Pharmacological Probes to Validate Biomarkers for Analgesic Drug Development
Abstract
:1. Introduction
- Confirmation of target engagement in preclinical studies. Preclinical evidence of anti-nociceptive efficacy from classical behavioural animal experiments may be strengthened by biomarker assessments.
- Estimates of the doses that must turn out to be safe and tolerated in early clinical trials and that are needed to achieve target engagement in humans. The possibility of early deselection of drugs with insufficient target engagement in Phase 1 clinical trials conducted in a limited number of healthy volunteers will reduce the failure rates of Phase 3 trials, which may make investments in analgesic development programs more attractive.
- Identification of patients who are likely to benefit from the candidate drug based on an early evaluation of target engagement, independently of PROs.
2. Methods
2.1. Criteria for Selecting Pharmacological Probes
- Evidence that the drug interacts with the central or peripheral nervous system, preferably with one specific compartment;
- Drug registered across the EU, preferably as an analgesic, and commercially available in an oral formulation;
- Sufficient evidence for target engagement and modulation of nociceptive processing in humans. This could be a marketing authorisation as an analgesic, literature data evidencing clinical analgesic efficacy or literature or in-house data indicating a relevant effect of the drug on at least some of the selected biomarkers of nociceptive processing;
- No active drug metabolites;
- Tmax not exceeding 2 h and, preferably, a t1/2 of about 2 to 12 h;
- No dependency of PK parameters on genetic polymorphism (e.g., CYP2D6). This dependency could be accounted for in pharmacometric analysis but would result in a relevantly increased cost and workload and would not have added value for biomarker validation;
- Tolerability profile indicating that side effects (e.g., sedation or nausea) would not interfere excessively with the biomarker assessments at the tested doses.
- NSAIDs (including dipyrone/metamizole, aspirin and cyclo-oxygenase-II inhibitors), propacetamol/paracetamol, corticosteroids, sulfasalazine, leflunomide, 5-amino-salicylic acid, resveratrol, triptans, tolterodine and diacerein because they primarily target processes distal to peripheral nociceptive nerve terminals (e.g., inflammatory processes);
- All local anaesthetics and all fentanyl analogues, buprenorphine, nalbuphine, pethidine, clonidine, dexmedetomidine, ketamine, anaesthetic gases and capsaicin because they are not available in an oral formulation across the EU;
- Hydrocodone, pentazocine, butorphanol, cebranopadol, levo-alpha-acetyl-methadol (LAAM), cannabinoids, curcumin, caffeine, calcitonin, palmitoylethanolamide, sucrose, substances without an international non-proprietary name (INN), herbal medicines and traditional Chinese medicines because they are not authorised medicines across the EU;
- Flupirtine and dextropropoxyphene because they were discontinued in the EU market for safety reasons;
- Retigabine because its worldwide manufacture was discontinued; and
- Controlled-release and abuse-deterrent formulations of known analgesics because formulations must be fast-acting, oral and commercially available.
2.2. Search for Candidate Pharmacological Probes
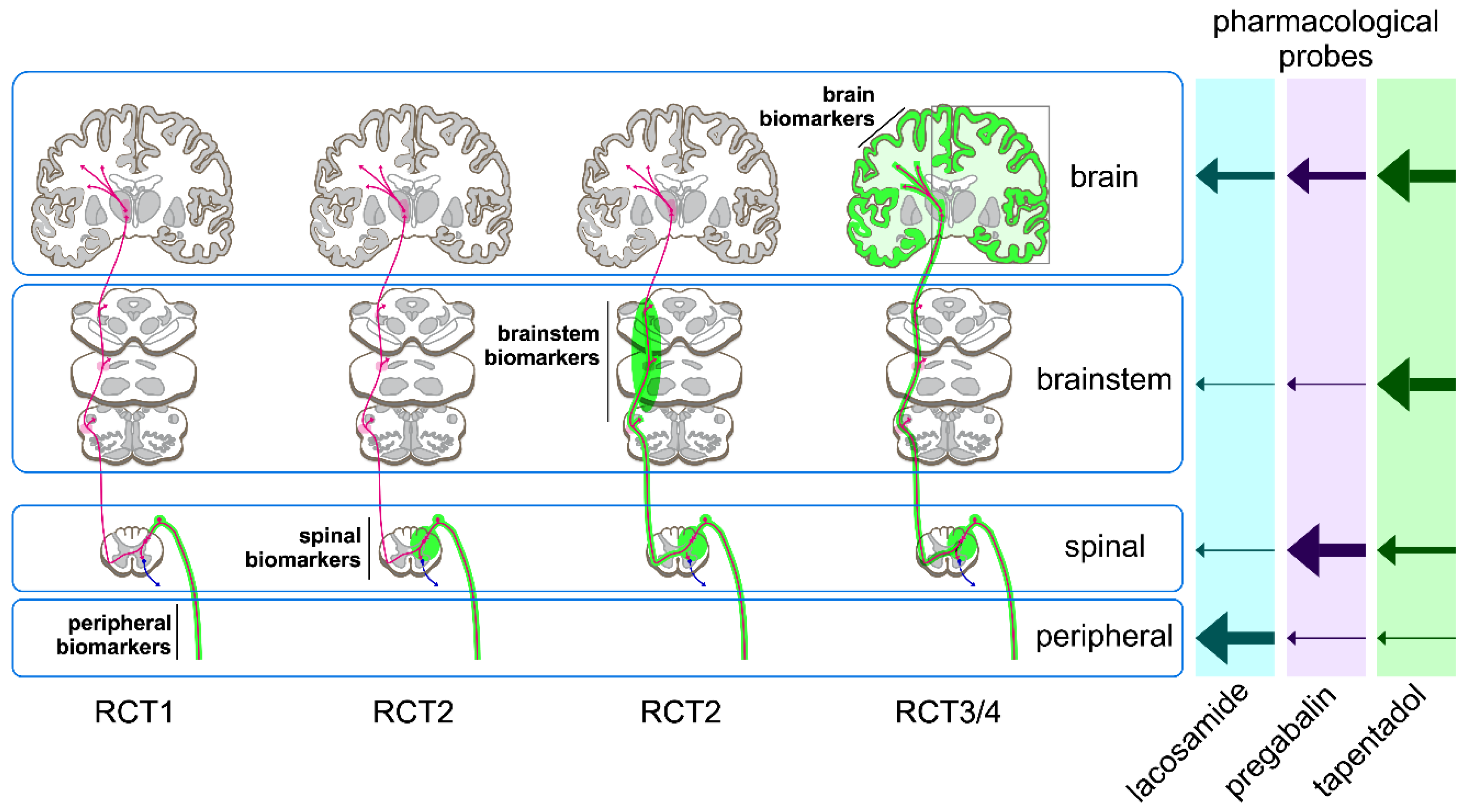
2.3. Additional Constraints for Preclinical/Clinical Study Design
3. Results
4. Discussion
4.1. Probes to Validate Biomarkers of Target Engagement at Peripheral Level
4.2. Probes to Validate Biomarkers of Nociceptive Processing at the Spinal Level
4.3. Probes to Validate Biomarkers of Nociceptive Processing at the Cortical Level
4.4. Proposed Pharmacometric Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CYP2D6 | iso-enzyme 2D6 of the cytochrome P450 system |
| EFPIA | European Federation of Pharmaceutical Industry Associations |
| EU | European Union |
| IASP | International Association for the Study of Pain |
| IMI | Innovative Medicines Initiative (of the EU) |
| iPSC | human induced pluripotent stem cell |
| MOR | µ-opioid receptor |
| NeuPSIG | Neuropathic Pain Special Interest Group (of the IASP) |
| PK | pharmacokinetic(s) |
| SmPC | Summary of Product Characteristics |
| Tmax | time to peak plasma concentration |
| t1/2 | elimination half-life |
| Nomenclature | |
| BioPain | a subproject of the IMI-PainCare project, which is a consortium of researchers from academia, hospitals, small-medium sized enterprises, patient organizations, pain societies and the pharmaceutical industry that combines and mutually shares expertise. Its goal is fulfilment of the objectives of Call H2020-JTI-IMI2-10, topic 3. |
| Call H2020-JTI-IMI2-10, topic 3 | a request for research proposals issued by the Innovative Medicines Initiative of the European Union and EFPIA in the framework of the Horizon 2020 pro-gramme. |
References
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017, 16, 545–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Colburn, W.; Degruttola, V.; Demets, D.; Downing, G.J.; Hoth, D.; Oates, J.A.; Peck, C.C.; Schooley, R.; Spilker, B.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. Available online: https://www.researchgate.net/publication/216211770_Biomarkers_and_surrogate_endpoints_Preferred_definitions_and_conceptual_framework (accessed on 9 September 2020).
- Tracey, I.; Woolf, C.J.; Andrews, N.A. Composite Pain Biomarker Signatures for Objective Assessment and Effective Treatment. Neuron 2019, 101, 783–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Call H2020-JTI-IMI2-2016-10, Topic 3: A Request for Research Proposals Issued by the Innovative Medicines Initiative of the European Union and EFPIA in the Framework of the Horizon 2020 Programme. Available online: https://www.imi.europa.eu/apply-funding/closed-calls/imi2-call-10 (accessed on 9 September 2020).
- EUClinicalTrialsRegister1. EU Clinical Trials Register (2019, June-). IMI2-PainCare-BioPain-RCT1. Identifier EuDRA-CT 2019-000942-36. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-000942-36/DK (accessed on 7 July 2022).
- EUClinicalTrialsRegister2. EU Clinical Trials Register (2020, November-). IMI2-PainCare-BioPain-RCT2. Identifier EuDRA-CT 2019-000755-14. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-000755-14/IT (accessed on 7 July 2022).
- EUClinicalTrialsRegister3. EU Clinical Trials Register (2019, June-). IMI2-PainCare-BioPain-RCT3. Identifier EuDRA-CT 2019-001204-37. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-001204-37/BE (accessed on 7 July 2022).
- EUClinicalTrialsRegister4. EU Clinical Trials Register (2019, July–). IMI2-PainCare-BioPain-RCT4. Identifier EuDRA-CT 2019-000908-15. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-000908-15/DK (accessed on 7 July 2022).
- Mouraux, A.; Bloms-Funke, P.; Boesl, I.; Caspani, O.; Chapman, S.C.; Di Stefano, G.; Finnerup, N.B.; Garcia-Larrea, L.; Goetz, M.; Kostenko, A.; et al. IMI2-PainCare-BioPain-RCT3: A randomized, double-blind, placebo-controlled, crossover, multi-center trial in healthy subjects to investigate the effects of lacosamide, pregabalin, and tapentadol on biomarkers of pain processing observed by electroencephalography (EEG). Trials 2021, 22, 404. [Google Scholar] [CrossRef]
- Nochi, Z.; Pia, H.; Bloms-Funke, P.; Boesl, I.; Caspani, O.; Chapman, S.C.; Fardo, F.; Genser, B.; Goetz, M.; Kostenko, A.V.; et al. IMI2-PainCare-BioPain-RCT1: Study protocol for a randomized, double-blind, placebo-controlled, crossover, multi-center trial in healthy subjects to investigate the effects of lacosamide, pregabalin, and tapentadol on biomarkers of pain processing observed by peripheral nerve excitability testing (NET). Trials 2022, 23, 163. [Google Scholar] [CrossRef] [PubMed]
- Leone, C.; Di Stefano, G.; Di Pietro, G.; Bloms-Funke, P.; Boesl, I.; Caspani, O.; Chapman, S.C.; Finnerup, N.B.; Garcia-Larrea, L.; Li, T.; et al. IMI2-PainCare-BioPain-RCT2 protocol: A randomized, double-blind, placebo-controlled, cross-over, multicenter trial in healthy subjects to investigate the effects of lacosamide, pregabalin and tapentadol on biomarkers of pain processing observed by non-invasive neurophysiological measurements of human spinal cord and brainstem activity. Trials, 2022; accepted for publication. [Google Scholar]
- More Information on the IMI-PainCare Project and Its Subtopic BioPain is Available on. Available online: http://www.imi-paincare.eu (accessed on 9 September 2020).
- SmPCs in English language Were Retrieved from the Website of the UK Drug Regulatory Authority. Available online: https://www.medicines.org.uk (accessed on 9 September 2020).
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Demant, D.T.; Lund, K.; Vollert, J.; Maier, C.; Segerdahl, M.; Finnerup, N.; Jensen, T.S.; Sindrup, S.H. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014, 155, 2263–2273. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Lunn, M.P.; Moore, R.A. Topiramate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2013, 8, CD008314. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Moore, R.A. Lamotrigine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2013, 2019, CD006044. [Google Scholar] [CrossRef] [PubMed]
- Hearn, L.; Derry, S.; Moore, R.A. Lacosamide for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, 2016, CD009318. [Google Scholar] [CrossRef]
- Tzschentke, T.M.; Christoph, T.; Kögel, B.; Schiene, K.; Hennies, H.-H.; Englberger, W.; Haurand, M.; Jahnel, U.; Cremers, T.I.F.H.; Friderichs, E.; et al. (–)-(1R,2R)-3-(3-Dimethylamino-1-ethyl-2-methyl-propyl)-phenol Hydrochloride (Tapentadol HCl): A Novel μ-Opioid Receptor Agonist/Norepinephrine Reuptake Inhibitor with Broad-Spectrum Analgesic Properties. J. Pharmacol. Exp. Ther. 2007, 323, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; Codd, E.E.; Vaught, J.L.; Jacoby, H.I.; Selve, N. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J. Pharmacol. Exp. Ther. 1993, 267, 331–340. [Google Scholar] [PubMed]
- Buvanendran, A.; Kroin, J.S.; Kari, M.; Tuman, K.J. Can a Single Dose of 300 mg of Pregabalin Reach Acute Antihyperalgesic Levels in the Central Nervous System? Reg. Anesth. Pain Med. 2010, 35, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Danhof, M.; de Lange, E.; Della Pasqua, O.E.; Ploeger, B.A.; Voskuyl, R.A. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol. Sci. 2008, 29, 186–191. [Google Scholar] [CrossRef]
- Xu, X.S.; Smit, J.W.; Lin, R.; Stuyckens, K.; Terlinden, R.; Nandy, P.; Xu, X.S. Population Pharmacokinetics of Tapentadol Immediate Release (IR) in Healthy Subjects and Patients with Moderate or Severe Pain. Clin. Pharmacokinet. 2010, 49, 671–682. [Google Scholar] [CrossRef]
- Antunes, N.D.; van Dijkman, S.C.; Lanchote, V.L.; Wichert-Ana, L.; Coelho, E.B.; Junior, V.A.; Takayanagui, O.M.; Tozatto, E.; Hasselt, J.G.C.; della Pasqua, O. Population pharmacokinetics of oxcarbazepine and its metabolite 10-hydroxycarbazepine in healthy subjects. Eur. J. Pharm. Sci. 2017, 109, S116–S123. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic Pain: A Maladaptive Response of the Nervous System to Damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Kellinghaus, C. Lacosamide as treatment for partial epilepsy: Mechanisms of action, pharmacology, effects, and safety. Ther. Clin. Risk Manag. 2009, 5, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, D.; Hidvegi, T.; Gurieva, I.; Bongardt, S.; Freynhagen, R.; Sen, D.; Sommerville, K. Efficacy and Safety of Lacosamide in Painful Diabetic Neuropathy. Diabetes Care 2010, 33, 839–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaibani, A.; Fares, S.; Selam, J.-L.; Arslanian, A.; Simpson, J.; Sen, D.; Bongardt, S. Lacosamide in Painful Diabetic Neuropathy: An 18-Week Double-Blind Placebo-Controlled Trial. J. Pain 2009, 10, 818–828. [Google Scholar] [CrossRef]
- Wymer, J.P.; Simpson, J.; Sen, D.; Bongardt, S. Efficacy and Safety of Lacosamide in Diabetic Neuropathic Pain An 18-week Double-blind Placebo-controlled Trial of Fixed-dose Regimens. Clin. J. Pain 2009, 25, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Rauck, R.L.; Shaibani, A.; Biton, V.; Simpson, J.; Koch, B. Lacosamide in Painful Diabetic Peripheral Neuropathy. A Phase 2 Double-blind Placebo-controlled Study. Clin. J. Pain 2007, 23, 150–158. [Google Scholar] [CrossRef] [PubMed]
- De Greef, B.T.; Hoeijmakers, J.G.J.; Geerts, M.; Oakes, M.; Church, T.J.E.; Waxman, S.G.; Dib-Hajj, S.D.; Faber, C.G.; Merkies, I.S.J. Lacosamide in patients with Nav1.7 mutations-related small fibre neuropathy: A randomized controlled trial. Brain 2019, 142, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Namer, B.; Schmidt, D.; Eberhardt, E.; Maroni, M.; Dorfmeister, E.; Kleggetveit, I.P.; Kaluza, L.; Meents, J.; Gerlach, A.; Lin, Z.; et al. Pain relief in a neuropathy patient by lacosamide: Proof of principle of clinical translation from patient-specific iPS cell-derived nociceptors. eBioMedicine 2018, 39, 401–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Esdonk, M.J.; Lindeman, I.; Okkerse, P.; de Kam, M.L.; Groeneveld, G.J.; Stevens, J. Population Pharmacokinetic/Pharmacodynamic Analysis of Nociceptive Pain Models Following an Oral Pregabalin Dose Administration to Healthy Subjects. CPT Pharmacomet. Syst. Pharm. 2018, 7, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Singh, N.; Jaggi, A.S. Pregabalin in Neuropathic Pain: Evidences and Possible Mechanisms. Curr. Neuropharmacol. 2014, 12, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Moy, J.K.; Hartung, J.E.; Duque, M.G.; Friedman, R.; Nagarajan, V.; Loeza-Alcocer, E.; Koerber, H.R.; Christoph, T.; Schröder, W.; Gold, M.S. Distribution of functional opioid receptors in human dorsal root ganglion neurons. Pain 2020, 161, 1636–1649. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Zhang, G.; Bouvier, C.; Saez, C.; Ronnekleiv, O.K.; Kelly, M.J.; Grandy, D.K. Characterization and Distribution of a Cloned Rat μ-Opioid Receptor. J. Neurochem. 2002, 64, 14–24. [Google Scholar] [CrossRef]
- Arvidsson, U.; Riedl, M.; Chakrabarti, S.; Lee, J.H.; Nakano, A.H.; Dado, R.J.; Loh, H.H.; Law, P.Y.; Wessendorf, M.W.; Elde, R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J. Neurosci. 1995, 15, 3328–3341. [Google Scholar] [CrossRef] [Green Version]
- Emery, E.C.; Luiz, A.P.; Wood, J.N. Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin. Ther. Targets 2016, 20, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Bennett, D.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef] [PubMed]
- May, T.W.; Brandt, C.; Helmer, R.; Bien, C.G.; Cawello, W. Comparison of lacosamide concentrations in cerebrospinal fluid and serum in patients with epilepsy. Epilepsia 2015, 56, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Michelhaugh, S.K.; Basha, B.; Rhoney, D.H.; Shah, A.K.; Mittal, S. Acute or chronic use of lacosamide does not alter its distribution between serum and cerebrospinal fluid. Epilepsia 2015, 56, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.-S.; Kim, S.-J.; Ha, D.-J.; Baek, M.; Moon, H. Pharmacokinetics, brain distribution, and plasma protein binding of the antiepileptic drug lacosamide in rats. Arch. Pharm. Res. 2011, 34, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.R.; Turluck, D.; Burleigh, J.; Lister, R.; Fan, C.; Middlebrook, A.; Taylor, C.; Su, T. Brain microdialysis and PK/PD correlation of pregabalin in rats. Eur. J. Drug Metab. Pharmacokinet. 2001, 26, 123–128. [Google Scholar] [CrossRef]
- Schröder, W.; Tzschentke, T.M.; Terlinden, R.; De Vry, J.; Jahnel, U.; Christoph, T.; Tallarida, R.J. Synergistic interaction between the two mechanisms of action of tapentadol in analgesia. J. Pharmacol. Exp. Ther. 2011, 337, 312–320, Erratum in J. Pharmacol. Exp. Ther. 2012, 342, 232; Erratum in J. Pharmacol. Exp. Ther. 2014, 348, 489. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Dickenson, A.H. Mechanisms of the gabapentinoids and α2δ-1 calcium channel subunit in neuropathic pain. Pharmacol. Res. Perspect. 2016, 4, e00205. [Google Scholar] [CrossRef]
- Di Lionardo, A.; Di Stefano, G.; Leone, C.; Di Pietro, G.; Sgro, E.; Malara, E.; Cosentino, C.; Mollica, C.; Blockeel, A.J.; Caspani, O.; et al. Modulation of the N13 component of the somatosensory evoked potentials in an experimental model of central sensitization in humans. Sci. Rep. 2021, 11, 20838. [Google Scholar] [CrossRef]
- Arikkath, J.; Campbell, K.P. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003, 13, 298–307. [Google Scholar] [CrossRef]
- Bian, F.; Li, Z.; Offord, J.; Davis, M.; McCormick, J.; Taylor, C. Calcium channel α(2)-δ type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: An ex vivo autoradiographic study in α(2)-δ type 1 genetically modified mice. Brain Res. 2006, 1075, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Belliotti, T.R.; Capiris, T.; Ekhato, I.V.; Kinsora, J.J.; Field, M.J.; Heffner, T.G.; Meltzer, L.T.; Schwarz, J.B.; Taylor, C.P.; Thorpe, A.J.; et al. Structure-activity relationships of pregabalin and analogues that target the α(2)-δ protein. J. Med. Chem. 2005, 48, 2294–2307. [Google Scholar] [CrossRef] [PubMed]
- Gazulla, J.; Tintoré, M. The P/Q-type voltage-dependent calcium channel as pharmacological target in spinocerebellar ataxia type 6: Gabapentin and pregabalin may be of therapeutic benefit. Med. Hypotheses 2007, 68, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Dooley, D.J.; Donovan, C.M.; Pugsley, T.A. Stimulus-dependent modulation of [(3)H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J. Pharmacol. Exp. Ther. 2000, 295, 1086–1093. [Google Scholar]
- Dooley, D.J.; Mieske, C.A.; Borosky, S.A. Inhibition of K+-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci. Lett. 2000, 280, 107–110. [Google Scholar] [CrossRef]
- Fink, K.; Dooley, D.J.; Meder, W.P.; Suman-Chauhan, N.; Duffy, S.; Clusmann, H.; Göthert, M. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 2002, 42, 229–236. [Google Scholar] [CrossRef]
- Errante, L.D.; Petroff, O.A. Acute effects of gabapentin and pregabalin on rat forebrain cellular GABA, glutamate, and glutamine concentrations. Seizure 2003, 12, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, M.; Woodhall, G.; Thompson, S.; Dooley, D.; Jones, R. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur. J. Neurosci. 2004, 20, 1566–1576. [Google Scholar] [CrossRef]
- Schiltmeyer, B.; Cawello, W.; Kropeit, D.; Horstmann, R. Population Pharmacokinetics of the New Antiepileptic Drug Lacosamide in Healthy Subjects with Different Age and Gender. In Proceedings of the Population Approach Group in Europe (PAGE) Conference, Pamplona, Spain, 16–17 June 2005; p. 14. [Google Scholar]
- Micheva, K.D.; Taylor, C.P.; Smith, S.J. Pregabalin Reduces the Release of Synaptic Vesicles from Cultured Hippocampal Neurons. Mol. Pharmacol. 2006, 70, 467–476. [Google Scholar] [CrossRef] [Green Version]


| Substance | Evidence for Use in Pain | Active Metabolites? 10 | Tmax (h) 4 | t1/2 (h) 3 | Polymor-phism? 9 | Mode of Action 8 | Active in Compartment | ||
|---|---|---|---|---|---|---|---|---|---|
| P 2 | S 2 | B 2I | |||||||
| Substances with marketing authorisation as an analgesic and without relevant active metabolites | |||||||||
| Tapentadol | Registered as an analgesic | No | 1.25 | 4 | no | M, NA | + | + | |
| Pregabalin | Registered for neuropathic pain | No | 1 | 6.3 | no | CC, NT | + | + | |
| Methadone | Registered as an analgesic | Not reported | 1.5–3 | 19–55 | minor, CYP2D6, CYP2B6 | M | + | + | |
| Gabapentin | Registered for neuropathic pain | No | 2–3 | 5–7 | no | CC, NT | + | + | |
| Duloxetine | Registered for neuropathic pain | No | 6 | 8–17 | CYP2D6 | SNRI | + | + | |
| Substances with marketing authorisation as an analgesic and with relevant active metabolites | |||||||||
| Hydromorphone | Registered as an analgesic | Hydromorphone-3-glucuronide | 0.5–1 | 2–3 | no | M | + | + | |
| Morphine | Registered as an analgesic | Morphine-6-glucuronide | 1 | 2 | no | M | + | + | |
| Diacetyl-morphine 5 | Registered as an analgesic | 6-acetyl-morphine, morphine | NA 5 | 0.03–0.05 | no | M | + | + | |
| Tramadol | Registered as an analgesic | (+)-O-demethyl-tramadol | 1–2 | 5–6 | CYP2D6 | M, NA, S | + | + | |
| Oxycodone | Registered as an analgesic | Oxymorphone, noroxycodone | 1–1.5 | 3 | CYP2D6 | M | + | + | |
| Codeine 6 | Registered as an analgesic | Morphine | - | - | CYP2D6 | ||||
| Amitriptyline | Registered for neuropathic pain | Nortriptyline | 4 | 25 | CYP2D6 | SNRI | + | + | + |
| Carbamazepine | Registered for trigeminal neuralgia | Yes | 12 | 36 | no | SC | + | + | + |
| Substances with evidence of analgesic activity but without marketing authorisation as an analgesic | |||||||||
| Lacosamide | yes | No | 0.5–4 | 12–13 | no | SC | + | + | + |
| Valproate | yes | Not reported | 3–5 | 14 | no | G, SC, HDI | + | ||
| Topiramate | yes | Not clinically relevant | 1.4–4.3 | 18–22 | no | SC, GA | + | + | |
| Lamotrigine | No positive studies | No | 2.5 | 33 | no | SC | + | + | |
| Oxcarbazepine | One positive study | 10-hydroxy-carbazepine | 4.5 | 1–3 | no | SC | + | + | + |
| Substances without substantial evidence of analgesic activity | |||||||||
| Baclofen | no | Not reported | 0.5–1.5 | 3–4 | no | GB, CC | + | ||
| Tizanidine | no | Not reported | 1 | 2–4 | no | α | + | + | + |
| Safinamide | no | No | 2–3 | 20–30 | no | MB | + | + | |
| Eslicarbazepine | no | Not clinically relevant | 2.5–3 | 9–11 | no | SC | + | + | |
| Rufinamide | no | No | 4–6 | 6–10 | no | SC | + | + | |
| Phenytoin | no | Not known | ? 7 | 7–42 | no | SC | + | ||
| Ivabradine | no | Yes | 1 | 2–11 | no | If | + | + | |
| Mexiletine | no | Yes | 3.0 | 9–11 | yes | SC, AA | + | + | |
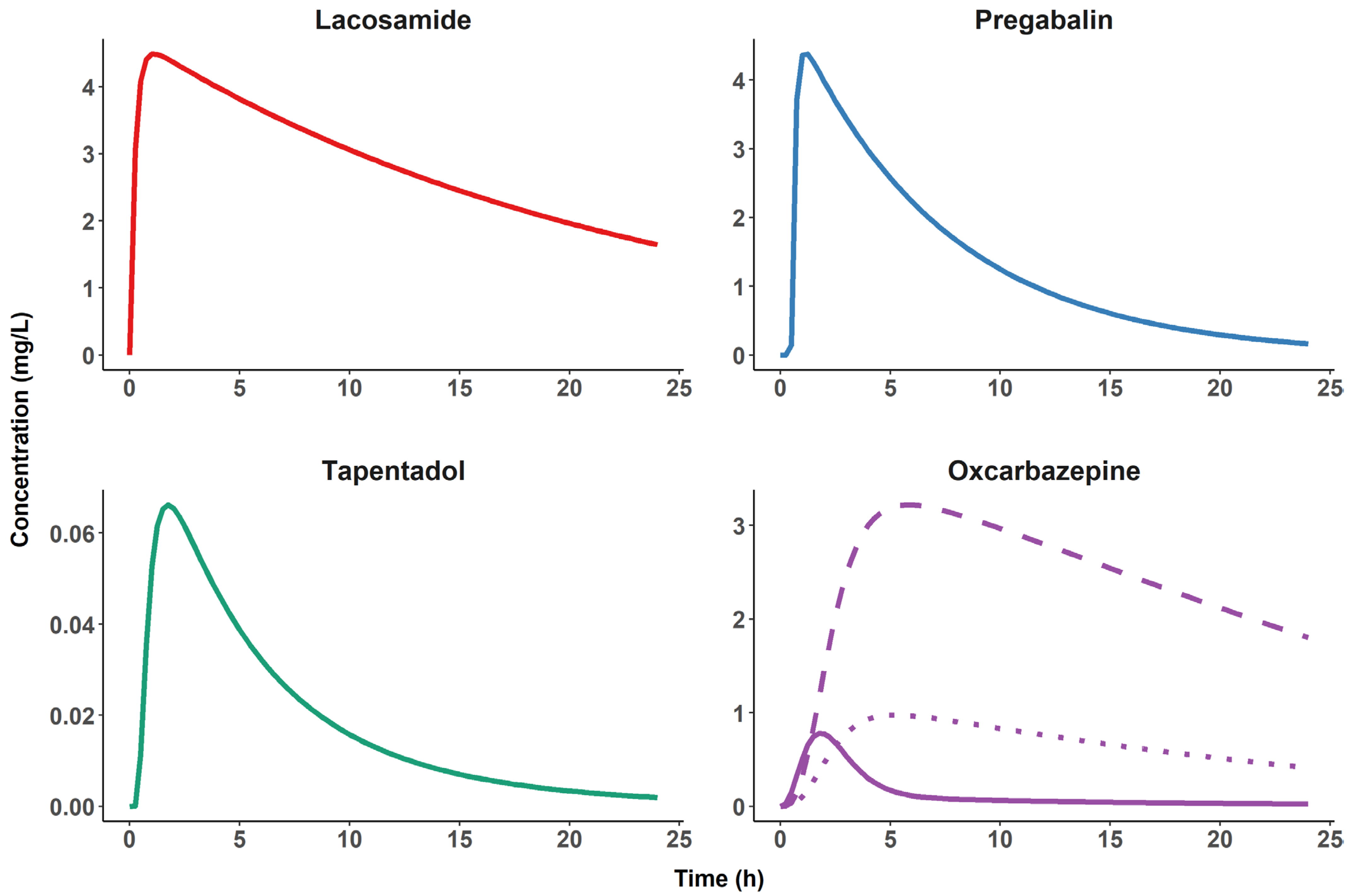
| Property | Lacosamide | Pregabalin | Tapentadol |
|---|---|---|---|
| MW (g/Mol) | 250.30 | 159.23 | 221.34 |
| Solubility (g/L) | 0.465 a | >30 b | 1.16 c |
| Lipophilicity (Log P) | 0.728 d | −1.35 | 2.87 |
| pKa | >12 e | 4.2//10.6 | 9.6//10.28 |
| BCS class | I | I | I |
| Bioavailability (%) | ≈100 | >90% | 32% |
| Fu | >0.85 | 1 | ≈0.8 |
| CL (L/h) | 1.92 # | 4.02–4.85 | 91.8 |
| V (L) | 42 # | 39.2 # | 540 |
| Unaltered fraction in urine | 0.4 | 1 | 0.03 |
| Metabolism | CYP2C9, CYP2C19 and CYP3A4 Relative contribution of each CYP still unknown | - | 70% conjugation 13% CYP2C9 and CYP2C19 2% CYP2D6 |
| CNS data | Concentration ratio: CSF/Serum † 0.85 f,, 0.641 g Brain/Plasma * 0.553 h | Concentration ratio: CSF/Plasma * ≈0.1 i | Concentration ratio: Brain/Plasma * ≈4 j |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Niel, J.; Bloms-Funke, P.; Caspani, O.; Cendros, J.M.; Garcia-Larrea, L.; Truini, A.; Tracey, I.; Chapman, S.C.; Marco-Ariño, N.; Troconiz, I.F.; et al. Pharmacological Probes to Validate Biomarkers for Analgesic Drug Development. Int. J. Mol. Sci. 2022, 23, 8295. https://doi.org/10.3390/ijms23158295
van Niel J, Bloms-Funke P, Caspani O, Cendros JM, Garcia-Larrea L, Truini A, Tracey I, Chapman SC, Marco-Ariño N, Troconiz IF, et al. Pharmacological Probes to Validate Biomarkers for Analgesic Drug Development. International Journal of Molecular Sciences. 2022; 23(15):8295. https://doi.org/10.3390/ijms23158295
Chicago/Turabian Stylevan Niel, Johannes, Petra Bloms-Funke, Ombretta Caspani, Jose Maria Cendros, Luis Garcia-Larrea, Andrea Truini, Irene Tracey, Sonya C. Chapman, Nicolás Marco-Ariño, Iñaki F. Troconiz, and et al. 2022. "Pharmacological Probes to Validate Biomarkers for Analgesic Drug Development" International Journal of Molecular Sciences 23, no. 15: 8295. https://doi.org/10.3390/ijms23158295
APA Stylevan Niel, J., Bloms-Funke, P., Caspani, O., Cendros, J. M., Garcia-Larrea, L., Truini, A., Tracey, I., Chapman, S. C., Marco-Ariño, N., Troconiz, I. F., Phillips, K., Finnerup, N. B., Mouraux, A., & Treede, R.-D. (2022). Pharmacological Probes to Validate Biomarkers for Analgesic Drug Development. International Journal of Molecular Sciences, 23(15), 8295. https://doi.org/10.3390/ijms23158295







