Metabolic Inflexibility as a Pathogenic Basis for Atrial Fibrillation
Abstract
:1. Introduction
2. Metabolic Flexibility in the Normal Heart
2.1. Substrate Availability
2.2. Metabolic Regulatory Network
3. Metabolic Flexibility Matters in AF Burst
3.1. Specificity of Atrial Metabolic Characteristics
3.2. AF Is an Energy Stress That Uses Fuel Selection to Meet High Energy Demand
3.3. Consequences of Atrial Metabolic Inflexibility
4. Metabolic heterogeneity among AF Stressors
4.1. Pro-FAO Stressors: Obesity, and Diabetes
4.2. Pro-GL Stressors: Aging, Physical Inactivity; Myocardial Infarction; Hypertension, and HF
- The energy-undemanding condition
- The energy-demanding condition
4.3. AF Classification Based on Cluster Analysis
4.4. Electrophysiological Properties under pro-FAO or pro-GL State
5. Metabolic Inflexibility as the Basis of Pathogenesis among AF Stressors
6. The Substrate-Metabolism Mechanism Underlying Metabolic Inflexibility
6.1. Substrate Metabolic Flexibility
6.1.1. Glucose Metabolic Inflexibility Underlying AF
- Glucose metabolic abnormalities and pathogenesis
- Insulin resistance and AF pathogenesis
- Mechanisms of glucose metabolic inflexibility underlying AF
6.1.2. FAs Metabolic Inflexibility Underlying AF Pathogenesis
- FAO and AF pathogenesis
- Mechanisms of FAs’ metabolic inflexibility underlying AF
6.1.3. Amino Acids’ Metabolic Flexibility
- BCAA and AF pathogenesis
- Mechanisms of BCAA metabolic inflexibility underlying AF
6.1.4. Ketones and Metabolic Flexibility
- Ketones and AF pathogenesis
- Mechanisms of ketone metabolic inflexibility underlying AF
6.2. Metabolism Regulatory Signaling and Metabolic Flexibility
6.3. The Substrate-Metabolism Mechanism Underlying Metabolic Inflexibility and AF Pathogenesis
7. Anti-AF Strategies Targeting Metabolic Inflexibility
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Benjamin, E.J.; Magnani, J.W. Atrial Fibrillation: Global Burdens and Global Opportunities. Heart 2021, 107, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Ruddox, V.; Sandven, I.; Munkhaugen, J.; Skattebu, J.; Edvardsen, T.; Otterstad, J.E. Atrial Fibrillation and the Risk for Myocardial Infarction, All-Cause Mortality and Heart Failure: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2017, 24, 1555–1566. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of Atrial Fibrillation on the Risk of Death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global Epidemiology of Atrial Fibrillation: An Increasing Epidemic and Public Health Challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef]
- Lau, D.H.; Nattel, S.; Kalman, J.M.; Sanders, P. Modifiable Risk Factors and Atrial Fibrillation. Circulation 2017, 136, 583–596. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wust, R.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [Green Version]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of Mitochondria in Human Skeletal Muscle in Type 2 Diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef] [Green Version]
- Abe, J.; Abriel, H.; Accili, E.A.; Acosta, D.; Allen, L.F.; Allen, T.J.; Antzelevitch, C.; Aoki, H.; Ardell, J.L.; Arita, M.; et al. Contributors. In Heart Physiology and Pathophysiology, 4th ed.; Sperelakis, N., Kurachi, Y., Terzic, A., Cohen, M.V., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. xiii–xvii. ISBN 978-0-12-656975-9. [Google Scholar]
- Suga, H. Ventricular Energetics. Physiol. Rev. 1990, 70, 247–277. [Google Scholar] [CrossRef]
- Kolwicz, S.J.; Purohit, S.; Tian, R. Cardiac Metabolism and Its Interactions with Contraction, Growth, and Survival of Cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neely, J.R.; Morgan, H.E. Relationship between Carbohydrate and Lipid Metabolism and the Energy Balance of Heart Muscle. Annu. Rev. Physiol. 1974, 36, 413–459. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Jaswal, J.S. Energy Metabolic Phenotype of the Cardiomyocyte during Development, Differentiation, and Postnatal Maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial Substrate Metabolism in the Normal and Failing Heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Wentz, A.E.; D’Avignon, D.A.; Weber, M.L.; Cotter, D.G.; Doherty, J.M.; Kerns, R.; Nagarajan, R.; Reddy, N.; Sambandam, N.; Crawford, P.A. Adaptation of Myocardial Substrate Metabolism to a Ketogenic Nutrient Environment. J. Biol. Chem. 2010, 285, 24447–24456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone Bodies: From Enemy to Friend and Guardian Angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef]
- McGarry, J.D.; Foster, D.W. Regulation of Hepatic Fatty Acid Oxidation and Ketone Body Production. Annu. Rev. Biochem. 1980, 49, 395–420. [Google Scholar] [CrossRef]
- Zaha, V.G.; Young, L.H. AMP-Activated Protein Kinase Regulation and Biological Actions in the Heart. Circ. Res. 2012, 111, 800–814. [Google Scholar] [CrossRef] [Green Version]
- Lehman, J.J.; Barger, P.M.; Kovacs, A.; Saffitz, J.E.; Medeiros, D.M.; Kelly, D.P. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Promotes Cardiac Mitochondrial Biogenesis. J. Clin. Invest. 2000, 106, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Francis, G.A.; Annicotte, J.S.; Auwerx, J. PPAR-Alpha Effects on the Heart and Other Vascular Tissues. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1–H9. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Murphy, A.N.; Brown, J.H. Akt Mediated Mitochondrial Protection in the Heart: Metabolic and Survival Pathways to the Rescue. J. Bioenerg. Biomembr. 2009, 41, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerychova, R.; Pavlinkova, G. HIF-1, Metabolism, and Diabetes in the Embryonic and Adult Heart. Front. Endocrinol. 2018, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Hypoxia-Inducible Factor 1: Regulator of Mitochondrial Metabolism and Mediator of Ischemic Preconditioning. Biochim. Biophys. Acta 2011, 1813, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Vassanelli, C. Left Atrium: No Longer Neglected. Ital. Heart J. 2005, 6, 881–885. [Google Scholar]
- Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the Adult Human Heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Burstein, B.; Libby, E.; Calderone, A.; Nattel, S. Differential Behaviors of Atrial versus Ventricular Fibroblasts: A Potential Role for Platelet-Derived Growth Factor in Atrial-Ventricular Remodeling Differences. Circulation 2008, 117, 1630–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, N.; Cardin, S.; Leung, T.-K.; Nattel, S. Differences in Atrial versus Ventricular Remodeling in Dogs with Ventricular Tachypacing-Induced Congestive Heart Failure. Cardiovasc. Res. 2004, 63, 236–244. [Google Scholar] [CrossRef]
- Cardin, S.; Pelletier, P.; Libby, E.; Le Bouter, S.; Xiao, L.; Kääb, S.; Demolombe, S.; Glass, L.; Nattel, S. Marked Differences between Atrial and Ventricular Gene-Expression Remodeling in Dogs with Experimental Heart Failure. J. Mol. Cell Cardiol. 2008, 45, 821–831. [Google Scholar] [CrossRef]
- Barth, A.S.; Merk, S.; Arnoldi, E.; Zwermann, L.; Kloos, P.; Gebauer, M.; Steinmeyer, K.; Bleich, M.; Kaab, S.; Pfeufer, A.; et al. Functional Profiling of Human Atrial and Ventricular Gene Expression. Pflugers Arch. 2005, 450, 201–208. [Google Scholar] [CrossRef]
- Barth, A.S.; Merk, S.; Arnoldi, E.; Zwermann, L.; Kloos, P.; Gebauer, M.; Steinmeyer, K.; Bleich, M.; Kääb, S.; Hinterseer, M.; et al. Reprogramming of the Human Atrial Transcriptome in Permanent Atrial Fibrillation: Expression of a Ventricular-like Genomic Signature. Circ. Res. 2005, 96, 1022–1029. [Google Scholar] [CrossRef]
- Shimura, D.; Nakai, G.; Jiao, Q.; Osanai, K.; Kashikura, K.; Endo, K.; Soga, T.; Goda, N.; Minamisawa, S. Metabolomic Profiling Analysis Reveals Chamber-Dependent Metabolite Patterns in the Mouse Heart. Am. J. Physiol. Heart. Circ. Physiol. 2013, 305, H494–H505. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.A.; Rubio, R.; Berne, R.M. Comparison of the Adenine Nucleotide Metabolism of Dog Atrial and Ventricular Myocardium. J. Mol. Cell Cardiol. 1975, 7, 115–123. [Google Scholar] [CrossRef]
- Bass, A.; Stejskalova, M.; Ostadal, B.; Samanek, M. Differences between Atrial and Ventricular Energy-Supplying Enzymes in Five Mammalian Species. Physiol. Res. 1993, 42, 1–6. [Google Scholar]
- Shimura, D.; Jiao, Q.; Kashikura, K.; Endo, K.; Soga, T.; Goda, N.; Minamisawa, S. The Manner of Metabolism Is Different between the Atrium and the Ventricle. FASEB J. 2012, 26, 1144.18. [Google Scholar] [CrossRef]
- Harada, M.; Melka, J.; Sobue, Y.; Nattel, S. Metabolic Considerations in Atrial Fibrillation―Mechanistic Insights and Therapeutic Opportunities. Circ. J. 2017, 81, 1749–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, P.; Nattel, S.; Nanthakumar, K. Linking Cellular Energy State to Atrial Fibrillation Pathogenesis: Potential Role of Adenosine Monophosphate–Activated Protein Kinase. Heart Rhythm 2020, 17, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Modrego, J.; Maroto, L.; Tamargo, J.; Azcona, L.; Mateos-Cáceres, P.; Segura, A.; Moreno-Herrero, R.; Pérez-Castellanos, N.; Delpón, E.; Pérez-Villacastín, J.; et al. Comparative Expression of Proteins in Left and Right Atrial Appendages from Patients with Mitral Valve Disease at Sinus Rhythm and Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2010, 21, 859–868. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Jansen, H.J.; Mackasey, M.; Rafferty, S.A.; Bogachev, O.; Sapp, J.L.; Howlett, S.E.; Rose, R.A. The Impacts of Age and Frailty on Heart Rate and Sinoatrial Node Function. J. Physiol. 2016, 594, 7105–7126. [Google Scholar] [CrossRef]
- McCauley, M.D.; Hong, L.; Sridhar, A.; Menon, A.; Perike, S.; Zhang, M.; Da, S.I.; Yan, J.; Bonini, M.G.; Ai, X.; et al. Ion Channel and Structural Remodeling in Obesity-Mediated Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, e008296. [Google Scholar] [CrossRef] [PubMed]
- Soltysinska, E.; Speerschneider, T.; Winther, S.V.; Thomsen, M.B. Sinoatrial Node Dysfunction Induces Cardiac Arrhythmias in Diabetic Mice. Cardiovasc. Diabetol. 2014, 13, 122. [Google Scholar] [CrossRef] [Green Version]
- Malagù, M.; Marchini, F.; Fiorio, A.; Sirugo, P.; Clò, S.; Mari, E.; Gamberini, M.R.; Rapezzi, C.; Bertini, M. Atrial Fibrillation in β-Thalassemia: Overview of Mechanism, Significance and Clinical Management. Biology 2022, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the Risk of New-Onset Atrial Fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedrow, U.B.; Conen, D.; Ridker, P.M.; Cook, N.R.; Koplan, B.A.; Manson, J.E.; Buring, J.E.; Albert, C.M. The Long- and Short-Term Impact of Elevated Body Mass Index on the Risk of New Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 55, 2319–2327. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.X.; Sullivan, T.; Sun, M.T.; Mahajan, R.; Pathak, R.K.; Middeldorp, M.; Twomey, D.; Ganesan, A.N.; Rangnekar, G.; Roberts-Thomson, K.C.; et al. Obesity and the Risk of Incident, Post-Operative, and Post-Ablation Atrial Fibrillation. JACC Clin. Electrophysiol. 2015, 1, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Shantsila, A.; Guo, P.; Potpara, T.S.; Zhan, X.; Fang, X.; Liao, H.; Liu, Y.; Wei, W.; Fu, L.; et al. A U-Shaped Relationship of Body Mass Index on Atrial Fibrillation Recurrence Post Ablation: A Report from the Guangzhou Atrial Fibrillation Ablation Registry. EBioMedicine 2018, 35, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, S.; Gupta, D.; Lip, G.Y.H. Obesity and Atrial Fibrillation: Making Inroads through Fat. Eur. Heart. J. Cardiovasc. Pharmacother. 2021, 7, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Aucouturier, J.; Duché, P.; Timmons, B.W. Metabolic Flexibility and Obesity in Children and Youth: Metabolic Flexibility in Youth. Obes. Rev. 2011, 12, e44–e53. [Google Scholar] [CrossRef]
- Dahlqvist, S.; Rosengren, A.; Gudbjörnsdottir, S.; Pivodic, A.; Wedel, H.; Kosiborod, M.; Svensson, A.-M.; Lind, M. Risk of Atrial Fibrillation in People with Type 1 Diabetes Compared with Matched Controls from the General Population: A Prospective Case-Control Study. Lancet Diabetes Endocrinol. 2017, 5, 799–807. [Google Scholar] [CrossRef]
- Bohne, L.J.; Johnson, D.; Rose, R.A.; Wilton, S.B.; Gillis, A.M. The Association Between Diabetes Mellitus and Atrial Fibrillation: Clinical and Mechanistic Insights. Front. Physiol. 2019, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Liu, T.; Tse, G.; Gong, M.; Gladding, P.A.; Smaill, B.H.; Stiles, M.K.; Gillis, A.M.; Zhao, J. A Machine Learning Aided Systematic Review and Meta-Analysis of the Relative Risk of Atrial Fibrillation in Patients With Diabetes Mellitus. Front. Physiol. 2018, 9, 835. [Google Scholar] [CrossRef] [Green Version]
- Huxley, R.R.; Filion, K.B.; Konety, S.; Alonso, A. Meta-Analysis of Cohort and Case-Control Studies of Type 2 Diabetes Mellitus and Risk of Atrial Fibrillation. Am. J. Cardiol. 2011, 108, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxley, R.R.; Alonso, A.; Lopez, F.L.; Filion, K.B.; Agarwal, S.K.; Loehr, L.R.; Soliman, E.Z.; Pankow, J.S.; Selvin, E. Type 2 Diabetes, Glucose Homeostasis and Incident Atrial Fibrillation: The Atherosclerosis Risk in Communities Study. Heart 2012, 98, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Schoen, T.; Pradhan, A.D.; Albert, C.M.; Conen, D. Type 2 Diabetes Mellitus and Risk of Incident Atrial Fibrillation in Women. J. Am. Coll. Cardiol. 2012, 60, 1421–1428. [Google Scholar] [CrossRef] [Green Version]
- Corpeleijn, E.; Saris, W.H.M.; Blaak, E.E. Metabolic Flexibility in the Development of Insulin Resistance and Type 2 Diabetes: Effects of Lifestyle. Obes. Rev. 2009, 10, 178–193. [Google Scholar] [CrossRef]
- Díez-Villanueva, P.; Alfonso, F. Atrial Fibrillation in the Elderly. J. Geriatr. Cardiol. 2019, 16, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, J.; van der Kuip, D.A.M.; Hofman, A.; Kors, J.A.; van Herpen, G.; Stricker, B.H.C.; Stijnen, T.; Lip, G.Y.H.; Witteman, J.C.M. Prevalence, Incidence and Lifetime Risk of Atrial Fibrillation: The Rotterdam Study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stull, A.J.; Galgani, J.E.; Johnson, W.D.; Cefalu, W.T. The Contribution of Race and Diabetes Status to Metabolic Flexibility in Humans. Metabolism 2010, 59, 1358–1364. [Google Scholar] [CrossRef] [Green Version]
- Ricci, C.; Gervasi, F.; Gaeta, M.; Smuts, C.M.; Schutte, A.E.; Leitzmann, M.F. Physical Activity Volume in Relation to Risk of Atrial Fibrillation. A Non-Linear Meta-Regression Analysis. Eur. J. Prev. Cardiol. 2018, 25, 857–866. [Google Scholar] [CrossRef]
- Mittal, S. Physical Activity and Incidence of Atrial Fibrillation in Older Adults: The Cardiovascular Health Study. J. Atr. Fibrillation 2008, 1, 132. [Google Scholar] [CrossRef] [Green Version]
- Middeldorp, M.E.; Ariyaratnam, J.; Lau, D.; Sanders, P. Lifestyle Modifications for Treatment of Atrial Fibrillation. Heart 2020, 106, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Duray, G.; Gersh, B.J.; Hohnloser, S.H. Atrial Fibrillation in Acute Myocardial Infarction: A Systematic Review of the Incidence, Clinical Features and Prognostic Implications. Eur. Heart. J. 2009, 30, 1038–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort. The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Lopez, F.L.; Folsom, A.R.; Agarwal, S.K.; Loehr, L.R.; Soliman, E.Z.; Maclehose, R.; Konety, S.; Alonso, A. Absolute and Attributable Risks of Atrial Fibrillation in Relation to Optimal and Borderline Risk Factors: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2011, 123, 1501–1508. [Google Scholar] [CrossRef] [Green Version]
- Nabauer, M.; Gerth, A.; Limbourg, T.; Schneider, S.; Oeff, M.; Kirchhof, P.; Goette, A.; Lewalter, T.; Ravens, U.; Meinertz, T.; et al. The Registry of the German Competence NETwork on Atrial Fibrillation: Patient Characteristics and Initial Management. Europace 2008, 11, 423–434. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F.; Reboldi, G. Hypertension and Atrial Fibrillation: Doubts and Certainties From Basic and Clinical Studies. Circ. Res. 2018, 122, 352–368. [Google Scholar] [CrossRef]
- Vallerie, S.N.; Bornfeldt, K.E. Metabolic Flexibility and Dysfunction in Cardiovascular Cells. Arter. Thromb. Vasc. Biol. 2015, 35, e37–e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deedwania, P.C.; Lardizabal, J.A. Atrial Fibrillation in Heart Failure: A Comprehensive Review. Am. J. Med. 2010, 123, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.; Lambiase, P. Obesity and Atrial Fibrillation: Epidemiology, Pathophysiology and Novel Therapeutic Opportunities. Arrhythm. Electrophysiol. Rev. 2019, 8, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Rider, O.J.; Cox, P.; Tyler, D.; Clarke, K.; Neubauer, S. Myocardial Substrate Metabolism in Obesity. Int. J. Obes. 2013, 37, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Carley, A.N.; Severson, D.L. Fatty Acid Metabolism Is Enhanced in Type 2 Diabetic Hearts. BBA Mol. Cell Biol. Lipids 2005, 1734, 112–126. [Google Scholar] [CrossRef]
- Sithara, T.; Drosatos, K. Metabolic Complications in Cardiac Aging. Front. Physiol. 2021, 12, 669497. [Google Scholar] [CrossRef]
- Bergouignan, A.; Rudwill, F.; Simon, C.; Blanc, S. Physical Inactivity as the Culprit of Metabolic Inflexibility: Evidence from Bed-Rest Studies. J. Appl. Physiol. 2011, 111, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Christe, M.E.; Rodgers, R.L. Altered Glucose and Fatty Acid Oxidation in Hearts of the Spontaneously Hypertensive Rat. J. Mol. Cell Cardiol. 1994, 26, 1371–1375. [Google Scholar] [CrossRef]
- Inohara, T.; Shrader, P.; Pieper, K.; Blanco, R.G.; Thomas, L.; Singer, D.E.; Freeman, J.V.; Allen, L.A.; Fonarow, G.C.; Gersh, B.; et al. Association of of Atrial Fibrillation Clinical Phenotypes With Treatment Patterns and Outcomes: A Multicenter Registry Study. JAMA Cardiol. 2018, 3, 54–63. [Google Scholar] [CrossRef]
- Inohara, T.; Piccini, J.P.; Mahaffey, K.W.; Kimura, T.; Katsumata, Y.; Tanimoto, K.; Inagawa, K.; Ikemura, N.; Ueda, I.; Fukuda, K.; et al. A Cluster Analysis of the Japanese Multicenter Outpatient Registry of Patients With Atrial Fibrillation. Am. J. Cardiol. 2019, 124, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Inoue, H.; Atarashi, H.; Okumura, K.; Yamashita, T.; Kodani, E.; Kiyono, K.; Origasa, H. J-RHYTHM Registry Investigators Clinical Phenotypes of Patients with Non-Valvular Atrial Fibrillation as Defined by a Cluster Analysis: A Report from the J-RHYTHM Registry. Int. J. Cardiol. Heart Vasc. 2021, 37, 100885. [Google Scholar] [CrossRef]
- Fujita, N.; Takei, Y. Alcohol Consumption and Metabolic Syndrome: Alcohol and Metabolic Syndrome. Hepatol. Res. 2011, 41, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, A.; Kalman, J.M.; De Silva, A.; Nicholls, T.; Costello, B.; Nanayakkara, S.; Prabhu, S.; Stub, D.; Azzopardi, S.; Vizi, D.; et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N. Engl. J. Med. 2020, 382, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Lau, D.H.; Brooks, A.G.; Shipp, N.J.; Manavis, J.; Wood, J.P.M.; Finnie, J.W.; Samuel, C.S.; Royce, S.G.; Twomey, D.J.; et al. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J. Am. Coll. Cardiol. 2015, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Y.; Jiang, T.; Liu, B.; Sun, H.; Zhang, Y.; Fan, B.; Li, X.; Qin, X.; Zheng, Q. Enhancing Fatty Acids Oxidation via L-Carnitine Attenuates Obesity-Related Atrial Fibrillation and Structural Remodeling by Activating AMPK Signaling and Alleviating Cardiac Lipotoxicity. Front. Pharmacol. 2021, 12, 771940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hartnett, S.; Sample, A.; Schnack, S.; Li, Y. High Fat Diet Induced Alterations of Atrial Electrical Activities in Mice. Am. J. Cardiovasc. Dis. 2016, 6, 1–9. [Google Scholar]
- Van Wagoner, D.R.; Pond, A.L.; McCarthy, P.M.; Trimmer, J.S.; Nerbonne, J.M. Outward K + Current Densities and Kv1.5 Expression Are Reduced in Chronic Human Atrial Fibrillation. Circ. Res. 1997, 80, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nunez, R.T.; Aaronson, P.I. K V 1.5 Channel Down-regulation in Pulmonary Hypertension Is Nothing Short of MiR-1-aculous! J. Physiol. 2019, 597, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Aromolaran, A.S.; Boutjdir, M. Cardiac Ion Channel Regulation in Obesity and the Metabolic Syndrome: Relevance to Long QT Syndrome and Atrial Fibrillation. Front. Physiol. 2017, 8, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dublin, S.; Glazer, N.L.; Smith, N.L.; Psaty, B.M.; Lumley, T.; Wiggins, K.L.; Page, R.L.; Heckbert, S.R. Diabetes Mellitus, Glycemic Control, and Risk of Atrial Fibrillation. J. Gen. Intern. Med. 2010, 25, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, W.; Zhang, N.; Korantzopoulos, P.; Letsas, K.P.; Cheng, M.; Di, F.; Tse, G.; Liu, T.; Li, G. Serum Glycated Hemoglobin Level as a Predictor of Atrial Fibrillation: A Systematic Review with Meta-Analysis and Meta-Regression. PLoS ONE 2017, 12, e0170955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Teshima, Y.; Fukui, A.; Kondo, H.; Nishio, S.; Nakagawa, M.; Saikawa, T.; Takahashi, N. Glucose Fluctuations Increase the Incidence of Atrial Fibrillation in Diabetic Rats. Cardiovasc. Res. 2014, 104, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gu, J. Impact of Long-Term Glycemic Variability on Development of Atrial Fibrillation in Type 2 Diabetic Patients. Anatol. J. Cardiol. 2017, 18, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-F.; Suenari, K.; Chang, S.-L.; Lin, Y.-J.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Tai, C.-T.; Tsao, H.-M.; Li, C.-H.; et al. Atrial Substrate Properties and Outcome of Catheter Ablation in Patients With Paroxysmal Atrial Fibrillation Associated With Diabetes Mellitus or Impaired Fasting Glucose. Am. J. Cardiol. 2010, 106, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Cha, S.J.; Park, J.H.; Shin, J.H.; Lim, Y.H.; Park, H.C.; Shin, J.; Kim, C.K.; Park, J.K. Association between Insulin Resistance and Risk of Atrial Fibrillation in Non-Diabetics. Eur. J. Prev. Cardiol. 2020, 27, 1934–1941. [Google Scholar] [CrossRef]
- Johnson, L.S.; Juhlin, T.; Engström, G.; Nilsson, P.M. Low Fasting Plasma Insulin Is Associated with Atrial Fibrillation in Men from a Cohort Study—The Malmö Preventive Project. BMC Cardiovasc. Disord. 2014, 14, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontes, J.D.; Lyass, A.; Massaro, J.M.; Rienstra, M.; Dallmeier, D.; Schnabel, R.B.; Wang, T.J.; Vasan, R.S.; Lubitz, S.A.; Magnani, J.W.; et al. Insulin Resistance and Atrial Fibrillation (from the Framingham Heart Study). Am. J. Cardiol. 2012, 109, 87–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, P.K.; Biggs, M.L.; Kaplan, R.; Kizer, J.R.; Heckbert, S.R.; Mukamal, K.J. Fasting and Post-Glucose Load Measures of Insulin Resistance and Risk of Incident Atrial Fibrillation: The Cardiovascular Health Study. Nutr. Metab. Cardiovasc. 2018, 28, 716–721. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Chang, G.-J.; Lai, Y.-J.; Chen, W.-J.; Chang, S.-H.; Hung, L.-M.; Kuo, C.-T.; Yeh, Y.-H. Atrial Fibrillation and Its Arrhythmogenesis Associated with Insulin Resistance. Cardiovasc. Diabetol. 2019, 18, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polina, I.; Jansen, H.J.; Li, T.; Moghtadaei, M.; Bohne, L.J.; Liu, Y.; Krishnaswamy, P.; Egom, E.E.; Belke, D.D.; Rafferty, S.A.; et al. Loss of Insulin Signaling May Contribute to Atrial Fibrillation and Atrial Electrical Remodeling in Type 1 Diabetes. Proc. Natl. Acad. Sci. USA 2020, 117, 7990–8000. [Google Scholar] [CrossRef]
- Maria, Z.; Campolo, A.R.; Scherlag, B.J.; Ritchey, J.W.; Lacombe, V.A. Insulin Treatment Reduces Susceptibility to Atrial Fibrillation in Type 1 Diabetic Mice. Front. Cardiovasc. Med. 2020, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Maria, Z.; Campolo, A.R.; Scherlag, B.J.; Ritchey, J.W.; Lacombe, V.A. Dysregulation of Insulin-Sensitive Glucose Transporters During Insulin Resistance-Induced Atrial Fibrillation. BBA Mol. Basis Dis. 2018, 1864, 987–996. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, F.; Liu, N.; Ouyang, F.; Liu, Q. The Warburg Effect: A New Insight into Atrial Fibrillation. Clin. Chim. Acta 2019, 499, 4–12. [Google Scholar] [CrossRef]
- Zhang, S.; Hulver, M.W.; McMillan, R.P.; Cline, M.A.; Gilbert, E.R. The Pivotal Role of Pyruvate Dehydrogenase Kinases in Metabolic Flexibility. Nutr. Metab. 2014, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Tao, R.; Xiong, X.; Harris, R.A.; White, M.F.; Dong, X.C. Genetic Inactivation of Pyruvate Dehydrogenase Kinases Improves Hepatic Insulin Resistance Induced Diabetes. PLoS ONE 2013, 8, e71997. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.J.; Zhang, C.; Tang, Z.H.; Qu, S.L.; Jiang, Z.S. Regulating the Warburg Effect on Metabolic Stress and Myocardial Fibrosis Remodeling and Atrial Intracardiac Waveform Activity Induced by Atrial Fibrillation. Biochem. Biophys. Res. Commun. 2019, 516, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, J.-A.; Kelley, D.E. Altered Glycolytic and Oxidative Capacities of Skeletal Muscle Contribute to Insulin Resistance in NIDDM. J. Appl. Physiol. 1997, 83, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kroger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the Prospective Association between Individual Plasma Phospholipid Saturated Fatty Acids and Incident Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Cheng, D.; Wang, Y.; Cao, Y.; Xiang, S.; Yu, Q. A Metabolomic Study for Chronic Heart Failure Patients Based on a Dried Blood Spot Mass Spectrometry Approach. RSC Adv. 2020, 10, 19621–19628. [Google Scholar] [CrossRef] [PubMed]
- Pararasa, C.; Ikwuobe, J.; Shigdar, S.; Boukouvalas, A.; Nabney, I.T.; Brown, J.E.; Devitt, A.; Bailey, C.J.; Bennett, S.J.; Griffiths, H.R. Age-Associated Changes in Long-Chain Fatty Acid Profile during Healthy Aging Promote pro-Inflammatory Monocyte Polarization via PPARgamma. Aging Cell 2016, 15, 128–139. [Google Scholar] [CrossRef]
- Fretts, A.M.; Mozaffarian, D.; Siscovick, D.S.; Djousse, L.; Heckbert, S.R.; King, I.B.; McKnight, B.; Sitlani, C.; Sacks, F.M.; Song, X.; et al. Plasma Phospholipid Saturated Fatty Acids and Incident Atrial Fibrillation: The Cardiovascular Health Study. J. Am. Heart Assoc. 2014, 3, e000889. [Google Scholar] [CrossRef] [Green Version]
- Jensen, P.N.; Fretts, A.M.; Hoofnagle, A.N.; Sitlani, C.M.; McKnight, B.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma Ceramides and Sphingomyelins in Relation to Atrial Fibrillation Risk: The Cardiovascular Health Study. J. Am. Heart Assoc. 2020, 9, e012853. [Google Scholar] [CrossRef]
- Steinbusch, L.K.; Schwenk, R.W.; Ouwens, D.M.; Diamant, M.; Glatz, J.F.; Luiken, J.J. Subcellular Trafficking of the Substrate Transporters GLUT4 and CD36 in Cardiomyocytes. Cell Mol. Life Sci. 2011, 68, 2525–2538. [Google Scholar] [CrossRef] [Green Version]
- Lenski, M.; Schleider, G.; Kohlhaas, M.; Adrian, L.; Adam, O.; Tian, Q.; Kaestner, L.; Lipp, P.; Lehrke, M.; Maack, C.; et al. Arrhythmia Causes Lipid Accumulation and Reduced Glucose Uptake. Basic Res. Cardiol. 2015, 110, 40. [Google Scholar] [CrossRef]
- Koonen, D.P.; Febbraio, M.; Bonnet, S.; Nagendran, J.; Young, M.E.; Michelakis, E.D.; Dyck, J.R. CD36 Expression Contributes to Age-Induced Cardiomyopathy in Mice. Circulation 2007, 116, 2139–2147. [Google Scholar] [CrossRef] [Green Version]
- Shingu, Y.; Yokota, T.; Takada, S.; Niwano, H.; Ooka, T.; Katoh, H.; Tachibana, T.; Kubota, S.; Matsui, Y. Decreased Gene Expression of Fatty Acid Binding Protein 3 in the Atrium of Patients with New Onset of Atrial Fibrillation in Cardiac Perioperative Phase. J. Cardiol. 2018, 71, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, T.; Zhou, S.; Liu, Z.; Li, X.; Liu, Q. Quantitative Proteomics of Changes in Energy Metabolism-Related Proteins in Atrial Tissue from Valvular Disease Patients with Permanent Atrial Fibrillation. Circ. J. 2014, 78, 993–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, K.; Nzirorera, C.; Kienesberger, P.C. Lipid Metabolism and Signaling in Cardiac Lipotoxicity. Biochim. Biophys. Acta 2016, 1861, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Suzuki, J.; Hirose, M.; Yamada, M.; Zenimaru, Y.; Nakaya, T.; Ichikawa, M.; Imagawa, M.; Takahashi, S.; Ikuyama, S.; et al. Cardiac Overexpression of Perilipin 2 Induces Atrial Steatosis, Connexin 43 Remodeling, and Atrial Fibrillation in Aged Mice. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1193–E1204. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.-B. Exercise and Obesity-Induced Insulin Resistance in Skeletal Muscle. Integr. Med. Res. 2013, 2, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Zhelev, Z.; Aoki, I.; Lazarova, D.; Vlaykova, T.; Higashi, T.; Bakalova, R. A “Weird” Mitochondrial Fatty Acid Oxidation as a Metabolic “Secret” of Cancer. Oxid. Med. Cell Longev. 2022, 2022, 1–38. [Google Scholar] [CrossRef]
- Taegtmeyer, H.; Harinstein, M.E.; Gheorghiade, M. More than Bricks and Mortar: Comments on Protein and Amino Acid Metabolism in the Heart. Am. J. Cardiol. 2008, 101, 3E–7E. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.-M.; Dong, X.; Zhao, J.-K.; Xu, Y.-L.; Xu, D.-Y.; Xue, X.-D.; Zhou, Z.-J.; Huang, Y.-T.; Zhao, Q.-S.; Luo, L.-Y.; et al. Activation of PKG-CREB-KLF15 by Melatonin Attenuates Angiotensin II-Induced Vulnerability to Atrial Fibrillation via Enhancing Branched-Chain Amino Acids Catabolism. Free Radical Biol. Med. 2022, 178, 202–214. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Xia, Y.; Zhao, S.; Yan, W.; Wang, H.; Lee, Y.; Li, C.; Zhang, L.; Lian, K.; et al. Defective Branched Chain Amino Acid Catabolism Contributes to Cardiac Dysfunction and Remodeling Following Myocardial Infarction. Am. J. Physiol. Heart C 2016, 311, H1160–H1169. [Google Scholar] [CrossRef]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.-Y.; Ren, S.; Liu, Y.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, C.J.; Adams, S.H. Branched-Chain Amino Acids in Metabolic Signalling and Insulin Resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Bing, R.J. The Metabolism of the Heart. Harvey Lect. 1954, 50, 27–70. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, W.; Maas, D.; Richter, J.; Hasinger, F.; Hofmann, H.; Dohrn, P. On the Significance of Acetoacetate and Beta-Hydroxybutyrate in Human Myocardial Metabolism. Klin. Wochenschr. 1965, 43, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Holcomb, L.E.; Kolwicz, S.J. Ketogenic Diets and Exercise Performance. Nutrients 2019, 11, 2296. [Google Scholar] [CrossRef] [Green Version]
- Lommi, J.; Koskinen, P.; Naveri, H.; Harkonen, M.; Kupari, M. Heart Failure Ketosis. J. Intern. Med. 1997, 242, 231–238. [Google Scholar] [CrossRef]
- Lommi, J.; Kupari, M.; Koskinen, P.; Naveri, H.; Leinonen, H.; Pulkki, K.; Harkonen, M. Blood Ketone Bodies in Congestive Heart Failure. J. Am. Coll. Cardiol. 1996, 28, 665–672. [Google Scholar] [CrossRef]
- Rudolph, W.; Schinz, A. Studies on Myocardial Blood Flow, Oxygen Consumption, and Myocardial Metabolism in Patients with Cardiomyopathy. Recent Adv. Stud. Cardiac. Struct. Metab. 1973, 2, 739–749. [Google Scholar]
- Mayr, M.; Yusuf, S.; Weir, G.; Chung, Y.L.; Mayr, U.; Yin, X.; Ladroue, C.; Madhu, B.; Roberts, N.; De Souza, A.; et al. Combined Metabolomic and Proteomic Analysis of Human Atrial Fibrillation. J. Am. Coll. Cardiol. 2008, 51, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Sasaguri, S.; Rajesh, K.G.; Suzuki, R. Dl-3-Hydroxybutyrate Administration Prevents Myocardial Damage after Coronary Occlusion in Rat Hearts. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1968–H1974. [Google Scholar] [CrossRef]
- Sato, K.; Kashiwaya, Y.; Keon, C.A.; Tsuchiya, N.; King, M.T.; Radda, G.K.; Chance, B.; Clarke, K.; Veech, R.L. Insulin, Ketone Bodies, and Mitochondrial Energy Transduction. FASEB J. 1995, 9, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ—Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef]
- Goday, A.; Bellido, D.; Sajoux, I.; Crujeiras, A.B.; Burguera, B.; García-Luna, P.P.; Oleaga, A.; Moreno, B.; Casanueva, F.F. Short-Term Safety, Tolerability and Efficacy of a Very Low-Calorie-Ketogenic Diet Interventional Weight Loss Program versus Hypocaloric Diet in Patients with Type 2 Diabetes Mellitus. Nutr. Diabetes 2016, 6, e230. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, J.H.; van Dijck, L.; Töns, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.E.P.B.; de Koning, E.J.P. Long-Term Ketogenic Diet Causes Glucose Intolerance and Reduced β- and α-Cell Mass but No Weight Loss in Mice. Am. J. Physiol. Endocr. Metab. 2014, 306, E552–E558. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-Chain Fatty Acids and Ketones Directly Regulate Sympathetic Nervous System via G Protein-Coupled Receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Tao, H.; Cao, W.; Cao, L.; Lin, Y.; Zhao, S.M.; Xu, W.; Cao, J.; Zhao, J.Y. Ketogenic Diets Inhibit Mitochondrial Biogenesis and Induce Cardiac Fibrosis. Signal Transduct. Target Ther. 2021, 6, 54. [Google Scholar] [CrossRef]
- Hasselbaink, D.M.; Glatz, J.F.; Luiken, J.J.; Roemen, T.H.; Van der Vusse, G.J. Ketone Bodies Disturb Fatty Acid Handling in Isolated Cardiomyocytes Derived from Control and Diabetic Rats. Biochem. J. 2003, 371, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Vanoverschelde, J.L.; Wijns, W.; Kolanowski, J.; Bol, A.; Decoster, P.M.; Michel, C.; Cogneau, M.; Heyndrickx, G.R.; Essamri, B.; Melin, J.A. Competition between Palmitate and Ketone Bodies as Fuels for the Heart: Study with Positron Emission Tomography. Am. J. Physiol. 1993, 264, H701–H707. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Björnholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F.P. Enhanced Muscle Insulin Sensitivity After Contraction/Exercise Is Mediated by AMPK. Diabetes 2017, 66, 598–612. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, C.; Battaglia, E.; Young, R.; Suzuki, G. LKB1 Knockout Mouse Develops Spontaneous Atrial Fibrillation and Provides Mechanistic Insights Into Human Disease Process. JAHA 2015, 4, e001733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, A.; Ghannam, M.; Liang, J.; Saeed, M.; Cunnane, R.; Ghanbari, H.; Latchamsetty, R.; Crawford, T.; Batul, S.A.; Chung, E.; et al. Effect of Metformin on Outcomes of Catheter Ablation for Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2021, 32, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Ostropolets, A.; Elias, P.A.; Reyes, M.V.; Wan, E.Y.; Pajvani, U.B.; Hripcsak, G.; Morrow, J.P. Metformin Is Associated With a Lower Risk of Atrial Fibrillation and Ventricular Arrhythmias Compared With Sulfonylureas: An Observational Study. Circ. Arrhythm. Electrophysiol. 2021, 14, e009115. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Z.; Hou, T.T.; Yuan, Y.; Hang, P.Z.; Zhao, J.J.; Sun, L.; Zhao, G.Q.; Zhao, J.; Dong, J.M.; Wang, X.B.; et al. Fenofibrate Inhibits Atrial Metabolic Remodelling in Atrial Fibrillation through PPAR-Alpha/Sirtuin 1/PGC-1alpha Pathway. Br. J. Pharmacol. 2016, 173, 1095–1109. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Li, W.; Li, Y.; Zhao, J.; Wang, L.; Dong, D.; Pan, Z.; Yang, B. Activation of Beta(3)-Adrenoceptor Promotes Rapid Pacing-Induced Atrial Electrical Remodeling in Rabbits. Cell Physiol. Biochem. 2011, 28, 87–96. [Google Scholar] [CrossRef]
- Bai, F.; Liu, Y.; Tu, T.; Li, B.; Xiao, Y.; Ma, Y.; Qin, F.; Xie, J.; Zhou, S.; Liu, Q. Metformin Regulates Lipid Metabolism in a Canine Model of Atrial Fibrillation through AMPK/PPAR-Alpha/VLCAD Pathway. Lipids Health Dis. 2019, 18, 109. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, C.; Dixit, G.; Li, Z. Activation of AMP-Activated Protein Kinases Prevents Atrial Fibrillation. J. Cardiovasc. Trans. Res. 2021, 14, 492–502. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middeldorp, M.E.; Pathak, R.K.; Meredith, M.; Mehta, A.B.; Elliott, A.D.; Mahajan, R.; Twomey, D.; Gallagher, C.; Hendriks, J.; Linz, D.; et al. PREVEntion and RegReSsive Effect of Weight-Loss and Risk Factor Modification on Atrial Fibrillation: The REVERSE-AF Study. Europace 2018, 20, 1929–1935. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of Atrial Fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef] [PubMed]

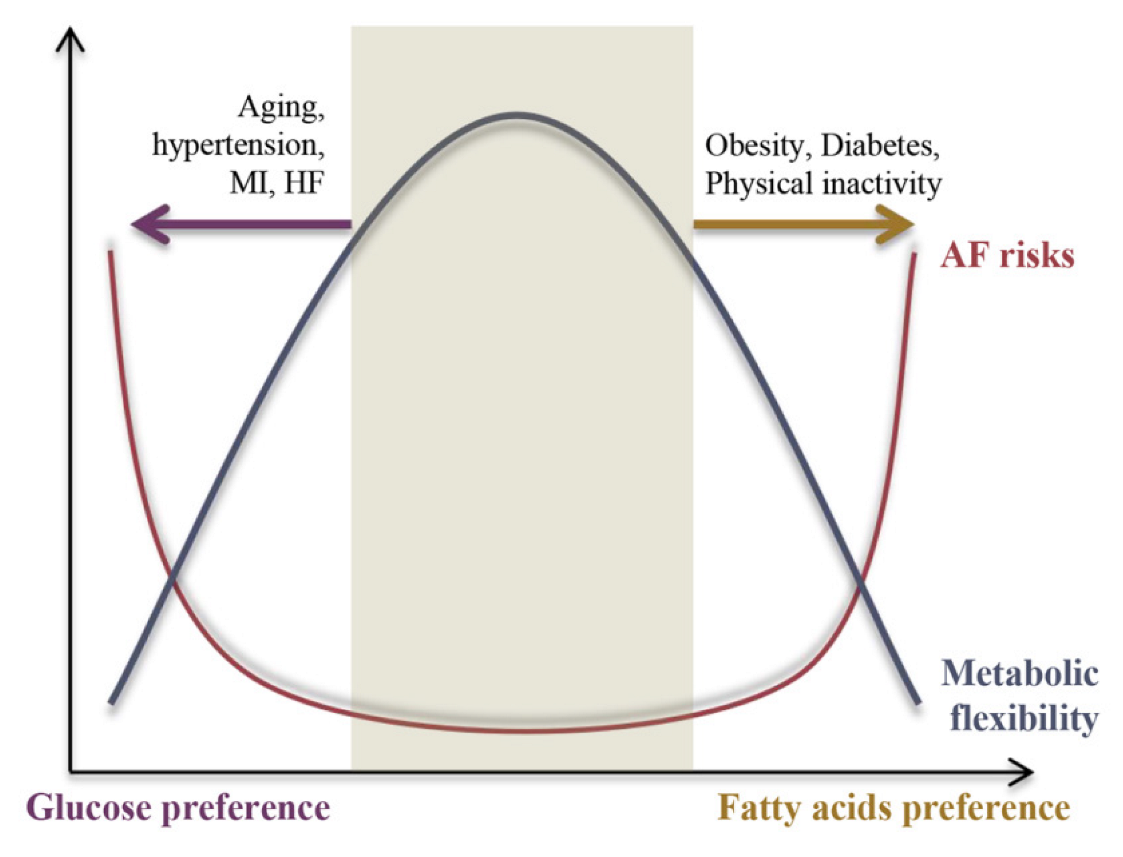
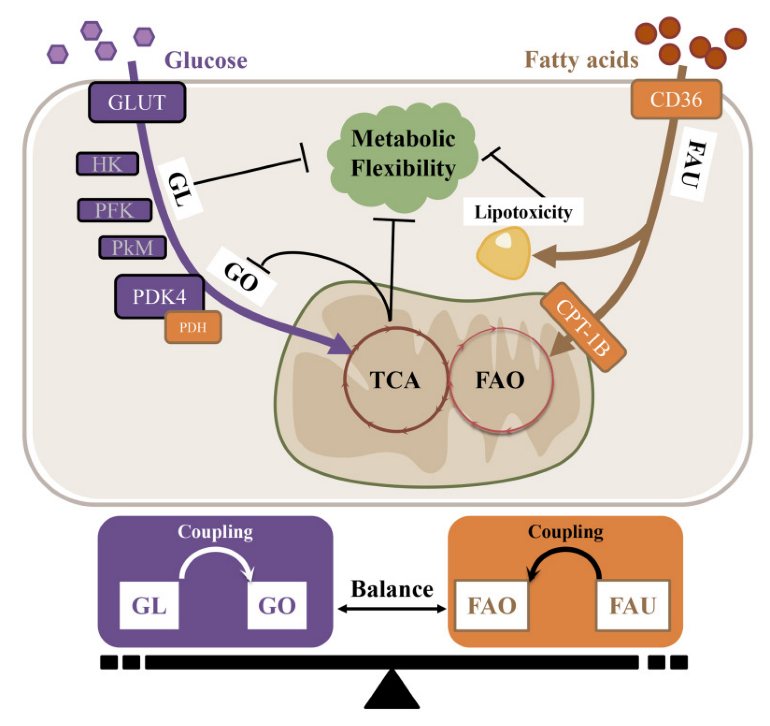
| AF Risk Factors | Clinical Relevance | Metabolic Abnormalities | Pro-FAO or Pro-GL | Metabolic Inflexibility |
|---|---|---|---|---|
| Obesity | The Framingham cohort and the Women’s Health Study revealed a tight correlation between BMI and AF risk [43,44]; a meta-analysis of 51 studies including 626,603 subjects revealed a 20%–30% increase risk in incident AF, a 10% risk of post-operative AF, and a 13% risk in post-ablation AF for 5-Unit increase in BMI [45]; Deng et al. revealed a U-shaped relationship between obesity and post-ablation AF recurrence [46]. The epidemiology of AF in obesity was reviewed in [47] | Increased fatty acids uptake; Increased fatty acids oxidation; Increased lipotoxicity; Decreased glucose oxidation; Decreased aerobic glycolysis; Increased insulin resistance; | pro-FAO | Yes [48] |
| T1DM | A prospective case-control study including 36,258 patients with T1DM and 179,980 controls showed T1DM was associated with a modest (13%) increase of AF risk in men, and a significant (50%) increase of AF risk in women [49]. The epidemiology of AF in diabetes was reviewed in [50]. | Increased fatty acids oxidation; Decreased glucose oxidation; Increased insulin resistance | pro-FAO | Yes |
| T2DM | T2DM was associated with increased AF risk in meta-analysis (39% or 49%) [51,52], the Atherosclerosis Risk in Communities Study (35%) [53] or the Women’s Health Study (37%) [54]. The epidemiology of AF in diabetes was reviewed in [50]. | Increased fatty acids oxidation; Decreased glucose oxidation; Increased insulin resistance | pro-FAO | Yes [55] |
| Aging | It is widely accepted that aging is the most important determinant of AF risk [56]. In the Rotterdam study, AF prevalence was 5.5%, rising from 0.7% in the age group 55–59 years to 17.8% in those aged 85 years and above [57]. | Increased fatty acids uptake; Decreased fatty acids oxidation; Increased glycolysis; Increased insulin resistance; | pro-GL | Yes [58] |
| Physical inactivity | Evidence revealed a J-shaped relationship between physical activity and AF risk [59]. The Cardiovascular Health Study demonstrated 26% of new AF cases were attributable to a lack of physiological activity, and moderate intensity exercise had a 28% lower AF risk compared with no regular exercise [60]. The Nord-Trøndelag Health Study 3 (HUNT3) showed that physical activity exerted an anti-AF effect independent of obesity [61]. | Increased glycolysis; Decreased fatty acids oxidation; Increased insulin resistance; | pro-GL | Yes |
| Myocardial infarction | AF incidence in patients admitted to hospital with AMI varied between 6.8–21%. The epidemiology of AF in acute myocardial infarction was reviewed in [62]. | Increased glycolysis; Decreased fatty acids oxidation; Increased insulin resistance; | pro-GL | Yes |
| Hypertension | In the Framingham Heart Study, hypertension portended an excess risk for AF by 50% in men and 40% in women [63]. In the Atherosclerosis Risk in Communities study, hypertension explained ~20% of new cases, and was the main contributor to AF burden [64]. Among AF patients, hypertension accounts for 60% to 80% of patients with established AF [65]. The epidemiology of AF in hypertension was reviewed in [66]. | Increased glycolysis; Decreased glucose oxidation; Increased insulin resistance; | pro-GL | Yes [67] |
| HF | HF is the strongest predictor for AF risk. In the Framingham Study, HF increased AF risk 5-fold in men and 6-fold in women [63]. AF risk increased dramatically with increasing HF severity. The epidemiology of AF in HF was reviewed in [68]. | Increased glycolysis; Decreased fatty acids oxidation; Increased insulin resistance; | pro-GL | Yes [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Zhang, Y.; Zheng, Q. Metabolic Inflexibility as a Pathogenic Basis for Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 8291. https://doi.org/10.3390/ijms23158291
Qin X, Zhang Y, Zheng Q. Metabolic Inflexibility as a Pathogenic Basis for Atrial Fibrillation. International Journal of Molecular Sciences. 2022; 23(15):8291. https://doi.org/10.3390/ijms23158291
Chicago/Turabian StyleQin, Xinghua, Yudi Zhang, and Qiangsun Zheng. 2022. "Metabolic Inflexibility as a Pathogenic Basis for Atrial Fibrillation" International Journal of Molecular Sciences 23, no. 15: 8291. https://doi.org/10.3390/ijms23158291
APA StyleQin, X., Zhang, Y., & Zheng, Q. (2022). Metabolic Inflexibility as a Pathogenic Basis for Atrial Fibrillation. International Journal of Molecular Sciences, 23(15), 8291. https://doi.org/10.3390/ijms23158291







