Endoplasmic Reticulum and Mitochondria Contacts Correlate with the Presence and Severity of NASH in Humans
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
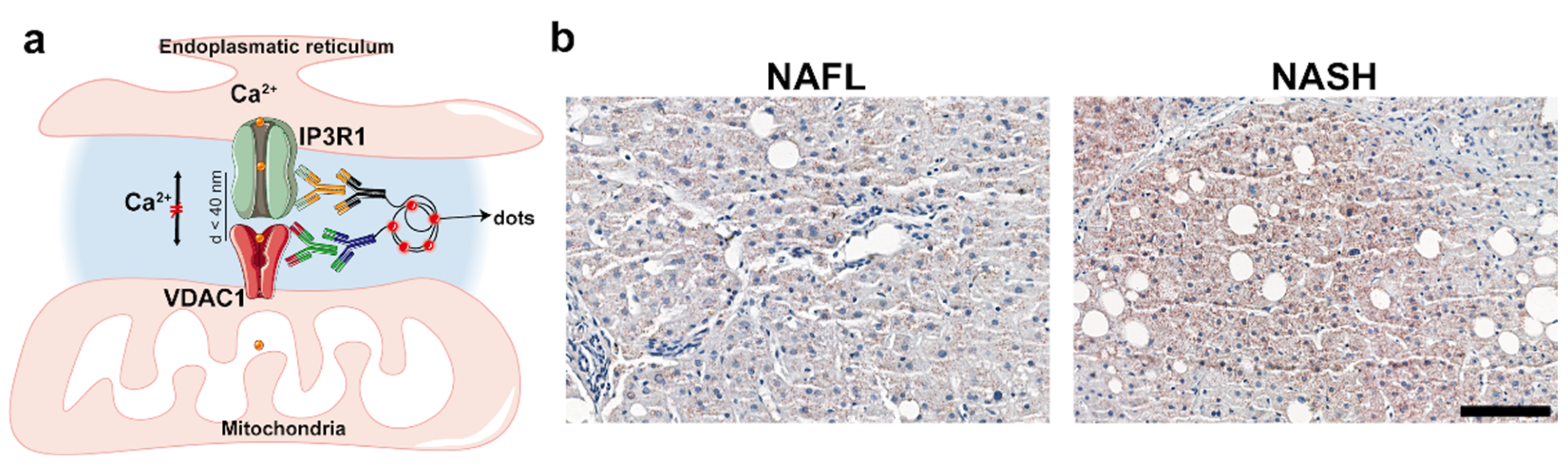
2.2. Analysis of ER–Mitochondria Contacts
2.2.1. NAFL vs. NASH
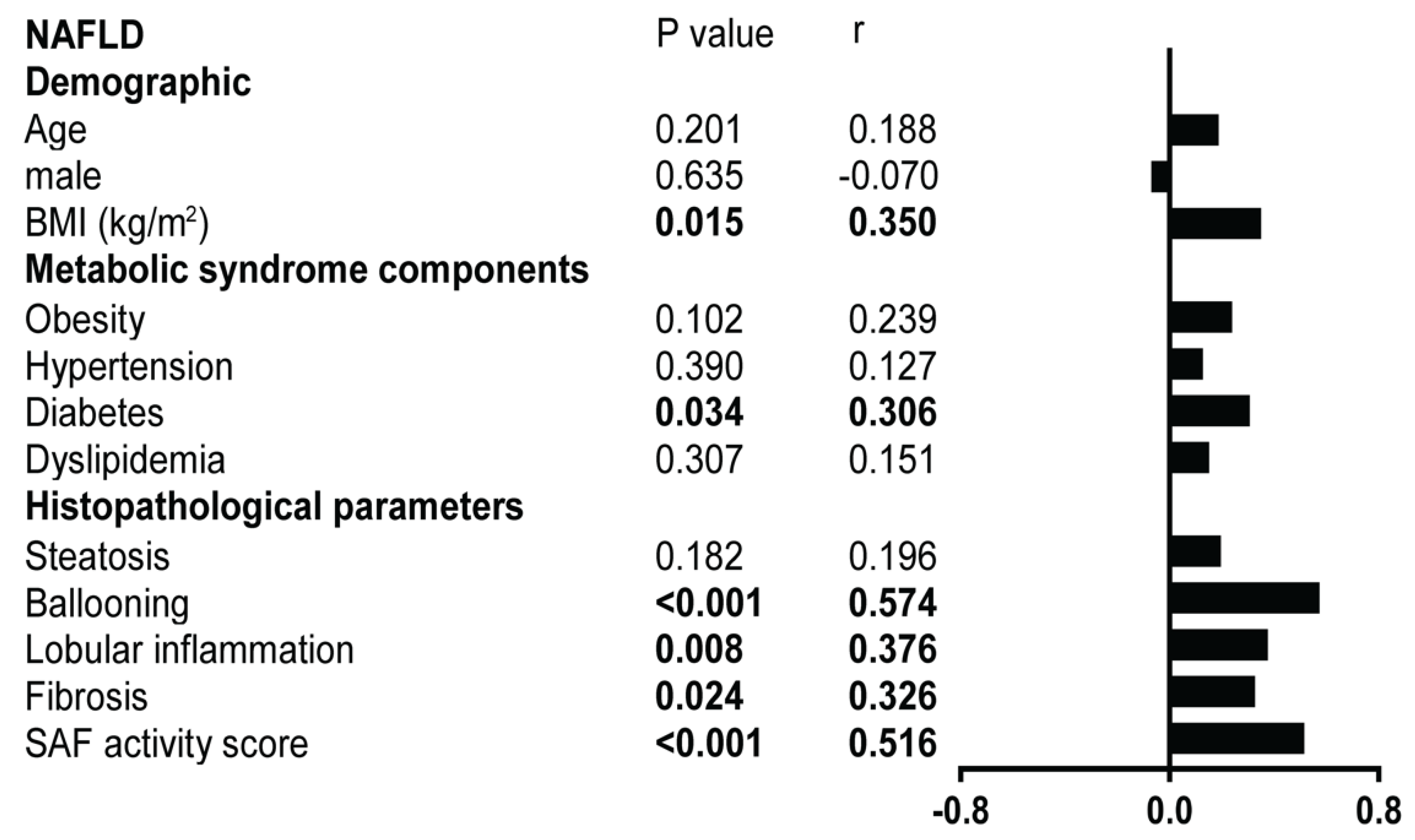
2.2.2. Metabolic Syndrome Contributions
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Population of the Study
4.3. Data Extraction
4.4. Histological Assessment
4.5. In Situ Proximity Ligation Assay
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
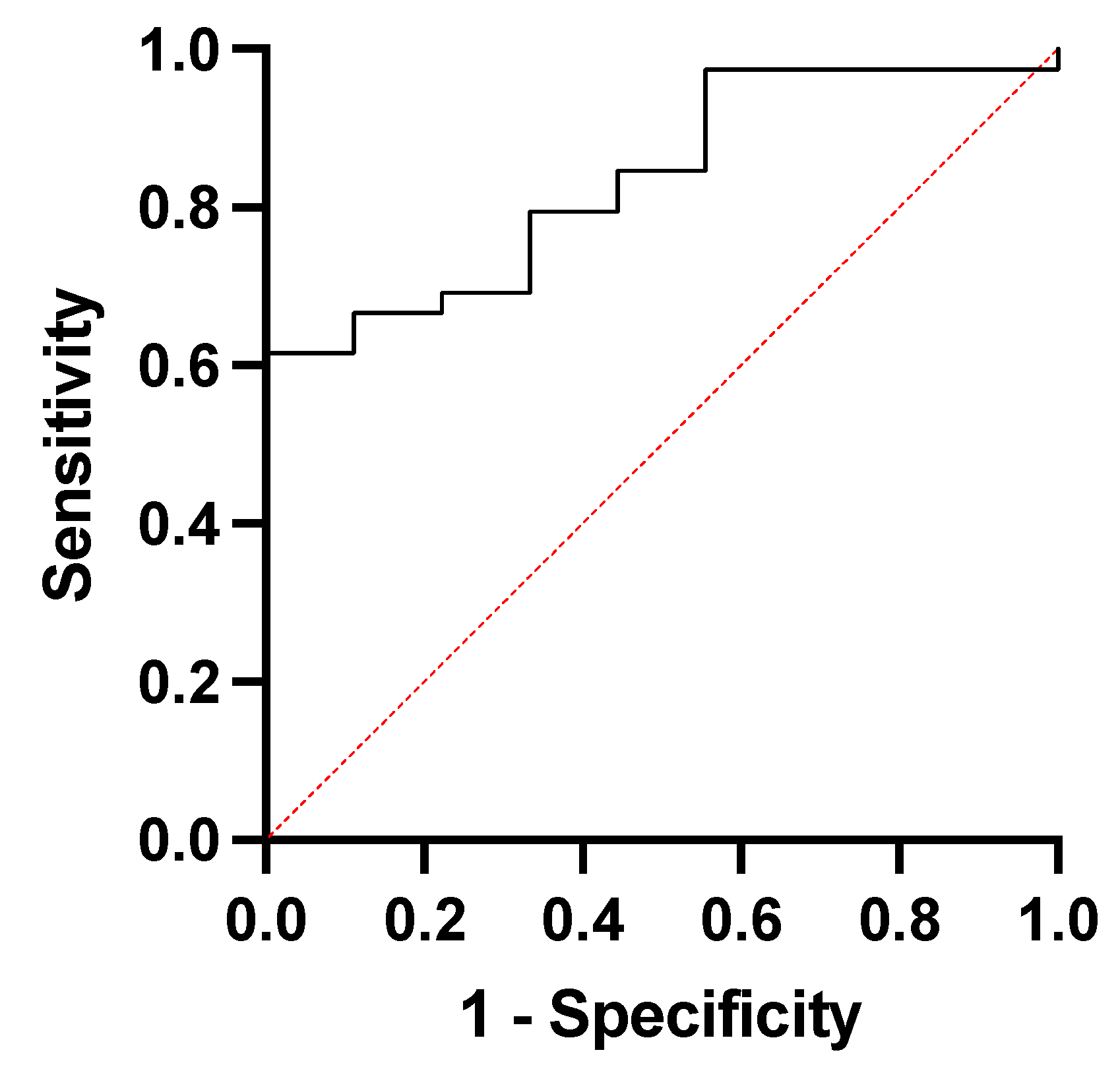
| Area under the ROC Curve | |
| Area | 0.8348 |
| Std. Error | 0.06255 |
| 95% Confidence Interval | 0.7122 to 0.9573 |
| pValue | 0.0019 |
| pValue Summary | *** |
References
- Arruda, A.P.; Pers, B.M.; Parlakgül, G.; Güney, E.; Inouye, K.; Hotamisligil, G.S. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 2014, 20, 1427–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubbs, E.; Theurey, P.; Vial, G.; Bendridi, N.; Bravard, A.; Chauvin, M.A.; Ji-Cao, J.; Zoulim, F.; Bartosch, B.; Ovize, M.; et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes 2014, 63, 3279–3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, G.; Goode, J.; Paz, J.C.; Ouyang, K.; Screaton, R.; Fischer, W.H.; Chen, J.; Tabas, I.; Montminy, M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 2012, 485, 128–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeyer, C.C.; McCullough, A.J. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin Liver Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rieusset, J.; Fauconnier, J.; Paillard, M.; Belaidi, E.; Tubbs, E.; Chauvin, M.A.; Durand, A.; Bravard, A.; Teixeira, G.; Bartosch, B.; et al. Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance. Diabetologia 2016, 59, 614–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubbs, E.; Axelsson, A.S.; Vial, G.; Wollheim, C.B.; Rieusset, J.; Rosengren, A.H. Sulforaphane improves disrupted ER-mitochondria interactions and suppresses exaggerated hepatic glucose production. Diabetes 2018, 461, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Theurey, P.; Tubbs, E.; Vial, G.; Jacquemetton, J.; Bendridi, N.; Chauvin, M.A.; Alam, M.R.; Le Romancer, M.; Vidal, H.; Rieusset, J. Mitochondria-associated endoplasmic reticulum membranes allow adaptation of mitochondrial metabolism to glucose availability in the liver. J. Mol. Cell Biol. 2016, 8, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacchiotti, A.; Favero, G.; Lavazza, A.; Garcia-Gomez, R.; Monsalve, M.; Rezzani, R. Perspective: Mitochondria-ER Contacts in Metabolic Cellular Stress Assessed by Microscopy. Cells 2018, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, L.K.; Brunetta, H.S.; Mori, M.A.S. Mitochondria-associated ER membranes in glucose homeostasis and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E1053–E1060. [Google Scholar] [CrossRef] [PubMed]
- Feriod, C.N.; Oliveira, A.G.; Guerra, M.T.; Nguyen, L.; Richards, K.M.; Jurczak, M.J.; Ruan, H.B.; Camporez, J.P.; Yang, X.; Shulman, G.I.; et al. Hepatic Inositol 1,4,5 Trisphosphate Receptor Type 1 Mediates Fatty Liver. Hepatol. Commun. 2017, 1, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef] [PubMed]
- Bedossa, P.; Poitou, C.; Veyrie, N.; Bouillot, J.L.; Basdevant, A.; Paradis, V.; Tordjman, J.; Clement, K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012, 56, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, F.; Bedossa, P.; Fedchuk, L.; Pais, R.; Charlotte, F.; Lebray, P.; Poynard, T.; Ratziu, V. Clinical validation of the FLIP algorithm and the SAF score in patients with non-alcoholic fatty liver disease. J. Hepatol. 2020, 72, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef] [PubMed]


| NAFL (n = 9) | NASH (n = 39) | p-Value | |
|---|---|---|---|
| Demographic | |||
| Age, years * | 38.7–58.8 | 50.2–66.8 | 0.075 |
| Male, n (%) ** | 7 (77.8) | 27 (69.2) | 0.611 |
| BMI (kg/m2) * | 27.9–30.8 | 29.1–34.6 | 0.129 |
| Comorbidities, n (%) | |||
| Obesity ** | 3 (33.3) | 25 (64.1) | 0.091 |
| Hypertension ** | 7 (77.8) | 30 (76.9) | 0.956 |
| T2D ** | 3 (33.3) | 17 (43.6) | 0.574 |
| Dyslipidemia ** | 6 (66.7) | 20 (51.3) | 0.404 |
| Biomedical parameters | |||
| Glycemia (mmol/L) * | 4.8–6.6 | 4.9–6.4 | 0.593 |
| Creatinine (µmol/L) *** | 82.3 ± 14.2 | 76.9 ± 15.0 | 0.330 |
| Albumin (g/L) *** | 40.1 ± 3.7 | 37.8 ± 3.8 | 0.134 |
| AST (IU/L) * | 30–60 | 40–73.5 | 0.130 |
| ALT (IU/L) *** | 72.3 ± 36.1 | 70.9 ± 39.8 | 0.756 |
| Total bilirubin (µmol/L) * | 10.0 (8.0–12.5) | 13.0 (10.0–18.5) | 0.047 |
| ALP (IU/L) *** | 88.2 ± 62.8 | 90.9 ± 27.1 | 0.844 |
| GGT (IU/L) * | 39–104.5 | 58.5–193.0 | 0.170 |
| Total cholesterol (mmol/L) *** | 5.0 ± 0.953 | 4.5 ± 1.075 | 0.288 |
| HDL cholesterol (mmol/L) *** | 1.2 ± 0.4 | 1.1 ± 0.3 | 0.448 |
| LDL cholesterol (mmol/L) *** | 2.6 ± 0.5 | 2.6 ± 1.0 | 0.961 |
| Triglycerides (mmol/L) * | 1.2–3.1 | 1.2–1.8 | 0.335 |
| HbA1c (%) * | 5.2–6.2 | 5.3–6.2 | 0.369 |
| Platelets (G/L) *** | 249.6 ± 50.5 | 209.5 ± 75.1 | 0.136 |
| INR * | 1–1.03 | 1–1.06 | 0.158 |
| PT (s) * | 10–11.0 | 10.7–11.9 | 0.008 |
| Insulin (µU/mL) * | 10.6–28.5 | 16.2–32.7 | 0.333 |
| Histological | |||
| Steatosis grade, n (%) | 0.032 | ||
| Steatosis 1 ** | 6 (66.67) | 9 (23.08) | |
| Steatosis 2 ** | 2 (22.22) | 12 (30.77) | |
| Steatosis 3 ** | 1 (11.11) | 18 (46.15) | |
| Ballooning grade, n (%) | <0.001 | ||
| Ballooning 0 ** | 6 (66.67) | 0 (00.00) | |
| Ballooning 1 ** | 3 (33.33) | 31 (79.49) | |
| Ballooning 2 ** | 0 (00.00) | 8 (20.51) | |
| Lobular inflammation grade, n (%) | <0.001 | ||
| Lobular inflammation 0 ** | 8 (88.89) | 1 (2.56) | |
| Lobular inflammation 1 ** | 1 (11.11) | 34 (87.18) | |
| Lobular inflammation 2 ** | 0 (00.00) | 4 (10.26) | |
| Fibrosis grade, n (%) | <0.001 | ||
| Fibrosis 0 ** | 5 (55.56) | 2 (5.13) | |
| Fibrosis 1 ** | 2 (22.22) | 9 (23.08) | |
| Fibrosis 2 ** | 2 (22.22) | 15 (38.46) | |
| Fibrosis 3 ** | 0 (00.00) | 13 (33.33) | |
| SAF activity scoreas continuous variable | 0–1 | 2–4 | <0.001 |
| SAF activity score, n (%) | <0.001 | ||
| SAF activity score 0 ** | 5 (55.56) | 0 (00.00) | |
| SAF activity score 1 ** | 4 (44.44) | 0 (00.00) | |
| SAF activity score 2 ** | 0 (00.00) | 30 (76.92) | |
| SAF activity score 3 ** | 0 (00.00) | 6 (15.38) | |
| SAF activity score 4 ** | 0 (00.00) | 3 (7.69) | |
| NAFL (n = 9) | NASH (n = 39) | p-value |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Felli, E.; Lange, N.F.; Berzigotti, A.; Gracia-Sancho, J.; Dufour, J.-F. Endoplasmic Reticulum and Mitochondria Contacts Correlate with the Presence and Severity of NASH in Humans. Int. J. Mol. Sci. 2022, 23, 8348. https://doi.org/10.3390/ijms23158348
Jin C, Felli E, Lange NF, Berzigotti A, Gracia-Sancho J, Dufour J-F. Endoplasmic Reticulum and Mitochondria Contacts Correlate with the Presence and Severity of NASH in Humans. International Journal of Molecular Sciences. 2022; 23(15):8348. https://doi.org/10.3390/ijms23158348
Chicago/Turabian StyleJin, Chaonan, Eric Felli, Naomi Franziska Lange, Annalisa Berzigotti, Jordi Gracia-Sancho, and Jean-François Dufour. 2022. "Endoplasmic Reticulum and Mitochondria Contacts Correlate with the Presence and Severity of NASH in Humans" International Journal of Molecular Sciences 23, no. 15: 8348. https://doi.org/10.3390/ijms23158348
APA StyleJin, C., Felli, E., Lange, N. F., Berzigotti, A., Gracia-Sancho, J., & Dufour, J.-F. (2022). Endoplasmic Reticulum and Mitochondria Contacts Correlate with the Presence and Severity of NASH in Humans. International Journal of Molecular Sciences, 23(15), 8348. https://doi.org/10.3390/ijms23158348








