Lysophosphatidylcholine: Potential Target for the Treatment of Chronic Pain
Abstract
:1. Introduction
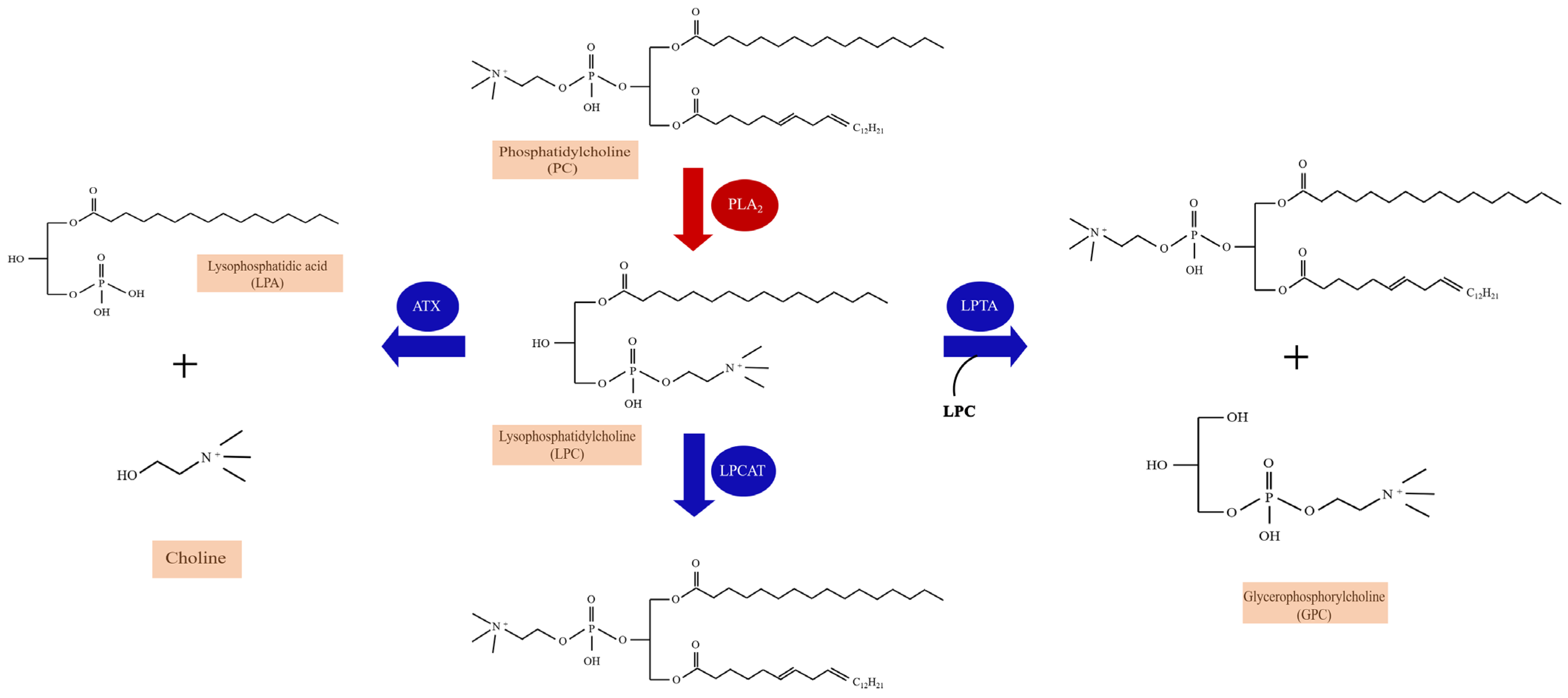
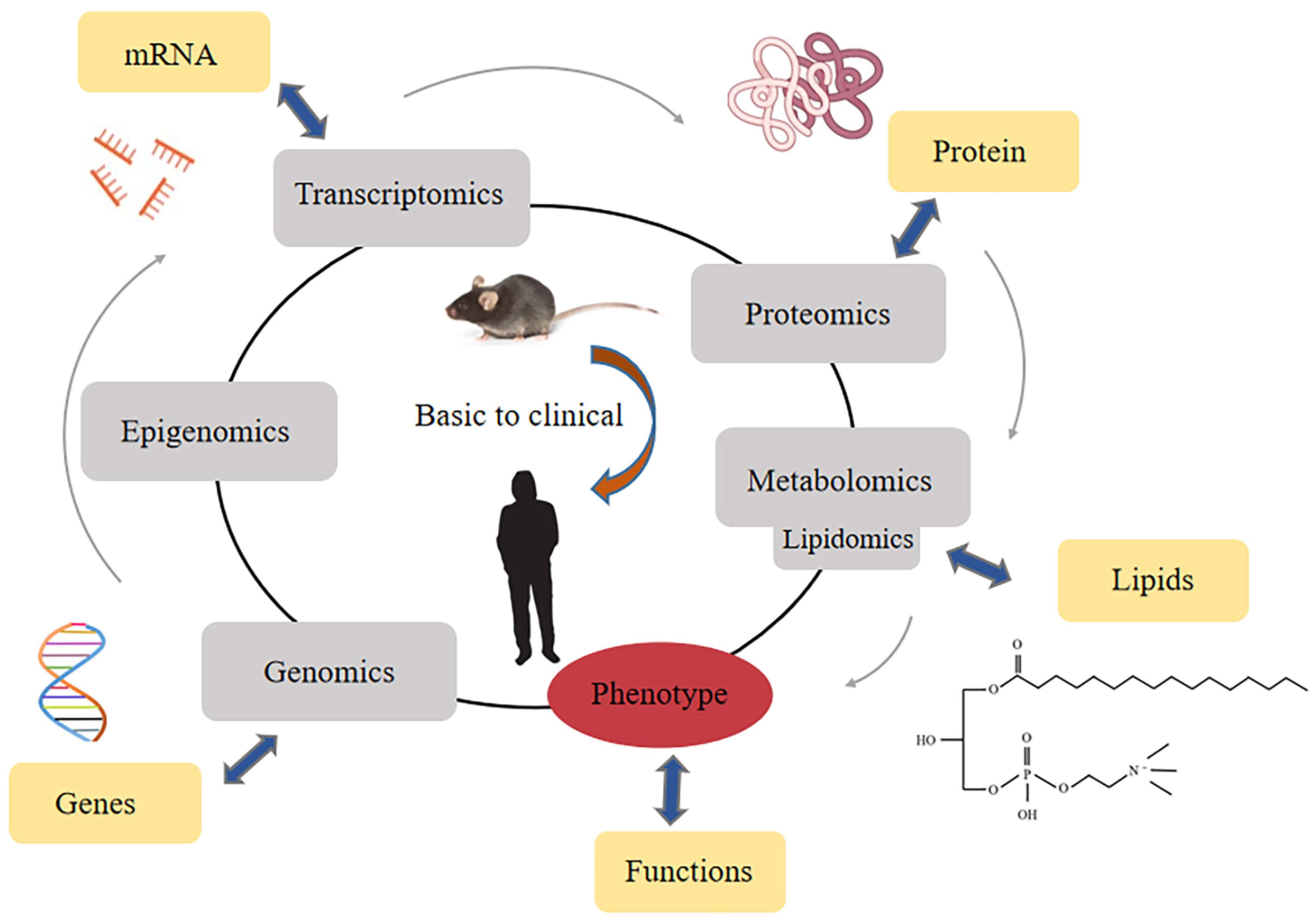
2. Lysophosphatidylcholine (LPC)
2.1. The Metabolism and Species of LPC
2.2. Detection Methods of LPC
2.2.1. NMR Spectroscopy
2.2.2. LC-MS
2.2.3. GC-MS
2.2.4. HPLC/UPLC
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| NMR spectroscopy | Great range of detectable molecular species; Simple sample preparation; Excellent reproducibility; High automation | Low sensitivity; Quantification of relatively high concentrations of metabolites/extensive | [57,58] |
| LC-MS | High sensitivity; Small sample volumes; Relatively low costs; Superior resolution | Matrix effects and ion suppression by co-eluting compounds; Limitation of detectable metabolites | [63,64,65,66,67] |
| GC-MS | High chromatographic resolution; Large databases of identified peaks; High sensitive; High throughput | A large number of unidentified peaks; Require additional analytical steps; Separate and identify low molecular weight | [70,71,72,73] |
| HPLC | Robustness; Convenience; Good selectivity; High sensitivity | Low throughput; Inability to observe non-electrochemically active species; Difficulties of metabolite identification; Lack of high efficiency | [75,76,77,78] |
| UPLC | Short analysis time; Improved peak efficiency; Better resolution; Decreased use of solvents | Less time life of columns | [79] |
| MALDI-MS | Suitability for solid samples; High sensitivity; Easy sample handling; Salt tolerance; High speed | Limitation of detectable metabolites | [43] |
2.2.5. MALDI Mass Spectrometry
3. Lysophosphatidylcholine and Chronic Pain Diseases
3.1. Inflammatory Pain
3.2. Chronic Joint Pain
3.3. Fibromyalgia and Multisite Musculoskeletal Pain (MSMP)
| Year | Author | Disease | Samples | Method | Observations | References |
|---|---|---|---|---|---|---|
| 2021 | Katelyn E Sadler et al. | CFA-induced inflammatory pain; skin incision-induced pain; chemotherapy-induced peripheral neuropathic pain | Mice hindpaw skin | LC-MS | CFA induced inflammatory pain, skin incision, and chemotherapy-induced peripheral neuropathy, all of which were characterized by elevated concentrations of LPC. | [81] |
| 2022 | Florian Jacquot et al. | Chronic joint pain | Synovial fluids from 50 patients (32 women and 18 men) | HDMS | The synovial fluid levels of LPC were significantly elevated, especially the LPC (16:0) species, compared with postmortem control subjects. | [82] |
| 2021 | Alexandra Jurczak et al. | B02/B09-induced pain | Bone marrow extracts of B02/B09-treated mice | HDMS | LPC (16:0) was the most abundant and significantly increased in the B02/B09 group compared with control. | [83] |
| 2020 | Chih-Hsien Hung et al. | Fibromyalgia | Serum from RISS mice; plasma from 31 fibromyalgia patients and 30 healthy controls | Untargeted lipidomic analysis/QqQ MS | LPC (16:0) in fibromyalgia mouse and patients were upregulated. | [16] |
| 2019 | Wei-Hsiang Hsu et al. | Fibromyalgia | Mice serum | 1H NMR and LC-MS | Impactful metabolites in the FM model including LPC (16:0), LPC (20:3) in serum. | [50] |
| 2014 | Pierluigi Caboni et al. | Fibromyalgia | Plasma from 22 females FM patients and 21 controls | LC-MS | Plasma of FM patients identified many lipid compounds, mainly including LPC. | [89] |
| 2021 | Ming Liu et al. | Multisite musculoskeletal pain (MSMP) | Plasma of 122 non-MSMP and 83 MSMP patients | Biocrates AbsoluteIDQ p180 kit | LPC (26:0) and LPC (28:1) are associated with MSMP. | [38] |
| 2021 | Baasanjav Uranbileg et al. | Cauda equina compression | CSF and plasma from CEC rats; CSF from lumbar spinal canal stenosis patients and controls | LC-MS/MS; UHPLC-MS/MS | Lots of LPC species were significantly increased, especially LPC (16:0), LPC (18:2), LPC (20:4). | [39] |
| 2020 | Vittoria Rimola et al. | Oxaliplatin-induced Peripheral Pain | Mice sciatic nerve, DRG, dorsal spinal cord | LC-MS/MS | LPC (18:1) and LPC (16:0) were significantly increased after oxaliplatin treatment. | [29] |
| 2011 | Jun Nagai et al. | Partial sciatic nerve injury (SCNI) | Mice spinal cord and dorsal root | NALDI-MS | The levels of LPC (16:0), LPC (18:0) and LPC (18:1) were increased after SCNI. | [84] |
3.4. Neuropathic Pain
3.5. The Enzymatic Pathways of Lysophosphatidylcholine (LPC) and Chronic Pain
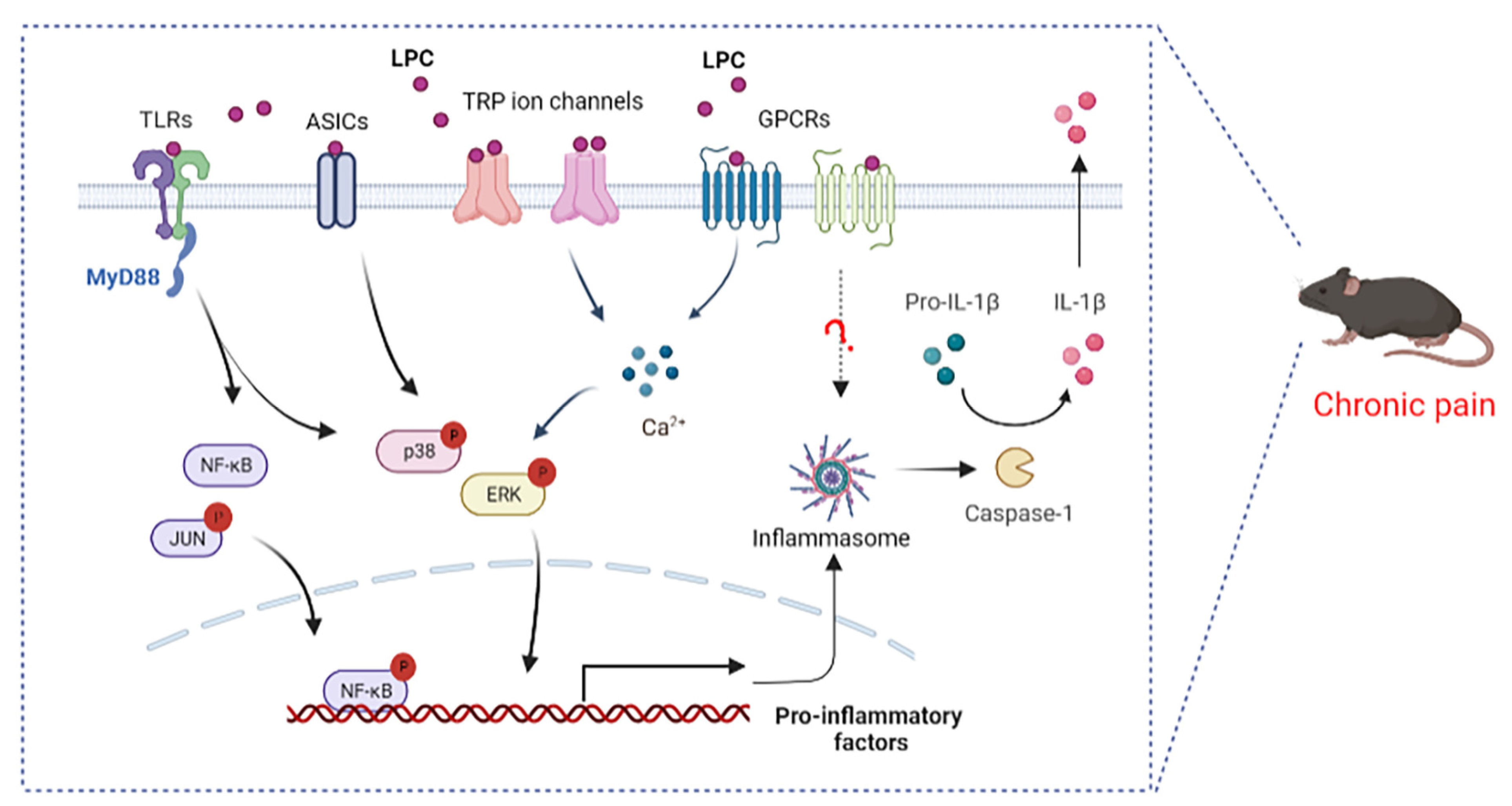
4. LPC-Related Receptor and Chronic Pain
4.1. LPC and G Protein Coupled Receptors
4.2. LPC and Toll-like Receptors
4.3. LPC and Ion Channels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Reckziegel, D.; Vachon-Presseau, E.; Petre, B.; Schnitzer, T.J.; Baliki, M.N.; Apkarian, A.V. Deconstructing biomarkers for chronic pain: Context- and hypothesis-dependent biomarker types in relation to chronic pain. Pain 2019, 160 (Suppl. 1), S37–S48. [Google Scholar] [CrossRef]
- Edwards, R.R.; Dworkin, R.H.; Sullivan, M.D.; Turk, D.C.; Wasan, A.D. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J. Pain 2016, 17, T70–T92. [Google Scholar] [CrossRef] [Green Version]
- Barroso, J.; Branco, P.; Apkarian, A.V. Brain mechanisms of chronic pain: Critical role of translational approach. Transl. Res. 2021, 238, 76–89. [Google Scholar] [CrossRef]
- McWilliams, D.F.; Walsh, D.A. Pain mechanisms in rheumatoid arthritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 94–101. [Google Scholar]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef]
- Gebhart, G.F.; Bielefeldt, K. Physiology of Visceral Pain. Compr. Physiol. 2016, 6, 1609–1633. [Google Scholar] [PubMed]
- Christo, P.J.; Mazloomdoost, D. Cancer pain and analgesia. Ann. N. Y. Acad. Sci. 2008, 1138, 278–298. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Averitt, D.L.; Maier, C.; Basu, A. The Effects of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (Nrf2) Activation in Preclinical Models of Peripheral Neuropathic Pain. Antioxidants 2022, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Cristescu, S.M.; Risby, T.H.; Marczin, N. Lipid peroxidation in cardiac surgery: Towards consensus on biomonitoring, diagnostic tools and therapeutic implementation. J. Breath Res. 2018, 12, 027109. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Ma, S.; Zhang, C.; Sun, J.; Zhang, D.; Chang, S.; Lin, Y.; Zhao, G. Higenamine Attenuates Neuropathic Pain by Inhibition of NOX2/ROS/TRP/P38 Mitogen-Activated Protein Kinase/NF-kB Signaling Pathway. Front. Pharmacol. 2021, 12, 716684. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Sudirman, S.; Yen, Y.W.; Mao, C.F.; Ong, A.D.; Kong, Z.L. Blue Mussel (Mytilus edulis) Water Extract Ameliorates Inflammatory Responses and Oxidative Stress on Osteoarthritis in Obese Rats. J. Pain Res. 2020, 13, 1109–1119. [Google Scholar] [CrossRef]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells. Exp. Neurol. 2012, 233, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Hassler, S.N.; Johnson, K.M.; Hulsebosch, C.E. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J. Neurochem. 2014, 131, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.H.; Lee, C.H.; Tsai, M.H.; Chen, C.H.; Lin, H.F.; Hsu, C.Y.; Lai, C.L.; Chen, C.C. Activation of acid-sensing ion channel 3 by lysophosphatidylcholine 16:0 mediates psychological stress-induced fibromyalgia-like pain. Ann. Rheum. Dis. 2020, 79, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.X.; Zhu, H.Y.; Hu, Y.H. Effects of lysophosphatidylcholine on beta-amyloid-induced neuronal apoptosis. Acta Pharmacol. Sin. 2009, 30, 388–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellenthal, K.E.M.; Brabenec, L.; Gross, E.R.; Wagner, N.M. TRP Channels as Sensors of Aldehyde and Oxidative Stress. Biomolecules 2021, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Nikolaou, A.; Kokotou, M.G.; Vasilakaki, S.; Kokotos, G. Small-molecule inhibitors as potential therapeutics and as tools to understand the role of phospholipases A2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 941–956. [Google Scholar] [CrossRef]
- Jianyong, Z.; Yanruo, H.; Xiaoju, T.; Yiping, W.; Fengming, L. Roles of Lipid Profiles in Human Non-Small Cell Lung Cancer. Technol. Cancer Res. Treat. 2021, 20, 15330338211041472. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Maity, S.; Patel, J.; Lupo, P.J.; Nembhard, W.N. Metabolomics Signatures and Subsequent Maternal Health among Mothers with a Congenital Heart Defect-Affected Pregnancy. Metabolites 2022, 12, 100. [Google Scholar] [CrossRef]
- Chiarini, A.; Armato, U.; Hu, P.; Dal Pra, I. Danger-Sensing/Patten Recognition Receptors and Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 9036. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.R.; Broste, S.K.; Frantz, R.P.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ 2016, 353, i1246. [Google Scholar] [CrossRef] [Green Version]
- Sasso, O.; Wagner, K.; Morisseau, C.; Inceoglu, B.; Hammock, B.D.; Piomelli, D. Peripheral FAAH and soluble epoxide hydrolase inhibitors are synergistically antinociceptive. Pharmacol. Res. 2015, 97, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Velasco, M.; O’Sullivan, C.; Sheridan, G.K. Lysophosphatidic acid receptors (LPARs): Potential targets for the treatment of neuropathic pain. Neuropharmacology 2017, 113, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Subbaiah, P.V.; Holian, O.; Zhang, J.; Johnson, A.; Gertzberg, N.; Lum, H. Lysophosphatidylcholine increases endothelial permeability: Role of PKCalpha and RhoA cross talk. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L176–L185. [Google Scholar] [CrossRef] [Green Version]
- Kabarowski, J.H.; Xu, Y.; Witte, O.N. Lysophosphatidylcholine as a ligand for immunoregulation. Biochem. Pharmacol. 2002, 64, 161–167. [Google Scholar] [CrossRef]
- Rimola, V.; Hahnefeld, L.; Zhao, J.; Jiang, C.; Angioni, C.; Schreiber, Y.; Osthues, T.; Pierre, S.; Geisslinger, G.; Ji, R.R.; et al. Lysophospholipids Contribute to Oxaliplatin-Induced Acute Peripheral Pain. J. Neurosci. 2020, 40, 9519–9532. [Google Scholar] [CrossRef]
- Vickers, K.C.; Castro-Chavez, F.; Morrisett, J.D. Lyso-phosphatidylcholine induces osteogenic gene expression and phenotype in vascular smooth muscle cells. Atherosclerosis 2010, 211, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Barbayianni, E.; Magrioti, V.; Moutevelis-Minakakis, P.; Kokotos, G. Autotaxin inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, K.M.; Schiller, J.; Galuska, C.E.; Fuchs, B. Phospholipases and Reactive Oxygen Species Derived Lipid Biomarkers in Healthy and Diseased Humans and Animals—A Focus on Lysophosphatidylcholine. Front. Physiol. 2021, 12, 732319. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saw, W.Y.; Tantoso, E.; Begum, H.; Zhou, L.; Zou, R.; He, C.; Chan, S.L.; Tan, L.W.; Wong, L.P.; Xu, W.; et al. Establishing multiple omics baselines for three Southeast Asian populations in the Singapore Integrative Omics Study. Nat. Commun. 2017, 8, 653. [Google Scholar] [CrossRef]
- Cao, B.; Wang, D.; Pan, Z.; McIntyre, R.S.; Brietzke, E.; Subramanieapillai, M.; Nozari, Y.; Wang, J. Metabolic profiling for water-soluble metabolites in patients with schizophrenia and healthy controls in a Chinese population: A case-control study. World J. Biol. Psychiatry 2020, 21, 357–367. [Google Scholar] [CrossRef]
- Bergqvist, F.; Ossipova, E.; Idborg, H.; Raouf, J.; Checa, A.; Englund, K.; Englund, P.; Khoonsari, P.E.; Kultima, K.; Wheelock, C.E.; et al. Inhibition of mPGES-1 or COX-2 Results in Different Proteomic and Lipidomic Profiles in A549 Lung Cancer Cells. Front. Pharmacol. 2019, 10, 636. [Google Scholar]
- Liu, M.; Xie, Z.; Costello, C.A.; Zhang, W.; Chen, L.; Qi, D.; Furey, A.; Randell, E.W.; Rahman, P.; Zhai, G. Metabolomic analysis coupled with extreme phenotype sampling identified that lysophosphatidylcholines are associated with multisite musculoskeletal pain. Pain 2021, 162, 600–608. [Google Scholar] [CrossRef]
- Uranbileg, B.; Ito, N.; Kurano, M.; Saigusa, D.; Saito, R.; Uruno, A.; Kano, K.; Ikeda, H.; Yamada, Y.; Sumitani, M.; et al. Alteration of the lysophosphatidic acid and its precursor lysophosphatidylcholine levels in spinal cord stenosis: A study using a rat cauda equina compression model. Sci. Rep. 2019, 9, 16578. [Google Scholar]
- Rohnisch, H.E.; Kyro, C.; Olsen, A.; Thysell, E.; Hallmans, G.; Moazzami, A.A. Identification of metabolites associated with prostate cancer risk: A nested case-control study with long follow-up in the Northern Sweden Health and Disease Study. BMC Med. 2020, 18, 187. [Google Scholar] [CrossRef]
- Gessner, D.K.; Winkler, A.; Koch, C.; Dusel, G.; Liebisch, G.; Ringseis, R.; Eder, K. Analysis of hepatic transcript profile and plasma lipid profile in early lactating dairy cows fed grape seed and grape marc meal extract. BMC Genom. 2017, 18, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, M.; Fu, T.; Li, Z.; Chen, Y.; He, T.; Feng, D.; Wang, Z.; Fan, Q.; Chen, M.; et al. Lipidomics Indicates the Hepatotoxicity Effects of EtOAc Extract of Rhizoma Paridis. Front. Pharmacol. 2022, 13, 799512. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bagarolo, G.I.; Thoroe-Boveleth, S.; Jankowski, J. “Lipidomics”: Mass spectrometric and chemometric analyses of lipids. Adv. Drug Deliv. Rev. 2020, 159, 294–307. [Google Scholar] [CrossRef]
- Shah, S.H.; Kraus, W.E.; Newgard, C.B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: Form and function. Circulation 2012, 126, 1110–1120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern analytical techniques in metabolomics analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Malatji, B.G.; Meyer, H.; Mason, S.; Engelke, U.F.H.; Wevers, R.A.; van Reenen, M.; Reinecke, C.J. A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Mickiewicz, B.; Kelly, J.J.; Ludwig, T.E.; Weljie, A.M.; Wiley, J.P.; Schmidt, T.A.; Vogel, H.J. Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. J. Orthop. Res. 2015, 33, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A.; van der Plas, A.A.; van Dasselaar, N.T.; Deelder, A.M.; van Hilten, J.J.; Mayboroda, O.A. 1H-NMR metabolic profiling of cerebrospinal fluid in patients with complex regional pain syndrome-related dystonia. Pain 2014, 155, 190–196. [Google Scholar] [CrossRef]
- Kim, J.W.; Ryu, S.H.; Kim, S.; Lee, H.W.; Lim, M.S.; Seong, S.J.; Kim, S.; Yoon, Y.R.; Kim, K.B. Pattern recognition analysis for hepatotoxicity induced by acetaminophen using plasma and urinary 1H NMR-based metabolomics in humans. Anal. Chem. 2013, 85, 11326–11334. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, C.H.; Chao, Y.M.; Kuo, C.H.; Ku, W.C.; Chen, C.C.; Lin, Y.L. ASIC3-dependent metabolomics profiling of serum and urine in a mouse model of fibromyalgia. Sci. Rep. 2019, 9, 12123. [Google Scholar] [CrossRef]
- Ciborowski, M.; Lipska, A.; Godzien, J.; Ferrarini, A.; Korsak, J.; Radziwon, P.; Tomasiak, M.; Barbas, C. Combination of LC-MS- and GC-MS-based metabolomics to study the effect of ozonated autohemotherapy on human blood. J. Proteome. Res. 2012, 11, 6231–6241. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malatji, B.G.; Mason, S.; Mienie, L.J.; Wevers, R.A.; Meyer, H.; van Reenen, M.; Reinecke, C.J. The GC-MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics 2019, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Wang, M.; Shi, W.; Du, X.; Sun, C. The toxicity of 3-chloropropane-1,2-dipalmitate in Wistar rats and a metabonomics analysis of rat urine by ultra-performance liquid chromatography-mass spectrometry. Chem. Biol. Interact. 2013, 206, 337–345. [Google Scholar] [CrossRef]
- Lan, K.; Zhang, Y.; Yang, J.; Xu, L. Simple quality assessment approach for herbal extracts using high performance liquid chromatography-UV based metabolomics platform. J. Chromatogr. A 2010, 1217, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Takahashi, M.; Sugiura, Y.; Izumi, Y.; Nishiyama, K.; Nishida, M.; Suematsu, M.; Bamba, T.; Yamada, K.I. Structural library and visualization of endogenously oxidized phosphatidylcholines using mass spectrometry-based techniques. Nat. Commun. 2021, 12, 6339. [Google Scholar] [CrossRef]
- Goodarzi, P.; Alavi-Moghadam, S.; Payab, M.; Larijani, B.; Rahim, F.; Gilany, K.; Bana, N.; Tayanloo-Beik, A.; Foroughi Heravani, N.; Hadavandkhani, M.; et al. Metabolomics Analysis of Mesenchymal Stem Cells. Int. J. Mol. Cell. Med. 2019, 8, 30–40. [Google Scholar] [PubMed]
- Guennec, A.L.; Giraudeau, P.; Caldarelli, S. Evaluation of fast 2D NMR for metabolomics. Anal. Chem. 2014, 86, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Wagen, C.C.; Ingoglia, B.T.; Buchwald, S.L. Unexpected Formation of Hexasubstituted Arenes through a 2-fold Palladium-Mediated Ligand Arylation. J. Org. Chem. 2019, 84, 12672–12679. [Google Scholar] [CrossRef] [PubMed]
- Kostara, C.E.; Ferrannini, E.; Bairaktari, E.T.; Papathanasiou, A.; Elisaf, M.; Tsimihodimos, V. Early Signs of Atherogenic Features in the HDL Lipidomes of Normolipidemic Patients Newly Diagnosed with Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 8835. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. Biosyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Loos, G.; Van Schepdael, A.; Cabooter, D. Quantitative mass spectrometry methods for pharmaceutical analysis. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2016, 374, 20150366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.S.; Shearer, J. Metabolomics and Type 2 Diabetes: Translating Basic Research into Clinical Application. J. Diabetes Res. 2016, 2016, 3898502. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Wu, Y.Q.; Xu, Y.S.; Wang, K.X.; Qin, X.M.; Lu, Y.F. LC-MS-Based Metabolomic Study of Oleanolic Acid-Induced Hepatotoxicity in Mice. Front. Pharmacol. 2020, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Gadepalli, S.G.; Deme, P.; Kuncha, M.; Sistla, R. Simultaneous determination of amlodipine, valsartan and hydrochlorothiazide by LC-ESI-MS/MS and its application to pharmacokinetics in rats. J. Pharm. Anal. 2014, 4, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostic, N.; Dotsikas, Y.; Jovic, N.; Stevanovic, G.; Malenovic, A.; Medenica, M. Quantitation of pregabalin in dried blood spots and dried plasma spots by validated LC-MS/MS methods. J. Pharm. Biomed. Anal. 2015, 109, 79–84. [Google Scholar] [CrossRef] [PubMed]
- de Meulder, M.; Waldron, M.P.; Li, L.; Peay, M.G.; Tingler, M.J.; Hidy, B.J.; Verhaeghe, T.; Jenkins, R.G. Development and validation of HILIC-ESI/MS/MS methods for simultaneous quantitation of several antipsychotics in human plasma and blood. Bioanalysis 2016, 8, 765–794. [Google Scholar] [CrossRef] [PubMed]
- Gakuubi, M.M.; Wagacha, J.M.; Dossaji, S.F.; Wanzala, W. Chemical Composition and Antibacterial Activity of Essential Oils of Tagetes minuta (Asteraceae) against Selected Plant Pathogenic Bacteria. Int. J. Microbiol. 2016, 2016, 7352509. [Google Scholar] [CrossRef] [Green Version]
- Zarate, E.; Boyle, V.; Rupprecht, U.; Green, S.; Villas-Boas, S.G.; Baker, P.; Pinu, F.R. Fully Automated Trimethylsilyl (TMS) Derivatisation Protocol for Metabolite Profiling by GC-MS. Metabolites 2016, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Kanani, H.; Chrysanthopoulos, P.K.; Klapa, M.I. Standardizing GC-MS metabolomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 871, 191–201. [Google Scholar] [CrossRef]
- Spagou, K.; Theodoridis, G.; Wilson, I.; Raikos, N.; Greaves, P.; Edwards, R.; Nolan, B.; Klapa, M.I. A GC-MS metabolic profiling study of plasma samples from mice on low- and high-fat diets. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.A.; Djukovic, D. Overview of mass spectrometry-based metabolomics: Opportunities and challenges. Methods Mol. Biol. 2014, 1198, 3–12. [Google Scholar] [PubMed] [Green Version]
- Mastrangelo, A.; Ferrarini, A.; Rey-Stolle, F.; Garcia, A.; Barbas, C. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 2015, 900, 21–35. [Google Scholar] [CrossRef]
- Lynch, K.B.; Chen, A.; Liu, S. Miniaturized high-performance liquid chromatography instrumentation. Talanta 2018, 177, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L. HPLC in natural product analysis: The detection issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Nahar, L.; Onder, A.; Sarker, S.D. A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010–2019). Phytochem. Anal. 2020, 31, 413–457. [Google Scholar] [CrossRef]
- Vigneau-Callahan, K.E.; Shestopalov, A.I.; Milbury, P.E.; Matson, W.R.; Kristal, B.S. Characterization of diet-dependent metabolic serotypes: Analytical and biological variability issues in rats. J. Nutr. 2001, 131, 924S–932S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Villiers, A.; Lestremau, F.; Szucs, R.; Gelebart, S.; David, F.; Sandra, P. Evaluation of ultra performance liquid chromatography. Part, I. Possibilities and limitations. J. Chromatogr. A 2006, 1127, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, I.; Gliszczynska-Swiglo, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef]
- Zaima, N.; Hayasaka, T.; Goto-Inoue, N.; Setou, M. Matrix-assisted laser desorption/ionization imaging mass spectrometry. Int. J. Mol. Sci. 2010, 11, 5040–5055. [Google Scholar] [CrossRef] [Green Version]
- Sadler, K.E.; Moehring, F.; Shiers, S.I.; Laskowski, L.J.; Mikesell, A.R.; Plautz, Z.R.; Brezinski, A.N.; Mecca, C.M.; Dussor, G.; Price, T.J.; et al. Transient receptor potential canonical 5 mediates inflammatory mechanical and spontaneous pain in mice. Sci. Transl. Med. 2021, 13, eabd7702. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, F.; Khoury, S.; Labrum, B.; Delanoe, K.; Pidoux, L.; Barbier, J.; Delay, L.; Bayle, A.; Aissouni, Y.; Barriere, D.A.; et al. Lysophosphatidylcholine 16: 0 mediates chronic joint pain associated to rheumatic diseases through acid-sensing ion channel 3. Pain 2022. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, A.; Delay, L.; Barbier, J.; Simon, N.; Krock, E.; Sandor, K.; Agalave, N.M.; Rudjito, R.; Wigerblad, G.; Rogoz, K.; et al. Antibody-induced pain-like behavior and bone erosion: Links to subclinical inflammation, osteoclast activity, and acid-sensing ion channel 3-dependent sensitization. Pain 2021, 163, 1542–1559. [Google Scholar] [CrossRef]
- Nagai, J.; Ueda, H. Pre-emptive morphine treatment abolishes nerve injury-induced lysophospholipid synthesis in mass spectrometrical analysis. J. Neurochem. 2011, 118, 256–265. [Google Scholar] [CrossRef]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical Assessment of Inflammatory Pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andres-Marin, N.; Fernandez-Eulate, G.; Abecia, L.; Lavin, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. Ebiomedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caboni, P.; Liori, B.; Kumar, A.; Santoru, M.L.; Asthana, S.; Pieroni, E.; Fais, A.; Era, B.; Cacace, E.; Ruggiero, V.; et al. Metabolomics analysis and modeling suggest a lysophosphocholines-PAF receptor interaction in fibromyalgia. PLoS ONE 2014, 9, e107626. [Google Scholar]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Kuffler, D.P. Injury-Induced Effectors of Neuropathic Pain. Mol. Neurobiol. 2020, 57, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Osthues, T.; Sisignano, M. Oxidized Lipids in Persistent Pain States. Front. Pharmacol. 2019, 10, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.Y.; Tsai, Y.J.; Chen, S.H.; Lin, C.T.; Lue, J.H. Lysophosphatidylcholine causes neuropathic pain via the increase of neuronal nitric oxide synthase in the dorsal root ganglion and cuneate nucleus. Pharmacol. Biochem. Behav. 2013, 106, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Chen, S.H.; Chang, C.F.; Lin, S.C.; Lue, J.H.; Tsai, Y.J. Melatonin reduces neuropathic pain behavior and glial activation through MT2 melatonin receptor modulation in a rat model of lysophosphatidylcholine-induced demyelination neuropathy. Neurochem. Int. 2020, 140, 104827. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Tanaka, H.; Sayanagi, J.; Iwahashi, T.; Suzuki, K.; Nishimoto, S.; Okada, K.; Murase, T.; Yoshikawa, H. Neurotropin((R)) Accelerates the Differentiation of Schwann Cells and Remyelination in a Rat Lysophosphatidylcholine-Induced Demyelination Model. Int. J. Mol. Sci. 2018, 19, 516. [Google Scholar] [CrossRef] [Green Version]
- Wallace, V.C.; Cottrell, D.F.; Brophy, P.J.; Fleetwood-Walker, S.M. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J. Neurosci. 2003, 23, 3221–3233. [Google Scholar] [CrossRef] [PubMed]
- Ousman, S.S.; David, S. MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J. Neurosci. 2001, 21, 4649–4656. [Google Scholar] [CrossRef]
- Serizawa, K.; Tomizawa-Shinohara, H.; Miyake, S.; Yogo, K.; Matsumoto, Y. Interleukin-6: Evolving role in the management of neuropathic pain in neuroimmunological disorders. Inflamm. Regen. 2021, 41, 34. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Li, Z.; Li, Y.; Yu, Y.; Zhang, L. The Involvement of Caspases in Neuroinflammation and Neuronal Apoptosis in Chronic Pain and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 898574. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, Y.H.; Lee, Y.; Jung, S.J.; Oh, S.B. TRPM2 contributes to LPC-induced intracellular Ca(2+) influx and microglial activation. Biochem. Biophys. Res. Commun. 2017, 485, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Ousman, S.S.; David, S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia 2000, 30, 92–104. [Google Scholar] [CrossRef]
- el Waly, B.; Buttigieg, E.; Karakus, C.; Brustlein, S.; Debarbieux, F. Longitudinal Intravital Microscopy Reveals Axon Degeneration Concomitant With Inflammatory Cell Infiltration in an LPC Model of Demyelination. Front. Cell. Neurosci. 2020, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, N.; Jeong, S.Y.; Lacroix, S.; David, S. T cells contribute to lysophosphatidylcholine-induced macrophage activation and demyelination in the CNS. Glia 2007, 55, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Xie, W.; Matsushita, Y.; Chun, J.; Aoki, J.; Ueda, H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience 2008, 152, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.L.; Yeo, M.; Zhang, Q.J.; Lopez-Romero, A.E.; Ding, H.P.; Zhang, X.; Zeng, Q.; Morales-Lazaro, S.L.; Moore, C.; et al. Epithelia-Sensory Neuron Cross Talk Underlies Cholestatic Itch Induced by Lysophosphatidylcholine. Gastroenterology 2021, 161, 301–317 e316. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Morioka, N.; Abdin, J.; Kitayama, S.; Nakata, Y.; Dohi, T. Development of tactile allodynia and thermal hyperalgesia by intrathecally administered platelet-activating factor in mice. Pain 2004, 111, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.; Chun, J.; Ueda, H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, K.; Swearingen, C.A.; Oskins, J.L.; Lin, C.; Bui, H.H.; Jones, S.B.; Pfeifer, L.A.; Norman, B.H.; Mitchell, P.G.; Chambers, M.G. Identification and pharmacological characterization of a novel inhibitor of autotaxin in rodent models of joint pain. Osteoarthr. Cartil. 2017, 25, 935–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uranbileg, B.; Ito, N.; Kurano, M.; Kano, K.; Uchida, K.; Sumitani, M.; Aoki, J.; Yatomi, Y. Inhibition of autotaxin activity ameliorates neuropathic pain derived from lumbar spinal canal stenosis. Sci. Rep. 2021, 11, 3984. [Google Scholar] [CrossRef]
- Nagai, J.; Uchida, H.; Matsushita, Y.; Yano, R.; Ueda, M.; Niwa, M.; Aoki, J.; Chun, J.; Ueda, H. Autotaxin and lysophosphatidic acid1 receptor-mediated demyelination of dorsal root fibers by sciatic nerve injury and intrathecal lysophosphatidylcholine. Mol. Pain 2010, 6, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herr, D.R.; Chew, W.S.; Satish, R.L.; Ong, W.Y. Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases. Mol. Neurobiol. 2020, 57, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.; Nakajima, S.; Gotoh, M.; Tanaka, S.; Murofushi, H.; Murakami-Murofushi, K. Qualitative and quantitative comparison of cyclic phosphatidic acid and its related lipid species in rat serum using hydrophilic interaction liquid chromatography with tandem-mass spectrometry. J. Chromatogr. A 2018, 1567, 177–184. [Google Scholar] [CrossRef]
- Tsuda, S.; Okudaira, S.; Moriya-Ito, K.; Shimamoto, C.; Tanaka, M.; Aoki, J.; Arai, H.; Murakami-Murofushi, K.; Kobayashi, T. Cyclic phosphatidic acid is produced by autotaxin in blood. J. Biol. Chem. 2006, 281, 26081–26088. [Google Scholar] [CrossRef] [Green Version]
- Kakiuchi, Y.; Nagai, J.; Gotoh, M.; Hotta, H.; Murofushi, H.; Ogawa, T.; Ueda, H.; Murakami-Murofushi, K. Antinociceptive effect of cyclic phosphatidic acid and its derivative on animal models of acute and chronic pain. Mol. Pain 2011, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, M.; Nagano, A.; Tsukahara, R.; Murofushi, H.; Morohoshi, T.; Otsuka, K.; Murakami-Murofushi, K. Cyclic phosphatidic acid relieves osteoarthritis symptoms. Mol. Pain 2014, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, L.; Li, S.; Jaffe, K.; Davis, L. Quantitative determination of cyclic phosphatidic acid in human serum by LC/ESI/MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 862, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Shindou, H.; Shiraishi, S.; Tokuoka, S.M.; Takahashi, Y.; Harayama, T.; Abe, T.; Bando, K.; Miyano, K.; Kita, Y.; Uezono, Y.; et al. Relief from neuropathic pain by blocking of the platelet-activating factor-pain loop. FASEB J. 2017, 31, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Murakami, N.; Yokomizo, T.; Okuno, T.; Shimizu, T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 2004, 279, 42484–42491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radu, C.G.; Yang, L.V.; Riedinger, M.; Au, M.; Witte, O.N. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.V.; Radu, C.G.; Wang, L.; Riedinger, M.; Witte, O.N. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 2005, 105, 1127–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osthues, T.; Zimmer, B.; Rimola, V.; Klann, K.; Schilling, K.; Mathoor, P.; Angioni, C.; Weigert, A.; Geisslinger, G.; Munch, C.; et al. The Lipid Receptor G2A (GPR132) Mediates Macrophage Migration in Nerve Injury-Induced Neuropathic Pain. Cells 2020, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Merlo, S.; Drago, F.; Caruso, G.; Parenti, C.; Sortino, M.A. Rescue of Noradrenergic System as a Novel Pharmacological Strategy in the Treatment of Chronic Pain: Focus on Microglia Activation. Front. Pharmacol. 2019, 10, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Wang, X.; Guo, F.; Sun, X.; Yuan, K.; Wang, Q.; Lan, C. Lysophosphatidylcholine induces apoptosis and inflammatory damage in brain microvascular endothelial cells via GPR4-mediated NLRP3 inflammasome activation. Toxicol. Vitr. 2021, 77, 105227. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Wang, H.; Fan, X.; An, N.; Li, J.; Song, H.; Kong, E.; Li, Y.; Yuan, H. BRD4 Inhibition Attenuates Inflammatory Pain by Ameliorating NLRP3 Inflammasome-Induced Pyroptosis. Front. Immunol. 2022, 13, 837977. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, A.; Okulus, M.; Rychlicka, M.; Biegala, L.; Gliszczynska, A.; Gendaszewska-Darmach, E. Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors. Nutrients 2020, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, A.; Kaminska, D.; Gliszczynska, A.; Gendaszewska-Darmach, E. Isoprenoid Derivatives of Lysophosphatidylcholines Enhance Insulin and GLP-1 Secretion through Lipid-Binding GPCRs. Int. J. Mol. Sci. 2021, 22, 5748. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; He, L. The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology 2017, 113, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Lingerfelt, M.A.; Zhao, P.; Sharir, H.P.; Hurst, D.P.; Reggio, P.H.; Abood, M.E. Identification of Crucial Amino Acid Residues Involved in Agonist Signaling at the GPR55 Receptor. Biochemistry 2017, 56, 473–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helley, M.P.; Abate, W.; Jackson, S.K.; Bennett, J.H.; Thompson, S.W. The expression of Toll-like receptor 4, 7 and co-receptors in neurochemical sub-populations of rat trigeminal ganglion sensory neurons. Neuroscience 2015, 310, 686–698. [Google Scholar] [CrossRef] [Green Version]
- Lacagnina, M.J.; Watkins, L.R.; Grace, P.M. Toll-like receptors and their role in persistent pain. Pharmacol. Ther. 2018, 184, 145–158. [Google Scholar] [CrossRef]
- Chen, R.X.; Dai, M.D.; Zhang, Q.Z.; Lu, M.P.; Wang, M.L.; Yin, M.; Zhu, X.J.; Wu, Z.F.; Zhang, Z.D.; Cheng, L. TLR Signaling Pathway Gene Polymorphisms, Gene-Gene and Gene-Environment Interactions in Allergic Rhinitis. J. Inflamm. Res. 2022, 15, 3613–3630. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, Y.J.; Ji, R.R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Acioglu, C.; Heary, R.F.; Elkabes, S. Roles of neuronal toll-like receptors in neuropathic pain and central nervous system injuries and diseases. Brain Behav. Immun. 2022, 102, 163–178. [Google Scholar] [CrossRef]
- Thakur, K.K.; Saini, J.; Mahajan, K.; Singh, D.; Jayswal, D.P.; Mishra, S.; Bishayee, A.; Sethi, G.; Kunnumakkara, A.B. Therapeutic implications of toll-like receptors in peripheral neuropathic pain. Pharmacol. Res. 2017, 115, 224–232. [Google Scholar] [CrossRef]
- Miller, R.E.; Scanzello, C.R.; Malfait, A.M. An emerging role for Toll-like receptors at the neuroimmune interface in osteoarthritis. Semin. Immunopathol. 2019, 41, 583–594. [Google Scholar] [CrossRef]
- Stokes, J.A.; Cheung, J.; Eddinger, K.; Corr, M.; Yaksh, T.L. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J. Neuroinflammation 2013, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrepf, A.; Bradley, C.S.; O’Donnell, M.; Luo, Y.; Harte, S.E.; Kreder, K.; Lutgendorf, S.; Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Toll-like receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: A multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav. Immun. 2015, 49, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Grace, P.M.; Strand, K.A.; Galer, E.L.; Urban, D.J.; Wang, X.; Baratta, M.V.; Fabisiak, T.J.; Anderson, N.D.; Cheng, K.; Greene, L.I.; et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 2016, 113, E3441–E3450. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Buzas, K.; Fan, H.; Cohen, J.I.; Wang, K.; Mont, E.; Klinman, D.; Oppenheim, J.J.; Howard, O.M. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J. Immunol. 2011, 186, 6417–6426. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Akhade, A.S.; Ismaeel, S.; Qadri, A. Serum-borne lipids amplify TLR-activated inflammatory responses. J. Leukoc. Biol. 2021, 109, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Socuellamos, P.G.; Olivos-Ore, L.A.; Barahona, M.V.; Cercos, P.; Perez Pascual, M.; Arribas-Blazquez, M.; Naranjo, J.R.; Valenzuela, C.; Gutierrez-Rodriguez, M.; Artalejo, A.R. IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain. Int. J. Mol. Sci. 2022, 23, 2142. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.S.; Brito, R.G.; Fusaro, M.; Sluka, K.A. ASIC3 Is Required for Development of Fatigue-Induced Hyperalgesia. Mol. Neurobiol. 2016, 53, 1020–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marra, S.; Ferru-Clement, R.; Breuil, V.; Delaunay, A.; Christin, M.; Friend, V.; Sebille, S.; Cognard, C.; Ferreira, T.; Roux, C.; et al. Non-acidic activation of pain-related Acid-Sensing Ion Channel 3 by lipids. EMBO J. 2016, 35, 414–428. [Google Scholar] [CrossRef] [Green Version]
- Sevastou, I.; Kaffe, E.; Mouratis, M.A.; Aidinis, V. Lysoglycerophospholipids in chronic inflammatory disorders: The PLA(2)/LPC and ATX/LPA axes. Biochim. Biophys. Acta 2013, 1831, 42–60. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, G.; Aitken, D.; Likhodii, S.; Liu, M.; Martin, G.; Furey, A.; Randell, E.; Rahman, P.; Jones, G.; et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology 2016, 55, 1566–1574. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Kurano, M.; Ohya, J.; Oichi, T.; Kano, K.; Nishikawa, M.; Uranbileg, B.; Kuwajima, K.; Sumitani, M.; Tanaka, S.; et al. Lysophosphatidic acids and their substrate lysophospholipids in cerebrospinal fluid as objective biomarkers for evaluating the severity of lumbar spinal stenosis. Sci. Rep. 2019, 9, 9144. [Google Scholar] [CrossRef]
- Rabini, R.A.; Galassi, R.; Fumelli, P.; Dousset, N.; Solera, M.L.; Valdiguie, P.; Curatola, G.; Ferretti, G.; Taus, M.; Mazzanti, L. Reduced Na(+)-K(+)-ATPase activity and plasma lysophosphatidylcholine concentrations in diabetic patients. Diabetes 1994, 43, 915–919. [Google Scholar] [CrossRef]
- Ren, C.; Liu, J.; Zhou, J.; Liang, H.; Wang, Y.; Sun, Y.; Ma, B.; Yin, Y. Lipidomic analysis of serum samples from migraine patients. Lipids Health Dis. 2018, 17, 22. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Qiu, M.; Xing, X.; Zhou, J.; Yao, H.; Li, M.; Yin, R.; Hou, Y.; Li, Y.; Pan, S.; et al. Lung cancer scRNA-seq and lipidomics reveal aberrant lipid metabolism for early-stage diagnosis. Sci. Transl. Med. 2022, 14, eabk2756. [Google Scholar] [CrossRef]
- Wolrab, D.; Jirasko, R.; Cifkova, E.; Horing, M.; Mei, D.; Chocholouskova, M.; Peterka, O.; Idkowiak, J.; Hrnciarova, T.; Kuchar, L.; et al. Lipidomic profiling of human serum enables detection of pancreatic cancer. Nat. Commun. 2022, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, X.; Zhang, X.; Han, F.; Lu, X.; Liu, L.; Zhang, J.; Dong, M.; Yang, H.; Li, H. Pharmacometabolomics Identifies 3-Hydroxyadipic Acid, d-Galactose, Lysophosphatidylcholine (P-16:0), and Tetradecenoyl-l-Carnitine as Potential Predictive Indicators of Gemcitabine Efficacy in Pancreatic Cancer Patients. Front. Oncol. 2019, 9, 1524. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, S.M.; Fernandes Silva, L.; Lankinen, M.A.; Schwab, U.; Laakso, M. Metabolite Signature of Physical Activity and the Risk of Type 2 Diabetes in 7271 Men. Metabolites 2022, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, H.; Yang, Y.; Yu, X.; Zhang, X.; Xing, Q.; Zhang, G. Using Metabolomics in Diabetes Management with Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2021, 49, 1813–1837. [Google Scholar] [CrossRef]
- Chen, S.; Zong, G.; Wu, Q.; Yun, H.; Niu, Z.; Zheng, H.; Zeng, R.; Sun, L.; Lin, X. Associations of plasma glycerophospholipid profile with modifiable lifestyles and incident diabetes in middle-aged and older Chinese. Diabetologia 2022, 65, 315–328. [Google Scholar] [CrossRef]
- Suvitaival, T.; Bondia-Pons, I.; Yetukuri, L.; Poho, P.; Nolan, J.J.; Hyotylainen, T.; Kuusisto, J.; Oresic, M. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism 2018, 78, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.H.; Koh, H.W.L.; Chua, J.Y.; Burla, B.; Ong, C.C.; Teo, L.S.L.; Yang, X.; Benke, P.I.; Choi, H.; Torta, F.; et al. Variability of the Plasma Lipidome and Subclinical Coronary Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 100–112. [Google Scholar] [CrossRef]
- Pena-Bautista, C.; Alvarez-Sanchez, L.; Canada-Martinez, A.J.; Baquero, M.; Chafer-Pericas, C. Epigenomics and Lipidomics Integration in Alzheimer Disease: Pathways Involved in Early Stages. Biomedicines 2021, 9, 1812. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Kim, H.S.; Cho, J.Y. Nicotinamide attenuates the decrease in dendritic spine density in hippocampal primary neurons from 5xFAD mice, an Alzheimer’s disease animal model. Mol. Brain 2020, 13, 17. [Google Scholar] [CrossRef]
- Masuda, R.; Lodge, S.; Whiley, L.; Gray, N.; Lawler, N.; Nitschke, P.; Bong, S.H.; Kimhofer, T.; Loo, R.L.; Boughton, B.; et al. Exploration of Human Serum Lipoprotein Supramolecular Phospholipids Using Statistical Heterospectroscopy in n-Dimensions (SHY-n): Identification of Potential Cardiovascular Risk Biomarkers Related to SARS-CoV-2 Infection. Anal. Chem. 2022, 94, 4426–4436. [Google Scholar] [CrossRef]
- Yoshioka, K.; Hirakawa, Y.; Kurano, M.; Ube, Y.; Ono, Y.; Kojima, K.; Iwama, T.; Kano, K.; Hasegawa, S.; Inoue, T.; et al. Lysophosphatidylcholine mediates fast decline in kidney function in diabetic kidney disease. Kidney Int. 2022, 101, 510–526. [Google Scholar] [CrossRef]
- Ismaiel, A.; Spinu, M.; Socaciu, C.; Budisan, L.; Leucuta, D.C.; Popa, S.L.; Chis, B.A.; Berindan-Neagoe, I.; Olinic, D.M.; Dumitrascu, D.L. Metabolic biomarkers related to cardiac dysfunction in metabolic-dysfunction-associated fatty liver disease: A cross-sectional analysis. Nutr. Diabetes 2022, 12, 4. [Google Scholar] [CrossRef] [PubMed]

| Year | Author | Administration | Species | Doses | Observations | References |
|---|---|---|---|---|---|---|
| 2020 | Chun-Ta Huang et al. | Intraneural injection | Sprague Dawley rats | 4% LPC 2 μL | The rats developed mechanical allodynia and thermal hyperalgesia on day 1 after LPC treatment. | [94] |
| 2021 | Yong Chen et al. | Intrathecal injection | C57BL/6J mice | 15 μg LPC | Intrathecal injection of LPC induced mechanical pain via activation of TRPV4-expressing DRG sensory neurons. | [105] |
| 2013 | Hsin-Ying Wang et al. | Intraneural injection | Male Wistar rats | 4% LPC 2 μL | LPC treatment caused mechanic allodynia and thermal hyperalgesia. | [93] |
| 2008 | M Inoue et al. | Intrathecal injection | Male mutant mice | 15 μg/50 μg LPC | A single injection of LPC at 15 μg showed significantly but slightly weaker mechanical allodynia on days 2–7. However, a higher dose of LPC (50 μg) caused abnormal behaviors. | [104] |
| 2018 | Hozo Matsuoka et al. | Intraneural injection | Wistar rats | 2% LPC 5 μL | Paw withdrawal thresholds were significantly higher in the LPC group compared with the Non-LPC group. | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Lin, J.; Yu, L.; Yan, M. Lysophosphatidylcholine: Potential Target for the Treatment of Chronic Pain. Int. J. Mol. Sci. 2022, 23, 8274. https://doi.org/10.3390/ijms23158274
Ren J, Lin J, Yu L, Yan M. Lysophosphatidylcholine: Potential Target for the Treatment of Chronic Pain. International Journal of Molecular Sciences. 2022; 23(15):8274. https://doi.org/10.3390/ijms23158274
Chicago/Turabian StyleRen, Jinxuan, Jiaqi Lin, Lina Yu, and Min Yan. 2022. "Lysophosphatidylcholine: Potential Target for the Treatment of Chronic Pain" International Journal of Molecular Sciences 23, no. 15: 8274. https://doi.org/10.3390/ijms23158274
APA StyleRen, J., Lin, J., Yu, L., & Yan, M. (2022). Lysophosphatidylcholine: Potential Target for the Treatment of Chronic Pain. International Journal of Molecular Sciences, 23(15), 8274. https://doi.org/10.3390/ijms23158274





