Blaze a New Trail: Plant Virus Xylem Exploitation
Abstract
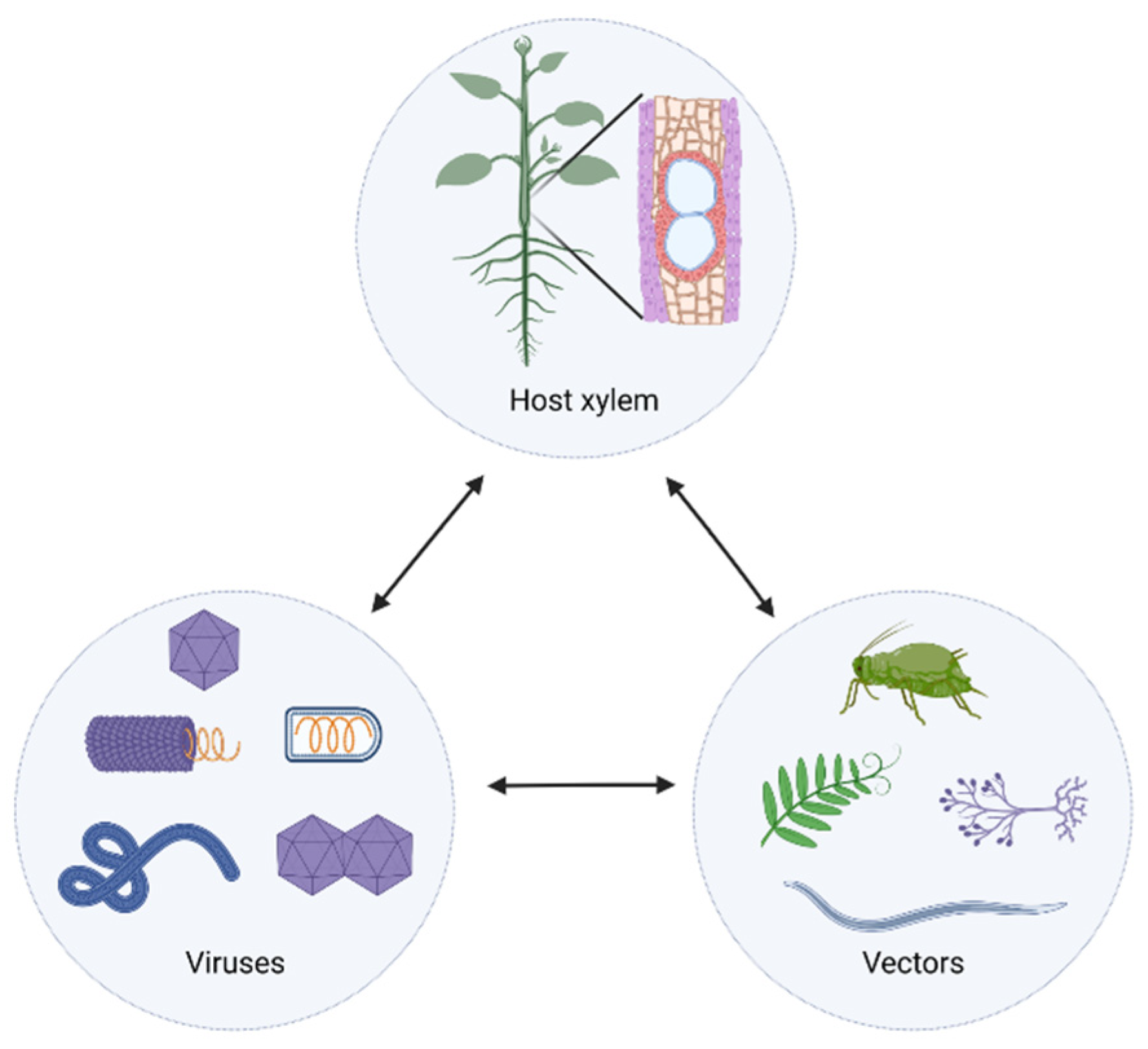
1. Introduction
2. Examples of Xylem-Invading Viruses
3. Techniques for Studying Virus Invasion into the Xylem
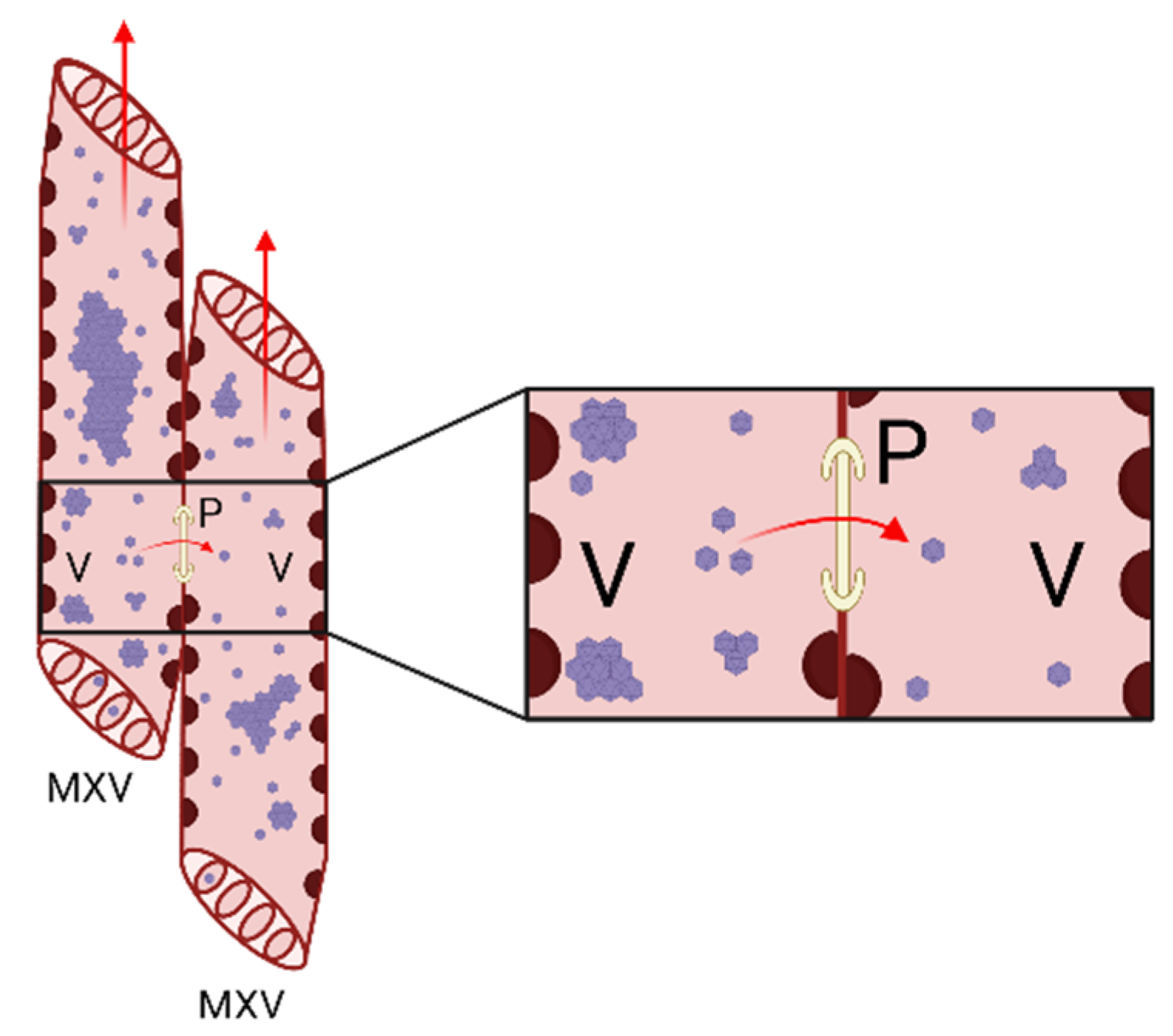
4. Viral Complexes in the Xylem
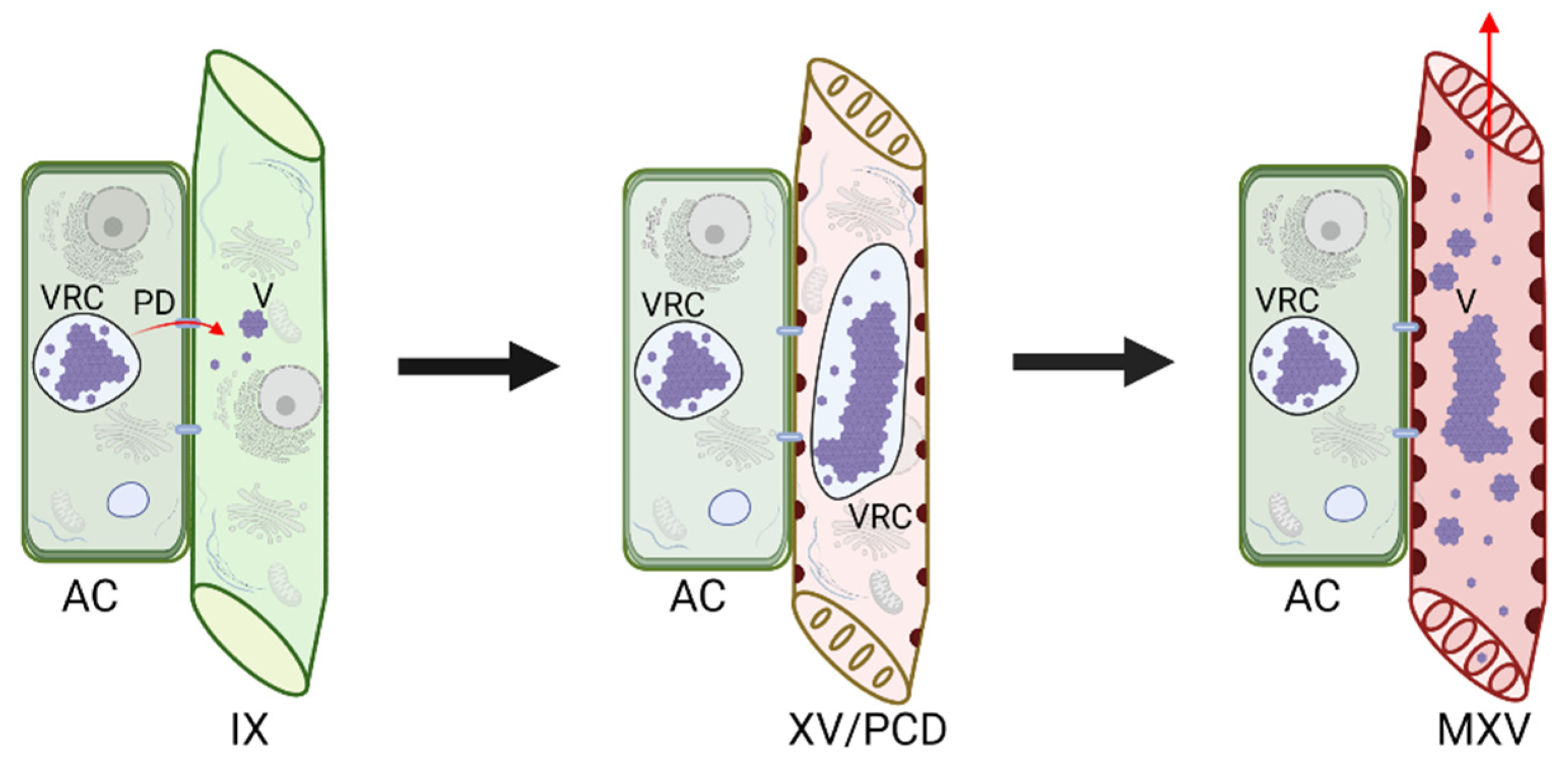
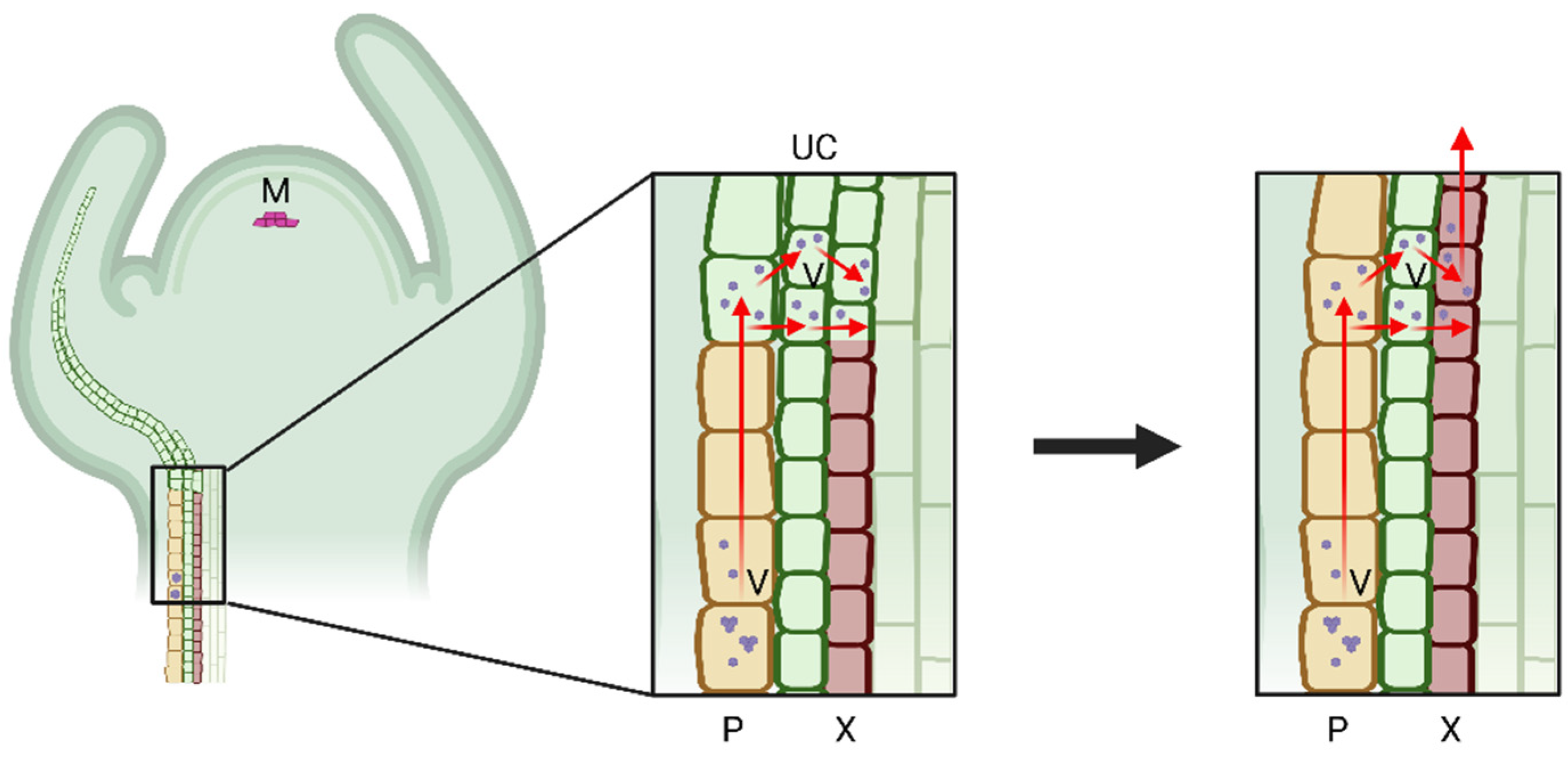
5. Possible Strategies for Virus Introduction into the Xylem
5.1. Vector-Mediated Introduction
5.2. Virus Movement through Plant Cell Connections
6. Factors Affecting Virus Xylem Colonization
6.1. Viral Factors Influencing Xylem Invasion
6.2. The Effect of Host on Virus Xylem Invasion
6.3. The Role of Insect Vectors
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schuetz, M.; Smith, R.; Ellis, B. Xylem tissue specification, patterning, and differentiation mechanisms. J. Exp. Bot. 2012, 64, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Růžička, K.; Ursache, R.; Hejátko, J.; Helariutta, Y. Xylem development—From the cradle to the grave. New Phytol. 2015, 207, 519–535. [Google Scholar] [CrossRef]
- Folimonova, S.Y.; Tilsner, J. Hitchhikers, highway tolls and roadworks: The interactions of plant viruses with the phloem. Curr. Opin. Plant Biol. 2018, 43, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kappagantu, M.; Collum, T.D.; Dardick, C.; Culver, J.N. Viral hacks of the plant vasculature: The role of phloem alterations in systemic virus infection. Annu. Rev. Virol. 2020, 7, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Knoblauch, M.; Turgeon, R. The phloem as an arena for plant pathogens. Annu. Rev. Phytopathol. 2022, 60, 4.1–4.20. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Nobuhara, S.; Nishimura, M.; Ryang, B.S.; Naoe, M.; Matsumoto, T.; Kosaka, Y.; Ohki, S.T. The entry of cucumber mosaic virus into cucumber xylem is facilitated by co-infection with zucchini yellow mosaic virus. Arch. Virol. 2016, 161, 2683–2692. [Google Scholar] [CrossRef]
- Sun, Y.D.; Folimonova, S.Y. Location matters: From changing a presumption about the Citrus tristeza virus tissue tropism to understanding the stem pitting disease. New Phytol. 2022, 233, 631–638. [Google Scholar] [CrossRef]
- Wan, J.; Cabanillas, D.G.; Zheng, H.; Laliberte, J.F. Turnip mosaic virus moves systemically through both phloem and xylem as membrane-associated complexes. Plant Physiol. 2015, 167, 1374–1388. [Google Scholar] [CrossRef]
- Chambers, T.C.; Francki, R.I. Localization and recovery of lettuce necrotic yellows virus from xylem tissues of Nicotiana glutinosa. Virology 1966, 29, 673–676. [Google Scholar] [CrossRef]
- Johnson, J. Factors relating to the control of ordinary tobacco mosaic. J. Agric. Res. 1937, 54, 239–273. [Google Scholar]
- Dubois, F.; Sangwan, R.S.; Sangwan-Norreel, B.S. Spread of beet necrotic yellow vein virus in infected seedlings and plants of sugar beet (Beta vulgaris). Protoplasma 1994, 179, 72–82. [Google Scholar] [CrossRef]
- French, C.J.; Elder, M.; Skelton, F. Recovering and Identifying Infectious Plant Viruses in Guttation Fluid. HortScience 1993, 28, 746–747. [Google Scholar] [CrossRef]
- French, C.J.; Elder, M. Virus particles in guttate and xylem of infected cucumber (Cucumis sativus L.). Ann. Appl. Biol. 1999, 134, 81–87. [Google Scholar] [CrossRef]
- Urban, L.A.; Ramsdell, D.C.; Klomparens, K.L.; Lynch, T.L.; Hancock, J.F. Detection of Blueberry Shoestring Virus in Xylem and Phloem Tissues of Highbush Blueberry. Phytopathology 1989, 79, 488–493. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.Y.; Song, J.; Li, J.J.; Klosterman, S.J.; Li, R.; Kong, Z.Q.; Subbarao, K.V.; Dai, X.F.; Zhang, D.D. Cytotoxic function of xylanase VdXyn4 in the plant vascular wilt pathogen Verticillium dahliae. Plant Physiol. 2021, 187, 409–429. [Google Scholar] [CrossRef]
- Paliwal, Y.C. Electron microscopy of Bromegrass mosaic virus in infected leaves. J. Ultrastruct. Res. 1970, 30, 491–502. [Google Scholar] [CrossRef]
- Sun, Y.D.; Folimonova, S.Y. The p33 protein of Citrus tristeza virus affects viral pathogenicity by modulating a host immune response. New Phytol. 2019, 221, 2039–2053. [Google Scholar] [CrossRef]
- Betti, C.; Lico, C.; Maffi, D.; D’Angeli, S.; Altamura, M.M.; Benvenuto, E.; Faoro, F.; Baschieri, S. Potato virus X movement in Nicotiana benthamiana: New details revealed by chimeric coat protein variants. Mol. Plant Pathol. 2012, 13, 198–203. [Google Scholar] [CrossRef]
- Giunchedi, L.; Poggi-Pollini, C. Immunogold-silver localization of Beet necrotic yellow vein virus antigen in susceptible and moderately resistant Sugar-beets. Phytopathol. Mediterr. 1988, 27, 1–6. [Google Scholar]
- Ding, X.S.; Boydston, C.M.; Nelson, R.S. Presence of Brome mosaic virus in barley guttation fluid and its association with localized cell death response. Phytopathology 2001, 91, 440–448. [Google Scholar] [CrossRef][Green Version]
- Kozieł, E.; Otulak, K.; Lockhart, B.E.L.; Garbaczewska, G. Subcelullar localization of proteins associated with Prune dwarf virus replication. Eur. J. Plant. Pathol. 2017, 149, 653–668. [Google Scholar] [CrossRef]
- Bar-Joseph, M.; Josephs, R.; Cohen, J. Carnation yellow fleck virus particles “in vivo”. A structural analysis. Virology 1977, 81, 144–151. [Google Scholar] [CrossRef]
- Khan, J.A.; Lohuis, D.; Bakardjieva, N.; Peters, D.; Goldbach, R.; Dijkstra, J. Interference between two strains of Bean common mosaic virus is accompanied by suppression of symptoms without affecting replication of the challenging virus. J. Phytopathol. 1994, 140, 260–268. [Google Scholar] [CrossRef]
- Khan, J.A.; Lohuis, H.; Goldbach, R.W.; Dijkstra, J. Distribution and localization of bean common mosaic virus and bean black root virus in stems of doubly infected bean plants. Arch. Virol. 1994, 138, 95–104. [Google Scholar] [CrossRef]
- Otulak, K.; Garbaczewska, G. Cytopathological potato virus Y structures during Solanaceous plants infection. Micron 2012, 43, 839–850. [Google Scholar] [CrossRef]
- Gergerich, R.C.; Scott, H.A. Evidence that virus translocation and virus infection of non-wounded cells are associated with transmissibility by leaf-feeding beetles. J. Gen. Virol. 1988, 69, 2935–2938. [Google Scholar] [CrossRef]
- Opalka, N.; Brugidou, C.; Bonneau, C.; Nicole, M.; Beachy, R.N.; Yeager, M.; Fauquet, C. Movement of rice yellow mottle virus between xylem cells through pit membranes. Proc. Natl. Acad. Sci. USA 1998, 95, 3323–3328. [Google Scholar] [CrossRef]
- Schneider, I.R.; Worley, J.F. Rapid entry of infectious particles of southern bean mosaic virus into living cells following transport of the particles in the water stream. Virology 1959, 8, 243–249. [Google Scholar] [CrossRef]
- Appiano, A.; D’Agostino, G. Distribution of tomato bushy stunt virus in root tips of systemically infected Gomphrena globosa. J. Ultrastruct. Res. 1983, 85, 239–248. [Google Scholar] [CrossRef]
- Manabayeva, S.A.; Shamekova, M.; Park, J.W.; Ding, X.S.; Nelson, R.S.; Hsieh, Y.C.; Omarov, R.T.; Scholthof, H.B. Differential requirements for Tombusvirus coat protein and P19 in plants following leaf versus root inoculation. Virology 2013, 439, 89–96. [Google Scholar] [CrossRef]
- Russo, M.; Martelli, G.P.; Quacquarelli, A. Occurrence of artichoke mottle crinkle virus in leaf vein xylem. Virology 1967, 33, 555–558. [Google Scholar] [CrossRef]
- Verchot, J.; Driskel, B.A.; Zhu, Y.; Hunger, R.M.; Littlefield, L.J. Evidence that soilborne wheat mosaic virus moves long distance through the xylem in wheat. Protoplasma 2001, 218, 57–66. [Google Scholar] [CrossRef]
- Jones, R.A.C. Systemic movement of Potato mop-top virus in tobacco may occur through the xylem. J. Phytopathol. 1975, 82, 352–355. [Google Scholar] [CrossRef]
- Moreno, I.M.; Thompson, J.R.; Garcia-Arenal, F. Analysis of the systemic colonization of cucumber plants by Cucumber green mottle mosaic virus. J. Gen. Virol. 2004, 85, 749–759. [Google Scholar] [CrossRef]
- Fribourg, C.E.; Koenig, R.; Lesemann, D.-E. A new tobamovirus from Passiflora edulis in Peru. Phytopathology 1987, 77, 486–491. [Google Scholar] [CrossRef]
- Garbaczewska, G.; Otulak, K.; Chouda, M.; Chrzanowska, M. Ultrastructural studies of plasmodesmatal and vascular translocation of tobacco rattle virus (TRV) in tobacco and potato. Acta Physiol. Plant. 2012, 34, 1229–1238. [Google Scholar] [CrossRef]
- Rasheed, M.S.; Selth, L.A.; Koltunow, A.M.G.; Randles, J.W.; Rezaian, M.A. Single-stranded DNA of Tomato leaf curl virus accumulates in the cytoplasm of phloem cells. Virology 2006, 348, 120–132. [Google Scholar] [CrossRef]
- Andrianifahanana, M.; Lovins, K.; Dute, R.; Sikora, E.; Murphy, J.F. Pathway for phloem-dependent movement of Pepper mottle potyvirus in the stem of Capsicum annuum. Phytopathology 1997, 87, 892–898. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Pina, M.A.; Gómez-Aix, C.; Méndez-López, E.; Bernal, B.G.; Aranda, M.A. Imaging techniques to study plant virus replication and vertical transmission. Viruses 2021, 13, 358. [Google Scholar] [CrossRef]
- Groover, A.; DeWitt, N.; Heidel, A.; Jones, A. Programmed cell death of plant tracheary elements differentiating in vitro. Protoplasma 1997, 196, 197–211. [Google Scholar] [CrossRef]
- Lehmann, K.; Hause, B.; Altmann, D.; Köck, M. Tomato Ribonuclease LX with the functional endoplasmic reticulum retention motif HDEF is expressed during programmed cell death processes, including xylem differentiation, germination, and senescence. Plant Physiol. 2001, 127, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Liu, L.; Wood, B.W. Nickel affects xylem sap RNase A and converts RNase A to a urease. BMC Plant. Biol. 2013, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Will, T.; van Bel, A.J.E. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 2006, 57, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Saheed, S.A.; Liu, L.; Jonsson, L.; Botha, C.E.J. Xylem—as well as phloem—sustains severe damage due to feeding by the Russian wheat aphid. S. Afr. J. Bot. 2007, 73, 593–599. [Google Scholar] [CrossRef]
- Levin, K.A.; Tucker, M.R.; Strock, C.F.; Lynch, J.P.; Mather, D.E. Three-dimensional imaging reveals that positions of cyst nematode feeding sites relative to xylem vessels differ between susceptible and resistant wheat. Plant Cell Rep. 2021, 40, 393–403. [Google Scholar] [CrossRef]
- Vaughn, K. Conversion of the searching hyphae of dodder into xylic and phloic hyphae: A cytochemical and immunocytochemical investigation. Int. J. Plant Sci. 2006, 167, 1099–1114. [Google Scholar] [CrossRef]
- Scholthof, H.B. Plant virus transport: Motions of functional equivalence. Trends Plant Sci. 2005, 10, 376–382. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, R.; Hyun, T.K.; Kim, J.Y. Cell-to-cell movement of viruses via plasmodesmata. J. Plant Res. 2015, 128, 37–47. [Google Scholar] [CrossRef]
- Niehl, A.; Heinlein, M. Cellular pathways for viral transport through plasmodesmata. Protoplasma 2011, 248, 75–99. [Google Scholar] [CrossRef]
- Van Alfen, N.K.; McMillan, B.D.; Turner, V.; Hess, W.M. Role of pit membranes in macromolecule-induced wilt of plants. Plant Physiol. 1983, 73, 1020–1023. [Google Scholar] [CrossRef]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and cellular factors involved in phloem transport of plant viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef]
- Tatineni, S.; Dawson, W.O. Enhancement or attenuation of disease by deletion of genes from Citrus tristeza virus. J. Virol. 2012, 86, 7850–7857. [Google Scholar] [CrossRef]
- Yadeta, K.A.; BP, J.T. The xylem as battleground for plant hosts and vascular wilt pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Collum, T.D.; Culver, J.N. Tobacco mosaic virus infection disproportionately impacts phloem associated translatomes in Arabidopsis thaliana and Nicotiana benthamiana. Virology 2017, 510, 76–89. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Nyalugwe, E.P.; Barbetti, M.J.; Clode, P.L.; Jones, R.A.C. Systemic hypersensitive resistance to Turnip mosaic virus in Brassica juncea is associated with multiple defense responses, especially phloem necrosis and xylem occlusion. Plant Dis. 2016, 100, 1261–1270. [Google Scholar] [CrossRef]
- Brlansky, R.H.; Howd, D.S.; Broadbent, P.; Damsteegt, V.D. Histology of sweet orange stem pitting caused by an Australian isolate of Citrus tristeza virus. Plant Dis. 2002, 86, 1169–1174. [Google Scholar] [CrossRef]
- Martelli, G.P.; Adams, M.J.; Kreuze, J.F.; Dolja, V.V. Family Flexiviridae: A case study in virion and genome plasticity. Annu. Rev. Phytopathol. 2007, 45, 73–100. [Google Scholar] [CrossRef]
- Smith, S.H.; Stouffer, R.F.; Soulen, D.M. Induction of stem pitting in peaches by mechanical inoculation with Tomato ringspot virus. Phytopathology 1973, 63, 1404–1406. [Google Scholar] [CrossRef]
- Welsh, M.F.; May, J. Virus-induced wood pitting in the root systems of apple seedlings, and its effects on tree vigor. Can. J. Plant Sci. 1966, 47, 51–59. [Google Scholar] [CrossRef]
| No. | Family | Genus | Species | Host Plants | References | |
|---|---|---|---|---|---|---|
| +ssRNA | 1 | Alphaflexiviridae | Potexvirus | Papaya mosaic virus | Cucumber (Cucumis surivus L.) | [13] |
| 2 | Alphaflexiviridae | Potexvirus | Potato virus X | Nicotiana benthamiana Domin | [8,18] | |
| 3 | Benyviridae | Benyvirus | Beet necrotic yellow vein virus | Sugar beet (Beta vulgaris L.) | [11,19] | |
| 4 | Bromoviridae | Alfamovirus | Alfalfa mosaic virus | Cucumber (Cucumis surivus L.) | [13] | |
| 5 | Bromoviridae | Bromovirus | Brome mosaic virus | Wheat (Triticum aestivum L.), Barley (Hordeum vulgare L.), Oat (Arena sativa L.) | [16,20] | |
| 6 | Bromoviridae | Bromovirus | Cowpea chlorotic mottle virus | Cucumber (Cucumis surivus L.) | [13] | |
| 7 | Bromoviridae | Cucumovirus | Cucumber mosaic virus | Cucumber (Cucumis surivus L.) | [6] | |
| 8 | Bromoviridae | Ilarvirus | Prune dwarf virus | Tobacco (Nicotiana tabacum L. cv. Samsun) | [21] | |
| 9 | Closteroviridae | Closterovirus | Carnation yellow fleck virus | Carnation (Dianthus caryophyllus L.) | [22] | |
| 10 | Closteroviridae | Closterovirus | Citrus tristeza virus | Citrus macrophylla Wester | [7] | |
| 11 | Potyviridae | Potyvirus | Bean common mosaic virus | Bean (Phaseolus vulgaris L. ‘Bataaf’) | [23,24] | |
| 12 | Potyviridae | Potyvirus | Papaya ringspot virus | Cucumber (Cucumis surivus L.) | [13] | |
| 13 | Potyviridae | Potyvirus | Potato virus Y | Potato (Solanum tuberosum L.) and tobacco (Nicotiana tabacum L.) | [25] | |
| 14 | Potyviridae | Potyvirus | Turnip mosaic virus | Nicotiana benthamiana Domin | [8] | |
| 15 | Potyviridae | Potyvirus | Zucchini yellow mosaic virus | Cucumber (Cucumis surivus L.) | [6,13] | |
| 16 | Secoviridae | Comovirus | Bean pod mottle virus | Black Valentine bean (Phaseolus vulgaris L.) | [26] | |
| 17 | Secoviridae | Comovirus | Cowpea severe mosaic virus | Monarch cowpea (Vigna unguiculata L.) | [26] | |
| 18 | Secoviridae | Comovirus | Squash mosaic virus | Cucumber (Cucumis surivus L.) | [13] | |
| 19 | Secoviridae | Nepovirus | Tobacco ringspot virus | Cucumber (Cucumis surivus L.) | [13] | |
| 20 | Secoviridae | Nepovirus | Tomato ringspot virus | Cucumber (Cucumis surivus L.) | [13] | |
| 21 | Solemoviridae | Sobemovirus | Blueberry shoestring virus | Highbush blueberry (Vaccinium corymbosum L.) | [14] | |
| 22 | Solemoviridae | Sobemovirus | Rice yellow mottle virus | Rice (Oryza sativa L.) | [27] | |
| 23 | Solemoviridae | Sobemovirus | Southern bean mosaic virus | Black Valentine bean, Pinto bean (Phaseolus vulgaris L.) | [26,28] | |
| 24 | Tombusviridae | Gammacarmovirus | Melon necrotic spot virus | Cucumber (Cucumis surivus L.) | [13] | |
| 25 | Tombusviridae | Tombusvirus | Cucumber necrosis virus | Cucumber (Cucumis surivus L.) | [13] | |
| 26 | Tombusviridae | Tombusvirus | Tomato bushy stunt virus | Nicotiana benthamiana Domin, Gomphrena globose L. | [29,30] | |
| 27 | Tombusviridae | Tombusvirus | Artichoke mottle crinkle virus | Artichoke (Cynara Cardunculus var. scolymus (L.) Benth.) | [31] | |
| 28 | Virgaviridae | Furovirus | Soilborne wheat mosaic virus | Red winter wheat (Triticum aestivum L. cv. Vona) | [32] | |
| 29 | Virgaviridae | Pomovirus | Potato mop-top virus | Tobacco (Nicotiana tabacum L. cv. Xanthi-nc) | [33] | |
| 30 | Virgaviridae | Tobamovirus | Cucumber green mottle mosaic virus | Cucumber (Cucumis surivus L.) | [13,34] | |
| 31 | Virgaviridae | Tobamovirus | Maracuja mosaic virus | Nicotiana benthamiana Domin | [35] | |
| 32 | Virgaviridae | Tobamovirus | Pepper mild mottle virus | Green pepper (Capsicum annuum L.) | [12] | |
| 33 | Virgaviridae | Tobamovirus | Tobacco mosaic virus | Tomato (Solanum lycopersicum L.) | [10] | |
| 34 | Virgaviridae | Tobravirus | Tobacco rattle virus | Potato (Solanum tuberosum L. cv. Glada), tobacco (Nicotiana tabacum L. cv. Samsun) | [36] | |
| 35 | Virgaviridae | Tobamovirus | Tomato mosaic virus | Tomato (Lycopersicon esculentum Mill.) | [12] | |
| −ssRNA | 36 | Rhabdoviridae | Cytorhabdovirus | Lettuce necrotic yellows virus | Tobacco (Nicotiana glutinosa L.) | [9] |
| ssDNA | 37 | Geminiviridae | Begomovirus | Tomato (yellow) leaf curl virus | Nicotiana benthamiana Domin | [37] |
| 38 | Geminiviridae | Begomovirus | Tomato yellow leaf curl Sardinia virus | Nicotiana benthamiana Domin | [37] | |
| 39 | Geminiviridae | Begomovirus | Tomato golden mosaic virus | Nicotiana benthamiana Domin | [37] |
| Genome Composition | +ssRNA | +ssRNA | +ssRNA | −ssRNA | ssDNA |
|---|---|---|---|---|---|
| Family | Bromoviridae | Benyviridae | Alphaflexiviridae | Rhabdoviridae | Geminiviridae |
| Tombusviridae | Virgaviridae | Closteroviridae | |||
| Solemoviridae | Potyviridae | ||||
| Secoviridae | |||||
| Tombusviridae | |||||
| Virion shape | icosahedral | short rigid rod-like | long flexuous | bullet-like | twinned-icosahedral |
| Virion size | 25–35 nm/Diameter | 65–350 nm/Length, 11~20 nm/Diameter | 500~2000 nm/Length, 12~13 nm/Diameter | 227 nm/Length, 66 nm/Diameter | ~30 nm/Length, 18~20 nm/Diameter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-D.; Spellman-Kruse, A.; Folimonova, S.Y. Blaze a New Trail: Plant Virus Xylem Exploitation. Int. J. Mol. Sci. 2022, 23, 8375. https://doi.org/10.3390/ijms23158375
Sun Y-D, Spellman-Kruse A, Folimonova SY. Blaze a New Trail: Plant Virus Xylem Exploitation. International Journal of Molecular Sciences. 2022; 23(15):8375. https://doi.org/10.3390/ijms23158375
Chicago/Turabian StyleSun, Yong-Duo, Arianna Spellman-Kruse, and Svetlana Y. Folimonova. 2022. "Blaze a New Trail: Plant Virus Xylem Exploitation" International Journal of Molecular Sciences 23, no. 15: 8375. https://doi.org/10.3390/ijms23158375
APA StyleSun, Y.-D., Spellman-Kruse, A., & Folimonova, S. Y. (2022). Blaze a New Trail: Plant Virus Xylem Exploitation. International Journal of Molecular Sciences, 23(15), 8375. https://doi.org/10.3390/ijms23158375







