Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (Ss)-DS-ONJ
Abstract
1. Introduction
2. Results
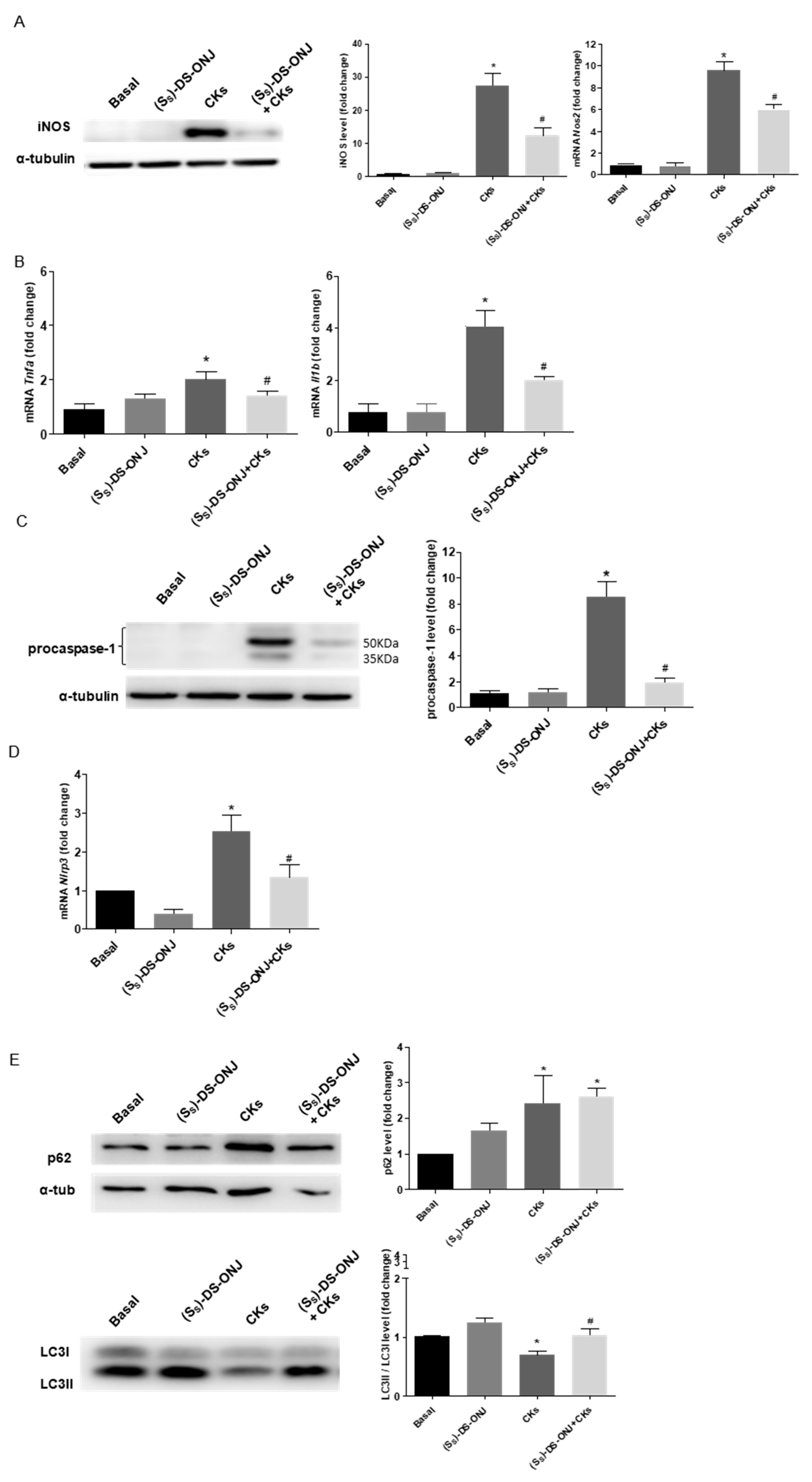
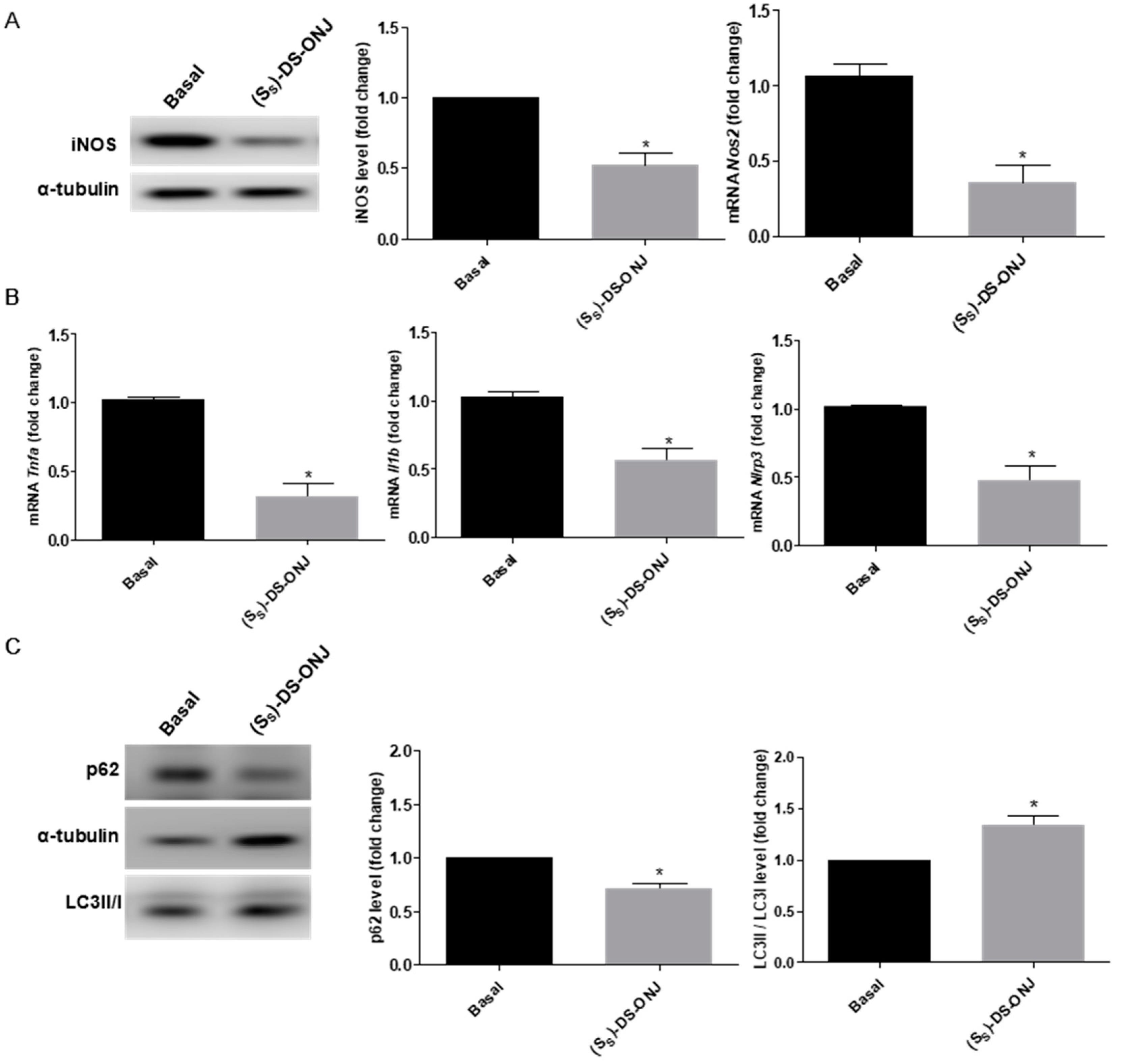
2.1. (SS)-DS-ONJ Treatment Reduces the Pro-Inflammatory Response in Mouse Cortical Ttbular Cells from Kidney (MCT) Cells under Diabetic Inflammatory Stimulus by Autophagy Flux Induction
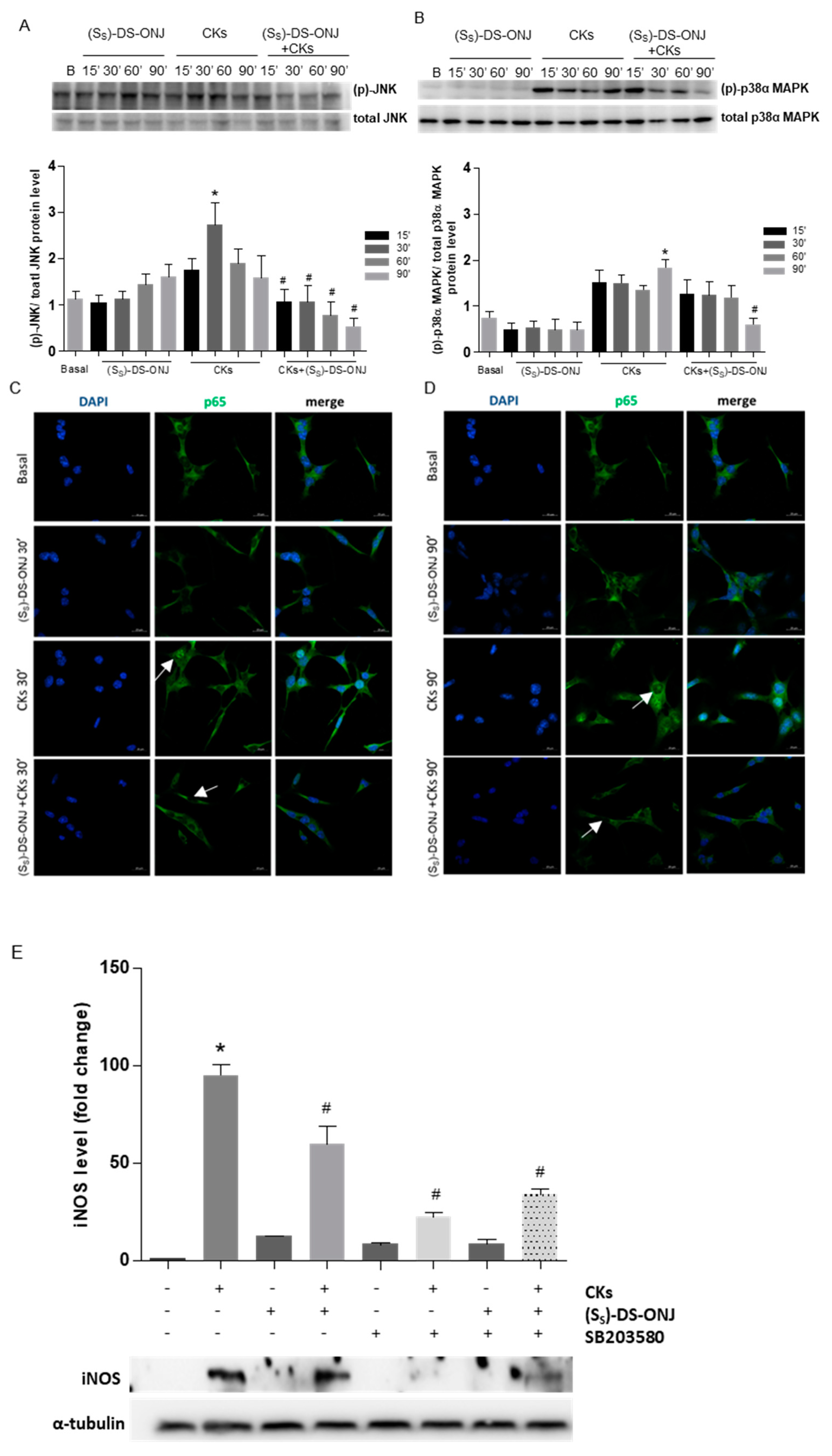
2.2. Stress Kinases Pathways Are Modulated by (Ss)-DS-ONJ in MCT Cells under Diabetic Conditions
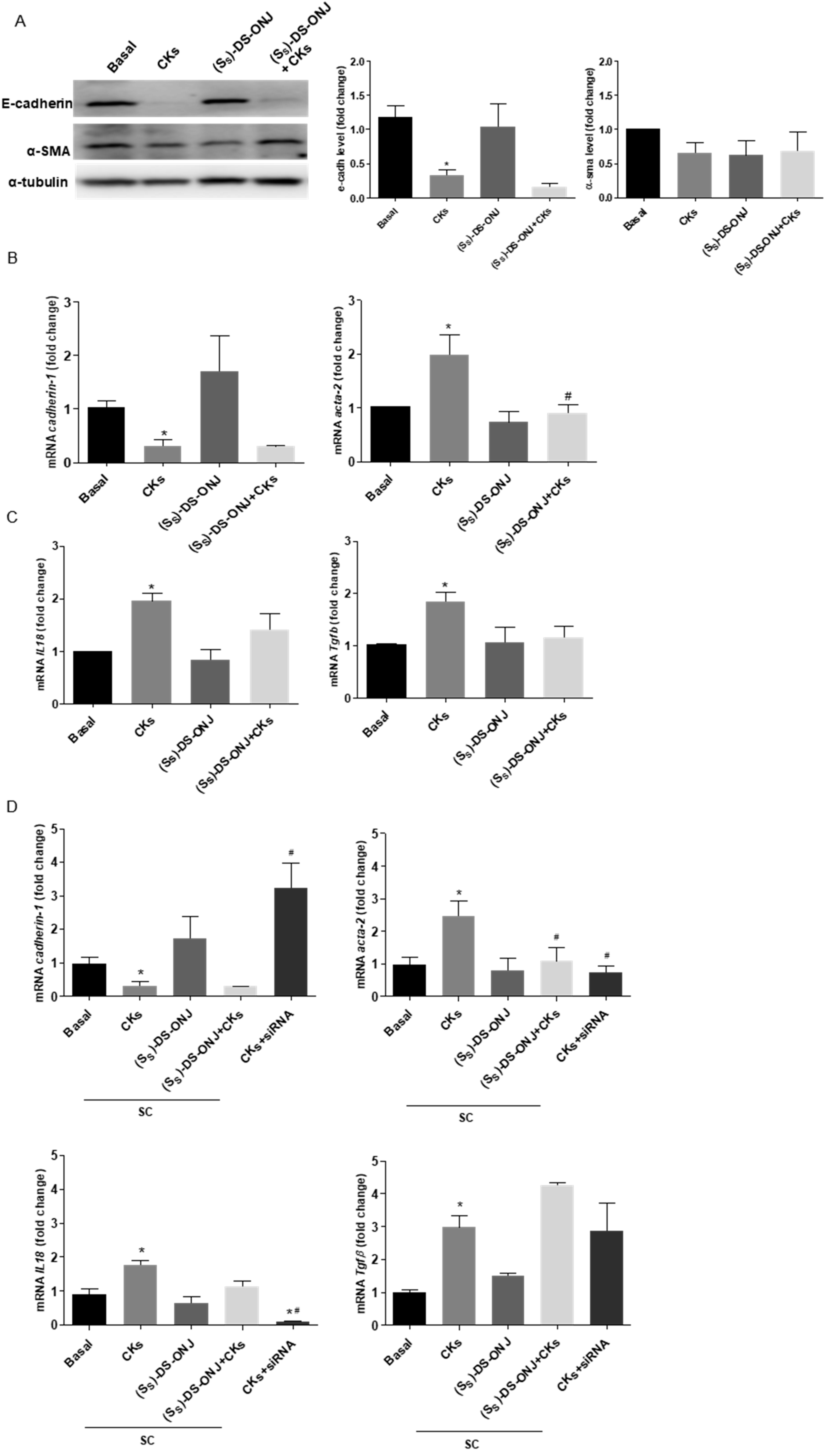
2.3. MCT Cells Cultured in a Diabetic Environment Show EMT Processes Mediated by IL18
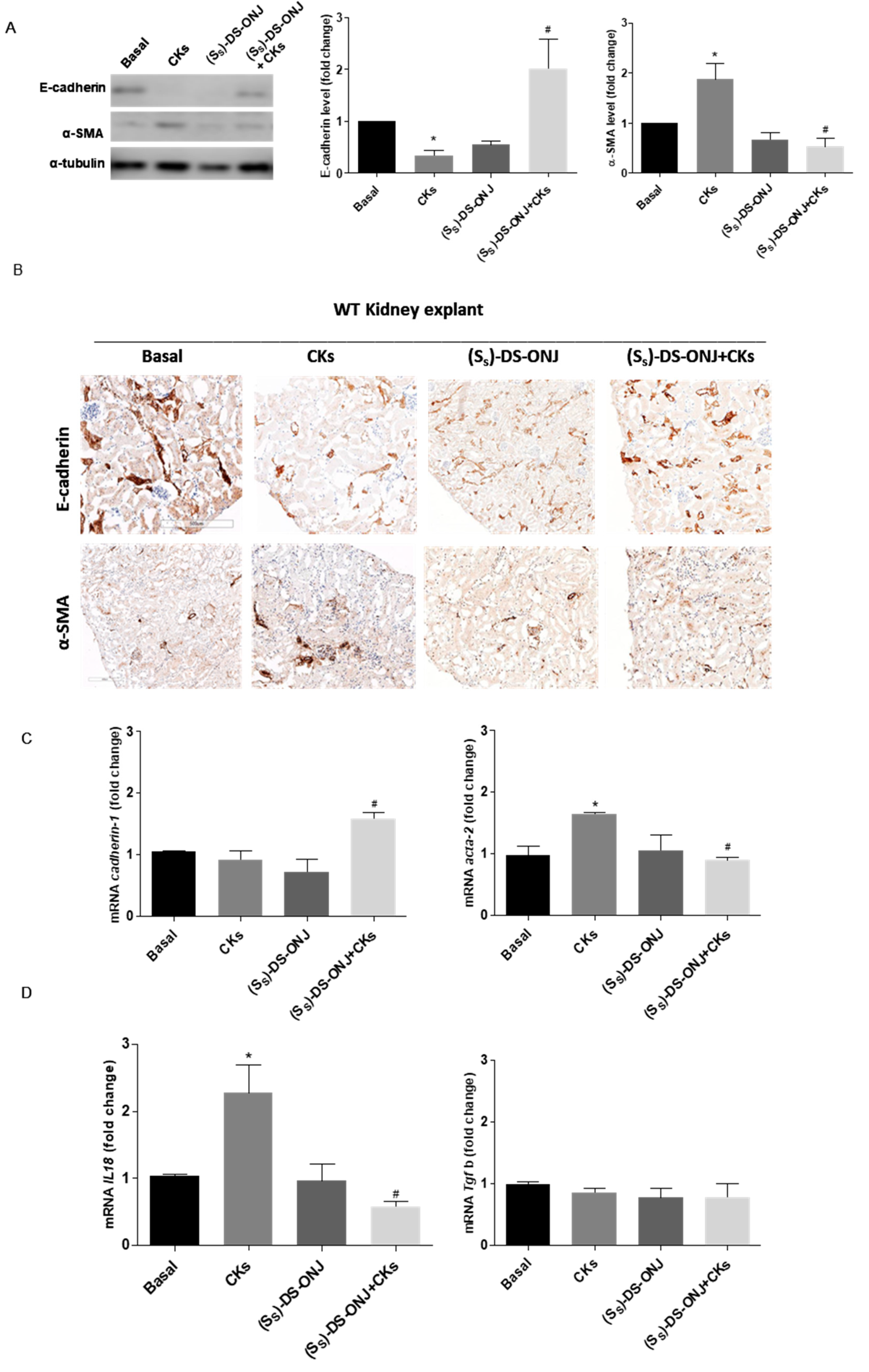
2.4. AK Explant Cultures from Non-Diabetic Rats Stimulated with CKs Reproduce the Molecular Events Observed in MCT Cells in the Diabetic Context and the (SS)-DS-ONJ Treatment Protects from Pro-Inflammatory Insults
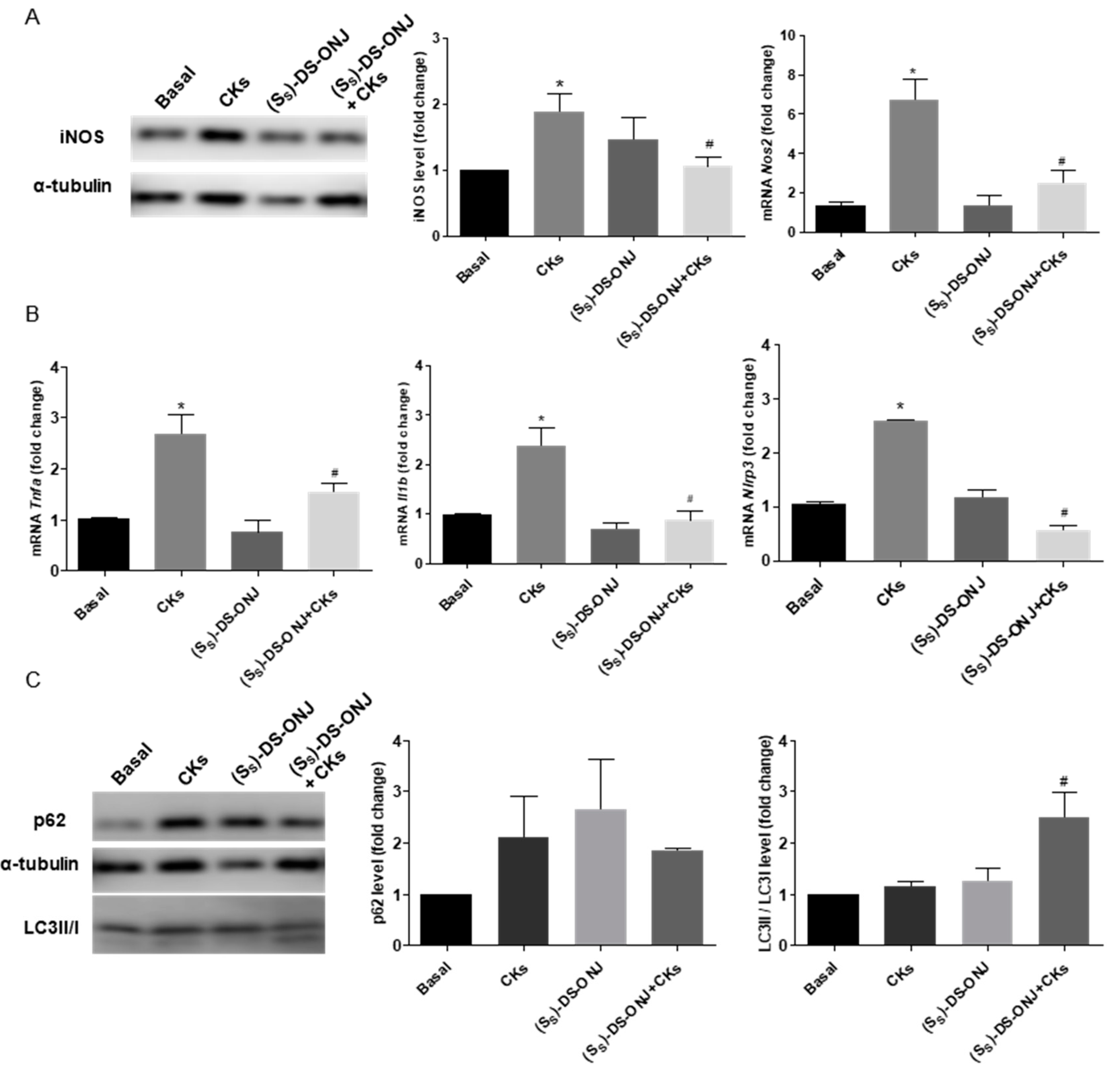
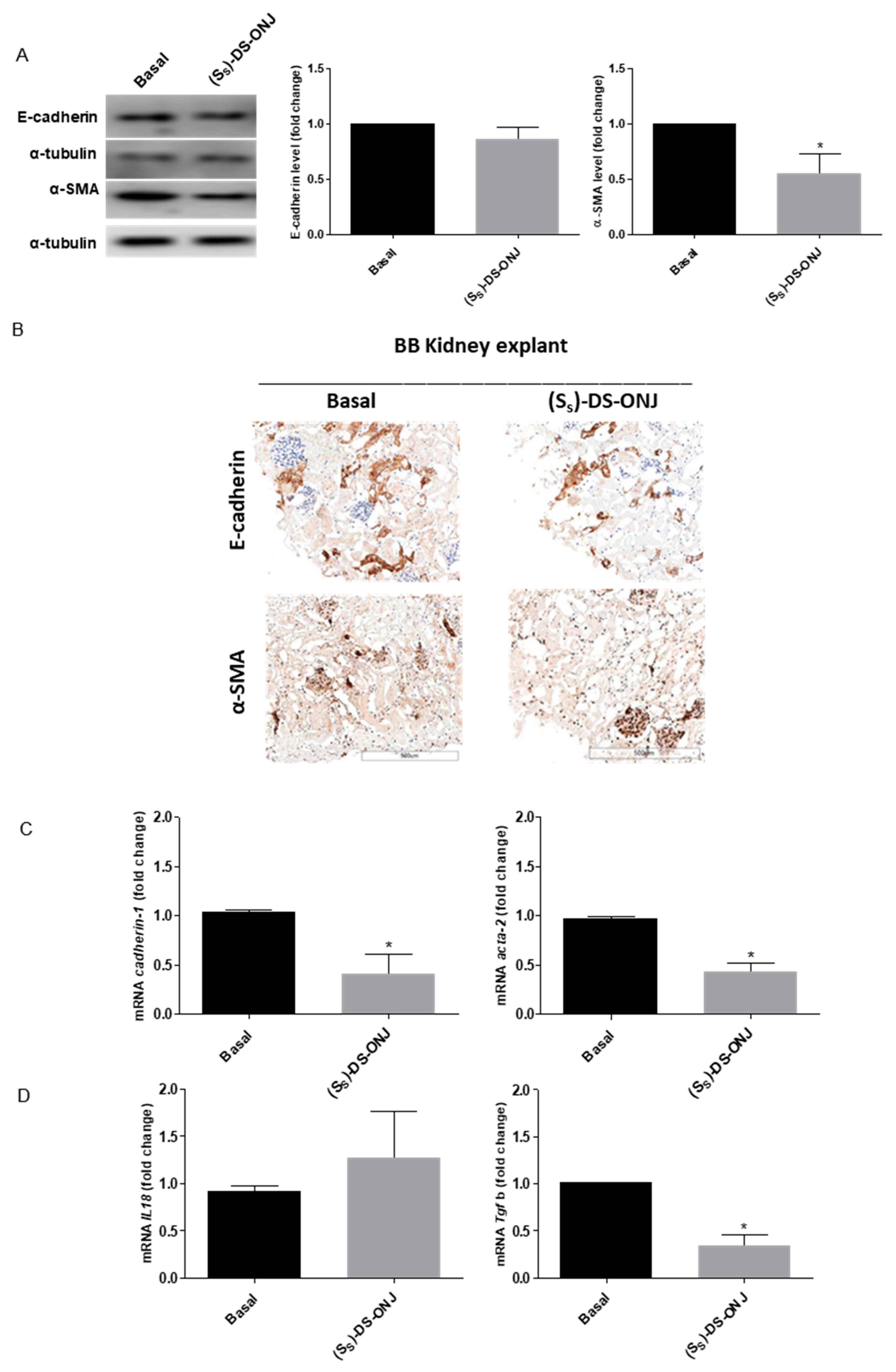
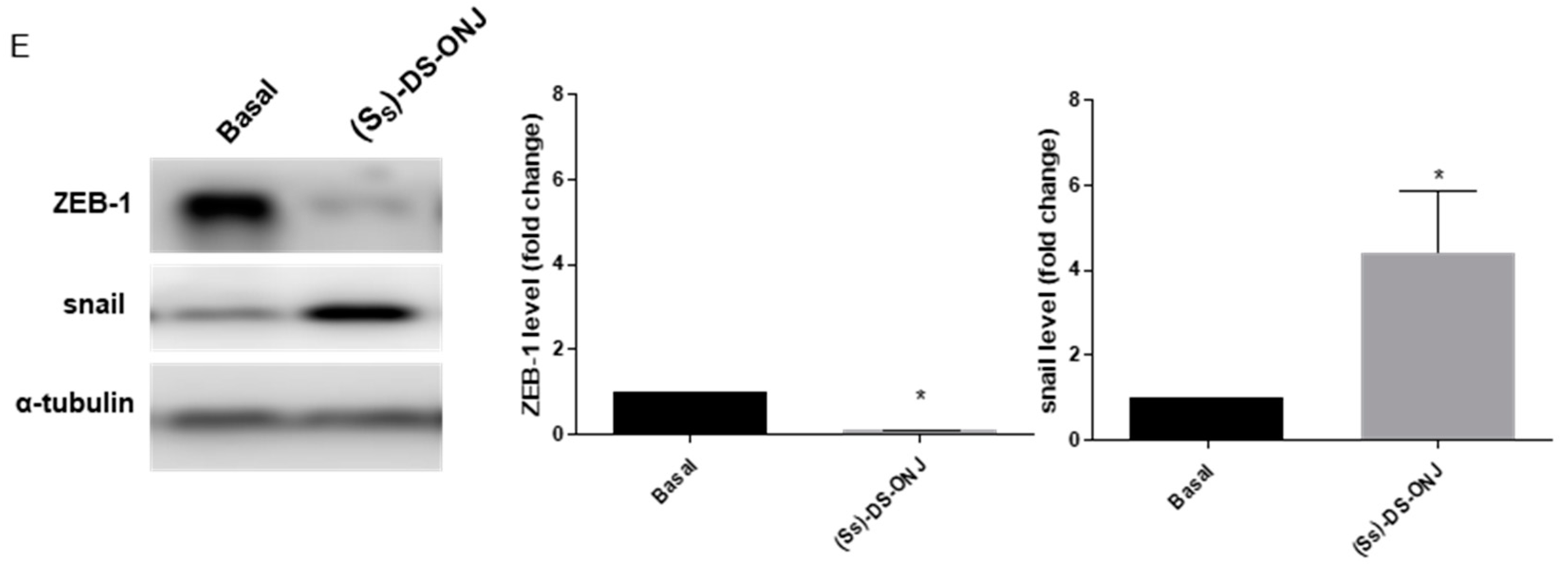
2.5. (SS)-DS-ONJ Abolishes Classic Hallmarks of DN in AK Explants from BB Rats
3. Discussion
4. Material and Methods
4.1. Reagents
4.2. Antibodies
4.3. Synthesis of the sp2-Iminosugar Glycolipid (SS)-DS-ONJ
4.4. Cell Culture
4.4.1. CKs Treatment
4.4.2. Chloroquine Treatment
4.4.3. SB203580 Treatment
4.5. Adult Kidney Explants
4.6. Analysis of the Cellular Viability by Crystal Violet Staining
4.7. Analysis of Nitrites (NO2−)
4.8. Western Blot
4.9. Immunofluorescence
4.10. Processing Samples for Histological Analysis Procedures
4.11. Quantitative Real-Time PCR (qRT-PCR)
4.12. siRNA Transfection
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pickup, J.C. Inflammation and Activated Innate Immunity in the Pathogenesis of Type 2 Diabletes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zheng, F. Immune Cells and Inflammation in Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 1841690. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef]
- Navarro, J.F.; Milena, F.J.; Mora, C.; León, C.; García, J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am. J. Nephrol. 2007, 26, 562–570. [Google Scholar] [CrossRef]
- Fantuzzi, G.; Reed, D.A.; Dinarello, C.A. IL-12-induced IFN-γ is dependent on caspase-1 processing of the IL-18 precursor. J. Clin. Investig. 1999, 104, 761–767. [Google Scholar] [CrossRef]
- Zhang, B.; Ramesh, G.; Norbury, C.C.; Reeves, W.B. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-α produced by renal parenchymal cells. Kidney Int. 2007, 72, 37–44. [Google Scholar] [CrossRef] [PubMed]
- DiPetrillo, K.; Coutermarsh, B.; Gesek, F.A. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am. J. Physiol. Ren. Physiol. 2003, 284, F113–F121. [Google Scholar] [CrossRef] [PubMed]
- Dipetrillo, K.; Gesek, F.A. Pentoxifylline ameliorates renal tumor necrosis factor expression, sodium retention, and renal hypertrophy in diabetic rats. Am. J. Nephrol. 2004, 24, 352–359. [Google Scholar] [CrossRef]
- Jones, S.; Jones, S.; Phillips, A.O. Regulation of renal proximal tubular epithelial cell hyaluronan generation: Implications for diabetic nephropathy. Kidney Int. 2001, 59, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, V.Y.; Ecder, T.; Fantuzzi, G.; Siegmund, B.; Lucia, M.S.; Dinarello, C.A.; Schrier, R.W.; Edelstein, C.L. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J. Clin. Investig. 2001, 107, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Shahzad, K.; Bock, F.; Dong, W.; Wang, H.; Kopf, S.; Kohli, S.; Al-Dabet, M.M.; Ranjan, S.; Wolter, J.; Wacker, C.; et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015, 87, 74–84. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.H.; Kim, Y.G.; Kim, S.Y.; Seo, J.W.; Choi, Y.W.; Kim, D.J.; Jeong, K.H.; Lee, T.W.; Ihm, C.G.; et al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2015, 308, F993–F1003. [Google Scholar] [CrossRef]
- Hutton, H.L.; Ooi, J.D.; Holdsworth, S.R.; Kitching, A.R. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology 2016, 21, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Choi, M.E. Autophagy in diabetic nephropathy. J. Endocrinol. 2015, 224, R15–R30. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Isaka, Y.; Yoshimori, T. Autophagy and kidney inflammation. Autophagy 2017, 13, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-A.; Wu, V.C.-C.; Wang, C.-Y. Autophagy in Chronic Kidney Diseases. Cells 2019, 8, 61. [Google Scholar] [CrossRef]
- Han, H.E.; Kim, T.K.; Son, H.J.; Park, W.J.; Han, P.L. Activation of autophagy pathway suppresses the expression of iNOS, IL6 and cell death of LPS-stimulated microglia cells. Biomol. Ther. 2013, 21, 21–28. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, I.; Wolf, G. Epithelial-to-Mesenchymal Transition in Diabetic Nephropathy: Fact or Fiction? Cells 2015, 4, 631–652. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Duffield, J.S. Resolved: EMT produces fibroblasts in the kidney. J. Am. Soc. Nephrol. 2010, 21, 1247–1253. [Google Scholar] [CrossRef]
- Fuxe, J.; Mayor, R.; Nieto, M.A.; Puisieux, A.; Runyan, R.; Savagner, P.; Thiery, J.P.; Thompson, E.W.; Theveneau, E.; Williams, E.D. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Gueder, N.; Allan, G.; Telliez, M.S.; Hague, F.; Fernandez, J.M.; Sanchez-Fernandez, E.M.; Ortiz-Mellet, C.; Ahidouch, A.; Ouadid-Ahidouch, H. sp2-Iminosugar α-glucosidase inhibitor 1-C-octyl-2-oxa-3-oxocastanospermine specifically affected breast cancer cell migration through Stim1, β1-integrin, and FAK signaling pathways. J. Cell. Physiol. 2017, 232, 3631–3640. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; Rísquez-Cuadro, R.; Chasseraud, M.; Ahidouch, A.; Mellet, C.O.; Ouadid-Ahidouch, H.; Fernández, J.M.G. Synthesis of N-, S-, and C-glycoside castanospermine analogues with selective neutral α-glucosidase inhibitory activity as antitumour agents. Chem. Commun. 2010, 46, 5328–5330. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; Gonçalves-Pereira, R.; Rísquez-Cuadro, R.; Plata, G.B.; Padrón, J.M.; García Fernández, J.M.; Ortiz Mellet, C. Influence of the configurational pattern of sp2-iminosugar pseudo N-, S-, O- and C-glycosides on their glycoside inhibitory and antitumor properties. Carbohydr. Res. 2016, 429, 113–122. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; Isabel García-Moreno, M.; García-Hernández, R.; Padrón, J.M.; García Fernández, J.M.; Gamarro, F.; Ortiz Mellet, C. Thiol-ene “click” synthesis and pharmacological evaluation of C-glycoside sp2-iminosugar glycolipids. Molecules 2019, 24, 2882. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; García-Moreno, M.I.; Arroba, A.I.; Aguilar-Diosdado, M.; Padrón, J.M.; García-Hernández, R.; Gamarro, F.; Fustero, S.; Sánchez-Aparicio, J.E.; Masgrau, L.; et al. Synthesis of polyfluoroalkyl sp2-iminosugar glycolipids and evaluation of their immunomodulatory properties towards anti-tumor, anti-leishmanial and anti-inflammatory therapies. Eur. J. Med. Chem. 2019, 182, 111604. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.; Ouadid-Ahidouch, H.; Sanchez-Fernandez, E.M.; Risquez-Cuadro, R.; Fernandez, J.M.G.; Ortiz-Mellet, C.; Ahidouch, A. New Castanospermine Glycoside Analogues Inhibit Breast Cancer Cell Proliferation and Induce Apoptosis without Affecting Normal Cells. PLoS ONE 2013, 8, e76411. [Google Scholar] [CrossRef]
- Alcalde-Estévez, E.; Arroba, A.I.; Sánchez-Fernández, E.M.; Mellet, C.O.; García Fernández, J.M.; Masgrau, L.; Valverde, Á.M. The sp2-iminosugar glycolipid 1-dodecylsulfonyl-5N,6O-oxomethylidenenojirimycin (DSO2-ONJ) as selective anti-inflammatory agent by modulation of hemeoxygenase-1 in Bv.2 microglial cells and retinal explants. Food Chem. Toxicol. 2018, 111, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Cano-Cano, F.; Alcalde-Estévez, E.; Gómez-Jaramillo, L.; Iturregui, M.; Sánchez-Fernández, E.M.; García Fernández, J.M.; Ortiz Mellet, C.; Campos-Caro, A.; López-Tinoco, C.; Aguilar-Diosdado, M.; et al. Anti-Inflammatory (M2) Response Is Induced by a sp2-Iminosugar Glycolipid Sulfoxide in Diabetic Retinopathy. Front. Immunol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Bao, Y.W.; Yuan, Y.; Chen, J.H.; Lin, W.Q. Kidney disease models: Tools to identify mechanisms and potential therapeutic targets. Zool. Res. 2018, 39, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; De La Rosa, L.R.; Murillo-Cuesta, S.; Vaquero-Villanueva, L.; Hurlé, J.M.; Varela-Nieto, I.; Valverde, Á.M. Autophagy resolves early retinal inflammation in Igf1-deficient mice. DMM Dis. Model. Mech. 2016, 9, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Zhang, Y.; Fei, C.; Zhang, J.; Wang, L.; Yi, Z.W.; Gao, G. Neutralization of IL-18 by IL-18 binding protein ameliorates bleomycin-induced pulmonary fibrosis via inhibition of epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2019, 508, 660–666. [Google Scholar] [CrossRef]
- Masaoka, T.; Vanuytsel, T.; Vanormelingen, C.; Kindt, S.; Salim Rasoel, S.; Boesmans, W.; De Hertogh, G.; Farré, R.; Vanden Berghe, P.; Tack, J. A spontaneous animal model of intestinal dysmotility evoked by inflammatory nitrergic dysfunction. PLoS ONE 2014, 9, e95879. [Google Scholar] [CrossRef]
- Gómez-jaramillo, L.; Cano-cano, F.; Campos, A.; Álcala, M.; Álvarez-gallego, F.; Arroba, A.I.; Aguilar-diosdado, M. Adult kidney explants is a physiologic model for studying diabetic nephropathy. Life Sci. 2022, 300, 120575. [Google Scholar] [CrossRef]
- Amoah-Apraku, B.; Chandler, L.J.; Harrison, J.K.; Tang, S.S.; Ingelfinger, J.R.; Guzman, N.J. NF-κB and transcriptional control of renal epithelial-inducible nitric oxide synthase. Kidney Int. 1995, 48, 674–682. [Google Scholar] [CrossRef][Green Version]
- Wolf, G.; Ziyadeh, F.N.; Schroeder, R.; Stahl, R.A.K. Angiotensin II inhibits inducible nitric oxide synthase in tubular MCT cells by a posttranscriptional mechanism. J. Am. Soc. Nephrol. 1997, 8, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Satriano, J.; Ishizuka, S.; Archer, D.C.; Blantz, R.C.; Kelly, C.J. Regulation of intracellular polyamine biosynthesis and transport by NO and cytokines TNF-α and IFN-γ. Am. J. Physiol. Cell Physiol. 1999, 276, C892–C899. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Tong, L.; Xie, C.; Wei, Y.; Qu, Y.; Liang, H.; Zhang, Y.; Xu, T.; Qian, X.; Qiu, H.; Deng, H. Antitumor effects of berberine on gliomas via inactivation of caspase-1mediated IL-1β and IL-18 release. Front. Oncol. 2019, 9, 364. [Google Scholar] [CrossRef]
- Grynberg, K.; Ma, F.Y.; Nikolic-Paterson, D.J. The JNK signaling pathway in renal fibrosis. Front. Physiol. 2017, 8, 829. [Google Scholar] [CrossRef]
- De Borst, M.H.; Prakash, J.; Melenhorst, W.B.W.H.; Van Den Heuvel, M.C.; Kok, R.J.; Navis, G.; Van Goor, H. Glomerular and tubular induction of the transcription factor c-Jun in human renal disease. J. Pathol. 2007, 213, 219–228. [Google Scholar] [CrossRef]
- De Borst, M.H.; Prakash, J.; Sandovici, M.; Klok, P.A.; Hamming, I.; Kok, R.J.; Navis, G.; Van Goor, H. c-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J. Pharmacol. Exp. Ther. 2009, 331, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Li, Y.; Jin, J.; Jiang, X.; Ren, Y.; He, Q. Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm. Biol. 2019, 57, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Maric-Bilkan, C.; Flynn, E.R.; Chade, A.R. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am. J. Physiol. Ren. Physiol. 2012, 302, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, Z.; Zhang, J.; Wang, J. Isoliquiritigenin attenuates acute renal injury through suppressing oxidative stress, fibrosis and JAK2/STAT3 pathway in streptozotocin-induced diabetic rats. Bioengineered 2021, 12, 11188–11200. [Google Scholar] [CrossRef]
- Xiong, M.; Jiang, L.; Zhou, Y.; Qiu, W.; Fang, L.; Tan, R.; Wen, P.; Yang, J. The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am. J. Physiol. Ren. Physiol. 2022, 302, 369–379. [Google Scholar] [CrossRef]
- Tang, O.; Chen, X.; Shen, S.; Hahn, M.; Pollock, C.A. MiRNA-200b represses transforming growth factor-β1-induced EMT and fibronectin expression in kidney proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2022, 1, 1266–1273. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; Gómez-Pérez, V.; García-Hernández, R.; García Fernández, J.M.; Plata, G.B.; Padrón, J.M.; Ortiz Mellet, C.; Castanys, S.; Gamarro, F. Antileishmanial activity of sp2-iminosugar derivatives. RSC Adv. 2015, 5, 21812–21822. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Jaramillo, L.; Cano-Cano, F.; Sánchez-Fernández, E.M.; Ortiz Mellet, C.; García-Fernández, J.M.; Alcalá, M.; Álvarez-Gallego, F.; Iturregui, M.; González-Montelongo, M.d.C.; Campos-Caro, A.; et al. Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (Ss)-DS-ONJ. Int. J. Mol. Sci. 2022, 23, 8450. https://doi.org/10.3390/ijms23158450
Gómez-Jaramillo L, Cano-Cano F, Sánchez-Fernández EM, Ortiz Mellet C, García-Fernández JM, Alcalá M, Álvarez-Gallego F, Iturregui M, González-Montelongo MdC, Campos-Caro A, et al. Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (Ss)-DS-ONJ. International Journal of Molecular Sciences. 2022; 23(15):8450. https://doi.org/10.3390/ijms23158450
Chicago/Turabian StyleGómez-Jaramillo, Laura, Fátima Cano-Cano, Elena M. Sánchez-Fernández, Carmen Ortiz Mellet, José M. García-Fernández, Martín Alcalá, Fabiola Álvarez-Gallego, Marta Iturregui, María del Carmen González-Montelongo, Antonio Campos-Caro, and et al. 2022. "Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (Ss)-DS-ONJ" International Journal of Molecular Sciences 23, no. 15: 8450. https://doi.org/10.3390/ijms23158450
APA StyleGómez-Jaramillo, L., Cano-Cano, F., Sánchez-Fernández, E. M., Ortiz Mellet, C., García-Fernández, J. M., Alcalá, M., Álvarez-Gallego, F., Iturregui, M., González-Montelongo, M. d. C., Campos-Caro, A., Arroba, A. I., & Aguilar-Diosdado, M. (2022). Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (Ss)-DS-ONJ. International Journal of Molecular Sciences, 23(15), 8450. https://doi.org/10.3390/ijms23158450






