Wasabi Component 6-(Methylsulfinyl)hexly Isothiocyanate and Derivatives Improve the Survival of Skin Allografts
Abstract
1. Introduction
2. Results
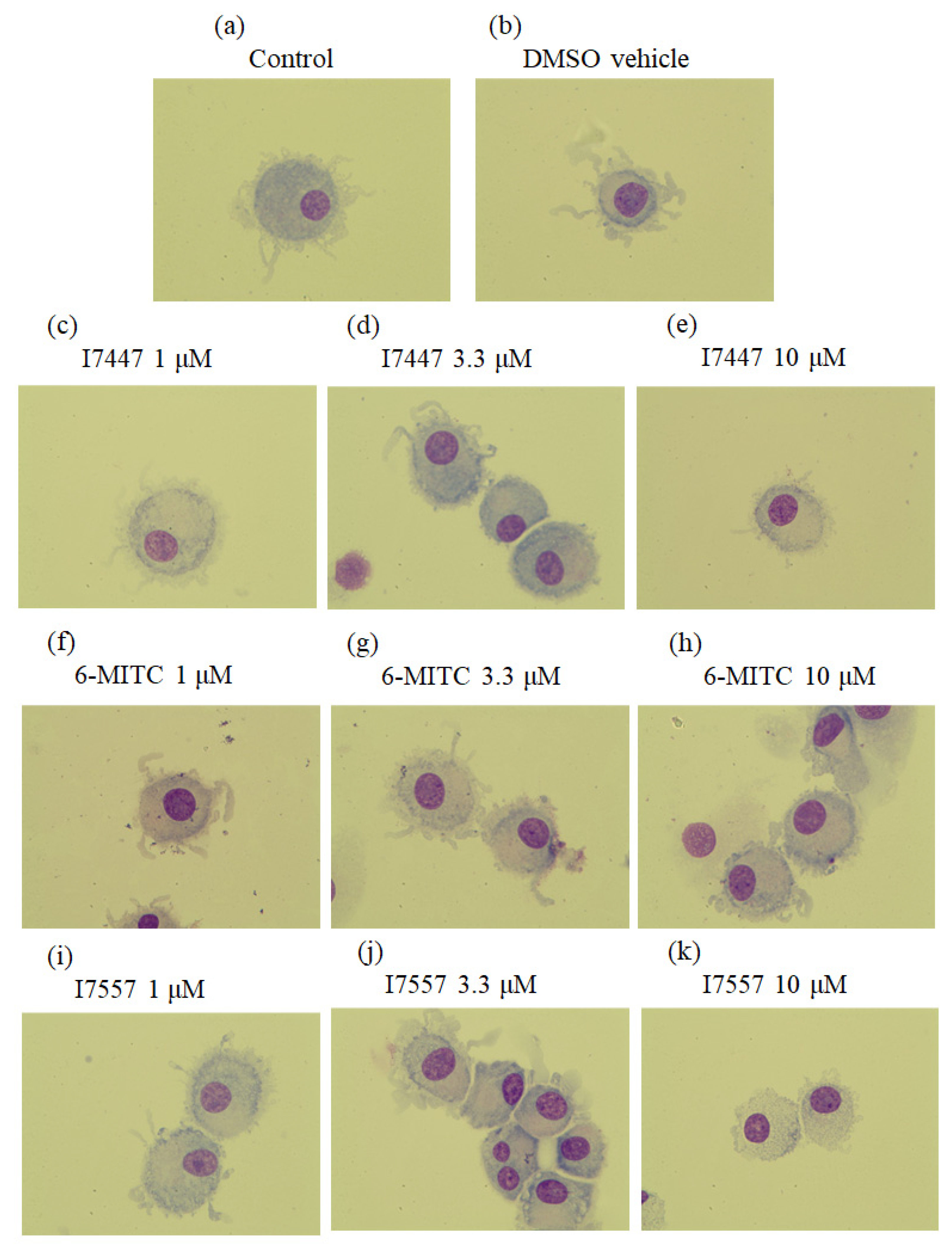
2.1. Morphological Features of 6-MITC and Its Derivatives on DCs
2.2. Effect of 6-MITC and Chemical Derivatives on DC Viability
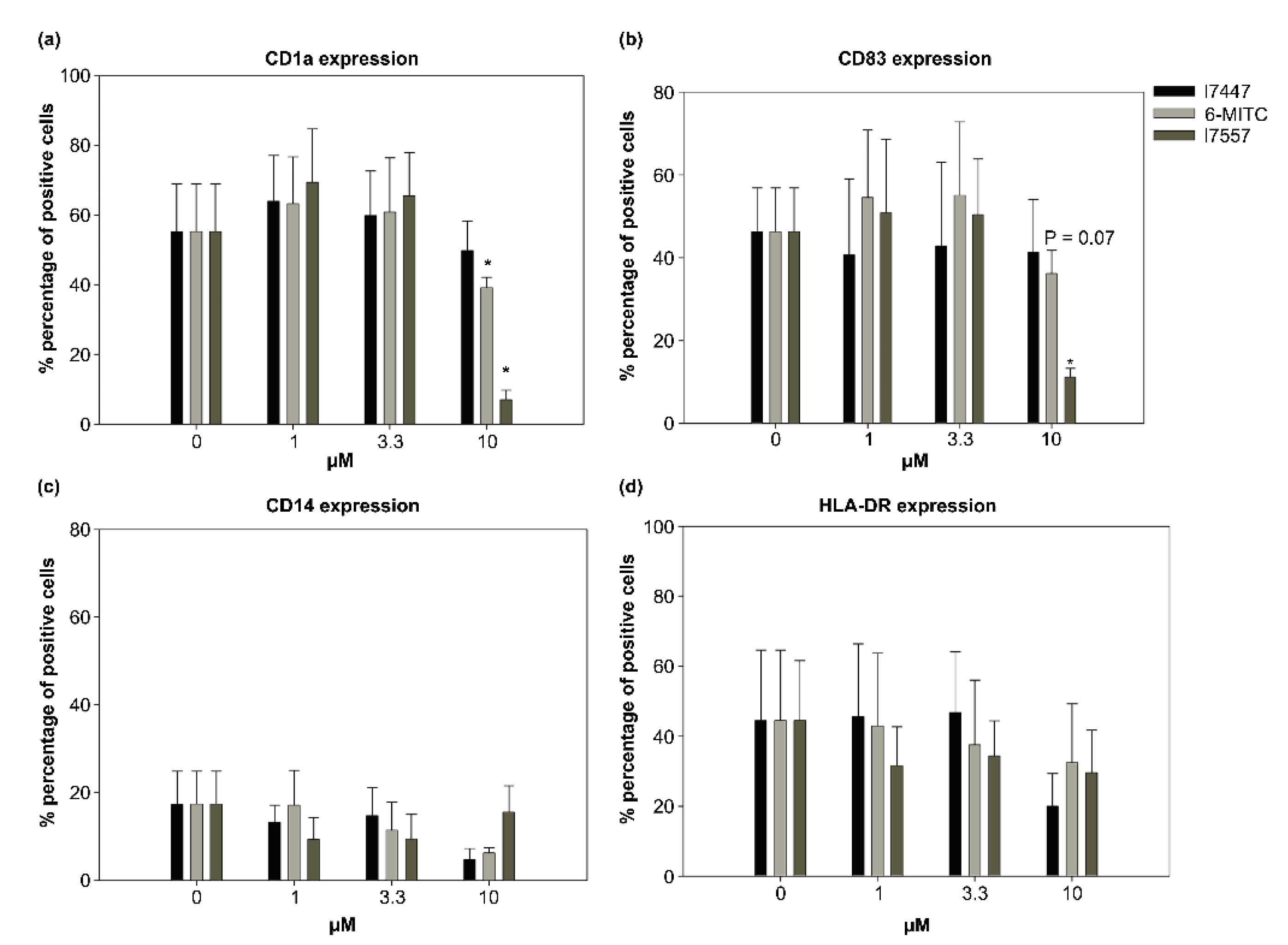
2.3. Modulation by 6-MITC and Its Derivatives on DC Surface Marker Expression
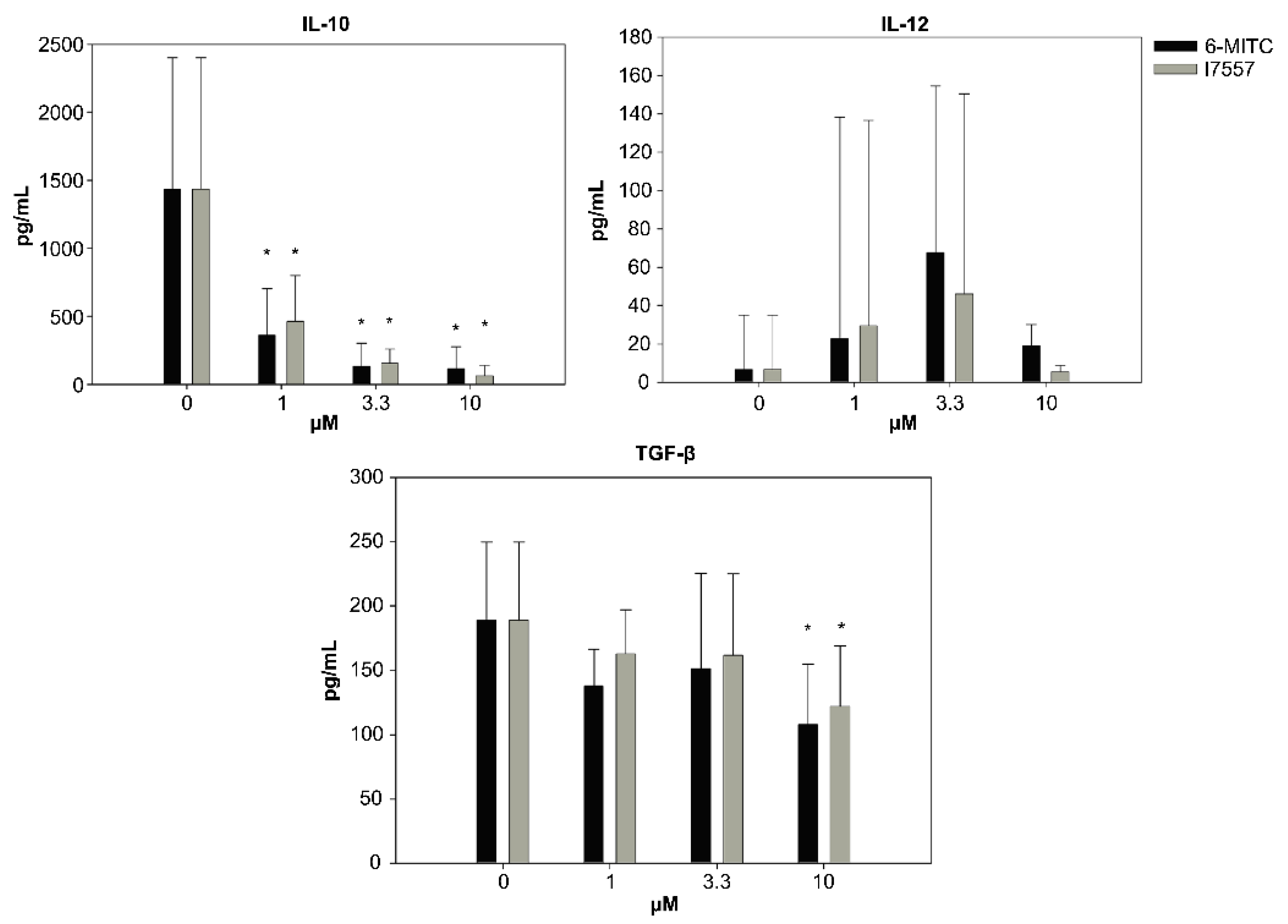
2.4. Secretion of Cytokines from DCs in the Presence of 6-MITC and Its Derivatives
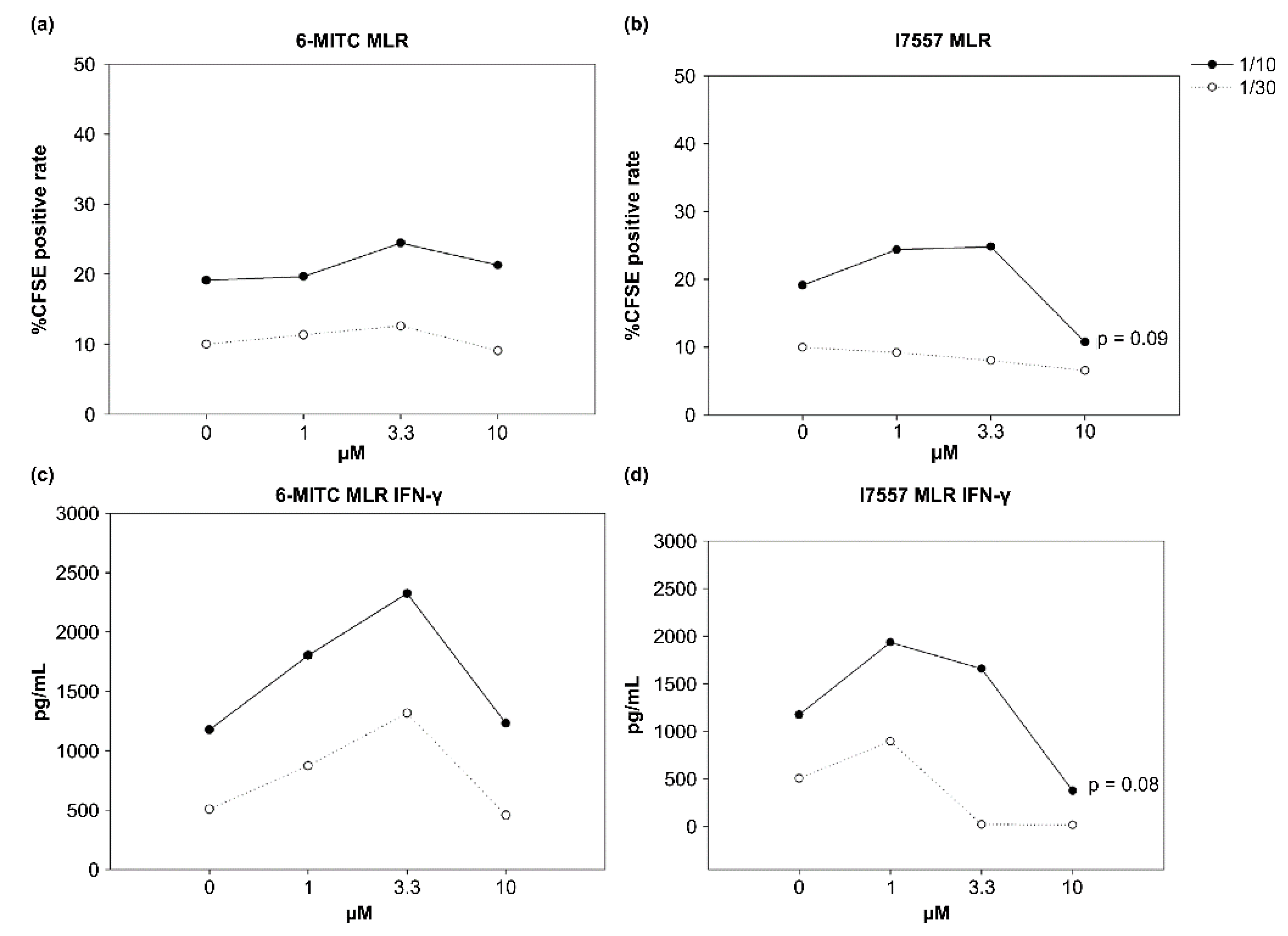
2.5. Effect of 6-MITC and I7557 on DC Stimulation of Allogeneic Naive T Cells
2.6. Effect of 6-MITC and I7557 on Allograft Survival
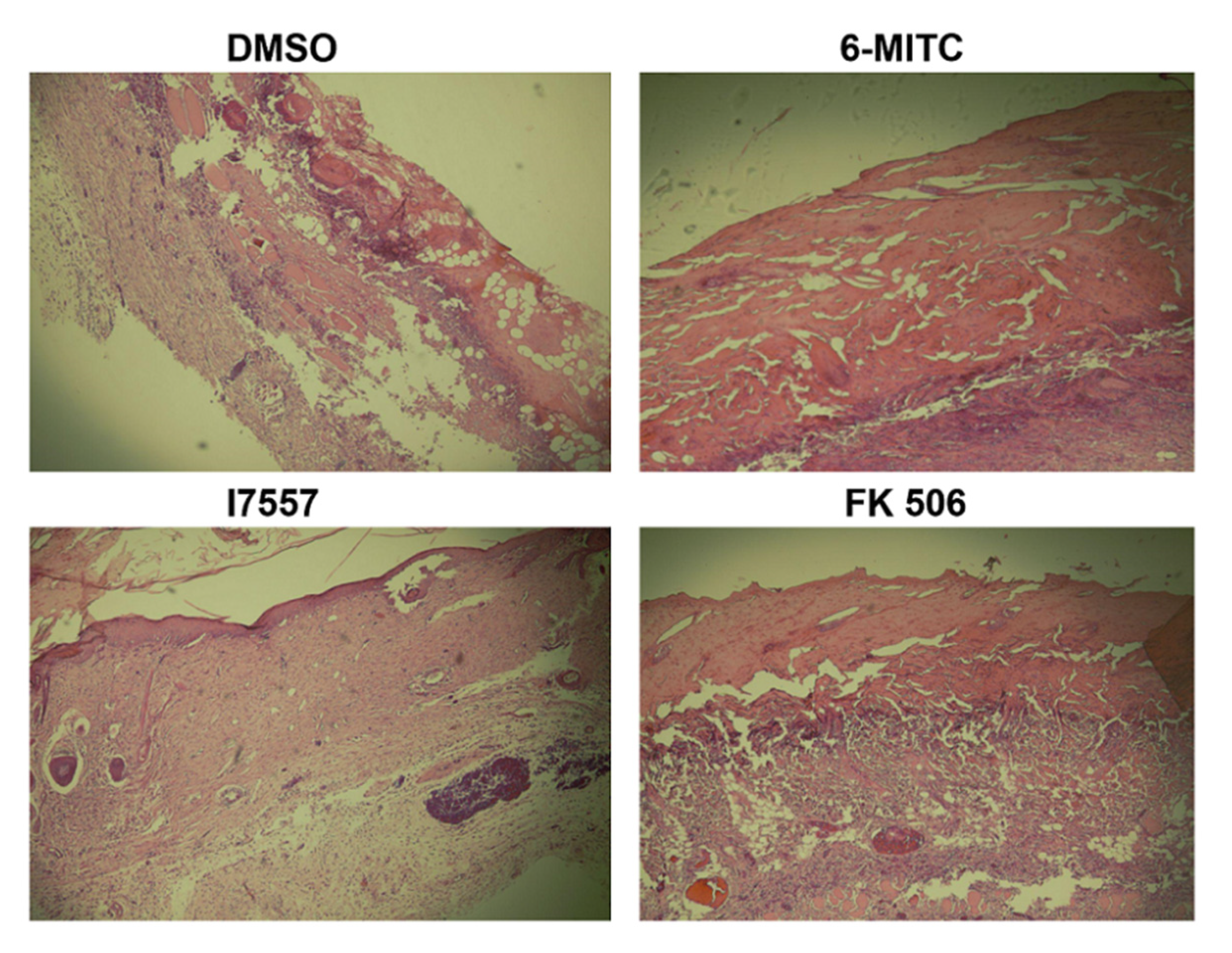
2.7. Histological Analysis
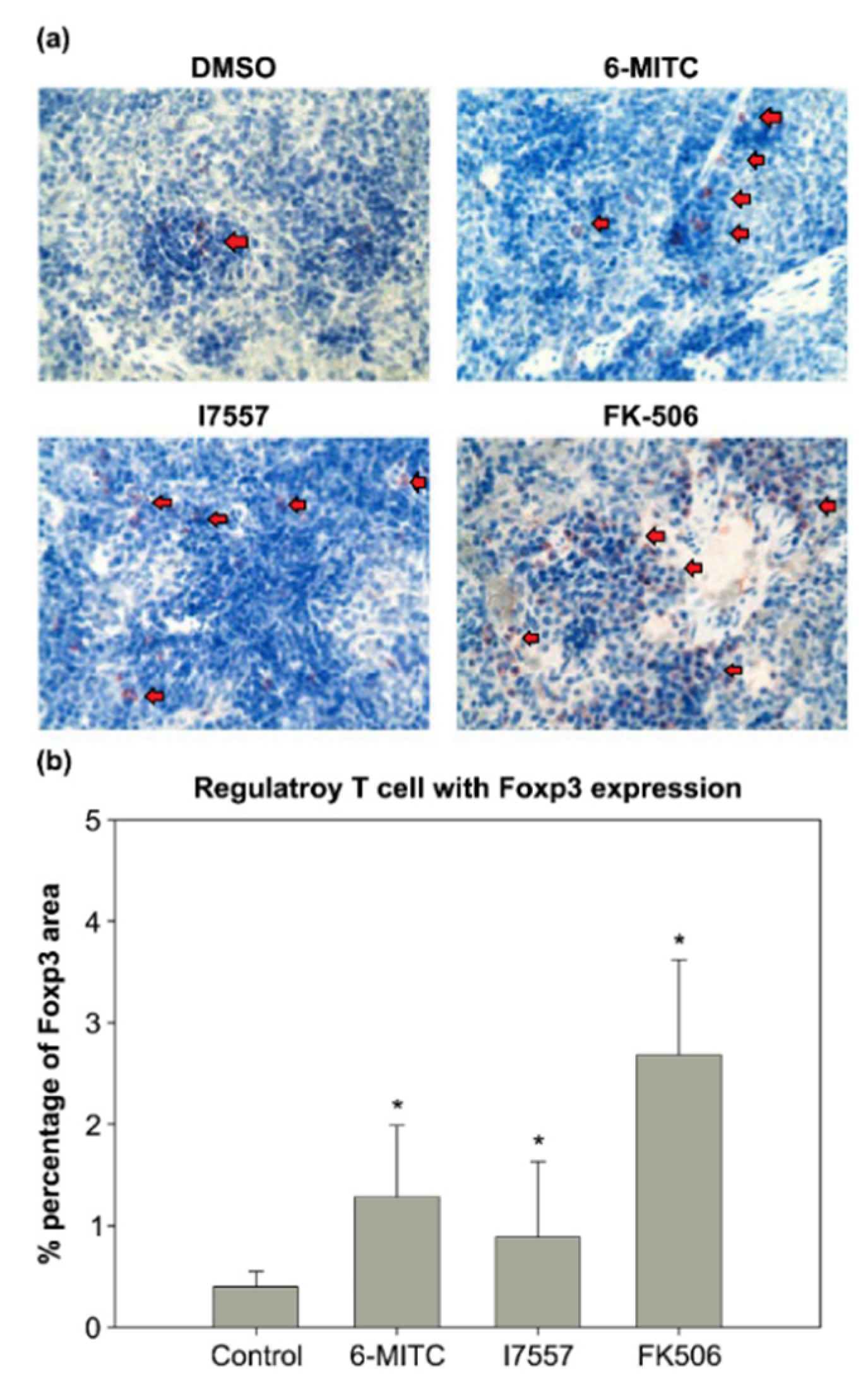
2.8. Immunohistochemical Study
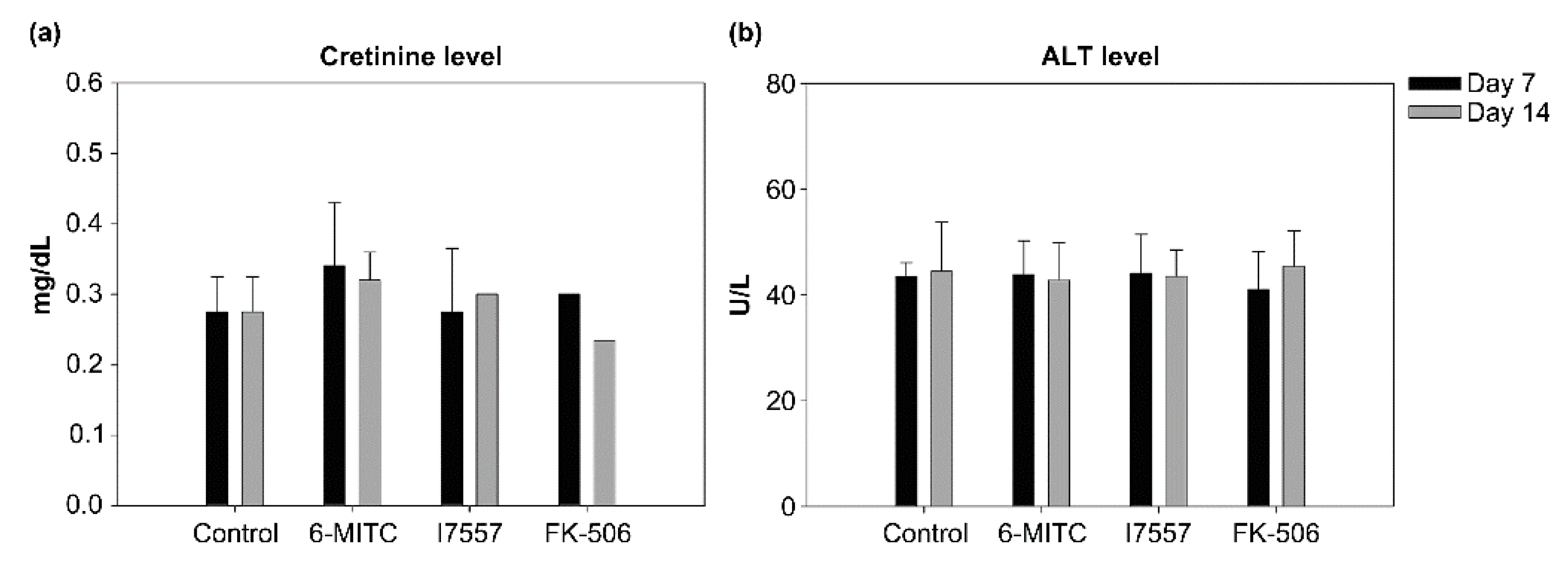
2.9. Evaluation of Hepatotoxicity and Nephrotoxicity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Generation of Dendritic Cells
4.3. Morphological Observation
4.4. Number of Viable Cells
4.5. Flow Cytometric Analysis
4.6. Allogeneic Naïve T-Cell Proliferation and Cytokine Secretion
4.7. Detection of Cytokines Produced by DC and Simulated Allogenic Naïve T cells
4.8. Animals
4.9. Skin Transplantation Model
4.10. Drug Preparation and Treatment
4.11. Histological Evaluation
4.12. Immunohistochemical Analysis
4.13. Evaluation of Leukocyte Count, Hepatic, and Renal Function
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandewalle, A.; Tourneur, E.; Bens, M.; Chassin, C.; Werts, C. Calcineurin/NFAT signaling and innate host defence: A role for NOD1-mediated phagocytic functions. Cell Commun. Signal. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Leas, B.F.; Uhl, S.; Sawinski, D.L.; Trofe-Clark, J.; Tuteja, S.; Kaczmarek, J.L.; Umscheid, C.A. AHRQ Comparative Effec-tiveness Reviews. In Calcineurin Inhibitors for Renal Transplant; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2016. [Google Scholar]
- Abdelmalek, M.F.; Humar, A.; Stickel, F.; Andreone, P.; Pascher, A.; Barroso, E.; Neff, G.W.; Ranjan, D.; Toselli, L.T.; Gane, E.J.; et al. Sirolimus Conversion Regimen Versus Continued Calcineurin Inhibitors in Liver Allograft Recipients: A Randomized Trial. Am. J. Transplant. 2012, 12, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 2007, 357, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.D.M.; Bier, J.; Paiatto, L.N.; Albuquerque, C.G.; Souza, C.L.; Fernandes, L.G.R.; Tamashiro, W.; Simioni, P.U. Tolerogenic Dendritic Cells on Transplantation: Immunotherapy Based on Second Signal Blockage. J. Immunol. Res. 2015, 2015, 856707. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Quintana, F.J. Tolerogenic dendritic cells. Semin. Immunopathol. 2017, 39, 113–120. [Google Scholar] [CrossRef]
- Morelli, A.E.; Thomson, A.W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 2007, 7, 610–621. [Google Scholar] [CrossRef]
- Hu, M.; Rogers, N.M.; Li, J.; Zhang, G.Y.; Wang, Y.M.; Shaw, K.; O’Connell, P.J.; Alexander, S.I. Antigen Specific Regulatory T Cells in Kidney Transplantation and Other Tolerance Settings. Front. Immunol. 2021, 12, 717594. [Google Scholar] [CrossRef] [PubMed]
- Ezzelarab, M.; Thomson, A.W. Tolerogenic dendritic cells and their role in transplantation. Semin. Immunol. 2011, 23, 252–263. [Google Scholar] [CrossRef]
- Uto, T.; Hou, D.-X.; Morinaga, O.; Shoyama, Y. Molecular Mechanisms Underlying Anti-Inflammatory Actions of 6-(Methylsulfinyl)hexyl Isothiocyanate Derived from Wasabi (Wasabia japonica). Adv. Pharmacol. Sci. 2012, 2012, 614046. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Huang, Y.-C.; Tsai, T.-H.; Liao, H.-F. Effect of Wasabi Component 6-(Methylsulfinyl)hexyl Isothiocyanate and Derivatives on Human Pancreatic Cancer Cells. Evid.-Based Complementary Altern. Med. 2014, 2014, 494739. [Google Scholar] [CrossRef]
- Wu, K.-M.; Liao, H.-F.; Chi, C.-W.; Kou, Y.R.; Chen, Y.-J. Wasabi Compound 6-(Methylsulfinyl) Hexyl Isothiocyanate Induces Cell Death with Coexisting Mitotic Arrest and Autophagy in Human Chronic Myelogenous Leukemia K562 Cells. Biomolecules 2019, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Tseng, W.-S.; Lai, J.C.-Y.; Shieh, H.-R.; Chi, C.-W.; Chen, Y.-J. Differential Pharmacological Activities of Oxygen Numbers on the Sulfoxide Moiety of Wasabi Compound 6-(Methylsulfinyl) Hexyl Isothiocyanate in Human Oral Cancer Cells. Molecules 2018, 23, 2427. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liao, H.F.; Kuo, C.D.; Huang, Y.C.; Shueng, P.W.; Hsu, Y.P.; Wang, L.Y.; Tsai, T.H.; Chen, Y.J. Norcan-tharidin modulates development of dendritic cells and prolongs skin allograft survival. Transplantation 2011, 92, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-P.; Lee, J.-J.; Chi, C.-W.; Chang, K.-M.; Chen, Y.-J. Platonin Improves Survival of Skin Allografts. J. Surg. Res. 2010, 164, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-C.; Huang, W.-P.; Liu, C.-L.; Lee, J.-J.; Liu, T.-P.; Ko, W.-C.; Huang, Y.-C.; Hsu, M.-L.; Wu, C.-H.; Chen, Y.-J. Sorafenib Induces Autophagy in Human Myeloid Dendritic Cells and Prolongs Survival of Skin Allografts. Transplantation 2013, 95, 791–800. [Google Scholar] [CrossRef]
- Li, D.-Y.; Gu, C.; Min, J.; Chu, Z.-H.; Ou, Q.-J. Maturation induction of human peripheral blood mononuclear cell-derived dendritic cells. Exp. Ther. Med. 2012, 4, 131–134. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; He, W.; Luo, G.; Wu, J. Fundamental Immunology of Skin Transplantation and Key Strategies for Tolerance Induction. Arch. Immunol. Ther. Exp. 2013, 61, 397–405. [Google Scholar] [CrossRef]
- Gardner, C.R. The pharmacology of immunosuppressant drugs in skin transplant rejection in mice and other rodents. Gen. Pharmacol. Vasc. Syst. 1995, 26, 245–271. [Google Scholar] [CrossRef]
- Binet, I.; Wood, K.J.; Winyard, P.G.; Willoughby, D.A. In Vivo Models of Inflammation Immune Rejection and Skin Transplantation In Vivo. Methods Mol. Biol. 2003, 225, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Metes, D.; Thomson, A.W. Dendritic cells and regulation of alloimmune responses: Relevance to outcome and therapy of organ transplantation. Expert Rev. Clin. Immunol. 2005, 1, 419–430. [Google Scholar] [CrossRef]
- Barratt-Boyes, S.M.; Thomson, A.W. Dendritic Cells: Tools and Targets for Transplant Tolerance. Am. J. Transplant. 2005, 5, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Turnquist, H.R.; Zahorchak, A.F.; Raimondi, G. Tolerogenic Dendritic Cell-Regulatory T-cell Interaction and the Promotion of Transplant Tolerance. Transplantation 2009, 87 (Suppl. 9), S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Shi, X.G.; Tang, W.; Xiong, S.M.; Zhu, J.; Cai, X.; Han, Z.G.; Ni, J.H.; Shi, G.Y.; Jia, P.M.; et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 1997, 89, 3345–3353. [Google Scholar] [PubMed]
- Denton, M.D.; Magee, C.C.; Sayegh, M.H. Immunosuppressive strategies in transplantation. Lancet 1999, 353, 1083–1091. [Google Scholar] [CrossRef]
- Lee, J.J.; Liao, H.F.; Yang, Y.C.; Liu, C.L.; Chen, Y.Y.; Lin, C.P.; Chen, Y.J. Platonin modulates differentiation and maturation of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2006, 6, 287–293. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.-S.; Ko, C.-J.; Lin, J.-C.; Hsu, M.-L.; Ko, C.-C.; Chi, C.-W.; Tsai, T.-H.; Chen, Y.-J. Wasabi Component 6-(Methylsulfinyl)hexly Isothiocyanate and Derivatives Improve the Survival of Skin Allografts. Int. J. Mol. Sci. 2022, 23, 8488. https://doi.org/10.3390/ijms23158488
Huang T-S, Ko C-J, Lin J-C, Hsu M-L, Ko C-C, Chi C-W, Tsai T-H, Chen Y-J. Wasabi Component 6-(Methylsulfinyl)hexly Isothiocyanate and Derivatives Improve the Survival of Skin Allografts. International Journal of Molecular Sciences. 2022; 23(15):8488. https://doi.org/10.3390/ijms23158488
Chicago/Turabian StyleHuang, Tun-Sung, Chih-Jung Ko, Jiunn-Chang Lin, Ming-Ling Hsu, Chun-Chuan Ko, Chih-Wen Chi, Tung-Hu Tsai, and Yu-Jen Chen. 2022. "Wasabi Component 6-(Methylsulfinyl)hexly Isothiocyanate and Derivatives Improve the Survival of Skin Allografts" International Journal of Molecular Sciences 23, no. 15: 8488. https://doi.org/10.3390/ijms23158488
APA StyleHuang, T.-S., Ko, C.-J., Lin, J.-C., Hsu, M.-L., Ko, C.-C., Chi, C.-W., Tsai, T.-H., & Chen, Y.-J. (2022). Wasabi Component 6-(Methylsulfinyl)hexly Isothiocyanate and Derivatives Improve the Survival of Skin Allografts. International Journal of Molecular Sciences, 23(15), 8488. https://doi.org/10.3390/ijms23158488







