Targeting Tumor Acidosis and Regulatory T Cells Unmasks Anti-Metastatic Potential of Local Tumor Ablation in Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Results
2.1. Combination of Bicarb, Cyclo, and ECE Ablation (BiCyclA) Reduced Tumor Growth and Increased Survival in 67NR and E0771 Tumors
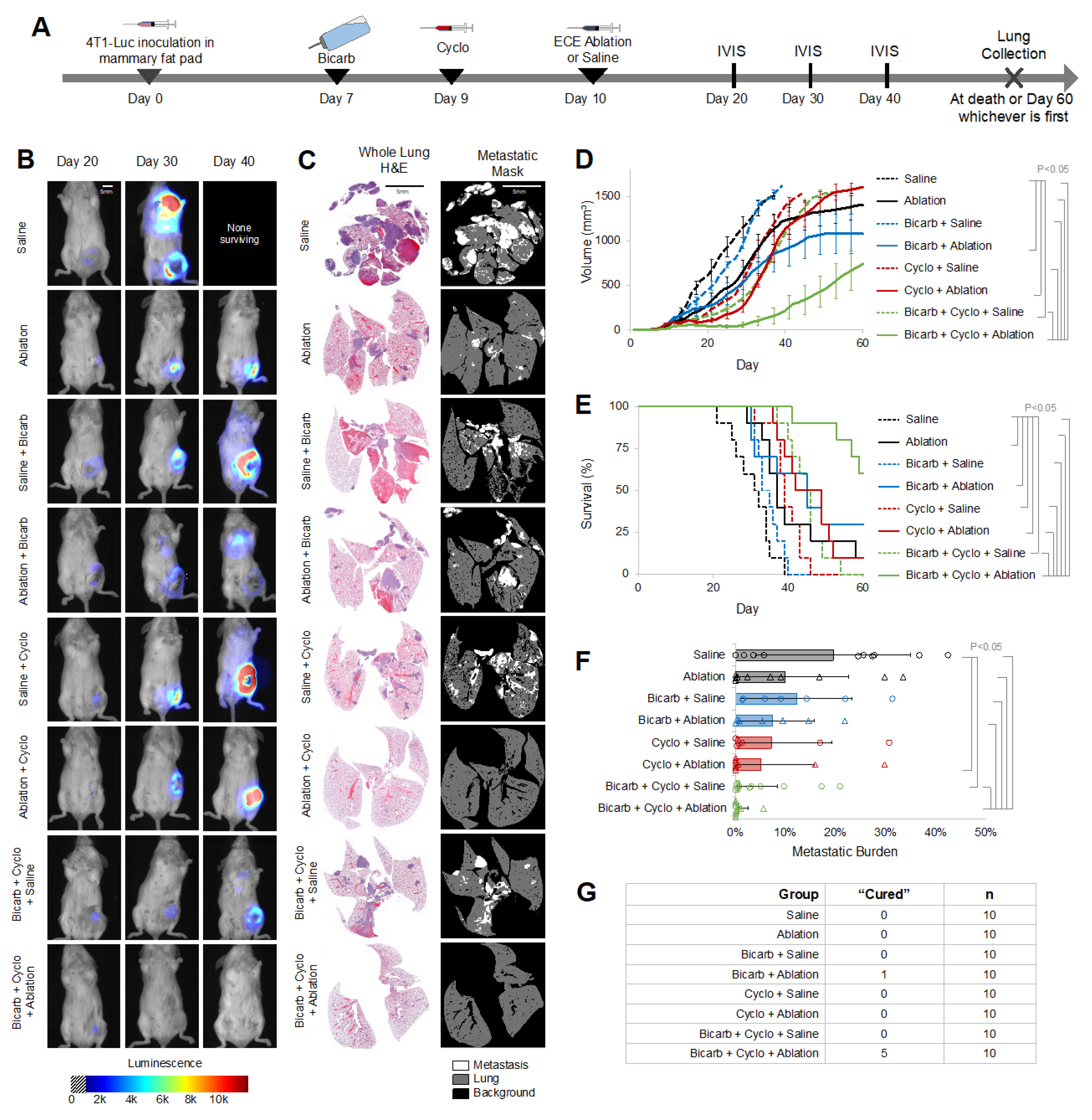
2.2. BiCyclA Therapy Cured Metastatic 4T1-Luc Tumors
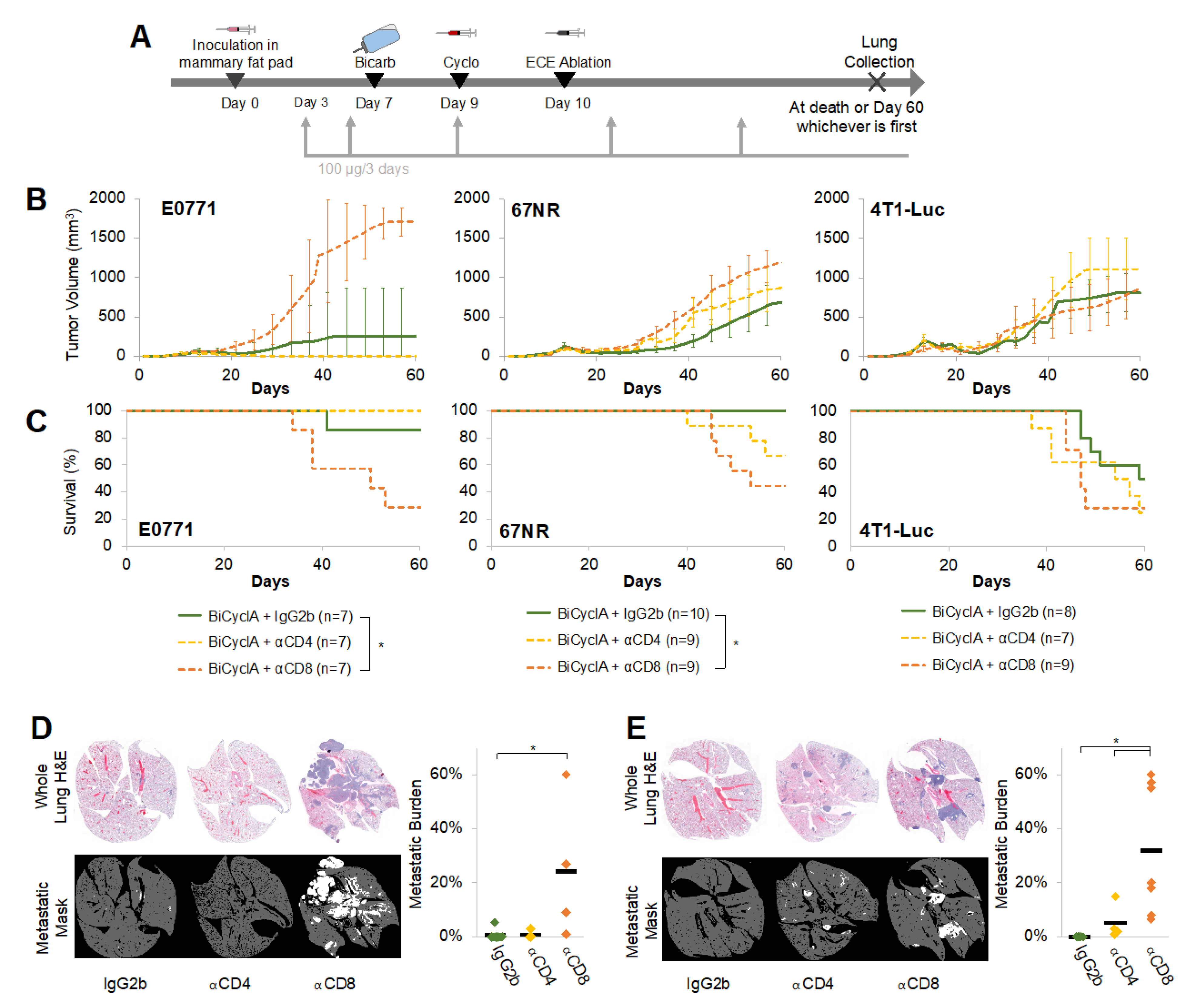
2.3. CD8+ Cells Contributed to the Anti-Metastatic Effects of BiCyclA Therapy
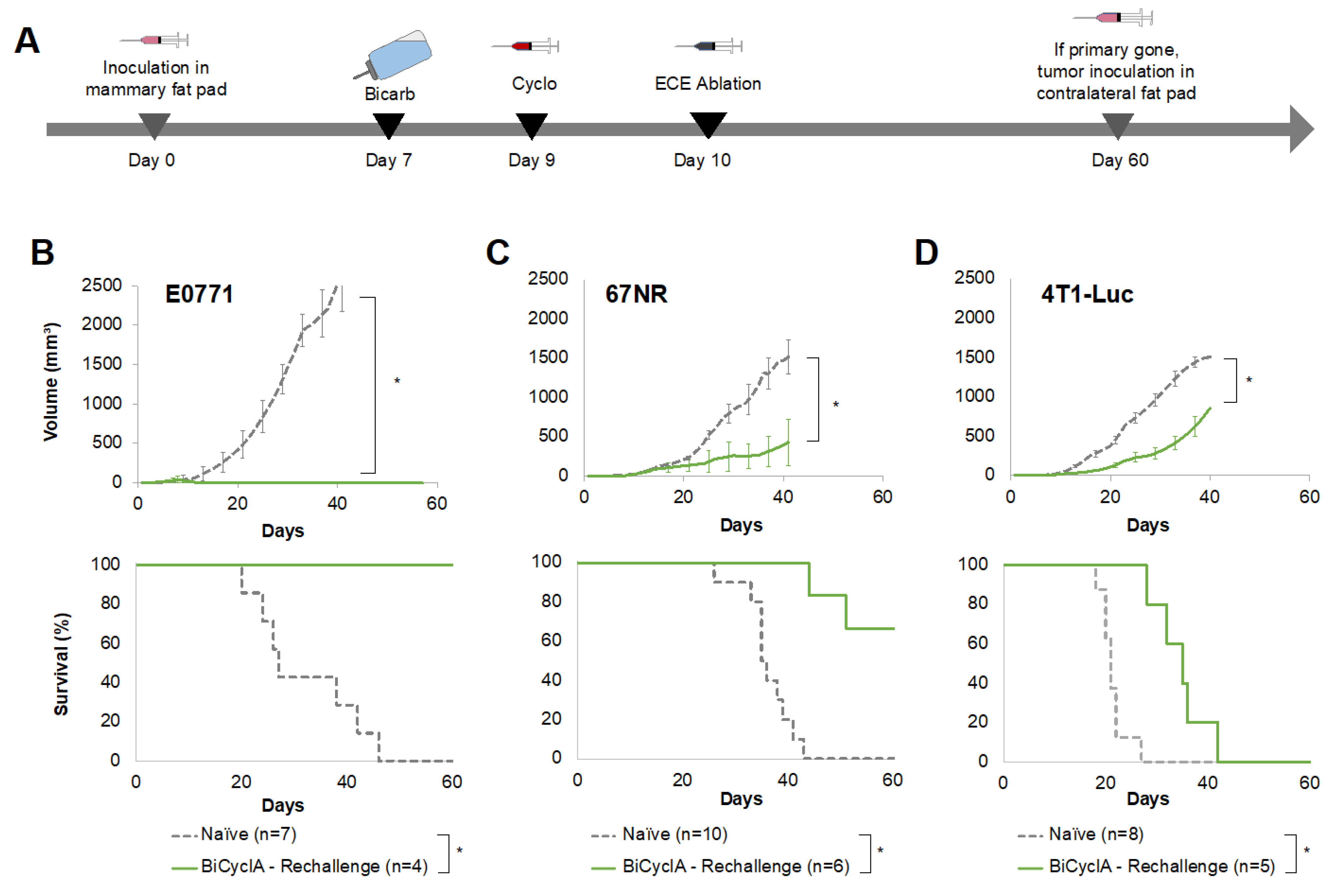
2.4. Mice Cured with BiCyclA Therapy Resisted Tumor Rechallenge
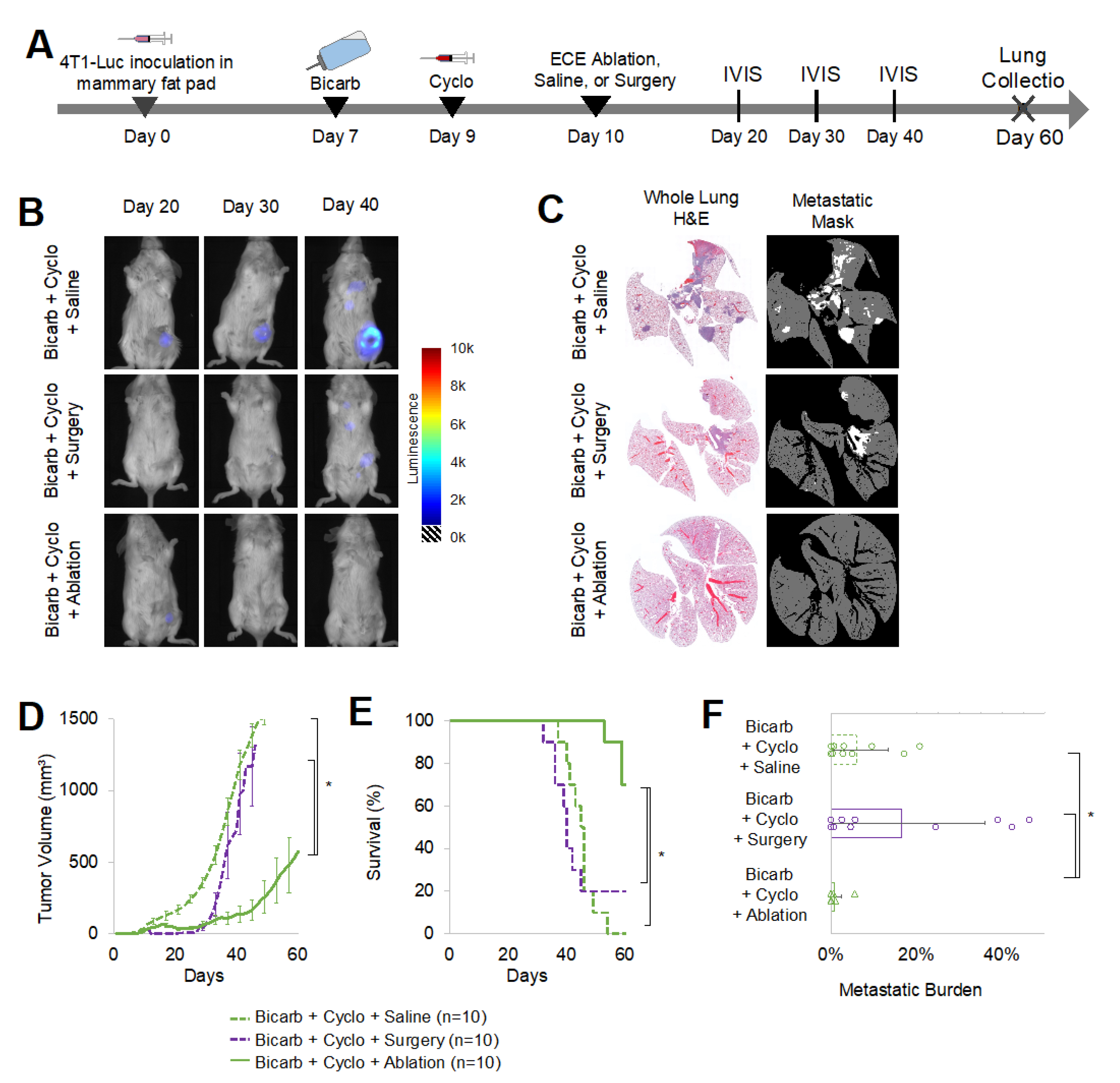
2.5. Tumor Ablation Was Crucial for Anti-Tumor and Anti-Metastatic Response in 4T1-Luc Tumors
3. Discussion
4. Methods
4.1. Tumor Cell Lines
4.2. Murine Mammary Tumor Models
4.3. Tumor Growth and Survival
4.4. 4T1-Luc Luminescent Imaging
4.5. Bicarbonate-Cyclophosphamide-Primed Ablation (BiCyclA)
4.6. Mammary Tumor Excision Surgery
4.7. Lung Metastases Quantification
4.8. T-Cell Depletion
4.9. Rechallenge
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Damgaci, S.; Ibrahim-Hashim, A.; Enríquez-Navas, P.M.; Pilon-Thomas, S.; Guvenis, A.; Gillies, R.J. Hypoxia and acidosis: Immune suppressors and therapeutic targets. Immunology 2018, 154, 354–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, R.; Rozeman, E.A.; Kreutz, M.; Renner, K.; Blank, C.U. Targeting tumor-associated acidity in cancer immunotherapy. Cancer Immunol. Immunother. 2018, 67, 1331–1348. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front. Immunol. 2021, 12, 741305. [Google Scholar] [CrossRef]
- Davern, M.; Donlon, N.E.; O’Connell, F.; Gaughan, C.; O’Donovan, C.; Habash, M.; Sheppard, A.D.; MacLean, M.; Dunne, M.R.; Moore, J.; et al. Acidosis significantly alters immune checkpoint expression profiles of T cells from oesophageal adenocarcinoma patients. Cancer Immunol. Immunother. 2022, 71, 1–17. [Google Scholar] [CrossRef]
- Stead, L.A.; Lash, T.L.; Sobieraj, J.E.; Chi, D.D.; Westrup, J.L.; Charlot, M.; Blanchard, R.A.; Lee, J.C.; King, T.C.; Rosenberg, C.L. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009, 11, R18-10. [Google Scholar] [CrossRef] [Green Version]
- Andrews, A. Treating with Checkpoint Inhibitors-Figure $1 Million per Patient. Am. Health Drug Benefits 2015, 8, 9. [Google Scholar]
- Meara, J.G.; Leather, A.J.M.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 2015, 386, 569–624. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Farmer, P.; Frenk, J.; Knaul, F.M.; Shulman, L.N.; Alleyne, G.; Armstrong, L.; Atun, R.; Blayney, D.; Chen, L.; Feachem, R.; et al. Expansion of cancer care and control in countries of low and middle income: A call to action. Lancet 2010, 376, 1186–1193. [Google Scholar] [CrossRef]
- Panieri, E. Breast cancer screening in developing countries. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The global burden of cancer: Priorities for prevention. Carcinogenesis 2010, 31, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-thermal ablations—Boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, A.; Pepple, A.; McGinnis, R.; Hubbard, R.; Felsted, A.; Worlikar, T.; Garavaglia, H.; Dib, J.; Guy, J.; Olszewski, M.; et al. 730 Histotripsy focused ultrasound ablation induces immunological cell death in treated and distant untreated tumors. J. Immunother. Cancer 2020, 8, 730. [Google Scholar] [CrossRef]
- Livraghi, T.; Benedini, V.; Lazzaroni, S.; Meloni, F.; Torzilli, G.; Vettori, C. Long term results of single session percutaneous ethanol injection in patients with large hepatocellular carcinoma. Cancer 1998, 83, 48–57. [Google Scholar] [CrossRef]
- Tapani, E.; Vehmas, T.; Taavitsainen, M. Ultrasound monitoring of experimental ethanol injection into pig liver. Acad. Radiol. 1994, 1, 21–24. [Google Scholar] [CrossRef]
- Koda, M.; Okamoto, K.; Miyoshi, Y.; Kawasaki, H. Hepatic vascular and bile duct injury after ethanol injection therapy for hepatocellular carcinoma. Gastrointest. Radiol. 1992, 17, 167–169. [Google Scholar] [CrossRef]
- Morhard, R.; Nief, C.; Castedo, C.B.; Hu, F.; Madonna, M.; Mueller, J.L.; Dewhirst, M.W.; Katz, D.F.; Ramanujam, N. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Morhard, R.; Mueller, J.L.; Tang, Q.; Nief, C.; Chelales, E.; Lam, C.T.; Alvarez, D.A.; Rubinstein, M.; Katz, D.F.; Ramanujam, N. Understanding Factors Governing Distribution Volume of Ethyl Cellulose-Ethanol to Optimize Ablative Therapy in the Liver. IEEE Trans. Biomed. Eng. 2020, 67, 2337–2348. [Google Scholar] [CrossRef]
- Nief, C.; Morhard, R.; Chelales, E.; Alvarez, D.A.; Bs, I.B.; Lam, C.T.; Sag, A.A.; Crouch, B.T.; Mueller, J.L.; Katz, D.; et al. Polymer-assisted intratumoral delivery of ethanol: Preclinical investigation of safety and efficacy in a murine breast cancer model. PLoS ONE 2021, 16, e0234535. [Google Scholar] [CrossRef]
- Nief, C.A.; Chelales, E.; Morhard, R.; Chem, M.; Everitt, J.; Mueller, J.; Yao, J.; Dewhirst, M.W.; Ramanujam, N. Abstract 5243: Averting tumor growth in rodent breast cancer models with a liquid ablation approach. Cancer Res. 2020, 80, 5243. [Google Scholar] [CrossRef]
- Lutsiak, M.E.C.; Semnani, R.T.; De Pascalis, R.; Kashmiri, S.V.S.; Schlom, J.; Sabzevari, H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005, 105, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.Y.; Sidana, A.; Chowdhury, W.H.; Solomon, S.B.; Drake, C.G.; Rodriguez, R.; Fuchs, E.J. Cyclophosphamide Unmasks an Antimetastatic Effect of Local Tumor Cryoablation. J. Pharmacol. Exp. Ther. 2009, 330, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano, A.P.; Mroz, P.; Wu, M.X.; Hamblin, M.R. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc. Natl. Acad. Sci. USA 2008, 105, 5495–5500. [Google Scholar] [CrossRef] [Green Version]
- Robey, I.F.; Baggett, B.K.; Kirkpatrick, N.D.; Roe, D.J.; Dosescu, J.; Sloane, B.F.; Hashim, A.I.; Morse, D.L.; Raghunand, N.; Gatenby, R.A.; et al. Bicarbonate Increases Tumor pH and Inhibits Spontaneous Metastases. Cancer Res. 2009, 69, 2260–2268. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wu, H.; Dai, C.; Pan, Q.; Ding, Z.; Hu, D.; Ji, B.; Luo, Y.; Hu, X. Beyond Warburg effect—Dual metabolic nature of cancer cells. Sci. Rep. 2014, 4, 4927. [Google Scholar] [CrossRef] [Green Version]
- Pilon-Thomas, S.; Kodumudi, K.N.; El-Kenawi, A.E.; Russell, S.; Weber, A.M.; Luddy, K.; Damaghi, M.; Wojtkowiak, J.W.; Mulé, J.J.; Ibrahim-Hashim, A.; et al. Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy. Cancer Res. 2016, 76, 1381–1390. [Google Scholar] [CrossRef] [Green Version]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- Hubert, P.; Roncarati, P.; Demoulin, S.; Pilard, C.; Ancion, M.; Reynders, C.; Lerho, T.; Bruyere, D.; Lebeau, A.; Radermecker, C.; et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J. Immunother. Cancer 2021, 9, e001966. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qian, W.; Mu, F.; Niu, L.; Du, D.; Xu, K. The future of cryoablation: An abscopal effect. Cryobiology 2020, 97, 1–4. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Garasa, S.; Barbes, B.; Solorzano, J.L.; Perez-Gracia, J.L.; Labiano, S.; Sanmamed, M.F.; Azpilikueta, A.; Bolaños, E.; et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res. 2016, 76, 5994–6005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey-Downs, L.C.; Thorpe, J.E.; Disch, B.C.; Bastian, A.; Hauser, P.J.; Farasyn, T.; Berry, W.L.; Hurst, R.E.; Ihnat, M.A. Development and Characterization of a Preclinical Model of Breast Cancer Lung Micrometastatic to Macrometastatic Progression. PLoS ONE 2014, 9, e98624. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, E.; Chen, C.; Li, C.; Ding, X.; Chen, J.; Xi, Q.; Zhou, S.; Liu, J.; Li, Z. Injectable and Biodegradable Chitosan Hydrogel-Based Drug Depot Contributes to Synergistic Treatment of Tumors. Biomacromolecules 2021, 22, 5339–5348. [Google Scholar] [CrossRef]
- Kim, I.S.; Gao, Y.; Welte, T.; Wang, H.; Liu, J.; Janghorban, M.; Sheng, K.; Niu, Y.; Goldstein, A.; Zhao, N.; et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat. Cell Biol. 2019, 21, 1113–1126. [Google Scholar] [CrossRef]
- Chao, M.; Wu, H.; Jin, K.; Li, B.; Wu, J.; Zhang, G.; Yang, G.; Hu, X. A nonrandomized cohort and a randomized study of local control of large hepatocarcinoma by targeting intratumoral lactic acidosis. eLife 2016, 5, e15691. [Google Scholar] [CrossRef]
- Finkel, J.; Tsochatzis, E.A. Targeting intra-tumoral lactic acidosis in hepatocellular carcinoma: A long way to go. J. Xiangya Med. 2017, 2, 32. [Google Scholar] [CrossRef]
- Aslakson, C.J.; Miller, F.R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992, 52, 1399–1405. [Google Scholar]
- Paschall, A.V.; Liu, K. An Orthotopic Mouse Model of Spontaneous Breast Cancer Metastasis. J. Vis. Exp. 2016, 114, e54040. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nief, C.A.; Gonzales, A.; Chelales, E.; Agudogo, J.S.; Crouch, B.T.; Nair, S.K.; Ramanujam, N. Targeting Tumor Acidosis and Regulatory T Cells Unmasks Anti-Metastatic Potential of Local Tumor Ablation in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 8479. https://doi.org/10.3390/ijms23158479
Nief CA, Gonzales A, Chelales E, Agudogo JS, Crouch BT, Nair SK, Ramanujam N. Targeting Tumor Acidosis and Regulatory T Cells Unmasks Anti-Metastatic Potential of Local Tumor Ablation in Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2022; 23(15):8479. https://doi.org/10.3390/ijms23158479
Chicago/Turabian StyleNief, Corrine A., Alana Gonzales, Erika Chelales, Júlia Sroda Agudogo, Brian T. Crouch, Smita K. Nair, and Nirmala Ramanujam. 2022. "Targeting Tumor Acidosis and Regulatory T Cells Unmasks Anti-Metastatic Potential of Local Tumor Ablation in Triple-Negative Breast Cancer" International Journal of Molecular Sciences 23, no. 15: 8479. https://doi.org/10.3390/ijms23158479
APA StyleNief, C. A., Gonzales, A., Chelales, E., Agudogo, J. S., Crouch, B. T., Nair, S. K., & Ramanujam, N. (2022). Targeting Tumor Acidosis and Regulatory T Cells Unmasks Anti-Metastatic Potential of Local Tumor Ablation in Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 23(15), 8479. https://doi.org/10.3390/ijms23158479






