The Crucial Roles of Bmi-1 in Cancer: Implications in Pathogenesis, Metastasis, Drug Resistance, and Targeted Therapies
Abstract
:1. Introduction
2. Molecular Features and Characteristics of Bmi-1
3. Upstream Regulatory Mechanisms of Bmi-1
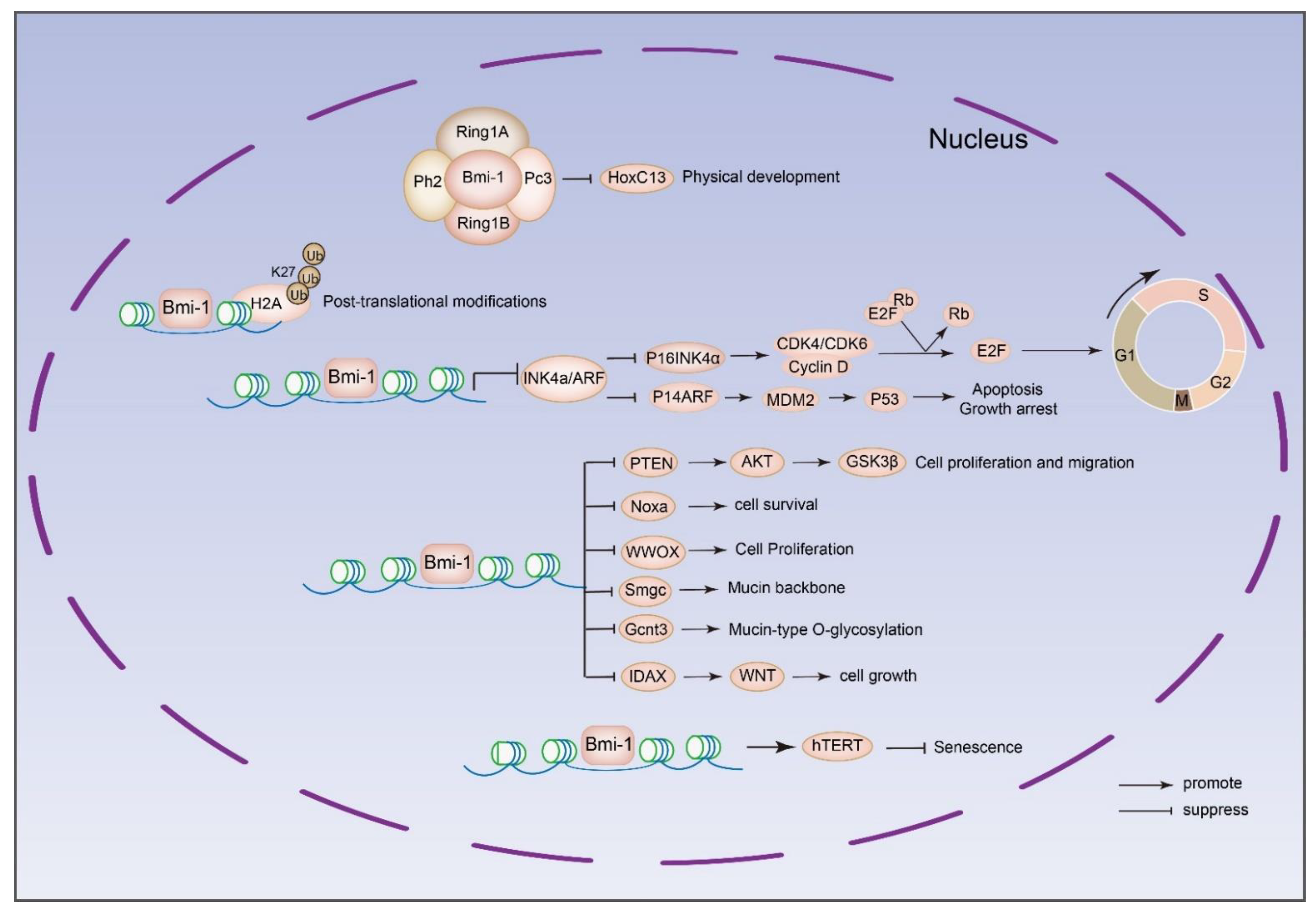
4. Bmi-1-Targeted Processes in Cancer
4.1. Bmi-1 in Cancer Proliferation
4.2. Bmi-1 in Cancer Apoptosis
4.3. Bmi-1 in Cancer Autophagy
4.4. Bmi-1 in Cancer EMT
4.5. Bmi-1 in Cancer DNA Damage Response
4.6. Bmi-1 in Cancer Inflammation
4.7. Bmi-1 in Cancer Stem Cells
4.8. Bmi-1 in Tumor Microenvironment
5. Clinical Characteristics and Cancer Therapy of Bmi-1
5.1. Protein Expression and Clinical Characteristics of Bmi-1
5.2. Bmi-1 in Chemoresistance and Cancer Therapy
6. Future Research and Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 4EBP1 | Eukaryotic Translation Initiation Factor 4E Binding Protein 1 |
| AKT | Protein kinase B |
| AML | Acute myeloid leukemia |
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine triphosphate |
| BCSCs | Breast cancer stem cells |
| Bmi-1 | B Lymphoma Mo-MLV Insertion Region 1 Homolog |
| CBX4 | Chromobox 4 |
| Chk2 | Checkpoint Kinase 2 |
| c-MYC | Myc proto-oncogene protein |
| CSCs | Cancer stem cells |
| DDR | DNA damage response |
| DKK1 | Dickkopf WNT Signaling Pathway Inhibitor 1 |
| DUB3 | Deubiquitinating Protein 3 |
| E2F1 | E2F Transcription Factor 1 |
| EMT | Epithelial-mesenchymal-like transformation |
| FoxM1 | Forkhead Box M1 |
| H2AX | H2A.X Variant Histone |
| HDACs | Histone Deacetylase |
| HoxC13 | Homeobox C13 |
| HSCs | Hematopoietic stem cells |
| hTERT | Human telomerase reverse transcriptase |
| HTH structure | Helix-turn-helix structure |
| IR | Ionizing radiation |
| KLF4 | Kruppel-like Factor 4 |
| Mcl-1 | Myeloid cell leukemia sequence 1 |
| MD-2 | Myeloid Differentiation Protein-2 |
| MDM2 | E3 ubiquitin-protein ligase Mdm2 |
| Mel18 | Polycomb Group RING Finger 2 |
| miRNAs | MicroRNAs |
| MM | Multiple myeloma |
| MM-MΦs | Myeloma-associated macrophages |
| MMP2 | Matrix Metallopeptidase2 |
| MMP9 | Matrix Metallopeptidase 9 |
| mTOR | mammalian target of rapamycin |
| MYC-N | MYCN Proto-Oncogene, BHLH Transcription Factor |
| MyD88 | MYD88 Innate Immune Signal Transduction Adaptor |
| Nanog | Homeobox protein NANOG |
| ncRNAs | Non-coding RNA |
| NF-κB | Nuclear factor kappa-B |
| NLRP3 | NACHT, LRR and PYD domains-containing protein 3 |
| NLRP3 | NOD-like receptors (NLRs) family member |
| NLS | Nuclear localization signal |
| Noxa | Phorbol-12-Myristate-13-Acetate-Induced Protein 1 |
| NSCs | Neural stem cells |
| NSPC | Neural stem cells and progenitor cells |
| P21 | Cyclin-dependent kinase inhibitor 1A |
| P53 | Tumor Protein P53 |
| PcG | Polycomb group |
| PRC1 | polycomb repressive complex1 |
| PRC2 | polycomb repressive complex 2 |
| PROS1 | Protein S |
| PTEN | Phosphatidylinositol3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN |
| PTMs | Post-translational modifications |
| RKIP | Raf kinase inhibitory protein |
| ROS | Proto-oncogene tyrosine-protein kinase ROS |
| SALL4 | Spalt Like Transcription Factor 4 |
| Slug | Snail Family Transcriptional Repressor 2 |
| Snail | Zinc finger protein SNAI1 |
| SP1 | Transcription factor Sp1 |
| Stat3 | Signal transducer and activator of transcription 3 |
| TAMs | Tumor-associated macrophages |
| TLR4 | Toll-like receptor 4 |
| TME | Tumor microenvironment |
| Twist1 | Twist Family BHLH Transcription Factor 1 |
| USP22 | Ubiquitin Specific Peptidase 22 |
| VEGF | Vascular Endothelial Growth Factor A |
| VEGF-C | Vascular Endothelial Growth Factor C |
| WWOX | WW Domain Containing Oxidoreductase |
| ZEB1 | Zinc Finger E-Box Binding Homeobox 1 |
References
- Wang, W.; Qin, J.J.; Voruganti, S.; Nag, S.; Zhou, J.; Zhang, R. Polycomb Group (PcG) Proteins and Human Cancers: Multifaceted Functions and Therapeutic Implications. Med. Res. Rev. 2015, 35, 1220–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, R.; Janakiraman, H.; Kanno, M. Epigenetic regulator polycomb group protein complexes control cell fate and cancer. Cancer Sci. 2008, 99, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Y.B.; Pirrotta, V. Polycomb complexes and epigenetic states. Curr. Opin. Cell Biol. 2008, 20, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Dontu, G.; Mantle, I.D.; Patel, S.; Ahn, N.S.; Jackson, K.W.; Suri, P.; Wicha, M.S. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006, 66, 6063–6071. [Google Scholar] [CrossRef] [Green Version]
- Park, I.K.; Qian, D.; Kiel, M.; Becker, M.W.; Pihalja, M.; Weissman, I.L.; Morrison, S.J.; Clarke, M.F. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003, 423, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, J.; Song, L. Bmi-1, stem cells and cancer. Acta Biochim. Biophys. Sin. 2009, 41, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.Q.; Nolasco, S.; Soares, H. Non-coding RNAs: Multi-tasking molecules in the cell. Int. J. Mol. Sci. 2013, 14, 16010–16039. [Google Scholar] [CrossRef]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics. Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.J.; Yan, Y.L.; Qian, L.; Gong, Z.C. Long non-coding RNAs act as regulators of cell autophagy in diseases (Review). Oncol. Rep. 2017, 37, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, R.A.; Fraczek, M.G.; Parker, S.; Delneri, D.; O’Keefe, R.T. Non-coding RNAs and disease: The classical ncRNAs make a comeback. Biochem. Soc. Trans. 2016, 44, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Zhang, X.; Hua, R.; Gan, L.; Huang, M.; Zhao, L.; Ni, S.; Guo, W. Bmi-1 regulates stem cell-like properties of gastric cancer cells via modulating miRNAs. J. Hematol. Oncol. 2016, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wu, H.; Fan, M.; Yu, R.; Zhang, Y.; Liu, J.; Zhou, X.; Cai, Y.; Huang, S.; Hu, Z.; et al. Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells. FASEB J. 2020, 34, 4266–4282. [Google Scholar] [CrossRef] [Green Version]
- Alkema, M.J.; Wiegant, J.; Raap, A.K.; Berns, A.; Vanlohuizen, M. Characterization And Chromosomal Localization Of the Human Protooncogene Bmi-1. Hum. Mol. Genet. 1993, 2, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabuddhe, A.A. BMI1: A Biomarker of Hematologic Malignancies. Biomark. Cancer 2016, 8, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voncken, J.W.; Schweizer, D.; Aagaard, L.; Sattler, L.; Jantsch, M.F.; van Lohuizen, M. Chromatin-association of the Polycomb group protein BMI1 is cell cycle-regulated and correlates with its phosphorylation status. J. Cell Sci. 1999, 112 Pt 24, 4627–4639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Yu, H.; Zhao, X.; Sashida, G.; Deblasio, A.; Harr, M.; She, Q.B.; Chen, Z.; Lin, H.K.; et al. Akt phosphorylates the transcriptional repressor bmi1 to block its effects on the tumor-suppressing ink4a-arf locus. Sci. Signal. 2012, 5, ra77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karvonen, H.; Barker, H.; Kaleva, L.; Niininen, W.; Ungureanu, D. Molecular Mechanisms Associated with ROR1-Mediated Drug Resistance: Crosstalk with Hippo-YAP/TAZ and BMI-1 Pathways. Cells 2019, 8, 812. [Google Scholar] [CrossRef] [Green Version]
- Voncken, J.W.; Niessen, H.; Neufeld, B.; Rennefahrt, U.; Dahlmans, V.; Kubben, N.; Holzer, B.; Ludwig, S.; Rapp, U.R. MAPKAP kinase 3pK phosphorylates and regulates chromatin association of the polycomb group protein Bmi1. J. Biol. Chem. 2005, 280, 5178–5187. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.H.; Gagné, J.P.; Caron, M.C.; McDonald, D.; Xu, Z.; Masson, J.Y.; Poirier, G.G.; Hendzel, M.J. CBX4-mediated SUMO modification regulates BMI1 recruitment at sites of DNA damage. Nucleic Acids Res. 2012, 40, 5497–5510. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Oh, M.; Sasaki, J.I.; Nör, J.E. Inverse and reciprocal regulation of p53/p21 and Bmi-1 modulates vasculogenic differentiation of dental pulp stem cells. Cell Death Dis. 2021, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Liu, J.; Zhang, P.; An, M.; Han, C.; Li, Y.; Guan, X.; Zhang, K. O-GlcNAcylation modulates Bmi-1 protein stability and potential oncogenic function in prostate cancer. Oncogene 2017, 36, 6293–6305. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Wang, D.; Liu, P.; Chu, Y.; Zhang, X.; Ding, X.; Li, X.; Mao, T.; Jing, X.; Tian, Z.; et al. MicroRNA-498 inhibits the proliferation, migration and invasion of gastric cancer through targeting BMI-1 and suppressing AKT pathway. Hum. Cell 2020, 33, 366–376. [Google Scholar] [CrossRef]
- Yu, W.W.; Jiang, H.; Zhang, C.T.; Peng, Y. The SNAIL/miR-128 axis regulated growth, invasion, metastasis, and epithelial-to-mesenchymal transition of gastric cancer. Oncotarget 2017, 8, 39280–39295. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zheng, X.; Li, X.; Fesler, A.; Hu, W.; Chen, L.; Xu, B.; Wang, Q.; Tong, A.; Burke, S.; et al. Reduction of gastric cancer proliferation and invasion by miR-15a mediated suppression of Bmi-1 translation. Oncotarget 2016, 7, 14522–14536. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Li, Y.; Yan, D.; He, J.; Liu, D. MicroRNA-183 inhibits gastric cancer proliferation and invasion via directly targeting Bmi-1. Oncol. Lett. 2014, 8, 2345–2351. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Tian, S.; Chen, Y.; Ji, M.; Qu, Y.; Hou, P. miR-218 inhibits gastric tumorigenesis through regulating Bmi-1/Akt signaling pathway. Pathol. Res. Pract. 2019, 215, 243–250. [Google Scholar] [CrossRef]
- Chai, Y.; Du, Y.; Zhang, S.; Xiao, J.; Luo, Z.; He, F.; Huang, K. MicroRNA-485-5p reduces O-GlcNAcylation of Bmi-1 and inhibits colorectal cancer proliferation. Exp. Cell Res. 2018, 368, 111–118. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dong, Y.; Wu, C.W.; Zhao, Z.; Ng, S.S.; Chan, F.K.; Sung, J.J.; Yu, J. MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 polycomb ring finger oncogene. Mol. Med. 2013, 18, 1491–1498. [Google Scholar] [CrossRef]
- Xu, Z.; Tao, J.; Chen, P.; Chen, L.; Sharma, S.; Wang, G.; Dong, Q. Sodium Butyrate Inhibits Colorectal Cancer Cell Migration by Downregulating Bmi-1 Through Enhanced miR-200c Expression. Mol. Nutr. Food Res. 2018, 62, e1700844. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W.G.; Wang, D.S.; Tao, K.S.; Song, W.J.; Dou, K.F. MicroRNA-183 is involved in cell proliferation, survival and poor prognosis in pancreatic ductal adenocarcinoma by regulating Bmi-1. Oncol. Rep. 2014, 32, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xu, X.; Tang, Y.; Zhang, C.; Li, J.; Ouyang, Y.; Ju, J.; Bie, P.; Wang, H. miR-15a inhibits cell proliferation and epithelial to mesenchymal transition in pancreatic ductal adenocarcinoma by down-regulating Bmi-1 expression. Cancer. Lett. 2014, 344, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, X.; Li, H.; Guo, L.; Jiang, W.; Lu, S.H. miR-203 inhibits the proliferation and self-renewal of esophageal cancer stem-like cells by suppressing stem renewal factor Bmi-1. Stem Cells Dev 2014, 23, 576–585. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, D.W.; Kim, C.S.; Yu, S.K.; Kim, H.J.; Go, D.S.; Lee, S.A.; Moon, S.M.; Kim, S.G.; Chun, H.S.; et al. MicroRNA-203 Induces Apoptosis by Targeting Bmi-1 in YD-38 Oral Cancer Cells. Anticancer Res. 2018, 38, 3477–3485. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Z.M.; Xie, Y.; He, X.Q.; Lin, X.H.; Hu, J.L.; Peng, Y.Z. miR-218 suppresses the growth of hepatocellular carcinoma by inhibiting the expression of proto-oncogene Bmi-1. J. BUON 2018, 23, 604–610. [Google Scholar]
- Yang, F.; Lv, L.Z.; Cai, Q.C.; Jiang, Y. Potential roles of EZH2, Bmi-1 and miR-203 in cell proliferation and invasion in hepatocellular carcinoma cell line Hep3B. World J. Gastroenterol. 2015, 21, 13268–13276. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, D.; Li, X.; Yang, J.; Chen, L.; Ning, Z.; Xu, Y.; Deng, G.; Tao, M.; Zhu, Y.; et al. MicroRNA-203 Increases Cell Radiosensitivity via Directly Targeting Bmi-1 in Hepatocellular Carcinoma. Mol. Pharm. 2018, 15, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.M.; Tang, L.P.; Zhu, X.; Lu, Y.F.; Zhang, Y.L.; Lee, W.Y.; Wang, H.; Yu, Y.; Liang, W.C.; Ko, C.H.; et al. MiR-218-targeting-Bmi-1 mediates the suppressive effect of 1,6,7-trihydroxyxanthone on liver cancer cells. Apoptosis 2015, 20, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Li, C.; Zheng, X.; Yang, W.; Yao, Y.; Liu, Q. Prognostic significance of miR-218 in human hepatocellular carcinoma and its role in cell growth. Oncol. Rep. 2014, 32, 1571–1577. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Li, J.; Zhou, C.; Lv, C.; Tian, M. MicroRNA-320a inhibits cell proliferation, migration and invasion by targeting BMI-1 in nasopharyngeal carcinoma. FEBS Lett. 2014, 588, 3732–3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.F.; Zhang, S.H.; Li, X.F.; Cao, L.Y.; Fu, Z.Z.; Yu, S.N. Overexpression of microRNA-132 enhances the radiosensitivity of cervical cancer cells by down-regulating Bmi-1. Oncotarget 2017, 8, 80757–80769. [Google Scholar] [CrossRef]
- Rong, X.; Gao, W.; Yang, X.; Guo, J. Downregulation of hsa_circ_0007534 restricts the proliferation and invasion of cervical cancer through regulating miR-498/BMI-1 signaling. Life Sci. 2019, 235, 116785. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Zhang, M.; Sun, W.; Shi, W.; Li, F. Circular RNA circ-0016068 Promotes the Growth, Migration, and Invasion of Prostate Cancer Cells by Regulating the miR-330-3p/BMI-1 Axis as a Competing Endogenous RNA. Front. Cell Dev. Biol. 2020, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Li, X.; Wang, W. Exosomal circRNA HIPK3 knockdown inhibited cell proliferation and metastasis in prostate cancer by regulating miR-212/BMI-1 pathway. J. Biosci. 2021, 46, 69. [Google Scholar] [CrossRef]
- Khan, A.P.; Poisson, L.M.; Bhat, V.B.; Fermin, D.; Zhao, R.; Kalyana-Sundaram, S.; Michailidis, G.; Nesvizhskii, A.I.; Omenn, G.S.; Chinnaiyan, A.M.; et al. Quantitative proteomic profiling of prostate cancer reveals a role for miR-128 in prostate cancer. Mol. Cell Proteom. 2010, 9, 298–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Zhang, T.; Liu, C.; Badeaux, M.A.; Liu, B.; Liu, R.; Jeter, C.; Chen, X.; Vlassov, A.V.; Tang, D.G. miRNA-128 suppresses prostate cancer by inhibiting BMI-1 to inhibit tumor-initiating cells. Cancer Res. 2014, 74, 4183–4195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, B.; Wang, R.; Chen, L.B. Review of miR-200b and cancer chemosensitivity. Biomed. Pharm. 2012, 66, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lu, Y.; Cui, D.; Li, E.; Zhu, Y.; Zhao, Y.; Zhao, F.; Xia, S. miR-200b suppresses cell proliferation, migration and enhances chemosensitivity in prostate cancer by regulating Bmi-1. Oncol. Rep. 2014, 31, 910–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, H.; Xue, W.; Pan, J.; Sha, J.; Dong, B.; Huang, Y. Downregulation of miR-221, -30d, and -15a contributes to pathogenesis of prostate cancer by targeting Bmi-1. Biochemistry 2015, 80, 276–283. [Google Scholar] [CrossRef]
- Ambs, S.; Prueitt, R.L.; Yi, M.; Hudson, R.S.; Howe, T.M.; Petrocca, F.; Wallace, T.A.; Liu, C.G.; Volinia, S.; Calin, G.A.; et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008, 68, 6162–6170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Liang, A.; Lv, Y.; Liu, G.; Jiang, A.; Liu, P. MicroRNA-200c Inhibits Epithelial-Mesenchymal Transition by Targeting the BMI-1 Gene Through the Phospho-AKT Pathway in Endometrial Carcinoma Cells In Vitro. Med. Sci. Monit. 2017, 23, 5139–5149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, P.; Kaneuchi, M.; Watari, H.; Hamada, J.; Sudo, S.; Ju, J.; Sakuragi, N. MicroRNA-194 inhibits epithelial to mesenchymal transition of endometrial cancer cells by targeting oncogene BMI-1. Mol. Cancer 2011, 10, 99. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, J.L.; Yu, L.; Liu, X.X.; Wu, H.M.; Lei, F.Y.; Wu, S.; Wang, X. Downregulated miR-495 [Corrected] Inhibits the G1-S Phase Transition by Targeting Bmi-1 in Breast Cancer. Medicine 2015, 94, e718. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, F.; Jiao, Y.; Feng, J.; Tang, W.; Yao, H.; Gong, C.; Chen, J.; Su, F.; Zhang, Y.; et al. Reduced miR-128 in breast tumor-initiating cells induces chemotherapeutic resistance via Bmi-1 and ABCC5. Clin. Cancer Res. 2011, 17, 7105–7115. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, R.; Nicoloso, M.; Arvizo, R.; Wang, E.; Cortez, A.; Rossi, S.; Calin, G.A.; Mukherjee, P. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 2009, 69, 9090–9095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.L.; Sun, B.L.; Tian, S.X.; Li, L.; Zhao, Y.C.; Shi, P.P. MicroRNA-132 reverses cisplatin resistance and metastasis in ovarian cancer by the targeted regulation on Bmi-1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3635–3644. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Wu, N.; Zhang, W.J.; Shang, L.X. Deregulation of miR-128 in ovarian cancer promotes cisplatin resistance. Int. J. Gynecol. Cancer 2014, 24, 1381–1388. [Google Scholar] [CrossRef]
- Godlewski, J.; Nowicki, M.O.; Bronisz, A.; Williams, S.; Otsuki, A.; Nuovo, G.; Raychaudhury, A.; Newton, H.B.; Chiocca, E.A.; Lawler, S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008, 68, 9125–9130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wei, C.; Li, J.; Liu, J.; Qu, J. MicroRNA-361-5p inhibits epithelial-to-mesenchymal transition of glioma cells through targeting Twist1. Oncol. Rep. 2017, 37, 1849–1856. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Gao, X.; Li, G.; Fu, H.; Cui, D.; Liu, H.; Jin, W.; Zhang, Y. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1. Cancer Res. 2013, 73, 6046–6055. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.G.; Zhao, Y.; Sethi, P.; Li, Y.Y.; Mahta, A.; Culicchia, F.; Lukiw, W.J. Micro-RNA-128 (miRNA-128) down-regulation in glioblastoma targets ARP5 (ANGPTL6), Bmi-1 and E2F-3a, key regulators of brain cell proliferation. J. Neurooncol. 2010, 98, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yu, G.; Wang, C.; Du, B.; Sun, D.; Liu, J.; Qi, T.; Yu, X.; Wei, W.; Cheng, J.; et al. MicroRNA-128a, BMI1 polycomb ring finger oncogene, and reactive oxygen species inhibit the growth of U-87 MG glioblastoma cells following exposure to X-ray radiation. Mol. Med. Rep. 2015, 12, 6247–6254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Chen, L.; He, H.; Huang, W.; Zhang, R.; Li, P.; Meng, Y.; Jiang, X. Up-regulation of microRNA-16 in Glioblastoma Inhibits the Function of Endothelial Cells and Tumor Angiogenesis by Targeting Bmi-1. Anticancer. Agents Med. Chem. 2016, 16, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Alimova, I.; Fan, R.; Harris, P.; Foreman, N.; Vibhakar, R. MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS ONE 2010, 5, e10748. [Google Scholar] [CrossRef]
- Lai, G.H.; Huang, A.L.; Zhao, Z.; Lu, X.H.; Zu, W.X. [MicroRNA-218 promotes osteosarcoma cell apoptosis by down-regulating oncogene B lymphoma mouse Moloney leukemia virus insertion region 1]. Nan Fang Yi Ke Da Xue Xue Bao 2018, 38, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Liang, D.; Li, B.; Liang, C.; Huang, W.; Lin, H. MicroRNA-320a protects against osteoarthritis cartilage degeneration by regulating the expressions of BMI-1 and RUNX2 in chondrocytes. Pharmazie 2017, 72, 223–226. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, M.; Tan, G.; Liang, Z.; Qin, Y.; Chen, L.; Chen, H.; Liu, J. miR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J. Transl. Med. 2014, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Yang, X.; Deng, X.; Zhang, X.; Li, P.; Tao, J.; Lu, Q. MicroRNA-218 inhibits bladder cancer cell proliferation, migration, and invasion by targeting BMI-1. Tumour Biol. 2015, 36, 8015–8023. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.Z.; Ma, M.; Shao, G.F. MiR-15 suppressed the progression of bladder cancer by targeting BMI1 oncogene via PI3K/AKT signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8813–8822. [Google Scholar] [CrossRef]
- Liu, J.F.; He, P.; Pan, D.F. [MiR-218 Targeting Bmi-1 Inhibits Proliferation of Acute Promyelocytic Leukemia Cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 815–820. [Google Scholar] [CrossRef]
- Xu, L.; Sun, H.B.; Xu, Z.N.; Han, X.L.; Yin, Y.Y.; Zheng, Y.; Zhao, Y.; Wang, Z.X. MicroRNA-218 regulates the epithelial-to-mesenchymal transition and the PI3K/Akt signaling pathway to suppress lung adenocarcinoma progression by directly targeting BMI-1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7978–7988. [Google Scholar] [CrossRef]
- Wu, S.Q.; Niu, W.Y.; Li, Y.P.; Huang, H.B.; Zhan, R. miR-203 inhibits cell growth and regulates G1/S transition by targeting Bmi-1 in myeloma cells. Mol. Med. Rep. 2016, 14, 4795–4801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, M.; Liang, Z.; Chen, L.; Tan, G.; Liu, L.; Wang, K.; Chen, H.; Liu, J. MicroRNA-200c suppresses cell growth and metastasis by targeting Bmi-1 and E2F3 in renal cancer cells. Exp. Ther. Med. 2017, 13, 1329–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Yang, Y.; Chen, L.; Liu, D.; Dong, H. MicroRNA-154 functions as a tumor suppressor in non-small cell lung cancer through directly targeting B-cell-specific Moloney murine leukemia virus insertion site 1. Oncol. Lett. 2018, 15, 10098–10104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Tetzlaff, M.T.; Cui, R.; Xu, X. miR-200c inhibits melanoma progression and drug resistance through down-regulation of BMI-1. Am. J. Pathol. 2012, 181, 1823–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassiouni, M.; Dos Santos, A.; Avci, H.X.; Löwenheim, H.; Müller, M. Bmi1 Loss in the Organ of Corti Results in p16ink4a Upregulation and Reduced Cell Proliferation of Otic Progenitors In Vitro. PLoS ONE 2016, 11, e0164579. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Tsukada, Y.; Zhang, Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 2005, 20, 845–854. [Google Scholar] [CrossRef]
- Gao, F.; Huang, W.; Zhang, Y.; Tang, S.; Zheng, L.; Ma, F.; Wang, Y.; Tang, H.; Li, X. Hes1 promotes cell proliferation and migration by activating Bmi-1 and PTEN/Akt/GSK3β pathway in human colon cancer. Oncotarget 2015, 6, 38667–38680. [Google Scholar] [CrossRef] [Green Version]
- Dimri, G.P.; Martinez, J.L.; Jacobs, J.J.; Keblusek, P.; Itahana, K.; Van Lohuizen, M.; Campisi, J.; Wazer, D.E.; Band, V. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002, 62, 4736–4745. [Google Scholar]
- Yamashita, M.; Kuwahara, M.; Suzuki, A.; Hirahara, K.; Shinnaksu, R.; Hosokawa, H.; Hasegawa, A.; Motohashi, S.; Iwama, A.; Nakayama, T. Bmi1 regulates memory CD4 T cell survival via repression of the Noxa gene. J. Exp. Med. 2008, 205, 1109–1120. [Google Scholar] [CrossRef]
- Kimura, M.; Takenobu, H.; Akita, N.; Nakazawa, A.; Ochiai, H.; Shimozato, O.; Fujimura, Y.; Koseki, H.; Yoshino, I.; Kimura, H.; et al. Bmi1 regulates cell fate via tumor suppressor WWOX repression in small-cell lung cancer cells. Cancer Sci. 2011, 102, 983–990. [Google Scholar] [CrossRef]
- Kameyama, A.; Nishijima, R.; Yamakoshi, K. Bmi-1 regulates mucin levels and mucin O-glycosylation in the submandibular gland of mice. PLoS ONE 2021, 16, e0245607. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, C.; Zeng, H.; Liu, Y.; Li, S. BMI1 activates WNT signaling in colon cancer by negatively regulating the WNT antagonist IDAX. Biochem. Biophys. Res. Commun. 2018, 496, 468–474. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: Preclinical and clinical evidences. Stem Cells 2012, 30, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Morrison, S.J.; Clarke, M.F. Bmi1, stem cells, and senescence regulation. J. Clin. Investig. 2004, 113, 175–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Du, P.; Ge, Z.; Jin, Y.; Ding, D.; Liu, X.; Zou, Q. TWIST1 and BMI1 in Cancer Metastasis and Chemoresistance. J. Cancer 2016, 7, 1074–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.Q.; Zhang, Y.H.; Ding, D.P.; Li, J.; Chen, L.L.; Tian, Y.Y.; Ao, K.J. Effect of Bmi-1-mediated NF-κB signaling pathway on the stem-like properties of CD133+ human liver cancer cells. Cancer Biomark. 2018, 22, 575–585. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Liu, B.; Liu, C.; Liu, D.; Zhu, J.; Yang, C.; Yan, J.; Liao, X.; Meng, X.; et al. Bmi-1-shRNA inhibits the proliferation of lung adenocarcinoma cells by blocking the G1/S phase through decreasing cyclin D1 and increasing p21/p27 levels. Nucleic Acid Ther. 2014, 24, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Jiang, S.X.; Yang, Y.M.; Xu, H.; Liu, J.L.; Wang, X.S. USP22 acts as an oncogene by the activation of BMI-1-mediated INK4a/ARF pathway and Akt pathway. Cell Biochem. Biophys. 2012, 62, 229–235. [Google Scholar] [CrossRef]
- Jiao, K.; Jiang, W.; Zhao, C.; Su, D.; Zhang, H. Bmi-1 in gallbladder carcinoma: Clinicopathology and mechanism of regulation of human gallbladder carcinoma proliferation. Oncol. Lett. 2019, 18, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Li, Y.; Wang, L.; Hu, L. Knockdown of BMI-1 causes cell-cycle arrest and derepresses p16INK4a, HOXA9 and HOXC13 mRNA expression in HeLa cells. Med. Oncol. 2011, 28, 1201–1209. [Google Scholar] [CrossRef]
- Wang, J.F.; Liu, Y.; Liu, W.J.; He, S.Y. Expression of Bmi-1 gene in esophageal carcinoma cell EC9706 and its effect on cell cycle, apoptosis and migration. Chin. J. Cancer 2010, 29, 689–696. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Q.; Tang, Q.; Zhou, H.; Liu, W.; Huang, J.; Liu, Y.; Wang, Q.; Zhang, J.; Zhou, M.; et al. CBX4 promotes the proliferation and metastasis via regulating BMI-1 in lung cancer. J. Cell Mol. Med. 2020, 24, 618–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.R.; Kwon, Y.; Rho, S.B. BMI-1 interacts with sMEK1 and inactivates sMEK1-induced apoptotic cell death. Oncol. Rep. 2017, 37, 579–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, D.; Ye, Y.; Fu, Y.; Wang, J.; Kuang, B.; Wang, H.; Wang, X.; Zu, L.; Xiao, G.; Hao, M.; et al. Bmi-1 expression modulates non-small cell lung cancer progression. Cancer Biol. Ther. 2015, 16, 756–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginjala, V.; Nacerddine, K.; Kulkarni, A.; Oza, J.; Hill, S.J.; Yao, M.; Citterio, E.; van Lohuizen, M.; Ganesan, S. BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Mol. Cell Biol. 2011, 31, 1972–1982. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Woo, S.M.; Seo, S.U.; Kwon, T.K. Inhibition of BMI-1 Induces Apoptosis through Downregulation of DUB3-Mediated Mcl-1 Stabilization. Int. J. Mol. Sci. 2021, 22, 10107. [Google Scholar] [CrossRef]
- Li, J.; Gong, L.Y.; Song, L.B.; Jiang, L.L.; Liu, L.P.; Wu, J.; Yuan, J.; Cai, J.C.; He, M.; Wang, L.; et al. Oncoprotein Bmi-1 renders apoptotic resistance to glioma cells through activation of the IKK-nuclear factor-kappaB Pathway. Am. J. Pathol. 2010, 176, 699–709. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Luo, B.; Zhao, M. Bmi-1-targeting suppresses osteosarcoma aggressiveness through the NF-κB signaling pathway. Mol Med. Rep. 2017, 16, 7949–7958. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Dey, A.; Mustafi, S.B.; Saha, S.; Kumar Dhar Dwivedi, S.; Mukherjee, P.; Bhattacharya, R. Inhibition of BMI1 induces autophagy-mediated necroptosis. Autophagy 2016, 12, 659–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Li, H.; Chen, S.; Wang, B.; Zhang, K. BMI1 promotes the proliferation and inhibits autophagy of breast cancer cells by activating COPZ1. Clin. Transl. Oncol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Griffith, J.; Andrade, D.; Mehta, M.; Berry, W.; Benbrook, D.M.; Aravindan, N.; Herman, T.S.; Ramesh, R.; Munshi, A. Silencing BMI1 radiosensitizes human breast cancer cells by inducing DNA damage and autophagy. Oncol. Rep. 2017, 37, 2382–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Wu, Z.; Yang, K.; Han, Y.; Chen, Y.; Zhao, W.; Huang, F.; Jin, Y.; Jin, W. BMI1 promotes cardiac fibrosis in ischemia-induced heart failure via the PTEN-PI3K/Akt-mTOR signaling pathway. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H61–H69. [Google Scholar] [CrossRef] [Green Version]
- Mourgues, L.; Imbert, V.; Nebout, M.; Colosetti, P.; Neffati, Z.; Lagadec, P.; Verhoeyen, E.; Peng, C.; Duprez, E.; Legros, L.; et al. The BMI1 polycomb protein represses cyclin G2-induced autophagy to support proliferation in chronic myeloid leukemia cells. Leukemia 2015, 29, 1993–2002. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.; Bao, Z.; Liu, Y.; Liu, G.; Li, F.; Li, L. BMI-1 promotes invasion and metastasis in endometrial adenocarcinoma and is a poor prognostic factor. Oncol. Rep. 2020, 43, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, Z.; Tan, Y.; Lian, G.; Chen, S.; Chen, S.; Li, J.; Li, X.; Huang, K.; Chen, Y. Bmi-1-induced miR-27a and miR-155 promote tumor metastasis and chemoresistance by targeting RKIP in gastric cancer. Mol. Cancer 2020, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, T.; Sun, W.; Liu, C.; Fang, X. Bmi-1 overexpression as an efficient prognostic marker in patients with nonsmall cell lung cancer. Medicine 2017, 96, e7346. [Google Scholar] [CrossRef]
- Pourjafar, M.; Samadi, P.; Karami, M.; Najafi, R. Assessment of clinicopathological and prognostic relevance of BMI-1 in patients with colorectal cancer: A meta-analysis. Biotechnol. Appl. Biochem. 2021, 68, 1313–1322. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Song, W.; Zhou, L.; Li, Q.; Tao, K.; Zhou, J.; Wang, X.; Zheng, Z.; You, N.; et al. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int. J. Oncol. 2013, 43, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Wu, J.; Yang, Y.; Liu, L.; Song, L.; Li, J.; Li, M. Bmi-1 promotes the aggressiveness of glioma via activating the NF-kappaB/MMP-9 signaling pathway. BMC Cancer 2012, 12, 406. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Chen, Q.W.; Sun, Y.F.; Lin, J.A.; Xu, J.H. Loss of BMI-1 dampens migration and EMT of colorectal cancer in inflammatory microenvironment through TLR4/MD-2/MyD88-mediated NF-κB signaling. J. Cell Biochem. 2018, 119, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Chagraoui, J.; Hébert, J.; Girard, S.; Sauvageau, G. An anticlastogenic function for the Polycomb Group gene Bmi1. Proc. Natl. Acad. Sci. USA 2011, 108, 5284–5289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gieni, R.S.; Ismail, I.H.; Campbell, S.; Hendzel, M.J. Polycomb group proteins in the DNA damage response: A link between radiation resistance and “stemness”. Cell Cycle 2011, 10, 883–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Cao, L.; Chen, J.; Song, S.; Lee, I.H.; Quijano, C.; Liu, H.; Keyvanfar, K.; Chen, H.; Cao, L.Y.; et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 2009, 459, 387–392. [Google Scholar] [CrossRef]
- Dong, Q.; Oh, J.E.; Chen, W.; Kim, R.; Kim, R.H.; Shin, K.H.; McBride, W.H.; Park, N.H.; Kang, M.K. Radioprotective effects of Bmi-1 involve epigenetic silencing of oxidase genes and enhanced DNA repair in normal human keratinocytes. J. Investig. Dermatol. 2011, 131, 1216–1225. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Ojo, D.; Wei, F.; Wong, N.; Gu, Y.; Tang, D. A Novel Aspect of Tumorigenesis-BMI1 Functions in Regulating DNA Damage Response. Biomolecules 2015, 5, 3396–3415. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.H.; Andrin, C.; McDonald, D.; Hendzel, M.J. BMI1-mediated histone ubiquitylation promotes DNA double-strand break repair. J. Cell Biol. 2010, 191, 45–60. [Google Scholar] [CrossRef]
- Wang, E.; Bhattacharyya, S.; Szabolcs, A.; Rodriguez-Aguayo, C.; Jennings, N.B.; Lopez-Berestein, G.; Mukherjee, P.; Sood, A.K.; Bhattacharya, R. Enhancing chemotherapy response with Bmi-1 silencing in ovarian cancer. PLoS ONE 2011, 6, e17918. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Papandreou, C.N. The Bmi-1/NF-κB/VEGF story: Another hint for proteasome involvement in glioma angiogenesis? J. Cell Commun. Signal. 2013, 7, 235–237. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Mu, Y.; Zhang, S. A novel NF-κB/MMP-3 signal pathway involves in the aggressivity of glioma promoted by Bmi-1. Tumour. Biol. 2014, 35, 12721–12727. [Google Scholar] [CrossRef]
- Jiang, L.; Song, L.; Wu, J.; Yang, Y.; Zhu, X.; Hu, B.; Cheng, S.Y.; Li, M. Bmi-1 promotes glioma angiogenesis by activating NF-κB signaling. PLoS ONE 2013, 8, e55527. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.Y.; Gu, X.; Chen, Y.L.; Liu, J.B.; Hou, C.X.; Lin, S.Y.; Hao, N.N.; Liang, Y.; Chen, W.; Meng, H.Y. Toll-like receptor 4 activates the NLRP3 inflammasome pathway and periodontal inflammaging by inhibiting Bmi-1 expression. Int. J. Mol. Med. 2021, 47, 137–150. [Google Scholar] [CrossRef]
- Spillane, J.B.; Henderson, M.A. Cancer stem cells: A review. ANZ J. Surg. 2007, 77, 464–468. [Google Scholar] [CrossRef]
- Raaphorst, F.M. Self-renewal of hematopoietic and leukemic stem cells: A central role for the Polycomb-group gene Bmi-1. Trends Immunol. 2003, 24, 522–524. [Google Scholar] [CrossRef]
- Bruggeman, S.W.; Valk-Lingbeek, M.E.; van der Stoop, P.P.; Jacobs, J.J.; Kieboom, K.; Tanger, E.; Hulsman, D.; Leung, C.; Arsenijevic, Y.; Marino, S.; et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005, 19, 1438–1443. [Google Scholar] [CrossRef] [Green Version]
- Zencak, D.; Lingbeek, M.; Kostic, C.; Tekaya, M.; Tanger, E.; Hornfeld, D.; Jaquet, M.; Munier, F.L.; Schorderet, D.F.; van Lohuizen, M.; et al. Bmi1 loss produces an increase in astroglial cells and a decrease in neural stem cell population and proliferation. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5774–5783. [Google Scholar] [CrossRef]
- Gargiulo, G.; Cesaroni, M.; Serresi, M.; de Vries, N.; Hulsman, D.; Bruggeman, S.W.; Lancini, C.; van Lohuizen, M. In Vivo RNAi Screen for BMI1 Targets Identifies TGF-beta/BMP-ER Stress Pathways as Key Regulators of Neural- and Malignant Glioma-Stem Cell Homeostasis. Cancer Cell 2013, 23, 660–676. [Google Scholar] [CrossRef] [Green Version]
- Molofsky, A.V.; He, S.; Bydon, M.; Morrison, S.J.; Pardal, R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005, 19, 1432–1437. [Google Scholar] [CrossRef] [Green Version]
- Zelentsova-Levytskyi, K.; Talmi, Z.; Abboud-Jarrous, G.; Capucha, T.; Sapir, T.; Burstyn-Cohen, T. Protein S Negatively Regulates Neural Stem Cell Self-Renewal through Bmi-1 Signaling. Front. Mol. Neurosci. 2017, 10, 124. [Google Scholar] [CrossRef]
- Lessard, J.; Sauvageau, G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003, 423, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Dimri, M.; Dimri, G.P. A Positive Feedback Loop Regulates the Expression of Polycomb Group Protein BMI1 via WNT Signaling Pathway. J. Biol. Chem. 2013, 288, 3406–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukacs, R.U.; Memarzadeh, S.; Wu, H.; Witte, O.N. Bmi-1 Is a Crucial Regulator of Prostate Stem Cell Self-Renewal and Malignant Transformation. Cell Stem Cell 2010, 7, 682–693. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.S.; Chia, L.A.; Li, X.N.; Ootani, A.; Su, J.; Lee, J.Y.; Su, N.; Luo, Y.L.; Heilshorn, S.C.; Amieva, M.R.; et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 2012, 109, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Qiao, C.; Xu, W.R.; Zhu, W.; Hu, J.B.; Qian, H.; Yin, Q.; Jiang, R.Q.; Yan, Y.M.; Mao, F.; Yang, H.; et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol. Int. 2008, 32, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [Green Version]
- Bolomsky, A.; Schlangen, K.; Schreiner, W.; Zojer, N.; Ludwig, H. Targeting of BMI-1 with PTC-209 shows potent anti-myeloma activity and impairs the tumour microenvironment. J. Hematol. Oncol. 2016, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Huang, J.; Wang, F.; Ding, H.; Cui, Y.; Yang, Y.; Xu, J.; Luo, H.; Gao, Y.; Pan, L.; et al. BMI1 regulates multiple myeloma-associated macrophage’s pro-myeloma functions. Cell Death Dis. 2021, 12, 495. [Google Scholar] [CrossRef]
- Sugihara, H.; Ishimoto, T.; Watanabe, M.; Sawayama, H.; Iwatsuki, M.; Baba, Y.; Komohara, Y.; Takeya, M.; Baba, H. Identification of miR-30e* regulation of Bmi1 expression mediated by tumor-associated macrophages in gastrointestinal cancer. PLoS ONE 2013, 8, e81839. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, X.D.; Da, C.L.; Xin, Y. Clinicopathological significance of B-cell-specific Moloney murine leukemia virus insertion site 1 expression in gastric carcinoma and its precancerous lesion. World J. Gastroenterol. 2009, 15, 2145–2150. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, S.O.; Jeong, W.Y.; Kim, H.K.; Kim, A.; Kim, B.H. Immunohistochemical analysis of polycomb group protein expression in advanced gastric cancer. Hum. Pathol. 2012, 43, 1704–1710. [Google Scholar] [CrossRef]
- Lu, Y.W.; Li, J.; Guo, W.J. Expression and clinicopathological significance of Mel-18 and Bmi-1 mRNA in gastric carcinoma. J. Exp. Clin. Cancer Res. 2010, 29, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, K.; Iseki, C.; Kikuchi, M.; Miyakawa, K.; Yoshizaki, M.; Yoshioka, H.; Watanabe, J. Bmi-1 Immunohistochemical Expression in Endometrial Carcinoma is Correlated with Prognostic Activity. Medicina 2020, 56, 72. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, K.; Jiang, X.; Wang, X.; Chen, Y.; Cui, X.; Pang, L.; Li, S.; Liu, C.; Zou, H.; et al. Clinicopathological significance of Bmi-1 overexpression in esophageal cancer: A meta-analysis. Biomark. Med. 2018, 12, 71–81. [Google Scholar] [CrossRef]
- Tong, Y.Q.; Liu, B.; Zheng, H.Y.; He, Y.J.; Gu, J.; Li, F.; Li, Y. Overexpression of BMI-1 is associated with poor prognosis in cervical cancer. Asia Pac. J. Clin. Oncol. 2012, 8, e55–e62. [Google Scholar] [CrossRef]
- Swelem, R.S.; Elneely, D.A.; Shehata, A.A.R. The Study of SALL4 Gene and BMI-1 Gene Expression in Acute Myeloid Leukemia Patients. Lab. Med. 2020, 51, 265–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.L.; Chen, H.M.; Pu, H.W.; Ma, W.J.; Li, X.M.; Ma, H.; Chen, X. Expression of Bmi-1 and PAI-1 in esophageal squamous cell carcinoma. World J. Gastroenterol. 2014, 20, 5533–5539. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Guo, X.Z.; Zhang, L.J.; Wang, J.Y.; Zhang, G.; Guan, S.; Chen, Y.M.; Kong, Q.L.; Xu, L.H.; Li, M.Z.; et al. Prognostic relevance of Bmi-1 expression and autoantibodies in esophageal squamous cell carcinoma. BMC Cancer 2010, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Li, D.W.; Tang, H.M.; Fan, J.W.; Yan, D.W.; Zhou, C.Z.; Li, S.X.; Wang, X.L.; Peng, Z.H. Expression level of Bmi-1 oncoprotein is associated with progression and prognosis in colon cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 997–1006. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.X.; Zhu, C.B.; Zhang, J.; Kan, S.F.; Du, L.T.; Li, W.; Wang, L.L.; Wang, S. Overexpression of Bmi-1 in uterine cervical cancer: Correlation with clinicopathology and prognosis. Int. J. Gynecol. Cancer 2010, 20, 1597–1603. [Google Scholar] [PubMed]
- Qin, Z.K.; Yang, J.A.; Ye, Y.L.; Zhang, X.; Xu, L.H.; Zhou, F.J.; Han, H.; Liu, Z.W.; Song, L.B.; Zeng, M.S. Expression of Bmi-1 is a prognostic marker in bladder cancer. BMC Cancer 2009, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.F.; He, W.P.; Cai, M.Y.; He, L.R.; Luo, J.H.; Deng, H.X.; Guan, X.Y.; Zeng, M.S.; Zeng, Y.X.; Xie, D. Intensive expression of Bmi-1 is a new independent predictor of poor outcome in patients with ovarian carcinoma. BMC Cancer 2010, 10, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd El Hafez, A.; El-Hadaad, H.A. Immunohistochemical expression and prognostic relevance of Bmi-1, a stem cell factor, in epithelial ovarian cancer. Ann. Diagn. Pathol. 2014, 18, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Wang, L.; Du, L.; Wang, S.; Zheng, G.; Li, W.; Zhuang, X.; Zhang, X.; Dong, Z. Detection of circulating Bmi-1 mRNA in plasma and its potential diagnostic and prognostic value for uterine cervical cancer. Int. J. Cancer 2012, 131, 165–172. [Google Scholar] [CrossRef]
- Yi, C.; Li, B.B.; Zhou, C.X. Bmi-1 expression predicts prognosis in salivary adenoid cystic carcinoma and correlates with epithelial-mesenchymal transition-related factors. Ann. Diagn. Pathol. 2016, 22, 38–44. [Google Scholar] [CrossRef]
- Allegra, E.; Puzzo, L.; Zuccalà, V.; Trapasso, S.; Vasquez, E.; Garozzo, A.; Caltabiano, R. Nuclear BMI-1 expression in laryngeal carcinoma correlates with lymph node pathological status. World J. Surg. Oncol. 2012, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Tao, K.; Li, H.; Jin, C.; Song, Z.; Li, J.; Shi, H.; Li, X.; Dang, Z.; Dou, K. Bmi-1 is related to proliferation, survival and poor prognosis in pancreatic cancer. Cancer Sci. 2010, 101, 1754–1760. [Google Scholar] [CrossRef]
- Häyry, V.; Mäkinen, L.K.; Atula, T.; Sariola, H.; Mäkitie, A.; Leivo, I.; Keski-Säntti, H.; Lundin, J.; Haglund, C.; Hagström, J. Bmi-1 expression predicts prognosis in squamous cell carcinoma of the tongue. Br. J. Cancer 2010, 102, 892–897. [Google Scholar] [CrossRef]
- Lozneanu, L.; Balan, R.A.; Păvăleanu, I.; Giuşcă, S.E.; Căruntu, I.D.; Amalinei, C. BMI-1 Expression Heterogeneity in Endometriosis-Related and Non-Endometriotic Ovarian Carcinoma. Int. J. Mol. Sci 2021, 22, 6082. [Google Scholar] [CrossRef]
- Cui, H.; Hu, B.; Li, T.; Ma, J.; Alam, G.; Gunning, W.T.; Ding, H.F. Bmi-1 is essential for the tumorigenicity of neuroblastoma cells. Am. J. Pathol. 2007, 170, 1370–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farivar, S.; Zati Keikha, R.; Shiari, R.; Jadali, F. Expression of bmi-1 in pediatric brain tumors as a new independent prognostic marker of patient survival. Biomed. Res. Int. 2013, 2013, 192548. [Google Scholar] [CrossRef] [Green Version]
- Huber, G.F.; Albinger-Hegyi, A.; Soltermann, A.; Roessle, M.; Graf, N.; Haerle, S.K.; Holzmann, D.; Moch, H.; Hegyi, I. Expression patterns of Bmi-1 and p16 significantly correlate with overall, disease-specific, and recurrence-free survival in oropharyngeal squamous cell carcinoma. Cancer 2011, 117, 4659–4670. [Google Scholar] [CrossRef]
- Mihic-Probst, D.; Kuster, A.; Kilgus, S.; Bode-Lesniewska, B.; Ingold-Heppner, B.; Leung, C.; Storz, M.; Seifert, B.; Marino, S.; Schraml, P.; et al. Consistent expression of the stem cell renewal factor BMI-1 in primary and metastatic melanoma. Int. J. Cancer 2007, 121, 1764–1770. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Y.; Yang, J.; Jin, C.; Zhao, X.; Cheng, J.; Liu, X.; Qi, X. Clinical implications of BMI-1 in cancer stem cells of laryngeal carcinoma. Cell Biochem. Biophys. 2015, 71, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lian, G.; Ou, G.; Yang, K.; Chen, J.; Li, H.; Chen, S.; Li, J.; Zeng, L.; Huang, K. Inverse association between Bmi-1 and RKIP affecting clinical outcome of gastric cancer and revealing the potential molecular mechanisms underlying tumor metastasis and chemotherapy resistance. Gastric Cancer 2016, 19, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.C.; Jing, R.N.; Pei, H.; Liu, F.; Zhao, B.X.; Meng, X.X. [Effect of Bmi-1 Expression on Chemotherapy Sensitivity in THP-1 Cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 363–368. [Google Scholar] [CrossRef]
- Herzog, A.E.; Warner, K.A.; Zhang, Z.; Bellile, E.; Bhagat, M.A.; Castilho, R.M.; Wolf, G.T.; Polverini, P.J.; Pearson, A.T.; Nör, J.E. The IL-6R and Bmi-1 axis controls self-renewal and chemoresistance of head and neck cancer stem cells. Cell Death Dis. 2021, 12, 988. [Google Scholar] [CrossRef]
- Jiang, M.; He, G.; Li, J.; Li, J.; Guo, X.; Gao, J. Hypoxic exposure activates the B cell-specific Moloney murine leukaemia virus integration site 1/PI3K/Akt axis and promotes EMT in leukaemia stem cells. Oncol. Lett. 2021, 21, 98. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Mihara, K.; Ohtsubo, M.; Yasunaga, S.; Takei, Y.; Yanagihara, K.; Sakai, A.; Hoshi, M.; Takihara, Y.; Kimura, A. Overexpression of BMI-1 correlates with drug resistance in B-cell lymphoma cells through the stabilization of survivin expression. Cancer Sci. 2012, 103, 34–41. [Google Scholar] [CrossRef]
- Wang, G.; Liu, L.; Sharma, S.; Liu, H.; Yang, W.; Sun, X.; Dong, Q. Bmi-1 confers adaptive radioresistance to KYSE-150R esophageal carcinoma cells. Biochem. Biophys. Res. Commun. 2012, 425, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, W.; Wang, C.Y. BMI1 Inhibition Eliminates Residual Cancer Stem Cells after PD1 Blockade and Activates Antitumor Immunity to Prevent Metastasis and Relapse. Cell Stem Cell 2020, 27, 238–253.e236. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; van Galen, P.; Pedley, N.M.; Lima-Fernandes, E.; Frelin, C.; Davis, T.; Cao, L.; Baiazitov, R.; Du, W.; Sydorenko, N.; et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat. Med. 2014, 20, 29–36. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Wu, Y.; Huang, R.; Zhu, Y.; Zhang, W.; Wang, Y.; Cheng, J. Pharmacological inhibition of Bmi1 by PTC-209 impaired tumor growth in head neck squamous cell carcinoma. Cancer Cell Int. 2017, 17, 107. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.A.; Vatapalli, R.; Lysy, B.; Mok, H.; Desouki, M.M.; Abdulkadir, S.A. The Role of Castration-Resistant Bmi1+Sox2+ Cells in Driving Recurrence in Prostate Cancer. J. Natl. Cancer Inst. 2019, 111, 311–321. [Google Scholar] [CrossRef]
- Alzrigat, M.; Párraga, A.A.; Majumder, M.M.; Ma, A.; Jin, J.; Österborg, A.; Nahi, H.; Nilsson, K.; Heckman, C.A.; Öberg, F.; et al. The polycomb group protein BMI-1 inhibitor PTC-209 is a potent anti-myeloma agent alone or in combination with epigenetic inhibitors targeting EZH2 and the BET bromodomains. Oncotarget 2017, 8, 103731–103743. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Ai, C.; Dong, F.; Xia, X.; Zhao, X.; Yang, C.; Kang, C.; Zhou, Y.; Zhao, Q.; Sun, X.; et al. Targeting of BMI-1 with PTC-209 inhibits glioblastoma development. Cell Cycle 2018, 17, 1199–1211. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Vangundy, Z.; Poi, M. PTC209, a Specific Inhibitor of BMI1, Promotes Cell Cycle Arrest and Apoptosis in Cervical Cancer Cell Lines. Anticancer Res. 2020, 40, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Xu, J.; Wang, M.; Liu, G.; Wang, J.; Zhao, X.; Qi, Y.; Shi, J.; Cheng, K.; et al. Reversing tumor stemness via orally targeted nanoparticles achieves efficient colon cancer treatment. Biomaterials 2019, 216, 119247. [Google Scholar] [CrossRef]
- Nishida, Y.; Maeda, A.; Kim, M.J.; Cao, L.; Kubota, Y.; Ishizawa, J.; AlRawi, A.; Kato, Y.; Iwama, A.; Fujisawa, M.; et al. The novel BMI-1 inhibitor PTC596 downregulates MCL-1 and induces p53-independent mitochondrial apoptosis in acute myeloid leukemia progenitor cells. Blood Cancer J. 2017, 7, e527. [Google Scholar] [CrossRef]
- Maeda, A.; Nishida, Y.; Weetall, M.; Cao, L.; Branstrom, A.; Ishizawa, J.; Nii, T.; Schober, W.D.; Abe, Y.; Matsue, K.; et al. Targeting of BMI-1 expression by the novel small molecule PTC596 in mantle cell lymphoma. Oncotarget 2018, 9, 28547–28560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolomsky, A.; Muller, J.; Stangelberger, K.; Lejeune, M.; Duray, E.; Breid, H.; Vrancken, L.; Pfeiffer, C.; Hübl, W.; Willheim, M.; et al. The anti-mitotic agents PTC-028 and PTC596 display potent activity in pre-clinical models of multiple myeloma but challenge the role of BMI-1 as an essential tumour gene. Br. J. Haematol. 2020, 190, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Flamier, A.; Abdouh, M.; Hamam, R.; Barabino, A.; Patel, N.; Gao, A.; Hanna, R.; Bernier, G. Off-target effect of the BMI1 inhibitor PTC596 drives epithelial-mesenchymal transition in glioblastoma multiforme. NPJ Precis. Oncol. 2020, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jernigan, F.; Branstrom, A.; Baird, J.D.; Cao, L.; Dali, M.; Furia, B.; Kim, M.J.; O’Keefe, K.; Kong, R.; Laskin, O.L.; et al. Preclinical and Early Clinical Development of PTC596, a Novel Small-Molecule Tubulin-Binding Agent. Mol. Cancer Ther. 2021, 20, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; O’Mara, E.; Laskin, O.L.; Gao, L.; Baird, J.D.; Spiegel, R.J.; Kaushik, D.; Weetall, M.; Colacino, J.; O’Keefe, K.; et al. Pharmacokinetics and Safety of PTC596, a Novel Tubulin-Binding Agent, in Subjects With Advanced Solid Tumors. Clin. Pharmacol. Drug Dev. 2021, 10, 940–949. [Google Scholar] [CrossRef]
- Bartucci, M.; Hussein, M.S.; Huselid, E.; Flaherty, K.; Patrizii, M.; Laddha, S.V.; Kui, C.; Bigos, R.A.; Gilleran, J.A.; El Ansary, M.M.S.; et al. Synthesis and Characterization of Novel BMI1 Inhibitors Targeting Cellular Self-Renewal in Hepatocellular Carcinoma. Target. Oncol. 2017, 12, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Xiong, X.; Crim, A.; Dwivedi, S.K.D.; Mustafi, S.B.; Mukherjee, P.; Cao, L.; Sydorenko, N.; Baiazitov, R.; Moon, Y.C.; et al. Evaluating the Mechanism and Therapeutic Potential of PTC-028, a Novel Inhibitor of BMI-1 Function in Ovarian Cancer. Mol. Cancer Ther. 2018, 17, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xing, Y.; Wang, Y.; He, Y.; Wang, L.; Peng, S.; Yang, L.; Xie, J.; Li, X.; Qiu, W.; et al. A novel BMI-1 inhibitor QW24 for the treatment of stem-like colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 422. [Google Scholar] [CrossRef]
- Shields, C.E.; Potlapalli, S.; Cuya-Smith, S.M.; Chappell, S.K.; Chen, D.; Martinez, D.; Pogoriler, J.; Rathi, K.S.; Patel, S.A.; Oristian, K.M.; et al. Epigenetic regulator BMI1 promotes alveolar rhabdomyosarcoma proliferation and constitutes a novel therapeutic target. Mol. Oncol. 2021, 15, 2156–2171. [Google Scholar] [CrossRef]
- Sulaiman, S.; Arafat, K.; Iratni, R.; Attoub, S. PTC-209 Anti-Cancer Effects Involved the Inhibition of STAT3 Phosphorylation. Front. Pharmacol. 2019, 10, 1199. [Google Scholar] [CrossRef]
- Shan, W.; Zhou, L.; Liu, L.; Lin, D.; Yu, Q. Polycomb group protein Bmi1 is required for the neuronal differentiation of mouse induced pluripotent stem cells. Exp. Ther. Med. 2021, 21, 619. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, K.; Cheng, M.; Wang, G.; Han, H.; Chen, F.; Liao, W.; Chen, Z.; Chen, J.; Bao, Y.; et al. Bmi1 Severs as a Potential Tumor-Initiating Cell Marker and Therapeutic Target in Esophageal Squamous Cell Carcinoma. Stem Cells Int. 2020, 2020, 8877577. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.; Deshpande, A.; Chen, B.R.; Ghosh, A.; Sun, Y.; Dutta, S.; Weetall, M.; Dixon, J.; Armstrong, S.A.; Bohlander, S.K.; et al. Acute myeloid leukemia driven by the CALM-AF10 fusion gene is dependent on BMI1. Exp. Hematol. 2019, 74, 42–51.e43. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, M.; Itoh, M.; Tohda, S. BMI1 Inhibitors Down-regulate NOTCH Signaling and Suppress Proliferation of Acute Leukemia Cells. Anticancer Res. 2017, 37, 6047–6053. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Maeda, A.; Chachad, D.; Ishizawa, J.; Qiu, Y.H.; Kornblau, S.M.; Kimura, S.; Andreeff, M.; Kojima, K. Preclinical activity of the novel B-cell-specific Moloney murine leukemia virus integration site 1 inhibitor PTC-209 in acute myeloid leukemia: Implications for leukemia therapy. Cancer Sci. 2015, 106, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Dimri, M.; Kang, M.; Dimri, G.P. A miR-200c/141-BMI1 autoregulatory loop regulates oncogenic activity of BMI1 in cancer cells. Oncotarget 2016, 7, 36220–36234. [Google Scholar] [CrossRef] [Green Version]
- Mayr, C.; Wagner, A.; Loeffelberger, M.; Bruckner, D.; Jakab, M.; Berr, F.; Di Fazio, P.; Ocker, M.; Neureiter, D.; Pichler, M.; et al. The BMI1 inhibitor PTC-209 is a potential compound to halt cellular growth in biliary tract cancer cells. Oncotarget 2016, 7, 745–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, Y.; Mimura, N.; Rizq, O.; Isshiki, Y.; Oshima, M.; Rizk, M.; Saraya, A.; Koide, S.; Nakajima-Takagi, Y.; Miyota, M.; et al. The combination of the tubulin binding small molecule PTC596 and proteasome inhibitors suppresses the growth of myeloma cells. Sci Rep. 2021, 11, 2074. [Google Scholar] [CrossRef]
- Eberle-Singh, J.A.; Sagalovskiy, I.; Maurer, H.C.; Sastra, S.A.; Olive, K.P. Effective Delivery of a Microtubule Polymerization Inhibitor Synergizes with Standard Regimens in Models of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 5548–5560. [Google Scholar] [CrossRef]
- Kumar, S.S.; Sengupta, S.; Zhu, X.; Mishra, D.K.; Phoenix, T.; Dyer, L.; Fuller, C.; Stevenson, C.B.; Dewire, M.; Fouladi, M. Diffuse Intrinsic Pontine Glioma Cells Are Vulnerable to Mitotic Abnormalities Associated with BMI-1 Modulation. Mol. Cancer Res. 2020, 18, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, I.; Danis, E.; Pierce, A.; Madhavan, K.; Wang, D.; Dahl, N.; Sanford, B.; Birks, D.K.; Davidson, N.; Metselaar, D.S.; et al. Senescence Induced by BMI1 Inhibition Is a Therapeutic Vulnerability in H3K27M-Mutant DIPG. Cell Rep. 2020, 33, 108286. [Google Scholar] [CrossRef]
- Bakhshinyan, D.; Venugopal, C.; Adile, A.A.; Garg, N.; Manoranjan, B.; Hallett, R.; Wang, X.; Mahendram, S.; Vora, P.; Vijayakumar, T.; et al. BMI1 is a therapeutic target in recurrent medulloblastoma. Oncogene 2019, 38, 1702–1716. [Google Scholar] [CrossRef] [PubMed]
- Buechel, M.; Dey, A.; Dwivedi, S.K.D.; Crim, A.; Ding, K.; Zhang, R.; Mukherjee, P.; Moore, K.N.; Cao, L.; Branstrom, A.; et al. Inhibition of BMI1, a Therapeutic Approach in Endometrial Cancer. Mol. Cancer Ther. 2018, 17, 2136–2143. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.F.; Xing, Y.; Xiao, J.X.; Xie, J.; Gao, W.; Xie, J.; Wang, L.T.; Wang, J.; Liu, M.; Yi, Z.; et al. Synthesis of Cyanoenone-Modified Diterpenoid Analogs as Novel Bmi-1-Mediated Antitumor Agents. ACS Med. Chem. Lett. 2018, 9, 1105–1110. [Google Scholar] [CrossRef]
- Wu, J.; Hu, D.; Yang, G.; Zhou, J.; Yang, C.; Gao, Y.; Zhu, Z. Down-regulation of BMI-1 cooperates with artemisinin on growth inhibition of nasopharyngeal carcinoma cells. J. Cell Biochem. 2011, 112, 1938–1948. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, Y.X.; Zhao, R.; Pan, S.T.; Zhe, H.; He, Z.X.; Duan, W.; Zhang, X.; Yang, T.; Qiu, J.X.; et al. Bardoxolone methyl induces apoptosis and autophagy and inhibits epithelial-to-mesenchymal transition and stemness in esophageal squamous cancer cells. Drug Des. Dev. Ther. 2015, 9, 993–1026. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Glia, A.; Deliorman, M.; Sukumar, P.; Janahi, F.K.; Samara, B.; Brimmo, A.T.; Qasaimeh, M.A. Herringbone Microfluidic Probe for Multiplexed Affinity-Capture of Prostate Circulating Tumor Cells. Adv. Mater. Technol. 2021, 6, 2100053. [Google Scholar] [CrossRef]
- Kure, K.; Hosoya, M.; Ueyama, T.; Fukaya, M.; Komiyama, H. Using the polymeric circulating tumor cell chip to capture circulating tumor cells in blood samples of patients with colorectal cancer. Oncol. Lett. 2020, 19, 2286–2294. [Google Scholar] [CrossRef] [Green Version]

| miRNAs | Efficacy for Cancer | Cancer Type | References |
|---|---|---|---|
| miR-34a, miR-15a, miR-218, miR-183, miR-498, miR-128 | Inhibits proliferation; inhibits metastasis; decreases chemoresistance | Gastric cancer | [23,24,25,26,27] |
| miR-218, miR-200c, miR-485-5p | Inhibits proliferation; inhibits migration; increases apoptosis | Colorectal cancer | [28,29,30] |
| miR-15a, miR-183 | Inhibits proliferation and EMT | Pancreatic ductal adenocarcinoma | [31,32] |
| miR-203 | Inhibits self-renewal | Esophageal cancer | [33] |
| miR-203 | Promotes apoptosis | Oral cancer | [34] |
| miR-218, miR-203 | Inhibits proliferation and invasion; introduces apoptosis; decreases radiosensitivity and chemosensitivity | Hepatocellular carcinoma | [35,36,37,38,39] |
| miR-218 | Increases chemosensitivity | Liver cancer | [38] |
| miR-320a | Inhibits proliferation and migration | Nosopharyngeal carcinoma | [40] |
| miR-132, miR-498 | Increases radiosensitivity; inhibits proliferation and invasion | Cervical cancer | [41,42] |
| miR-128, miR-200b, miR-221, miR-30d, miR-15a, miR-330-3p, miR-212 | Inhibits proliferation and migration; increases chemosensitivity | Prostate cancer | [43,44,45,46,47,48,49,50] |
| miR-200c, miR-194 | Inhibits proliferation; inhibits EMT | Endometrial carcinoma | [51,52] |
| miR-128, miR-495 | Inhibits proliferation; introduces apoptosis; increases chemosensitivity and DNA damage | Breast cancer | [53,54] |
| miR-132, miR-15a, miR-16, miR-128 | Inhibits metastasis; increases chemosensitivity | Ovarian cancer | [55,56,57] |
| miR-361-5p, miR-218, miR-128 | Inhibits proliferation; inhibits EMT | Glioma | [58,59,60] |
| miR-128, miR-16, miR-128a | Inhibits proliferation and angiogenesis; introduces radiosensitivity | Glioblastoma | [61,62,63] |
| miR-128a | Inhibits ROS | Medulloblastoma | [64] |
| miR-218 | Inhibits proliferation, inhibits migration, inhibits apoptosis | Osteosarcoma | [65,66] |
| miR-200c, miR-139-5p, miR-218, miR-15 | Inhibits proliferation; inhibits metastasis; inhibits apoptosis; inhibits autophagy | Bladder cancer | [13,67,68,69] |
| miR-218 | Inhibits proliferation | Acute Promyelocytic Leukemia | [70] |
| miR-218 | Inhibits proliferation and metastasis | Lung adenocarcinoma | [71] |
| miR-203 | Inhibits proliferation | Myeloma | [72] |
| miR-200C | Inhibits proliferation and metastasis | Renal cancer | [73] |
| miR-154 | Inhibits proliferation and migration | Non-small cell lung cancer | [74] |
| miR-200c | Inhibits proliferation and migration; increases chemosensitivity | Melanoma | [75] |
| Cancer Type | mRNA/Protein | High/Low Expression | Positive Percentage | Clinical Characteristics | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Gastric cancer | Protein | High | GC162 (52.5%) | Associated with Lauren’s and Borrmann’s classification and clinical stage | Mainly in nucleus | [142] |
| Protein | High | GC 178 (70.8%) | Associated with sex, gross type, and histologic type | Mainly in nucleus | [143] | |
| mRNA | High | 71 | Associated with tumor size, depth of invasion, lymph node metastasis, and clinical stage | Not involved | [144] | |
| Nonsmall cell lung cancer | Protein and mRNA | High | Associated with tumor size, poor differentiation, and distant metastasis | Mainly in nucleus | [108] | |
| Endometrial Carcinoma | Protein | High | 48 | A significant positive relationship between Bmi-1 and Ki-67, cyclin A, or p53 | Mainly in nucleus | [145] |
| Esophageal cancer | Protein | High | 1523 | Associated with differentiation, tumor/node/metastasis stage, depth of invasion, and lymph node metastasis | Mainly in nucleus | [146] |
| Cervical cancer | Protein and mRNA | High | 302 (55.3%) | Correlated with clinical stage, lymph node metastasis, vascular invasion, and human papillomavirus (HPV) infection | Mainly in nucleus | [147] |
| Acute myeloid leukemia (AML) | mRNA | High | 60 | Showed a strong association with failure to achieve complete remission (CR) or with relapse | Not involved | [148] |
| Esophageal squamous cell carcinoma (ESCC) | Protein | High | 80 (78.7%) | Correlated with depth of invasion and lymph node metastasis, but not with patient age, tumor size, or nationality | Not involved | [149] |
| ESCC | Protein and mRNA | High | 171 (64.3%) | Correlated with stage and pN classification. | Mainly in nucleus | [150] |
| Endometrial adenocarcinoma | Protein | High | 60 | Correlated with FIGO stage, myometrial invasion, and lymph node metastasis | both the nucleus and cytoplasm | [106] |
| Colon cancer | Protein and mRNA | High | 203 (66.5%) | Correlated with clinical stage, depth of invasion, nodal involvement, distant metastasis, and Ki67 level | Mainly in nucleus | [151] |
| Uterine cervical cancer | Protein | High | 152 | Correlated with tumor size, clinical stage, and regional lymph nodes metastasis | Mainly in nucleus | [152] |
| Bladder cancer | Protein | High | 137 | Correlated withhistopathological classification, clinical stage, recurrence, and patient survival | Mainly in nucleus | [153] |
| Ovarian carcinoma | Protein | Low | 179 | Correlated with tumors’ histological type, grade, pT/pN/pM status, and FIGO stage | both the nucleus and cytoplasm | [154] |
| Epithelial ovarian cancer | Protein | High | 40 (72.5%) | Associated with advanced International Federation of Gynecology and Obstetrics stages, bilaterality, and higher Gynecologic Oncology Group grades and carcinomas of serous histology | Mainly in nucleus | [155] |
| Uterine cervical cancer | mRNA | High | 109 | Correlated with clinical stage and lymph nodes metastasis | Not involved | [156] |
| Salivary adenoid cystic carcinoma | Protein | High | 10 | Associated with tumor metastasis, Snail, Slug, and E-cadherin, serves as a highrisk for AdCC | Not involved | [157] |
| Laryngeal carcinoma | Protein | High | 64 (84.4%) | Correlated with distant metastasis, N pathological status, T classification | B oth the nucleus and cytoplasm | [158] |
| Pancreatic cancer | Protein | High | 72 (48.61%) | Correlated with the presence of lymph node metastases and negatively correlated with patient survival rates | Mainly in nucleus | [159] |
| Squamous cell carcinoma of the tongue | Protein | High | 73 (82%) | Correlated with recurrence | in nucleus | [160] |
| ovarian Carcinoma | mRNA | High | 47(72.34%) | Correlated with tumor grade | both the nucleus and cytoplasm | [161] |
| Neuroblastoma | Protein | High | 45 | Correlated with MYCN | in nucleus | [162] |
| Pediatric brain tumors | mRNA | High | 56 | Expression of Bmi-1 showed significant differences between high-grade tumors and low-grade tumors | Not involved | [163] |
| Inhibitor Name | Regulatory for Bmi-1 | Tumor Type | Regulatory Mechanism | References |
|---|---|---|---|---|
| PTC-209 | Reduces transcript levels | Cervical cancer | Promotes cell G0/G1 arrest and apoptosis | [179] |
| Colon cancer | Developed an orally active, easily synthesized PTC209 nanomedicine | [180] | ||
| Alveolar rhabdomyosarcoma | Activates the Hippo pathway | [190] | ||
| Glioblastoma | Inhibits glioblastoma cell proliferation and migration | [178] | ||
| Ovarian cancer | Induces autophagy through ATP depletion | [101] | ||
| Lung cancer cells, breast cancer cells and colon cancer cells | Inhibits STAT3 Phosphorylation | [191] | ||
| Pluripotent stem cells | Reduces the expression of neuronal markers, such as Nestin | [192] | ||
| Prostate cancer | Efficiently targets Bmi-1 and Sox2 | [176] | ||
| ESCC | Inhibits ESCC progression when combined with cisplatin | [193] | ||
| Acute myeloid leukemia | Inhibits proliferation and induce apoptosis | [194] | ||
| Acute Leukemia Cells | Down-regulates the expression of Notch signaling proteins Notch1, Hes1, and MYC | [195] | ||
| Acute myeloid leukemia | Reduces protein level of Bmi-1 and its downstream target mono-ubiquitinated histone H2A and induces apoptosis | [196] | ||
| Breast cancer | Transcriptionally upregulates expression of miR-200c/141 cluster | [197] | ||
| Biliary tract cancer cells | Causes down-regulation of cell cycle-promoting genes, DNA synthesis gene and DNA repair gene | [198] | ||
| Chronic myeloid leukemia cells | Triggers CCNG2 expression | [105] | ||
| MM | Down-regulates the expression of Bmi-1 protein and the associated repressive histone mark H2AK119ub | [177] | ||
| HNSCC | Inhibits proliferation, migration and invasiveness, increases cell apoptosis and chemosensitivity | [175] | ||
| PTC596 | Reduces protein levels of BMI-1 | Myeloma | Induces cell cycle arrest at G2/M phase followed by apoptotic cell death | [199] |
| AML | Downregulates Mcl-1 and induces p53-independent mitochondrial apoptosis | [181] | ||
| Glioblastoma | Targets both Bmi-1 and EZH2, prevents GBM colony growth and CSC self-renewal | [184] | ||
| Mantle cell lymphoma | Induces mitochondrial apoptosis, loss of mitochondrial membrane potential, C-caspase-3, Bax activation, and phosphatidylserine externalization | [182] | ||
| Cancer stem-like cells | Induces apoptosis through DUB3-mediated Mcl-1 degradation | [99] | ||
| Pancreatic ductal adenocarcinoma (PDA) | Induces mitotic arrest and apoptosis | [200] | ||
| Diffuse intrinsic pontine glioma (DIPG) | Decreases tumor volume and growth kinetics, increases intertumoral apoptosis, and sustains animal survival benefit. | [201] | ||
| RU-A1 | Bind to the Bmi-1 mRNA | Hepatocellular carcinoma | Impairs cell viability, reduces cell migration, enhances HCC cell sensitivity to 5-fluorouracil (5-FU) in vitro | [187] |
| PTC-028 | Posttranslational modification | multiple myeloma | Impairs MYC and Akt signalling activity; induces cell cycle arrest at G2/M phase and apoptotic | [183] |
| Diffuse intrinsic pontine glioma (DIPG) | Decreases the expression of E2F1, KRAS, Nestin, SOX2 while increases the expression of p21 and differentiation markers (GFAP) | [202] | ||
| Hyperphosphorylation | Ovarian cancer | Decreases ATP and a compromised mitochondrial redox balance potentiate caspase-dependent apoptosis | [188] | |
| Medulloblastoma (MB) | Abolishes the self-renewal capacity of MB stem cells, reduces tumor initiation ability of recurrent MB cells | [203] | ||
| Endometrial cancer | Reduces cell invasive capacity and enhances caspase-dependent apoptosis | [204] | ||
| Alveolar rhabdomyosarcoma | Inhibits proliferation and causes tumor growth delay in vivo | [190] | ||
| QW24 | Autophagy-lysosome degradation pathway | Stem-like colorectal cancer | Inhibits self-renewal of colorectal cancer-initiating cells (CICs) | [189] |
| SH498 | Colorectal cancer | Reduces PRC1 complex activity by down-regulating Bmi-1 and ub-H2A | [205] | |
| Artemisinin | Protein and transcript levels | Nasopharyngeal carcinoma | Induces G1 cell cycle arrest via the Bmi-1-p16/CDK4 axis | [206] |
| PRT4165 | Down-regulating Bmi-1/RING1A self-ubiquitination | Acute leukemia | Increases cell apoptosis | [195] |
| CDDO-Me | ESCC | Induces autophagy via suppression of PI3K/Akt/mTOR signaling pathway | [207] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, L.; Shi, P.; Cui, H.; Yang, L. The Crucial Roles of Bmi-1 in Cancer: Implications in Pathogenesis, Metastasis, Drug Resistance, and Targeted Therapies. Int. J. Mol. Sci. 2022, 23, 8231. https://doi.org/10.3390/ijms23158231
Xu J, Li L, Shi P, Cui H, Yang L. The Crucial Roles of Bmi-1 in Cancer: Implications in Pathogenesis, Metastasis, Drug Resistance, and Targeted Therapies. International Journal of Molecular Sciences. 2022; 23(15):8231. https://doi.org/10.3390/ijms23158231
Chicago/Turabian StyleXu, Jie, Lin Li, Pengfei Shi, Hongjuan Cui, and Liqun Yang. 2022. "The Crucial Roles of Bmi-1 in Cancer: Implications in Pathogenesis, Metastasis, Drug Resistance, and Targeted Therapies" International Journal of Molecular Sciences 23, no. 15: 8231. https://doi.org/10.3390/ijms23158231






