressed in rat taste buds. Biochim. Biophys. Acta 1998, 1403, 265–272. [Google Scholar] [CrossRef]
- Sukumaran, S.K.; Lewandowski, B.C.; Kaulin, Y.; Jiang, P.; Beauchamp, G.; Bachmanov, A.; Huang, L.; Margolskee, R.F. Transcriptome Analysis of Individual Taste Receptor Cells. In Proceedings of the 35th Annual Meeting of AChemS, Huntington Beach, CA, USA, 17–20 April 2013; Chem Senses: Huntington Beach, CA, USA, 2013; p. A645. [Google Scholar] [PubMed]
- Rossler, P.; Boekhoff, I.; Tareilus, E.; Beck, S.; Breer, H.; Freitag, J. G protein betagamma complexes in circumvallate taste cells involved in bitter transduction. Chem. Senses 2000, 25, 413–421. [Google Scholar] [CrossRef]
- Dutta Banik, D.; Martin, L.E.; Freichel, M.; Torregrossa, A.M.; Medler, K.F. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc. Natl. Acad. Sci. USA 2018, 115, E772–E781. [Google Scholar] [CrossRef]
- Tordoff, M.G.; Aleman, T.R.; Ellis, H.T.; Ohmoto, M.; Matsumoto, I.; Shestopalov, V.I.; Mitchell, C.H.; Foskett, J.K.; Poole, R.L. Normal Taste Acceptance and Preference of PANX1 Knockout Mice. Chem. Senses 2015, 40, 453–459. [Google Scholar] [CrossRef][Green Version]
- Hennigs, J.K.; Burhenne, N.; Stahler, F.; Winnig, M.; Walter, B.; Meyerhof, W.; Schmale, H. Sweet taste receptor interacting protein CIB1 is a general inhibitor of InsP3-dependent Ca2+ release in vivo. J. Neurochem. 2008, 106, 2249–2262. [Google Scholar] [CrossRef]
- Fenech, C.; Patrikainen, L.; Kerr, D.S.; Grall, S.; Liu, Z.; Laugerette, F.; Malnic, B.; Montmayeur, J.P. Ric-8A, a Galpha protein guanine nucleotide exchange factor potentiates taste receptor signaling. Front. Cell. Neurosci. 2009, 3, 11. [Google Scholar] [CrossRef]
- von Buchholtz, L.; Elischer, A.; Tareilus, E.; Gouka, R.; Kaiser, C.; Breer, H.; Conzelmann, S. RGS21 is a novel regulator of G protein signalling selectively expressed in subpopulations of taste bud cells. Eur J. Neurosci. 2004, 19, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Buckley, B.K.; Kosloff, M.; Garland, A.L.; Bosch, D.E.; Cheng, G., Jr.; Radhakrishna, H.; Brown, M.D.; Willard, F.S.; Arshavsky, V.Y.; et al. Regulator of G-protein signaling-21 (RGS21) is an inhibitor of bitter gustatory signaling found in lingual and airway epithelia. J. Biol. Chem. 2012, 287, 41706–41719. [Google Scholar] [CrossRef] [PubMed]
- Schroer, A.B.; Branyan, K.W.; Gross, J.D.; Chantler, P.D.; Kimple, A.J.; Vandenbeuch, A.; Siderovski, D.P. The stability of tastant detection by mouse lingual chemosensory tissue requires Regulator of G protein Signaling-21 (RGS21). Chem. Senses 2021, 46, bjab048. [Google Scholar] [CrossRef] [PubMed]
- Schroer, A.B.; Gross, J.D.; Kaski, S.W.; Wix, K.; Siderovski, D.P.; Vandenbeuch, A.; Setola, V. Development of Full Sweet, Umami, and Bitter Taste Responsiveness Requires Regulator of G protein Signaling-21 (RGS21). Chem. Senses 2018, 43, 367–378. [Google Scholar] [CrossRef]
- Dando, R.; Pereira, E.; Kurian, M.; Barro-Soria, R.; Chaudhari, N.; Roper, S.D. A permeability barrier surrounds taste buds in lingual epithelia. Am. J. Physiol.-Cell Physiol. 2015, 308, C21–C32. [Google Scholar] [CrossRef]
- Gao, N.; Lu, M.; Echeverri, F.; Laita, B.; Kalabat, D.; Williams, M.E.; Hevezi, P.; Zlotnik, A.; Moyer, B.D. Voltage-gated sodium channels in taste bud cells. BMC Neurosci. 2009, 10, 20. [Google Scholar] [CrossRef]
- Murciano-Calles, J. The Conformational Plasticity Vista of PDZ Domains. Life 2020, 10, 123. [Google Scholar] [CrossRef]
- Liu, Z.; Fenech, C.; Cadiou, H.; Grall, S.; Tili, E.; Laugerette, F.; Wiencis, A.; Grosmaitre, X.; Montmayeur, J.P. Identification of new binding partners of the chemosensory signaling protein Ggamma13 expressed in taste and olfactory sensory cells. Front. Cell. Neurosci. 2012, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Benard, O.; Margolskee, R.F. Ggamma13 interacts with PDZ domain-containing proteins. J. Biol. Chem. 2006, 281, 11066–11073. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Sugimoto, K.; Nakashima, K.; Miura, H.; Ninomiya, Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11044–11049. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, N.; Miura, H.; Kusakabe, Y.; Hino, A.; Ninomiya, Y. Expression of leptin receptor (Ob-R) isoforms and signal transducers and activators of transcription (STATs) mRNAs in the mouse taste buds. Arch. Histol. Cytol. 2003, 66, 253–260. [Google Scholar] [CrossRef]
- Yoshida, R.; Noguchi, K.; Shigemura, N.; Jyotaki, M.; Takahashi, I.; Margolskee, R.F.; Ninomiya, Y. Leptin Suppresses Mouse Taste Cell Responses to Sweet Compounds. Diabetes 2015, 64, 3751–3762. [Google Scholar] [CrossRef]
- Yoshida, R.; Margolskee, R.F.; Ninomiya, Y. Phosphatidylinositol-3 kinase mediates the sweet suppressive effect of leptin in mouse taste cells. J. Neurochem. 2021, 158, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Sanematsu, K.; Ohta, R.; Shirosaki, S.; Koyano, K.; Nonaka, K.; Shigemura, N.; Ninomiya, Y. Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes 2008, 57, 2661–2665. [Google Scholar] [CrossRef]
- Yoshida, R.; Ohkuri, T.; Jyotaki, M.; Yasuo, T.; Horio, N.; Yasumatsu, K.; Sanematsu, K.; Shigemura, N.; Yamamoto, T.; Margolskee, R.F.; et al. Endocannabinoids selectively enhance sweet taste. Proc. Natl. Acad. Sci. USA 2010, 107, 935–939. [Google Scholar] [CrossRef]
- Jyotaki, M.; Shigemura, N.; Ninomiya, Y. Modulation of sweet taste sensitivity by orexigenic and anorexigenic factors. Endocr. J. 2010, 57, 467–475. [Google Scholar] [CrossRef]
- Leo, L.M.; Abood, M.E. CB1 Cannabinoid Receptor Signaling and Biased Signaling. Molecules 2021, 26, 5413. [Google Scholar] [CrossRef]
- Shigemura, N.; Iwata, S.; Yasumatsu, K.; Ohkuri, T.; Horio, N.; Sanematsu, K.; Yoshida, R.; Margolskee, R.F.; Ninomiya, Y. Angiotensin II modulates salty and sweet taste sensitivities. J. Neurosci. 2013, 33, 6267–6277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.L.; Shen, T.; Kaya, N.; Lu, S.G.; Cao, Y.; Herness, S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc. Natl. Acad. Sci. USA 2005, 102, 11100–11105. [Google Scholar] [CrossRef]
- Hajnal, A.; Covasa, M.; Bello, N.T. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 289, R1675–R1686. [Google Scholar] [CrossRef] [PubMed]
- Herness, S.; Zhao, F.L. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol. Behav. 2009, 97, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Martin, B.; Golden, E.; Dotson, C.D.; Maudsley, S.; Kim, W.; Jang, H.J.; Mattson, M.P.; Drucker, D.J.; Egan, J.M.; et al. Modulation of taste sensitivity by GLP-1 signaling. J. Neurochem. 2008, 106, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Dotson, C.D.; Shin, Y.K.; Ji, S.; Drucker, D.J.; Maudsley, S.; Munger, S.D. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann. N. Y. Acad. Sci. 2009, 1170, 98–101. [Google Scholar] [CrossRef]
- Takai, S.; Yasumatsu, K.; Inoue, M.; Iwata, S.; Yoshida, R.; Shigemura, N.; Yanagawa, Y.; Drucker, D.J.; Margolskee, R.F.; Ninomiya, Y. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015, 29, 2268–2280. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukumaran, S.K.; Palayyan, S.R. Sweet Taste Signaling: The Core Pathways and Regulatory Mechanisms. Int. J. Mol. Sci. 2022, 23, 8225. https://doi.org/10.3390/ijms23158225
Sukumaran SK, Palayyan SR. Sweet Taste Signaling: The Core Pathways and Regulatory Mechanisms. International Journal of Molecular Sciences. 2022; 23(15):8225. https://doi.org/10.3390/ijms23158225
Chicago/Turabian StyleSukumaran, Sunil Kumar, and Salin Raj Palayyan. 2022. "Sweet Taste Signaling: The Core Pathways and Regulatory Mechanisms" International Journal of Molecular Sciences 23, no. 15: 8225. https://doi.org/10.3390/ijms23158225
APA StyleSukumaran, S. K., & Palayyan, S. R. (2022). Sweet Taste Signaling: The Core Pathways and Regulatory Mechanisms. International Journal of Molecular Sciences, 23(15), 8225. https://doi.org/10.3390/ijms23158225





