Microglia Remodelling and Neuroinflammation Parallel Neuronal Hyperactivation Following Acute Organophosphate Poisoning
Abstract
:1. Introduction
2. Results
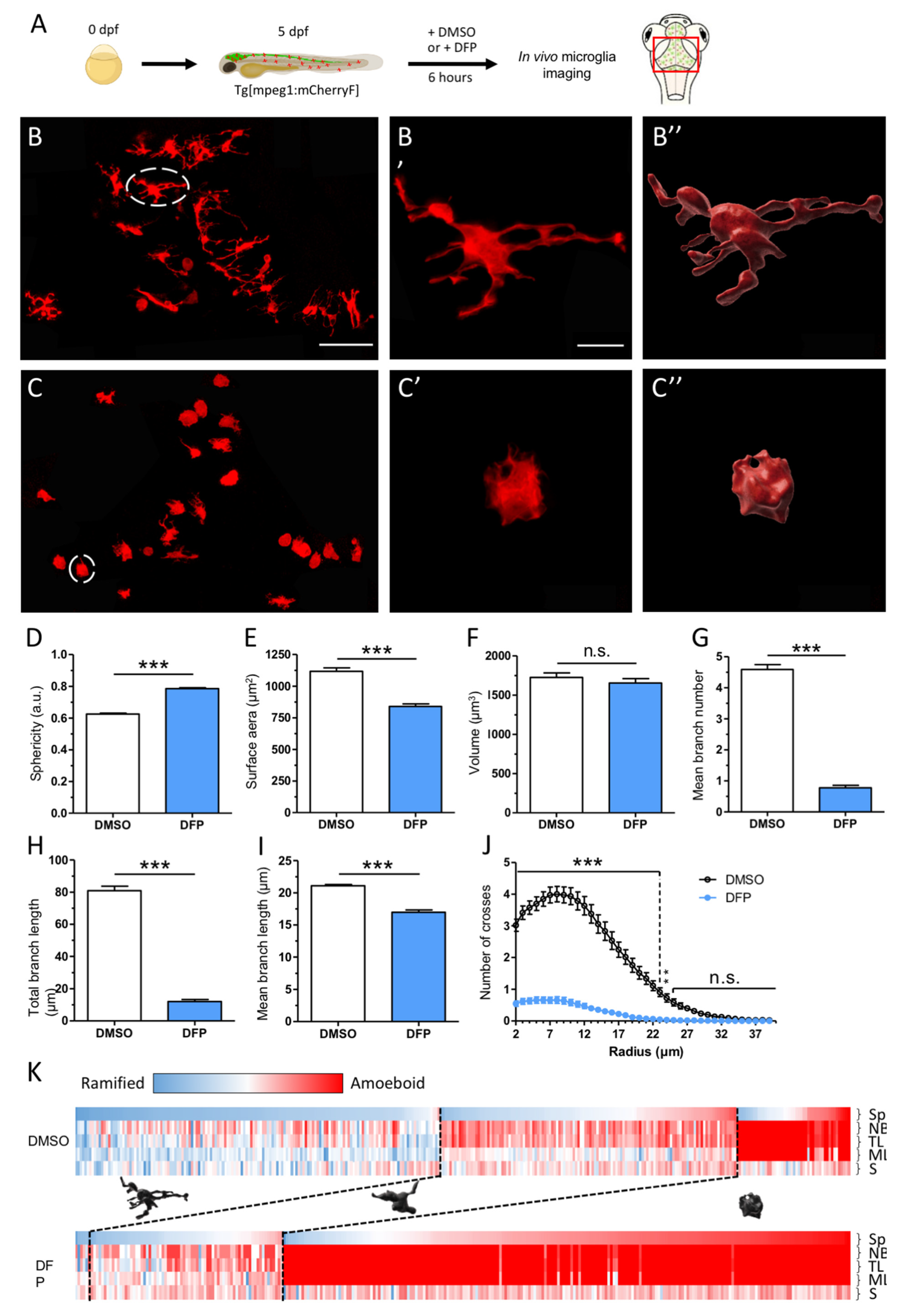
2.1. DFP Exposure Induced Dramatic Phenotypic Remodelling of Microglia
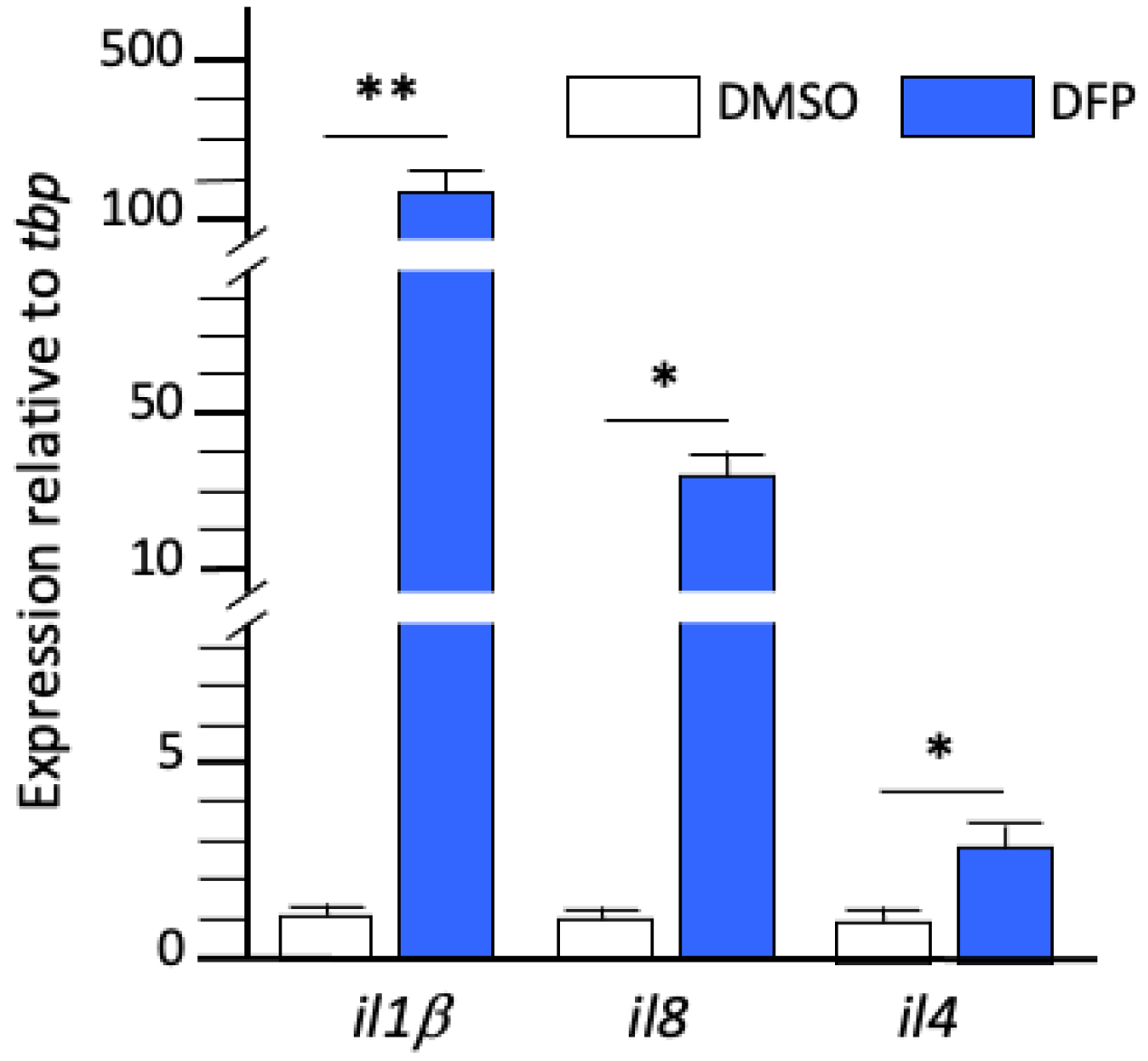
2.2. DFP Exposure Induced Microglia-Mediated Overexpression of Inflammatory Cytokines
2.3. Kinetics of Neuronal Activity in DFP-Exposed Larvae
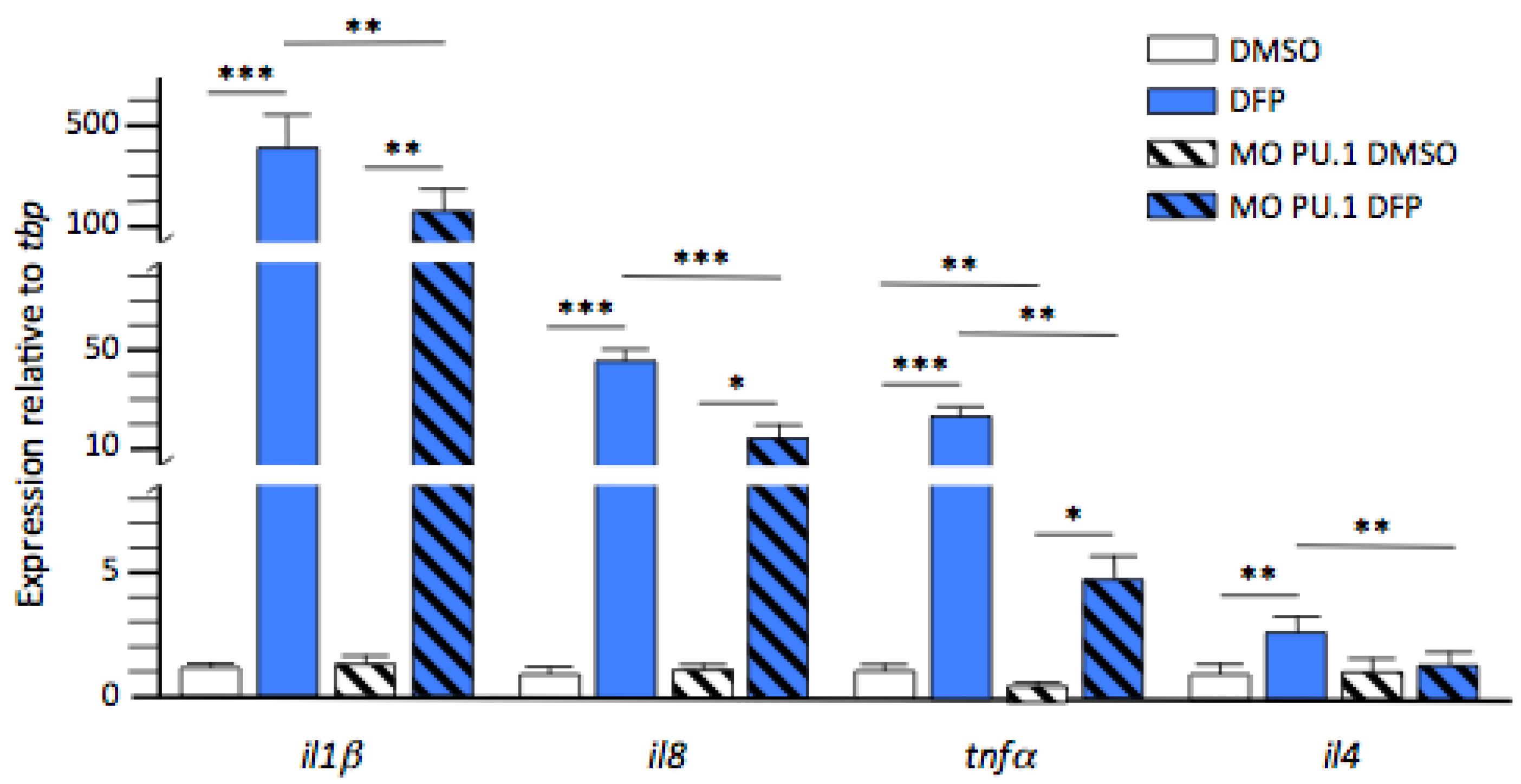
2.4. Inflammatory Cytokines Expression in DFP-Treated Larvae without Microglia
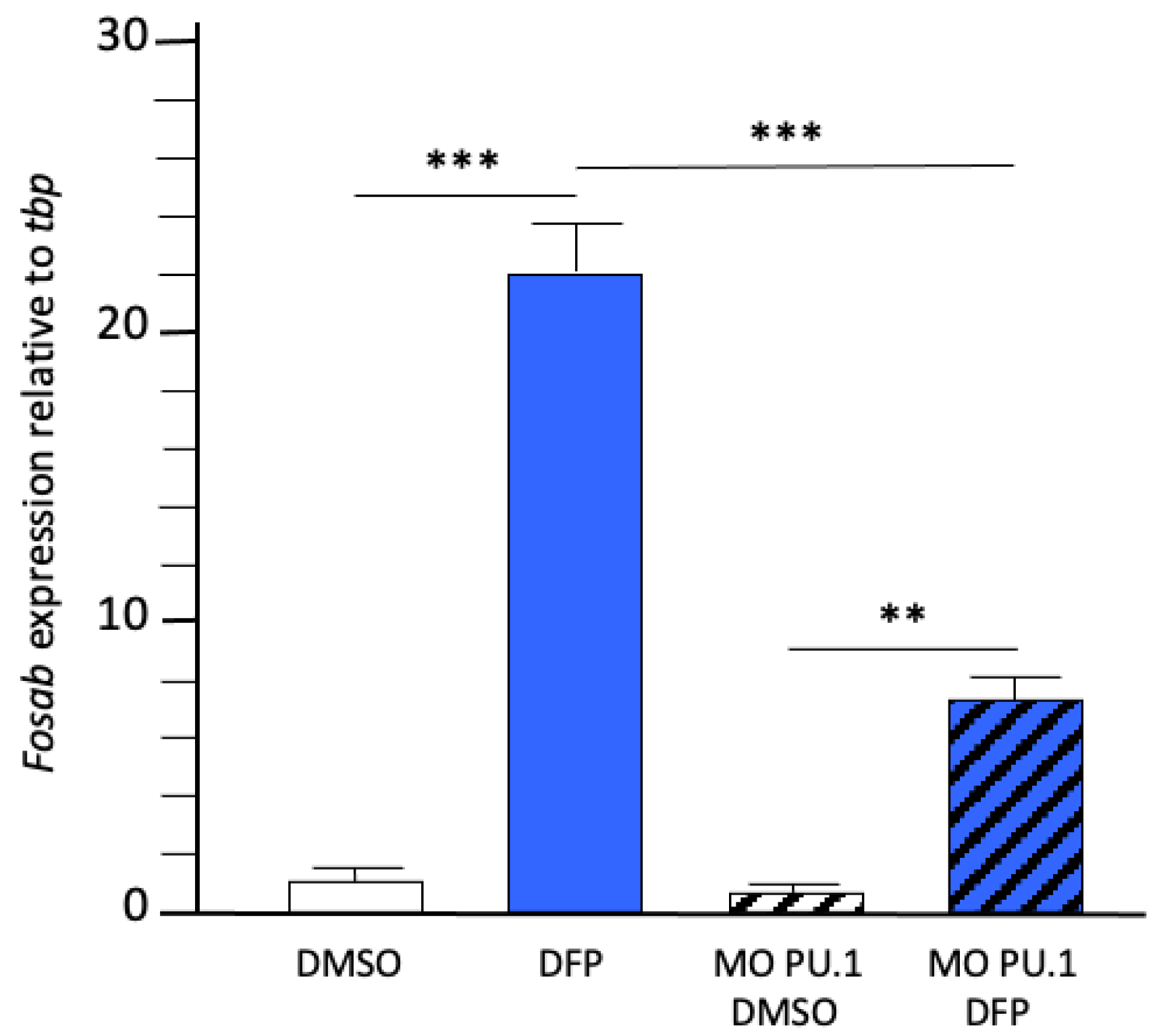
2.5. DFP-Induced Neuronal Hyperactivation Was Markedly Reduced in Larvae without Microglia
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eddleston, M.; Singh, S.; Buckley, N. Organophosphorus poisoning (acute). Clin. Evid. 2004, 12, 1941–1953. [Google Scholar]
- Eddleston, M.; Eyer, P.; Worek, F.; Rezvi Sheriff, M.H.; Buckley, N.A. Predicting outcome using butyrylcholinesterase activity in organophosphorus pesticide self-poisoning. QJM 2008, 101, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissman, B.A.; Raveh, L. Therapy against organophosphate poisoning: The importance of anticholinergic drugs with antiglutamatergic properties. Toxicol. Appl. Pharmacol. 2008, 232, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, M.S.; Cowan, M.L.; Balint, C.A.; Sun, C.; Kapur, J. Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res. 2012, 101, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokanović, M.; Kosanović, M. Neurotoxic effects in patients poisoned with organophosphorus pesticides. Environ. Toxicol. Pharmacol. 2010, 29, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Jett, D.A. Neurological aspects of chemical terrorism. Ann. Neurol. 2007, 61, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.A.; Ennis, M.; Shipley, M.T. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J. Comp. Neurol. 1997, 378, 482–492. [Google Scholar] [CrossRef]
- Li, Y.; Lein, P.J.; Ford, G.D.; Liu, C.; Stovall, K.C.; White, T.E.; Bruun, D.A.; Tewolde, T.; Gates, A.S.; Distel, T.J.; et al. Neuregulin-1 inhibits neuroinflammatory responses in a rat model of organophosphate-nerve agent-induced delayed neuronal injury. J. Neuroinflamm. 2015, 12, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souders, C.L.; Liang, X.; Wang, X.; Ector, N.; Zhao, Y.H.; Martyniuk, C.J. High-throughput assessment of oxidative respiration in fish embryos: Advancing adverse outcome pathways for mitochondrial dysfunction. Aquat. Toxicol. 2018, 199, 162–173. [Google Scholar] [CrossRef]
- Sisó, S.; Hobson, B.A.; Harvey, D.J.; Bruun, D.A.; Rowland, D.J.; Garbow, J.R.; Lein, P.J. Editor’s Highlight: Spatiotemporal Progression and Remission of Lesions in the Rat Brain Following Acute Intoxication With Diisopropylfluorophosphate. Toxicol. Sci. 2017, 157, 330–341. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, E.A.; Calsbeek, J.J.; Tsai, Y.H.; Tang, M.Y.; Andrew, P.; Vu, J.; Berg, E.L.; Saito, N.H.; Harvey, D.J.; Supasai, S.; et al. Sex-specific acute and chronic neurotoxicity of acute diisopropylfluorophosphate (DFP)-intoxication in juvenile Sprague-Dawley rats. Curr. Res. Toxicol. 2021, 2, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, W.; Bealer, S.L.; Roach, B.; Dudek, F.E. A rodent model of human organophosphate exposure producing status epilepticus and neuropathology. Neurotoxicology 2016, 56, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenet, A.; Somkhit, J.; Hassan-Abdi, R.; Yanicostas, C.; Romain, C.; Bar, O.; Igert, A.; Saurat, D.; Taudon, N.; Dal-Bo, G.; et al. Organophosphorus diisopropylfluorophosphate (DFP) intoxication in zebrafish larvae causes behavioral defects, neuronal hyperexcitation and neuronal death. Sci. Rep. 2020, 10, 19228. [Google Scholar] [CrossRef] [PubMed]
- Travnickova, J.; Chau, V.T.; Julien, E.; Mateos-Langerak, J.; Gonzalez, C.; Lelièvre, E.; Lutfalla, G.; Tavian, M.; Kissa, K. Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat. Commun. 2015, 6, 6227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan-Abdi, R.; Brenet, A.; Bennis, M.; Yanicostas, C.; Soussi-Yanicostas, N. Neurons Expressing Pathological Tau Protein Trigger Dramatic Changes in Microglial Morphology and Dynamics. Front. Neurosci. 2019, 13, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, N.J.; Dorman, L.C.; Vainchtein, I.D.; Horneck, N.C.; Molofsky, A.V. In situ and transcriptomic identification of microglia in synapse-rich regions of the developing zebrafish brain. Nat. Commun. 2021, 12, 5916. [Google Scholar] [CrossRef]
- Morin-Brureau, M.; Milior, G.; Royer, J.; Chali, F.; LeDuigou, C.; Savary, E.; Blugeon, C.; Jourdren, L.; Akbar, D.; Dupont, S.; et al. Microglial phenotypes in the human epileptic temporal lobe. Brain 2018, 141, 3343–3360. [Google Scholar] [CrossRef]
- Flannery, B.M.; Bruun, D.A.; Rowland, D.J.; Banks, C.N.; Austin, A.T.; Kukis, D.L.; Li, Y.; Ford, B.D.; Tancredi, D.J.; Silverman, J.L.; et al. Persistent neuroinflammation and cognitive impairment in a rat model of acute diisopropylfluorophosphate intoxication. J. Neuroinflamm. 2016, 13, 267. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lein, P.J.; Liu, C.; Bruun, D.A.; Tewolde, T.; Ford, G.; Ford, B.D. Spatiotemporal pattern of neuronal injury induced by DFP in rats: A model for delayed neuronal cell death following acute OP intoxication. Toxicol. Appl. Pharmacol. 2011, 253, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Maupu, C.; Enderlin, J.; Igert, A.; Oger, M.; Auvin, S.; Hassan-Abdi, R.; Soussi-Yanicostas, N.; Brazzolotto, X.; Nachon, F.; Bo, G.D.; et al. Diisopropylfluorophosphate-induced status epilepticus drives complex glial cell phenotypes in adult male mice. Neurobiol. Dis. 2021, 152, 105276. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Ganesh, T.; Wang, W.; Wang, J.; Dingledine, R. A rat model of organophosphate-induced status epilepticus and the beneficial effects of EP2 receptor inhibition. Neurobiol. Dis. 2020, 133, 104399. [Google Scholar] [CrossRef]
- Szyndler, J.; Maciejak, P.; Turzyńska, D.; Sobolewska, A.; Taracha, E.; Skórzewska, A.; Lehner, M.; Bidziński, A.; Hamed, A.; Wisłowska-Stanek, A.; et al. Mapping of c-Fos expression in the rat brain during the evolution of pentylenetetrazol-kindled seizures. Epilepsy Behav. 2009, 16, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Gage, M.; Golden, M.; Putra, M.; Sharma, S.; Thippeswamy, T. Sex as a biological variable in the rat model of diisopropylfluorophosphate-induced long-term neurotoxicity. Ann. N. Y. Acad. Sci. 2020, 1479, 44–64. [Google Scholar] [CrossRef]
- Supasai, S.; González, E.A.; Rowland, D.J.; Hobson, B.; Bruun, D.A.; Guignet, M.A.; Soares, S.; Singh, V.; Wulff, H.; Saito, N.; et al. Acute administration of diazepam or midazolam minimally alters long-term neuropathological effects in the rat brain following acute intoxication with diisopropylfluorophosphate. Eur. J. Pharmacol. 2020, 11, 886. [Google Scholar] [CrossRef]
- Peri, F.; Nüsslein-Volhard, C. Live Imaging of Neuronal Degradation by Microglia Reveals a Role for v0-ATPase a1 in Phagosomal Fusion In Vivo. Cell 2008, 133, 916–927. [Google Scholar] [CrossRef] [Green Version]
- Van-Vliet, E.A.; Aronica, E.; Vezzani, A.; Ravizza, T. Review: Neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: Emerging evidence from preclinical and clinical studies. Neuropathol. Appl. Neurobiol. 2018, 44, 91–111. [Google Scholar] [CrossRef]
- Kuruba, R.; Wu, X.; Reddy, D.S. Benzodiazepine-refractory status epilepticus, neuroinflammation, and interneuron neurodegeneration after acute organophosphate intoxication. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2845–2858. [Google Scholar] [CrossRef]
- Li, Y.; Du, X.; Liu, C.; Wen, Z.; Du, J. Reciprocal Regulation between Resting Microglial Dynamics and Neuronal Activity In Vivo. Dev. Cell 2012, 23, 1189–1202. [Google Scholar] [CrossRef] [Green Version]
- Rojas, A.; Wang, W.; Glover, A.; Manji, Z.; Fu, Y.; Dingledine, R. Beneficial Outcome of Urethane Treatment Following Status Epilepticus in a Rat Organophosphorus Toxicity Model. eNeuro 2018, 5, 2. [Google Scholar] [CrossRef]
- Wu, X.; Kuruba, R.; Reddy, D.S. Midazolam-resistant seizures and brain injury after acute intoxication of diisopropylfluorophosphate, an organophosphate pesticide and surrogate for nerve agents. J. Pharmacol. Exp. Ther. 2018, 367, 302–321. [Google Scholar] [CrossRef] [Green Version]
- Hobson, B.A.; Sisó, S.; Rowland, D.J.; Harvey, D.J.; Bruun, D.A.; Garbow, J.R.; Lein, P.J. From the Cover: Magnetic Resonance Imaging Reveals Progressive Brain Injury in Rats Acutely Intoxicated With Diisopropylfluorophosphate. Toxicol. Sci. 2017, 157, 342–353. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef]
- Ravizza, T.; Gagliardi, B.; Noé, F.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–160. [Google Scholar] [CrossRef]
- Enderlin, J.; Igert, A.; Auvin, S.; Nachon, F.; Dal Bo, G.; Dupuis, N. Characterization of organophosphate-induced brain injuries in a convulsive mouse model of diisopropylfluorophosphate exposure. Epilepsia 2020, 61, 54–59. [Google Scholar] [CrossRef]
- Benson, M.J.; Manzanero, S.; Borges, K. Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia 2015, 56, 895–905. [Google Scholar] [CrossRef]
- Vezzani, A.; Moneta, D.; Richichi, C.; Aliprandi, M.; Burrows, S.J.; Ravizza, T.; Perego, C.; Simoni, M.G.D. Functional Role of Inflammatory Cytokines and Antiinflammatory Molecules in Seizures and Epileptogenesis. Epilepsia 2002, 43, 30–35. [Google Scholar] [CrossRef]
- Rodgers, K.M.; Hutchinson, M.R.; Northcutt, A.; Maier, S.F.; Watkins, L.R.; Barth, D.S. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain 2009, 132, 2478–2486. [Google Scholar] [CrossRef] [Green Version]
- Vezzani, A.; Friedman, A.; Dingledine, R.J. The role of inflammation in epileptogenesis. Neuropharmacology 2013, 69, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Chiavegato, A.; Zurolo, E.; Losi, G.; Aronica, E.; Carmignoto, G. The inflammatory molecules IL-1Î2 and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front. Cell. Neurosci. 2014, 8, 155. [Google Scholar] [CrossRef]
- DeSena, A.D.; Do, T.; Schulert, G.S. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J. Neuroinflamm. 2018, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noe, F.M.; Polascheck, N.; Frigerio, F.; Bankstahl, M.; Ravizza, T.; Marchini, S.; Beltrame, L.; Banderó, C.R.; Löscher, W.; Vezzani, A. Pharmacological blockade of IL-1β/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol. Dis. 2013, 59, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Moneta, D.; Conti, M.; Richichi, C.; Ravizza, T.; De Luigi, A.; De Simoni, M.G.; Sperk, G.; Andell-Jonsson, S.; Lundkvist, J.; et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11534–11539. [Google Scholar] [CrossRef] [Green Version]
- Młodzikowska-Albrecht, J.; Steinborn, B.; Zarowski, M. Cytokines, epilepsy, and antiepileptic drugs—Is there a mutual influence? Pharmacol. Rep. 2007, 59, 129–138. [Google Scholar] [PubMed]
- Viviani, B.; Bartesaghi, S.; Gardoni, F.; Vezzani, A.; Behrens, M.M.; Bartfai, T.; Binaglia, M.; Corsini, E.; Di Luca, M.; Galli, C.L.; et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003, 23, 8692–8700. [Google Scholar] [CrossRef]
- Roseti, C.; Van Vliet, E.A.; Cifelli, P.; Ruffolo, G.; Baayen, J.C.; Di Castro, M.A.; Bertollini, C.; Limatola, C.; Aronica, E.; Vezzani, A.; et al. GABAA currents are decreased by IL-1β in epileptogenic tissue of patients with temporal lobe epilepsy: Implications for ictogenesis. Neurobiol. Dis. 2015, 82, 311–320. [Google Scholar] [CrossRef]
- Westerfield, M.; Doerry, E.; Kirkpatrick, A.E.; Driever, W.; Douglas, S.A. An on-line database for zebrafish development and genetics research. Semin. Cell Dev. Biol. 1997, 8, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, J.; Chen, T.W.; Wardill, T.J.; Tian, L.; Marvin, J.S.; Mutlu, S.; Calderón, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012, 32, 13819–13840. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somkhit, J.; Yanicostas, C.; Soussi-Yanicostas, N. Microglia Remodelling and Neuroinflammation Parallel Neuronal Hyperactivation Following Acute Organophosphate Poisoning. Int. J. Mol. Sci. 2022, 23, 8240. https://doi.org/10.3390/ijms23158240
Somkhit J, Yanicostas C, Soussi-Yanicostas N. Microglia Remodelling and Neuroinflammation Parallel Neuronal Hyperactivation Following Acute Organophosphate Poisoning. International Journal of Molecular Sciences. 2022; 23(15):8240. https://doi.org/10.3390/ijms23158240
Chicago/Turabian StyleSomkhit, Julie, Constantin Yanicostas, and Nadia Soussi-Yanicostas. 2022. "Microglia Remodelling and Neuroinflammation Parallel Neuronal Hyperactivation Following Acute Organophosphate Poisoning" International Journal of Molecular Sciences 23, no. 15: 8240. https://doi.org/10.3390/ijms23158240
APA StyleSomkhit, J., Yanicostas, C., & Soussi-Yanicostas, N. (2022). Microglia Remodelling and Neuroinflammation Parallel Neuronal Hyperactivation Following Acute Organophosphate Poisoning. International Journal of Molecular Sciences, 23(15), 8240. https://doi.org/10.3390/ijms23158240






