Sexual Dimorphism in Brown Adipose Tissue Activation and White Adipose Tissue Browning
Abstract
:1. Introduction
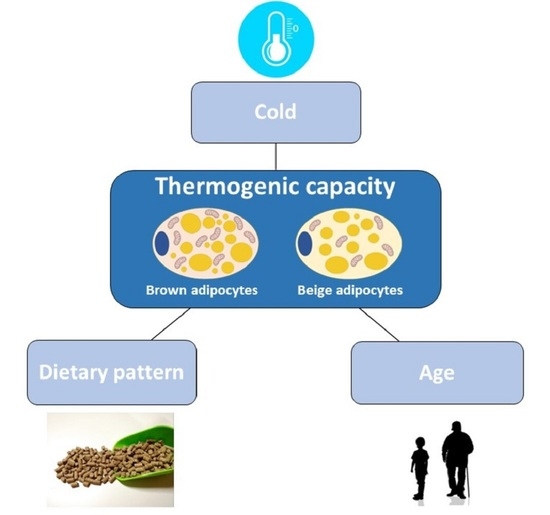
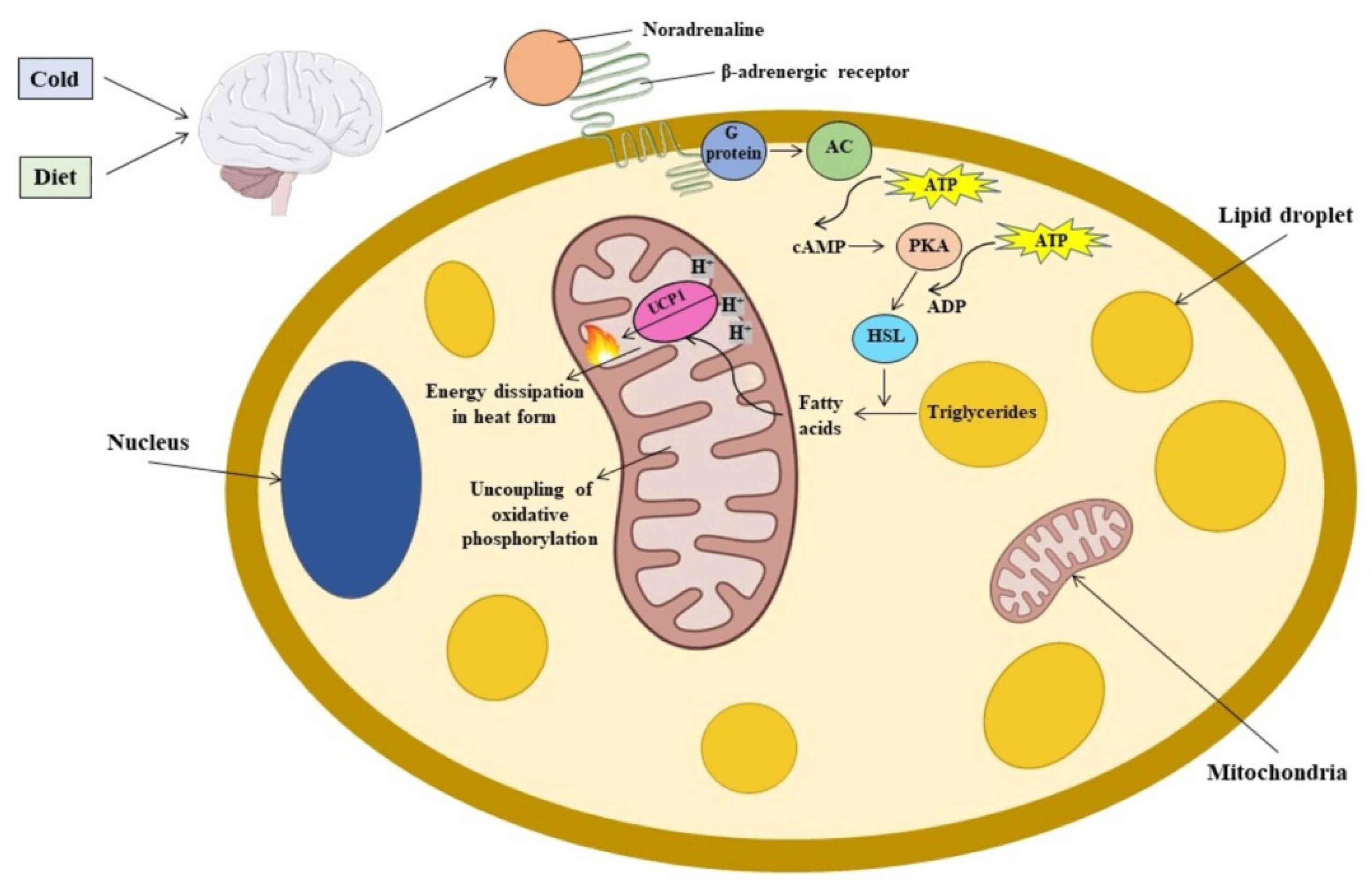
2. Sexual Dimorphism in BAT Activation Induced by Cold
2.1. Preclinical Studies
2.2. Human Studies: Child and Adolescents
2.3. Human Studies: Adults
2.4. Summary
3. Sexual Dimorphism in BAT Activation Induced by Diet
Summary
4. Sexual Dimorphism in BAT Activation: Effect of Age
Summary
5. Sexual Dimorphism in Browning
5.1. Preclinical Studies
5.2. Human Studies
5.3. Summary
6. The Role of Bioactive Compounds in Browning Sexual Dimorphism
Summary
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Liu, W.; Li, D.; Cao, H.; Li, H.; Wang, Y. Expansion and Inflammation of White Adipose Tissue—Focusing on Adipocyte Progenitors. Biol. Chem. 2020, 402, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and Glucose Metabolism in White Adipocytes: Pathways, Dysfunction and Therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeren, J.; Scheja, L. Brown Adipose Tissue and Lipid Metabolism. Curr. Opin. Lipidol. 2018, 29, 180–185. [Google Scholar] [CrossRef]
- Herz, C.T.; Kiefer, F.W. Adipose Tissue Browning in Mice and Humans. J. Endocrinol. 2019, 241, R97–R109. [Google Scholar] [CrossRef] [PubMed]
- Kaisanlahti, A.; Glumoff, T. Browning of White Fat: Agents and Implications for Beige Adipose Tissue to Type 2 Diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scime, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 Controls a Brown Fat/Skeletal Muscle Switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Gurmaches, J.; Hung, C.M.; Sparks, C.A.; Tang, Y.; Li, H.; Guertin, D.A. PTEN Loss in the Myf5 Lineage Redistributes Body Fat and Reveals Subsets of White Adipocytes that Arise from Myf5 Precursors. Cell Metab. 2012, 16, 348–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenwald, M.; Wolfrum, C. The Origin and Definition of Brite Versus White and Classical Brown Adipocytes. Adipocyte 2014, 3, 4–9. [Google Scholar] [CrossRef]
- Maurer, S.; Harms, M.; Boucher, J. The Colorful Versatility of Adipocytes: White-to-Brown Transdifferentiation and its Therapeutic Potential in Humans. FEBS J. 2021, 288, 3628–3646. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Li, M.; Niu, C.; Wang, G.; Li, T.; Krol, E.; Jin, W.; Speakman, J.R. Brown Adipocytes can Display a Mammary Basal Myoepithelial Cell Phenotype in Vivo. Mol. Metab. 2017, 6, 1198–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinti, S. Pink Adipocytes. Trends Endocrinol. Metab. 2018, 29, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown Adipose Tissue as a Secretory Organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef]
- Gavaldà-Navarro, A.; Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. The Endocrine Role of Brown Adipose Tissue: An Update on Actors and Actions. Rev. Endocr. Metab. Disord. 2021, 23, 31–41. [Google Scholar] [CrossRef]
- Schiebinger, L.; Stefanick, M.L. Gender Matters in Biological Research and Medical Practice. J. Am. Coll. Cardiol. 2016, 67, 136–138. [Google Scholar] [CrossRef] [Green Version]
- Corella, D.; Coltell, O.; Portoles, O.; Sotos-Prieto, M.; Fernandez-Carrion, R.; Ramirez-Sabio, J.B.; Zanon-Moreno, V.; Mattei, J.; Sorli, J.V.; Ordovas, J.M. A Guide to Applying the Sex-Gender Perspective to Nutritional Genomics. Nutrients 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keuper, M.; Jastroch, M. The Good and the BAT of Metabolic Sex Differences in Thermogenic Human Adipose Tissue. Mol. Cell. Endocrinol. 2021, 533, 111337. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Chen, Y. Fat Biology and Metabolic Balance: On the Significance of Sex. Mol. Cell. Endocrinol. 2021, 533, 111336. [Google Scholar] [CrossRef] [PubMed]
- Kaikaew, K.; Grefhorst, A.; Visser, J.A. Sex Differences in Brown Adipose Tissue Function: Sex Hormones, Glucocorticoids, and their Crosstalk. Front. Endocrinol. 2021, 12, 652444. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quevedo, S.; Roca, P.; Pico, C.; Palou, A. Sex-Associated Differences in Cold-Induced UCP1 Synthesis in Rodent Brown Adipose Tissue. Pflug. Arch. 1998, 436, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Roca, P.; Rodriguez, A.M.; Oliver, P.; Bonet, M.L.; Quevedo, S.; Pico, C.; Palou, A. Brown Adipose Tissue Response to Cafeteria Diet-Feeding Involves Induction of the UCP2 Gene and is Impaired in Female Rats as Compared to Males. Pflug. Arch. 1999, 438, 628–634. [Google Scholar] [CrossRef]
- Harshaw, C.; Culligan, J.J.; Alberts, J.R. Sex Differences in Thermogenesis Structure Behavior and Contact within Huddles of Infant Mice. PLoS ONE 2014, 9, e87405. [Google Scholar] [CrossRef]
- Grefhorst, A.; van den Beukel, J.C.; van Houten, E.L.; Steenbergen, J.; Visser, J.A.; Themmen, A.P. Estrogens Increase Expression of Bone Morphogenetic Protein 8b in Brown Adipose Tissue of Mice. Biol. Sex Differ. 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Robinson, L.J.; Law, J.; Astle, V.; Gutierrez-Garcia, M.; Ojha, S.; Symonds, M.E.; Pitchford, N.; Budge, H. Sexual Dimorphism of Brown Adipose Tissue Function. J. Pediatr. 2019, 210, 166–172.e1. [Google Scholar] [CrossRef] [PubMed]
- Malpique, R.; Gallego-Escuredo, J.M.; Sebastiani, G.; Villarroya, J.; Lopez-Bermejo, A.; de Zegher, F.; Villarroya, F.; Ibanez, L. Brown Adipose Tissue in Prepubertal Children: Associations with Sex, Birthweight, and Metabolic Profile. Int. J. Obes. 2019, 43, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Brychta, R.J.; Linderman, J.D.; Smith, S.; Courville, A.; Dieckmann, W.; Herscovitch, P.; Millo, C.M.; Remaley, A.; Lee, P.; et al. Brown Fat Activation Mediates Cold-Induced Thermogenesis in Adult Humans in Response to a Mild Decrease in Ambient Temperature. J. Clin. Endocrinol. Metab. 2013, 98, E1218–E1223. [Google Scholar] [CrossRef] [PubMed]
- Mengel, L.A.; Seidl, H.; Brandl, B.; Skurk, T.; Holzapfel, C.; Stecher, L.; Claussnitzer, M.; Hauner, H. Gender Differences in the Response to Short-Term Cold Exposure in Young Adults. J. Clin. Endocrinol. Metab. 2020, 105, dgaa110. [Google Scholar] [CrossRef]
- Fletcher, L.A.; Kim, K.; Leitner, B.P.; Cassimatis, T.M.; O’Mara, A.E.; Johnson, J.W.; Halprin, M.S.; McGehee, S.M.; Brychta, R.J.; Cypess, A.M.; et al. Sexual Dimorphisms in Adult Human Brown Adipose Tissue. Obesity 2020, 28, 241–246. [Google Scholar] [CrossRef]
- Herz, C.T.; Kulterer, O.C.; Prager, M.; Marculescu, R.; Langer, F.B.; Prager, G.; Kautzky-Willer, A.; Haug, A.R.; Kiefer, F.W. Sex Differences in Brown Adipose Tissue Activity and Cold-Induced Thermogenesis. Mol. Cell. Endocrinol. 2021, 534, 111365. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Morentin, P.B.; Gonzalez-Garcia, I.; Martins, L.; Lage, R.; Fernandez-Mallo, D.; Martinez-Sanchez, N.; Ruiz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell. Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, T.E. Thermal, Metabolic, and Cardiovascular Changes in Men and Women during Cold Stress. Med. Sci. Sports Exerc. 1988, 20, S185–S192. [Google Scholar] [CrossRef]
- Martinez-Tellez, B.; Sanchez-Delgado, G.; Alcantara, J.M.A.; Acosta, F.M.; Amaro-Gahete, F.; Osuna-Prieto, F.; Perez-Bey, A.; Jimenez-Pavon, D.; Llamas-Elvira, J.; Gil, A.; et al. Evidence of High 18F-Fluorodeoxyglucose Uptake in the Subcutaneous Adipose Tissue of the Dorsocervical Area in Young Adults. Exp. Physiol. 2019, 104, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Puigserver, P.; Llado, I.; Palou, A.; Gianotti, M. Evidence for Masking of Brown Adipose Tissue Mitochondrial GDP-Binding Sites in Response to Fasting in Rats made Obese by Dietary Manipulation. Effects of Reversion to Standard Diet. Biochem. J. 1991, 279 Pt 2, 575–579. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Fisler, J.S.; Fukushima, M.; Bray, G.A.; Schemmel, R.A. Diet, Lighting, and Food Intake Affect Norepinephrine Turnover in Dietary Obesity. Am. J. Physiol. 1987, 252, R402–R408. [Google Scholar] [CrossRef]
- van Baak, M.A. Meal-Induced Activation of the Sympathetic Nervous System and its Cardiovascular and Thermogenic Effects in Man. Physiol. Behav. 2008, 94, 178–186. [Google Scholar] [CrossRef]
- Vijgen, G.H.; Bouvy, N.D.; Leenen, L.; Rijkers, K.; Cornips, E.; Majoie, M.; Brans, B.; van Marken Lichtenbelt, W.D. Vagus Nerve Stimulation Increases Energy Expenditure: Relation to Brown Adipose Tissue Activity. PLoS ONE 2013, 8, e77221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, E.; Monjo, M.; Rodriguez-Cuenca, S.; Pujol, E.; Amengual, B.; Roca, P.; Palou, A. Sexual Dimorphism in the Adrenergic Control of Rat Brown Adipose Tissue Response to Overfeeding. Pflug. Arch. 2001, 442, 396–403. [Google Scholar] [CrossRef]
- Rodriguez, E.; Ribot, J.; Rodriguez, A.M.; Palou, A. PPAR-Gamma2 Expression in Response to Cafeteria Diet: Gender- and Depot-Specific Effects. Obes. Res. 2004, 12, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.K.; Oh, T.S.; Choi, J.W.; Mukherjee, R.; Wang, X.; Liu, H.; Yun, J.W. Gender Difference in Proteome of Brown Adipose Tissues between Male and Female Rats Exposed to a High Fat Diet. Cell Physiol. Biochem. 2011, 28, 933–948. [Google Scholar] [CrossRef]
- MacCannell, A.D.V.; Futers, T.S.; Whitehead, A.; Moran, A.; Witte, K.K.; Roberts, L.D. Sexual Dimorphism in Adipose Tissue Mitochondrial Function and Metabolic Flexibility in Obesity. Int. J. Obes. 2021, 45, 1773–1781. [Google Scholar] [CrossRef]
- Valle, A.; Catala-Niell, A.; Colom, B.; Garcia-Palmer, F.J.; Oliver, J.; Roca, P. Sex-Related Differences in Energy Balance in Response to Caloric Restriction. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E15–E22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, A.; Garcia-Palmer, F.J.; Oliver, J.; Roca, P. Sex Differences in Brown Adipose Tissue Thermogenic Features during Caloric Restriction. Cell Physiol. Biochem. 2007, 19, 195–204. [Google Scholar] [CrossRef]
- Monjo, M.; Rodriguez, A.M.; Palou, A.; Roca, P. Direct Effects of Testosterone, 17 Beta-Estradiol, and Progesterone on Adrenergic Regulation in Cultured Brown Adipocytes: Potential Mechanism for Gender-Dependent Thermogenesis. Endocrinology 2003, 144, 4923–4930. [Google Scholar] [CrossRef] [Green Version]
- Gilsanz, V.; Smith, M.L.; Goodarzian, F.; Kim, M.; Wren, T.A.; Hu, H.H. Changes in Brown Adipose Tissue in Boys and Girls during Childhood and Puberty. J. Pediatr. 2012, 160, 604–609.e1. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Beltran, C.; Cereijo, R.; Plou, C.; Gavaldà-Navarro, A.; Malpique, R.; Villarroya, J.; López-Bermejo, A.; de Zegher, F.; Ibáñez, L.; Villarroya, F. Posterior Cervical Brown Fat and CXCL14 Levels in the First Year of Life: Sex Differences and Association with Adiposity. J. Clin. Endocrinol. Metab. 2022, 107, e1148–e1158. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Santandreu, F.M.; Garcia-Palmer, F.J.; Roca, P.; Oliver, J. The Serum Levels of 17beta-Estradiol, Progesterone and Triiodothyronine Correlate with Brown Adipose Tissue Thermogenic Parameters during Aging. Cell Physiol. Biochem. 2008, 22, 337–346. [Google Scholar] [CrossRef]
- Yasui, T.; Uemura, H.; Irahara, M.; Arai, M.; Kojimahara, N.; Okabe, R.; Yasutomo, I.; Tashiro, S.; Sato, H. Differences in Sensitivity to Cold in Japanese Men and Postmenopausal Women Aged ≥ 50 Years. Gend. Med. 2007, 4, 359–366. [Google Scholar] [CrossRef]
- Pfannenberg, C.; Werner, M.K.; Ripkens, S.; Stef, I.; Deckert, A.; Schmadl, M.; Reimold, M.; Haring, H.U.; Claussen, C.D.; Stefan, N. Impact of Age on the Relationships of Brown Adipose Tissue with Sex and Adiposity in Humans. Diabetes 2010, 59, 1789–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persichetti, A.; Sciuto, R.; Rea, S.; Basciani, S.; Lubrano, C.; Mariani, S.; Ulisse, S.; Nofroni, I.; Maini, C.L.; Gnessi, L. Prevalence, Mass, and Glucose-Uptake Activity of (1)(8)F-FDG-Detected Brown Adipose Tissue in Humans Living in a Temperate Zone of Italy. PLoS ONE 2013, 8, e63391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, J.M.; Valencak, T.G. Sex Differences and Aging: Is there a Role of Brown Adipose Tissue? Mol. Cell. Endocrinol. 2021, 531, 111310. [Google Scholar] [CrossRef]
- Cao, Q.; Jing, J.; Cui, X.; Shi, H.; Xue, B. Sympathetic Nerve Innervation is Required for Beigeing in White Fat. Physiol. Rep. 2019, 7, e14031. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.N.; Jung, Y.S.; Kwon, H.J.; Seong, J.K.; Granneman, J.G.; Lee, Y.H. Sex Differences in Sympathetic Innervation and Browning of White Adipose Tissue of Mice. Biol. Sex Differ. 2016, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Nayantai, E.; Komatsu, T.; Hayashi, H.; Mori, R.; Shimokawa, I. NPY Deficiency Prevents Postmenopausal Adiposity by Augmenting Estradiol-Mediated Browning. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1042–1049. [Google Scholar] [CrossRef]
- Miao, Y.; Su, W.; Dai, Y.-B.; Wu, W.-F.; Huang, B.; Barros, R.P.A.; Nguyen, H.; Maneix, L.; Guan, Y.-F.; Warner, M.; et al. An ERβ Agonist Induces Browning of Subcutaneous Abdominal Fat Pad in Obese Female Mice. Sci. Rep. 2016, 6, 38579. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, B.; Gomez, N.A.; de Avila, J.M.; Zhu, M.J.; Du, M. Even a Low Dose of Tamoxifen Profoundly Induces Adipose Tissue Browning in Female Mice. Int. J. Obes. 2020, 44, 226–234. [Google Scholar] [CrossRef]
- Servera, M.; Lopez, N.; Serra, F.; Palou, A. Expression of “Brown-in-White” Adipocyte Biomarkers shows Gender Differences and the Influence of Early Dietary Exposure. Genes Nutr. 2014, 9, 372. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Kim, S.H.; Kim, S.N.; Kwon, H.J.; Kim, J.D.; Oh, J.Y.; Jung, Y.S. Sex-Specific Metabolic Interactions between Liver and Adipose Tissue in MCD Diet-Induced Non-Alcoholic Fatty Liver Disease. Oncotarget 2016, 7, 46959–46971. [Google Scholar] [CrossRef] [Green Version]
- Norheim, F.; Hasin-Brumshtein, Y.; Vergnes, L.; Chella Krishnan, K.; Pan, C.; Seldin, M.M.; Hui, S.T.; Mehrabian, M.; Zhou, Z.; Gupta, S.; et al. Gene-by-Sex Interactions in Mitochondrial Functions and Cardio-Metabolic Traits. Cell Metab. 2019, 29, 932–949.e4. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, P.; Shou, Q.; Lu, Y.; Wang, G.; Qiu, J.; Wang, J.; He, L.; Chen, J.; Jiao, J.; Zhang, Y. Arachidonic Acid Sex-Dependently Affects Obesity through Linking Gut Microbiota-Driven Inflammation to Hypothalamus-Adipose-Liver Axis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Asnani-Kishnani, M.; Rodriguez, A.M.; Palou, A.; Ribot, J.; Bonet, M.L. Programming of the Beige Phenotype in White Adipose Tissue of Adult Mice by Mild Resveratrol and Nicotinamide Riboside Supplementations in Early Postnatal Life. Mol. Nutr. Food Res. 2018, 62, e1800463. [Google Scholar] [CrossRef] [PubMed]
- Asnani-Kishnani, M.; Rodríguez, A.M.; Serrano, A.; Palou, A.; Bonet, M.L.; Ribot, J. Neonatal Resveratrol and Nicotinamide Riboside Supplementations Sex-Dependently Affect Beige Transcriptional Programming of Preadipocytes in Mouse Adipose Tissue. Front. Physiol. 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzolini, M.; La Spina, M.; Mattarei, A.; Paradisi, C.; Zoratti, M.; Biasutto, L. Pharmacokinetics and Tissue Distribution of Pterostilbene in the Rat. Mol. Nutr. Food Res. 2014, 58, 2122–2132. [Google Scholar] [CrossRef]
- van den Beukel, J.C.; Grefhorst, A.; Hoogduijn, M.J.; Steenbergen, J.; Mastroberardino, P.G.; Dor, F.J.M.F.; Themmen, A.P.N. Women have More Potential to Induce Browning of Perirenal Adipose Tissue than Men. Obesity 2015, 23, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yu, L. Natural Bioactive Compounds as Potential Browning Agents in White Adipose Tissue. Pharm. Res. 2021, 38, 549–567. [Google Scholar] [CrossRef]

| Author | Animal Model | Experimental Model | Effects | Mechanism of Actions |
|---|---|---|---|---|
| Preclinical Studies | ||||
| Quevedo et al. (1998) [23] | Male and female Wistar rats | Acute exposure (4 °C for 24 h) to rats previously acclimated to 22 °C Chronic acclimation from 22 °C to 28 °C or 18 °C for 7 days | Higher increase in thermogenic capacity in males than in females At 22 °C and 18 °C higher thermogenic capacity in females than in males At 28 °C no differences between sexes | ↑ Ucp1 gene expression ↑ UCP1 protein levels ↑ UCP1 protein levels ↑ activity (GDP binding to mitochondria) |
| Roca et al. (1999) [24] | Male and female Wistar rats | Exposure at 22 °C for 100 days | Higher BAT thermogenic capacity in females than in males | ↑ Mitochondrial proteins ↑ COX activity and UCP1 ↑ Ucp1 and Ucp2 gene expression |
| Harshaw et al. (2014) [25] | Male and female C57BL/6 mice pups | Cooling temperature from 35.4 °C to 22.5 °C | Greater temperature in the interscapular and rump regions in females than in males | Not explained |
| Grefhorst et al. (2015) [26] | Male and female C57Bl/6J mice | Exposure at 23 °C or 4 °C for 24 h | __ | 2.5-fold higher BAT Fgf1 in males than in females 35-fold lower BAT Bmp8b in males than in females |
| Clinical studies in children and adolescents | ||||
| Robinson et al. (2019) [27] | 36 adolescents (16 boys and 20 girls) Age: 8.5–11.8 | Hand immersion in a moderately cold water for 5 min. | Higher temperature in the BAT area in boys than in girls, measured by infrared thermography | Not explained |
| Malpique et al. (2019) [28] | Infants: 39 boys and 47 girls Age: 8.5 years ± 0.1 years BMI: 17.5 ± 0.4 kg/m2, distributed by born small-for-gestational age (SGA) or appropriate-for-gestational age (AGA) | Hand immersion in a moderately cold water (17–18 °C) for 5 min. | In AGA: ↑ BAT activity higher in girls than in boys, measured by infrared thermography. In SGA: no differences between sexes | Not explained |
| Clinical studies in adults | ||||
| Chen et al. (2013) [29] | 14 men and 10 women Age: 28.1 ± 7.3 years BMI: 20.0–27.0 kg/m2 | Exposure at 19 °C after staying at 24 °C for 36 h | Higher energy expenditure in women than in men No differences in BAT activity measured by 18F-FDG PET/CT | Not explained |
| Mengel et al. (2020) [30] | 58 men and 59 women Age: 25.1 ± 3.6 years BMI: 22.3 ± 1.7 kg/m2 | Perfusion of cold water (lowered from 32 °C to shivering threshold) for 120 min. | ↓ Supraclavicular skin temperature only in men, measured by thermosensors in iBAT skin. No differences between sexes in energy expenditure | ↑ T3 levels in men |
| Fletcher et al. (2020) [31] | 12 men and 12 women Age: 18–35 years BMI: 18.5–25.0 kg/m2 | Cold exposure (16 °C) for 5 h | No differences between women and men, with the exception of: ↑ BAT activity in dorsocervical BAT in women vs. men, measured by 18F-FDG uptake (PET) | Not explained |
| Herz et al. (2021) [32] | 95 adults Age: 18–50 years BMI: 20–55 kg/m2 | Perfusion of cold water (to shivering threshold) for 60 min | ↑ Thermogenesis in women vs. men ↓ Thermogenesis during the menstrual cycle | ↑ oestradiol |
| Author | Animal Model | Experimental Model (Diet) | Effects | Mechanism of Actions |
|---|---|---|---|---|
| Overfeeding | ||||
| Roca et al. (1999) [24] | Male and female Wistar rats | Ad libitum feeding with control diet or cafeteria diet for 100 days | Cafeteria diet: Higher BAT thermogenic capacity in males than in females | Cafeteria diet: ↑ Ucp1 and Ucp2 gene expression |
| Rodríguez et al. (2001) [40] Rodriguez et al. (2004) [41] | Male and female Wistar rats | Ad libitum feeding with control diet or cafeteria diet for 15 days | Control diet: Higher BAT thermogenic capacity in females than in males Cafeteria diet: Higher BAT thermogenesis in males than in females | Control diet: ↑ UCP1 protein expression Cafeteria diet: ↓ β3 AR protein expression. ↓ α2A-AR protein expression ↑ Gene expression of Pparɣ2 |
| Choi et al. (2011) [42] | Male and female Sprague–Dawley rats | Ad libitum feeding with control diet or high-fat diet for 8 weeks. | High-fat diet: ↑ Body weight only in males Females vs. males: ↑ Fatty acid β-oxidation ↑ Energy expenditure | ↑ UCP1 protein expression ↑ Oestrogens |
| McCannell et al. (2021) [43] | Male and female C57BL6/N mice | Ad libitum feeding with control diet or high.fat diet for 10 weeks | High fat diet: ↑ BAT mass ↑ sWAT in males High fat diet, females vs. males: ↑ Energy expenditure | ↑ Complex I and II respiration |
| Energy restriction | ||||
| Valle et al. (2005) [44] Valle et al. (2007) [45] | Male and female Wistar rats | Ad libitum feeding or restricted feeding (60% of ad libitum intake) for 100 days. | Ad libitum feeding: ↑ Energy expenditure in females vs. males Restricted diet: Females vs. males ↓ Energy expenditure ↓ BAT mass. | ↑ UCP1 protein expression ↓ UCP1 protein expression ↓ Mitochondrial protein. ↓ Mitochondial DNA content ↓ UCP1, LPL, HSL and TFAM protein expression. ↑ α2A/β3 adrenergic receptor ratio |
| Author | Animal Model | Experimental Model | Effects | Mechanism of Actions |
|---|---|---|---|---|
| Preclinical Studies | ||||
| Valle et al. (2008) [49] | Male and female Wistar rats | Ad libitum feeding maintained under 22 °C and sacrificed at 6, 18 and 24 months of age. | Higher thermogenesis in female than in male | Females vs. males: ↑ UCP1 and COX protein expression ↑ T3 levels |
| Clinical studies in children and adolescents | ||||
| Gilsanz et al. (2012) [47] | Pediatric patients: 38 males and 35 females Age: 4–19.9 years BMI: 25.4–106.4 kg/m2 | BAT presence and activity was measured by 18F-FDG PET/CT. | Lower thermogenesis and BAT depot size during puberty in female than in male | Not explained |
| Clinical studies in adults | ||||
| Yasui et al. (2007) [50] | 154 healthy men Age: 50 to 84 years BMI: 20.7–24.1 kg/m2 and 180 post-menopausal women Age: 51 to 85 years BMI: 23.0–23.8 kg/m2 | Questionnaire-based allotted to “Sensitive to cold” group. | Age was significantly associated with sensitivity to cold only in men | Not explained |
| Pfannenberg et al. (2010) [51] | 124 men and 136 women Age: 11–82 years BMI: 15.5–40.8 kg/m2 | BAT mass and activity was measured by 18F-FDG PET/CT in thermoneutral conditions | Higher BAT thermogenesis in premenopausal women (43–56 years-old) than in men | Not explained |
| Persichetti et al. (2013) [52] | 168 men and 477 women Mean age: 56.25 ± 15.96 years BMI: 25.23 ± 64.73 kg/m2 | BAT presence and activity was measured by 18F-FDG PET/CT scan at 24 °C | Inverse trend between age and BAT mass; and between age and BAT activity | Not explained |
| Author | Animal Model | Experimental Model (Diet) | Effects | Mechanism of Actions |
|---|---|---|---|---|
| Preclinical Studies (Miscelanea) | ||||
| Kim et al. (2016) [55] | Female and male C57BL/6 mice. | Daily intraperitoneally administration of CL316,243 (a β3-adrenergic receptor agonist; 1 mg/kg d) for 5 days. Intraperitoneal injection of 4-vinylcyclohexene (150 mg/kg) for 15 consecutive days in order to create ovarian failure. | Females vs. males: ↑ Browning of gWAT. | ↑ UCP1 and PGC-1α protein expression ↑COX8 protein expression ↑ TH protein expression ↑ NGF and BDNF protein expression ↓TH and UCP1protein expression ↓NGF and BDNF protein expression |
| Seongjoon et al. (2020) [56] | Male and female NPY−/− and NPY+/+ mice | NPY knock-out Letrozole administration (0.02 or 0.1 mg/kg in drinking water) for 4 months to inhibit oestrogen production | Females vs. males: ↑ Thermogenenic capacity in iWAT ↓ Thermogenenic capacity in iWAT | ↑ UCP1 protein expression ↑Ucp1, Cox7a1, Cox8b, Pparα, and Dio2 gene expression ↓ UCP1 protein expression ↓Ucp1, Cox7a1, Cox8b, Pparα, and Dio2 gene expression |
| Miao et al. (2016) [57] | Female and male C57BL/6 mice 3-month-old 1-year-old | Mice treated with LY3201 a ERβ agonist, (0.04 mg/d) for 3 days. | One-year-old females vs. males ↑ Browning in mammary WAT. Three-month-old females vs. males No differences between sexes | ↑ ERβ (immunochemistry) ↑ TH and β3-adrenoceptor (immunochemistry). |
| Zhao et al. (2019) [58] | Female and male C57BL/6 mice | Intraperitoneal injection of tamoxifen, an oestrogen receptor ligand, (25 mg/kg/d) for 3 alternative days. Cold temperature and cold exposure | Room temperature: Females vs. males: ↓ igWAT and gWAT weight in females. ↑ Browning markers in igWAT Cold exposure: Females vs. males: ↑ Browning markers in igWAT and gWAT | ↑ UCP1 protein expression in iWAT ↑ UCP1 and cytochrome C proteins in iWAT and gWAT |
| Preclinical studies (dietary treatments) | ||||
| Servera et al. (2014) [59] | Female and male rats | Standard diet supplemented or not with leucine (2%) while lactation until weaning at 21 days of age. Then, at the age of 6 months high- fat diet or control diet for 3 additional months. | Males vs. females: ↑ Browning capacity | ↑ Cidea, Hoxc9 and Shox2 gene expression. |
| Lee et al. (2016) [60] | Female and male C57BL/6 Mice | Diet deficient in methionine and choline (MCD) for 2 weeks | Females vs. males: ↑Activation of mitochondrial oxidation. ↑ Browning in gWAT. | ↑ UCP1 protein expression ↑ Cox8b, Ucp1, and Elovl3 gene expression ↑ COX8b protein expression ↑ FGF21 protein expression in liver ↑ FGF21 in plasma |
| Norheim et al. (2019) [61] | Female and male inbred and recombinant inbred mouse | High-fat high-sucrose diet for 8 weeks | Females vs. males ↑ Browning of gWAT. | ↑ UCP1 protein expression |
| Zhuang et al. (2017) [62] | Female and male C57BL/6J mice | High-fat diet for 10 weeks, and arachidonic acid (AA; 10g/kg) for 15 additional weeks. | No differences between sexes | |
| Preclinical studies (bioactive compounds). | ||||
| Serrano et al. (2018) [63] | NMRI female and male mice | RSV (2 mg/kg) supplementation from day 2 to 20 (orally). On day 90, half the animals were assigned to a high-fat diet for 10 additional weeks. | Males vs. females ↑ Browning of iWAT. | ↑ Prdm16, Pgc-1a, Pgc-1b, Hoxc9, Slc27a1 and Pparɣ gene expression. |
| Asnani-Kishnani et al. (2019) [64] | NMRI female and male mice Primary cultures of iWAT from mice treated with RSV | RSV included in a standard diet 2 mg/kg body weight/day) for 18 days. | Males vs. females ↓ Browning markers in iWAT. | ↓ Ucp2 gene expression in males. ↑ Ucp1 gene expression in iWAT adipocytes from males ↓ Ucp1 gene expression in iWAT adipocytes from females |
| La Spina et al. (2019) [65] | Female and male C57BL/6 mice | PT included in a high-fat diet (352 mol/kg body weight/day) for 30 weeks. | Females vs. males ↑ Browing in iWAT | ↑ UCP1 protein expression |
| Cold Influence | ||
| Preclinical studies | Females vs. males: ↑ Thermogenic capacity [23,24,25,26] | Both in humans and animals, females seem to be more sensitive to cold |
| Clinical studies (adolescents) | Controversial [27,28] | |
| Clinical studies (adults) | Women vs. men: ↑ Thermogenic capacity [29,30,31,32] | |
| Diet Influence | ||
| Cafeteria diet | Females vs. males: ↓ Thermogenic capacity [24,40,41] | Thermogenic capacity induction could depend on the dietary intervention |
| High-fat diet | Females vs. males: ↑ Thermogenic capacity [42,43] | |
| Energy restriction | Females vs. males: ↓ Thermogenic capacity [44,45] | |
| Age Influence | ||
| Preclinical studies | Females vs. males: ↑ Thermogenic capacity [49] | With the exception of adolescents, females seem to be more prone to activate thermogenesis than males |
| Clinical studies (adolescents) | Boys vs. girls: ↑ Thermogenic capacity [47] | |
| Clinical studies (adults) | Women vs. men: ↑ Thermogenic capacity [50,51,52] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-García, I.; Trepiana, J.; Fernández-Quintela, A.; Giralt, M.; Portillo, M.P. Sexual Dimorphism in Brown Adipose Tissue Activation and White Adipose Tissue Browning. Int. J. Mol. Sci. 2022, 23, 8250. https://doi.org/10.3390/ijms23158250
Gómez-García I, Trepiana J, Fernández-Quintela A, Giralt M, Portillo MP. Sexual Dimorphism in Brown Adipose Tissue Activation and White Adipose Tissue Browning. International Journal of Molecular Sciences. 2022; 23(15):8250. https://doi.org/10.3390/ijms23158250
Chicago/Turabian StyleGómez-García, Iker, Jenifer Trepiana, Alfredo Fernández-Quintela, Marta Giralt, and María P. Portillo. 2022. "Sexual Dimorphism in Brown Adipose Tissue Activation and White Adipose Tissue Browning" International Journal of Molecular Sciences 23, no. 15: 8250. https://doi.org/10.3390/ijms23158250
APA StyleGómez-García, I., Trepiana, J., Fernández-Quintela, A., Giralt, M., & Portillo, M. P. (2022). Sexual Dimorphism in Brown Adipose Tissue Activation and White Adipose Tissue Browning. International Journal of Molecular Sciences, 23(15), 8250. https://doi.org/10.3390/ijms23158250