Comparative Investigation of the Chemiluminescent Properties of a Dibrominated Coelenterazine Analog
Abstract
:1. Introduction
2. Results and Discussion
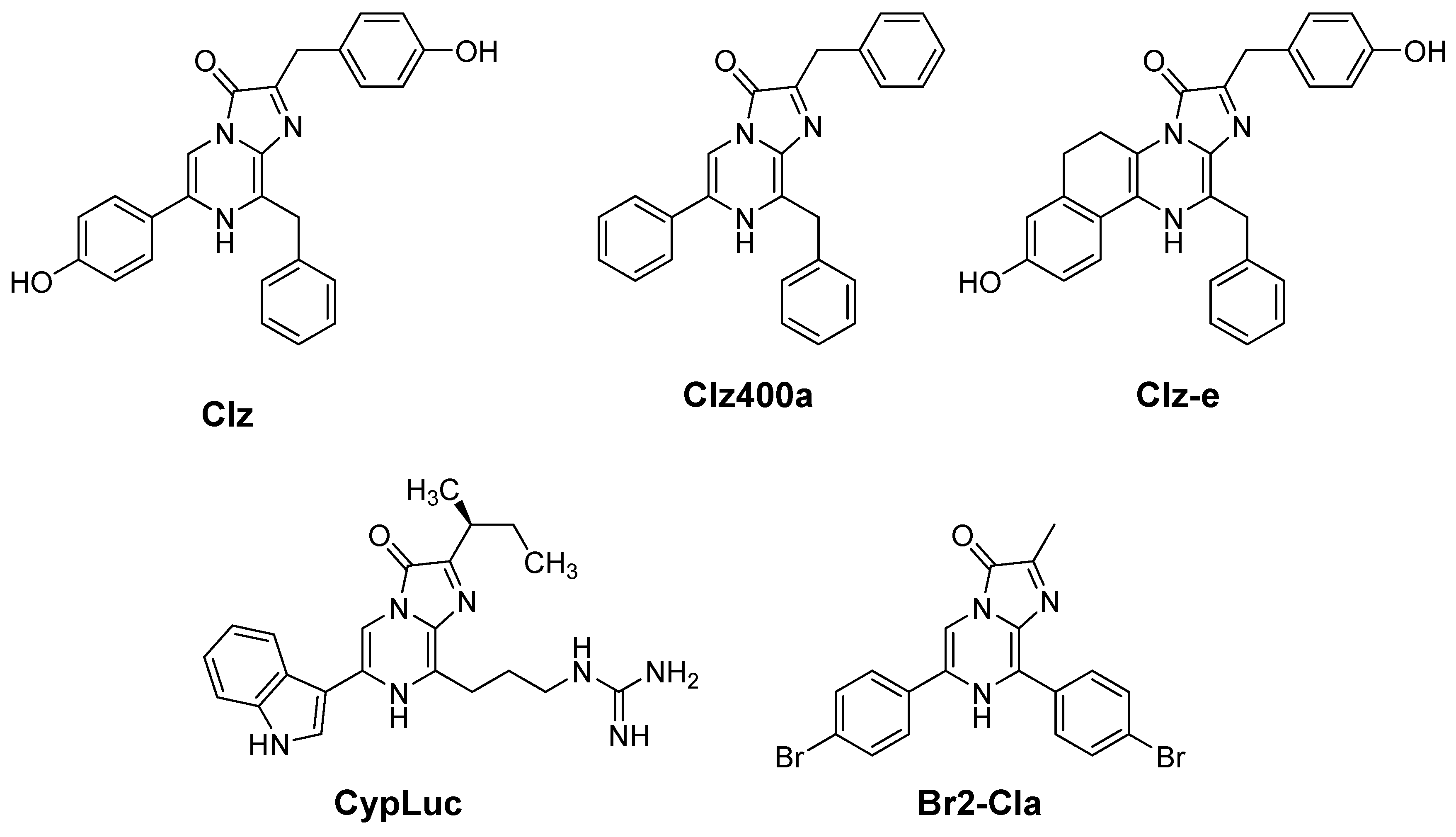
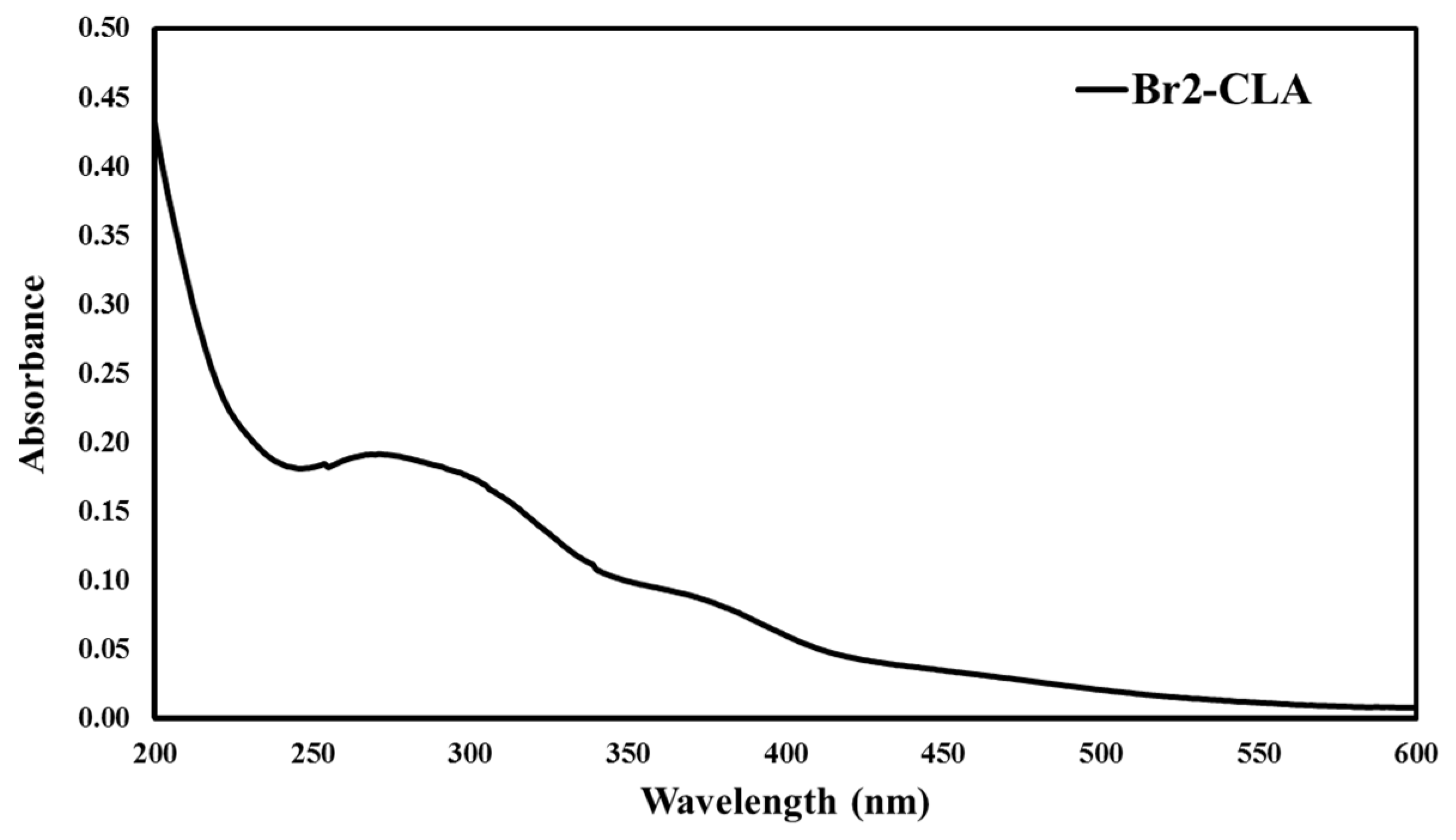
2.1. Chemistry and Spectroscopic Properties of Br2-Cla
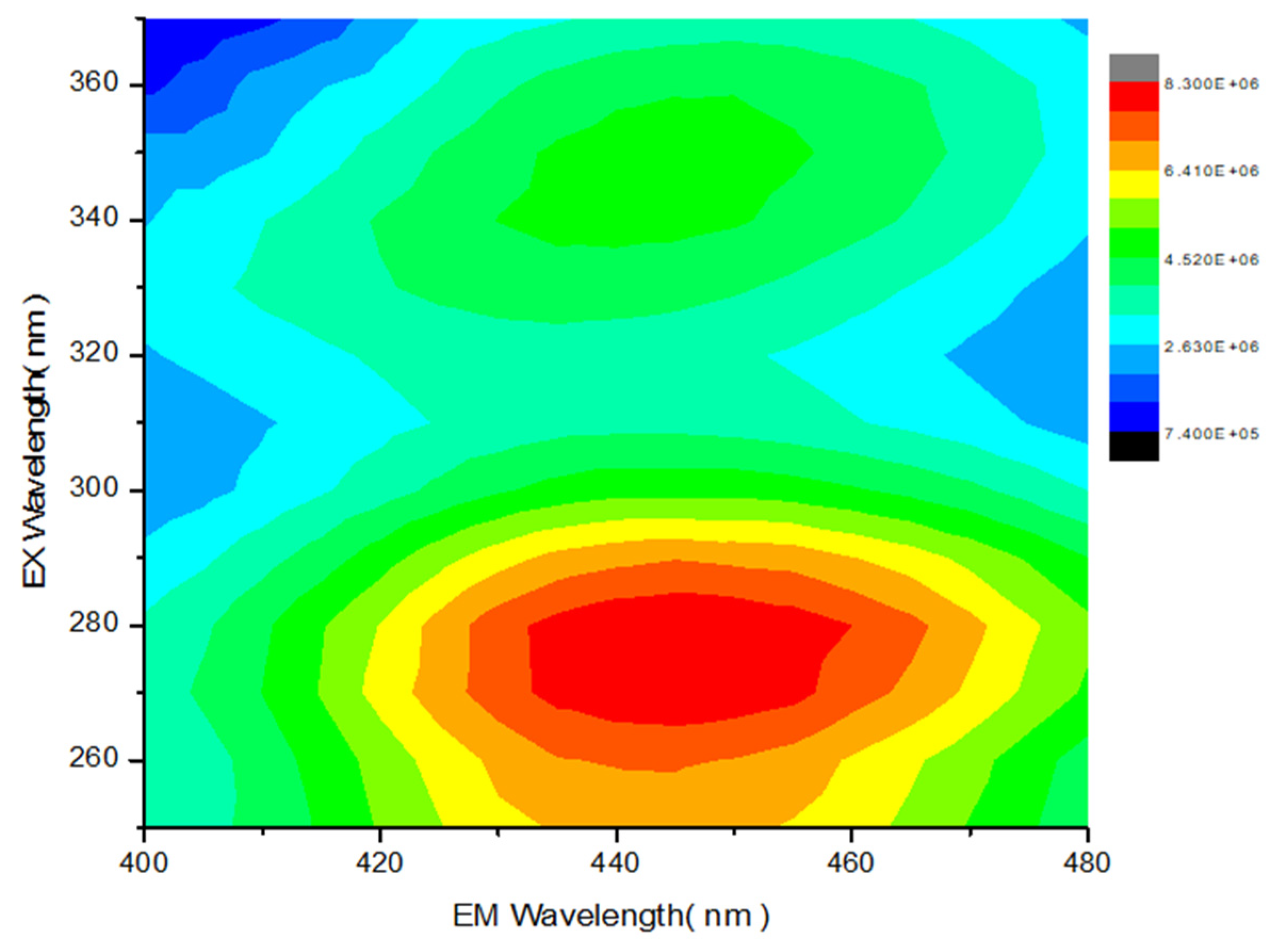
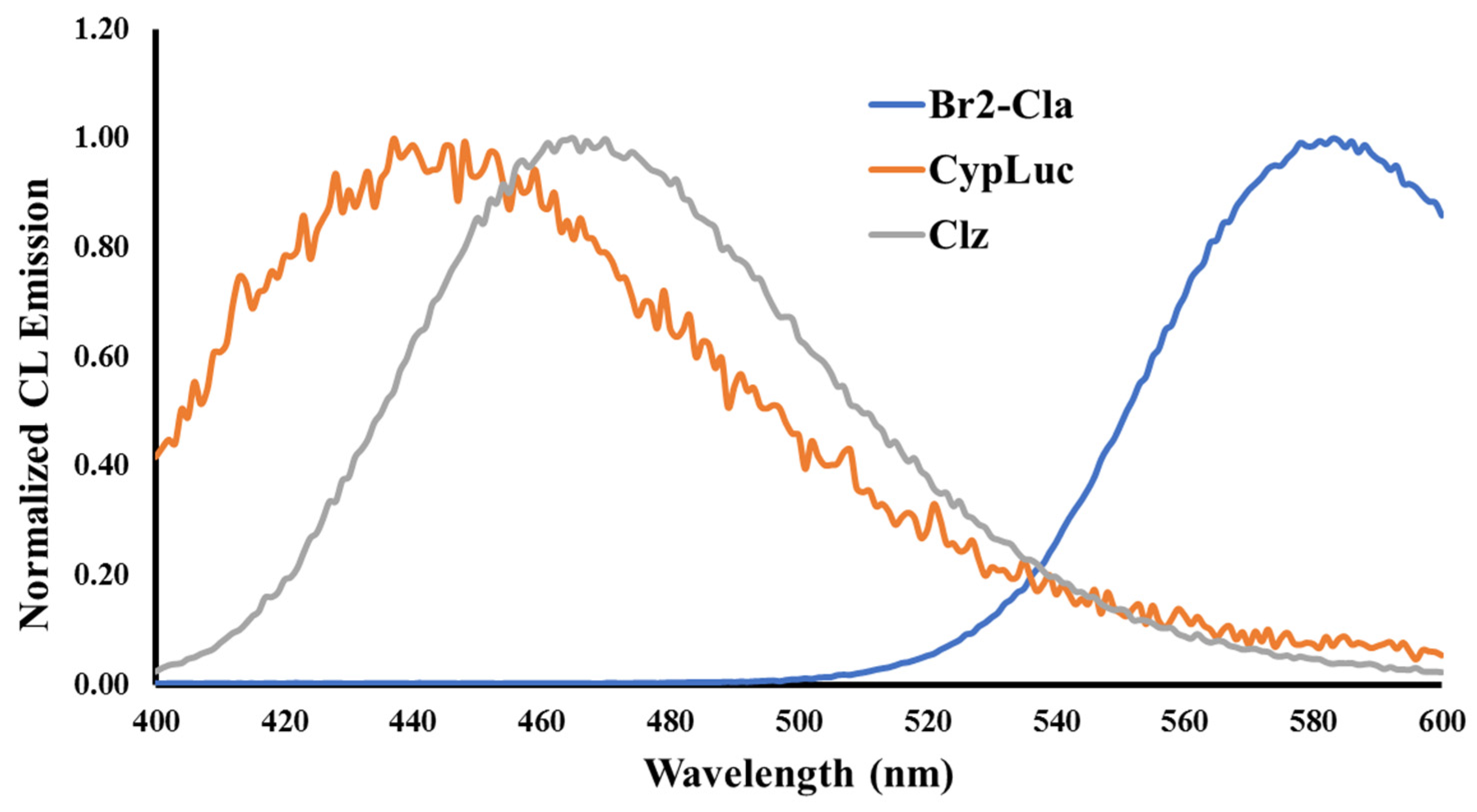
2.2. Investigation of Chemiluminescence in Aprotic Solvents
2.3. Investigation of Chemiluminescence in Protic Solvents
3. Materials and Methods
3.1. Synthesis of Br2-Cla
3.2. Clz, Clz400a, Clz-e and CypLuc
3.3. Luminometric and Spectroscopy Characterization
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Chemiluminescence and Bioluminescence as an Excitation Source in the Photodynamic Therapy of Cancer: A Critical Review. Chem. Phys. Chem. 2016, 17, 2286–2294. [Google Scholar] [CrossRef]
- Silva, J.P.; GonzálezBerdullas, P.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Development of a Coelenterazine Derivative with Enhanced Superoxide AnionTriggered Chemiluminescence in Aqueous Solution. Chemosensors 2022, 10, 174. [Google Scholar] [CrossRef]
- Vacher, M.; Galván, I.F.; Ding, B.W.; Schramm, S.; Berraud-Pache, R.; Naumov, P.; Ferré, N.; Liu, Y.J.; Navizet, I.; Roca-Sanjuán, D.; et al. Chemi- and bioluminescence of cyclic peroxides. Chem. Rev. 2018, 118, 6927–6974. [Google Scholar] [CrossRef] [PubMed]
- Pinto da Silva, L.; Magalhães, C.M.; Esteves da Silva, J.C.G. Interstate Crossing-Induced Chemiexcitation Mechanism as the Basis for Imidazopyrazinone Bioluminescence. ChemistrySelect 2016, 1, 3343–3356. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Comparative study of the chemiluminescence of coelenterazine, coelenterazine-e and Cypridina luciferin with an experimental and theoretical approach. J. Photochem. Photobiol. B 2019, 190, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Grinstead, K.M.; Rowe, L.; Ensor, C.M.; Joel, S.; Daftarian, P.; Dikici, E.; Zingg, J.-M.; Daunert, S. Red-Shifted Aequorin Variants Incorporating Non-Canonical Amino Acids: Applications in In Vivo Imaging. PLoS ONE 2016, 11, e0158579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimmer, T.; Lorenz, B.; Stieger, K. Quantification of the vascular endothelial growth factor with a bioluminescence resonance energy transfer (BRET) based single molecule biosensor. Biosens. Bioelectron. 2016, 86, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.; Akin, A.R.; Francis, K.P.; Tangney, M. In vivo bioluminescence imaging of intratumoral bacteria. Methods Mol. Biol. 2016, 1409, 69–77. [Google Scholar] [PubMed]
- Zhang, Y.; Pang, L.; Ma, C.; Tu, Q.; Zhang, R.; Saeed, E.; Mahmoud, A.E.; Wang, J. Small Molecule-Initiated Light-Activated Semiconducting Polymer Dots: An Integrated Nanoplatform for Targeted Photodynamic Therapy and Imaging of Cancer Cells. Anal. Chem. 2014, 86, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Pinto da Silva, L.; Núnez-Montenegro, A.; Magalhães, C.M.; Ferreira, P.J.O.; Duarte, D.; González-Berdullas, P.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C.G. Single-molecule chemiluminescent photosensitizer for a self-activating and tumor-selective photodynamic therapy of cancer. Eur. J. Med. Chem. 2019, 183, 111683. [Google Scholar] [CrossRef] [PubMed]
- Pinto da Silva, L.; Magalhães, C.M.; Núnez-Montenegro, A.; Ferreira, P.J.O.; Duarte, D.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C.G. Study of the Combination of Self-Activating Photodynamic Therapy and Chemotherapy for Cancer Treatment. Biomolecules 2019, 9, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, C.M.; González-Berdullas, P.; Duarte, D.; Correia, A.S.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Target-Oriented Synthesis of Marine Coelenterazine Derivatives with Anticancer Activity by Applying the Heavy-Atom Effect. Biomedicines 2021, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Shelef, O.; Sedgwick, A.C.; Pozzi, S.; Green, O.; Satchi-Fainaro, R.; Shabat, D.; Sessler, J.L. Turn on chemiluminescence-basedprobes for monitoring tyrosinase activity in conjunction with biological thiols. Chem. Commun. 2021, 57, 11386–11389. [Google Scholar] [CrossRef] [PubMed]
- Berneschi, S.; Trono, C.; Mirasoli, M.; Giannetti, A.; Zangheri, M.; Guardigli, M.; Tombelli, S.; Marchgiani, E.; Baldini, F.; Roda, A. In-Parallel Polar Monitoring of Chemiluminescence Emission Anisotropy at the Solid-Liquid Interface by an Optical Fiber Radial Array. Chemosensors 2020, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Ievtukhov, V.; Zadykowicz, B.; Blazheyevskiy, M.Y.; Krzyminski, K. New luminometric method for quantification of biological sulfur nucleophiles with the participation of 9-cyano-10-methylacridinium salt. Luminescence 2022, 37, 208–219. [Google Scholar] [CrossRef]
- Min, C.G.; Pinto da Silva, L.; Esteves da Silva, J.C.G.; Yang, X.K.; Huang, S.J.; Ren, A.M.; Zhu, Y.Q. A Computational Investigation of the Equilibrium Constants for the Fluorescent and Chemiluminescent States of Coelenteramide. Chem. Phys. Chem. 2017, 18, 117–123. [Google Scholar] [CrossRef]
- Jiang, T.; Du, L.; Li, M. Lighting up bioluminescence with coelenterazine: Strategies and applications. Photochem. Photobiol. Sci. 2016, 15, 466–480. [Google Scholar] [CrossRef]
- Krasitskaya, V.V.; Bashmakova, E.E.; Frank, L.A. Coelenterazine-dependent luciferases as a powerful analytical tool for research and biomedical applications. Int. J. Mol. Sci. 2020, 21, 7465. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tanaka, M.; Nishihara, R.; Hiruta, Y.; Citterio, D.; Suzuki, K.; Niwa, K. Quantitative evaluation of luminescence intensity from enzymatic luminescence reaction of coelenterazine analogues. J. Photochem. Photobiol. A 2020, 394, 112459. [Google Scholar] [CrossRef]
- Min, C.G.; Ferreira, P.J.O.; Pinto da Silva, L. Theoretically obtained insight into the mechanism and dioxetanone species responsible for the singlet chemiexcitation of Coelenterazine. J. Photochem. Photobiol. B 2017, 174, 18–26. [Google Scholar] [CrossRef]
- Lindberg, E.; Mizukami, S.; Ibata, K.; Miyawaki, A.; Kikuchi, K. Development of luminescent Coelenterazine derivatives activatable by b-galactosidase for monitoring dual gene expression. Chem. Eur. J. 2013, 19, 13970–14976. [Google Scholar] [CrossRef] [PubMed]
- Haddock, S.H.D.; Moline, M.A.; Case, J.F. Bioluminescence in the sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teranishi, K. Luminescence of imidazo[1,2-a]pyrazin-3(7H)-one compounds. Bioorg. Chem. 2007, 35, 82–111. [Google Scholar] [CrossRef] [PubMed]
- Kanie, S.; Komatsu, M.; Mitani, Y. Luminescence of Cypridina Luciferin in the Presence of Human Plasma Alpha 1-Acid Glycoprotein. Int. J. Mol. Sci. 2020, 21, 7516. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Dong, G.; Yan, C.; Cui, Y.; Zhang, Z.; Du, L.; Li, M. Novel furimazine derivatives for nanoluciferase bioluminescence with various C-6 and C-8 substituents. Org. Biomol. Chem. 2021, 19, 7930. [Google Scholar] [CrossRef]
- Yuan, M.L.; Jiang, T.Y.; Du, L.P.; Li, M.Y. Luminescence of coelenterazine derivatives with C-8 extended electronic conjugation. Chin. Chem. Lett. 2016, 27, 550–554. [Google Scholar] [CrossRef]
- Shimomura, O.; Musicki, B.; Kishi, Y. Semi-synthetic aequorins with improved sensitivitiy to Ca2+ ions. Biochem. J. 1989, 261, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Gagnot, G.; Hervin, V.; Coutant, E.P.; Goyard, S.; Jacob, Y.; Rose, T.; Hibti, F.E.; Quatela, A.; Janin, Y.L. Core-Modified Coelenterazine Luciferin Analogues: Synthesis and Chemiluminescence Properties. Chem. Eur. J. 2021, 27, 2112–2123. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, X.; Zhou, Y.; Yampolsky, I.; Du, L.; Li, M. New Bioluminescent Coelenterazine Derivatives with Various C-6 substitutions. Org. Biomol. Chem. 2017, 15, 7008. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T.; Hayashi, C.; Ishii, Y. Chemiluminescent 2,6-diphenylimidazo[1,2-a]pyrazin-3(7H)-ones: A new entry to Cypridina luciferin analogues. Photochem. Photobiol. Sci. 2014, 13, 182–189. [Google Scholar]
- Hirano, T.; Takahashi, Y.; Kondo, H.; Maki, S.; Kojima; Ikeda, H.; Niwa, H. The reaction mechanism for the high quantum yield of Cypridina (Vargula) bioluminescence supported by the chemiluminescence of 6-aryl-2-methylimidazo[1,2-a]pyrazin-3(7H)-ones (Cypridina luciferin analogues). Photochem. Photobiol. Sci. 2008, 7, 197–207. [Google Scholar] [CrossRef]
- Giuliani, G.; Molinari, P.; Ferreti, G.; Cappelli, A.; Anzini, M.; Vomero, S.; Costa, T. New red-shifted coelenterazine analogues with an extended electronic conjugation. Tetrahedron Lett. 2012, 53, 5114–5118. [Google Scholar] [CrossRef]
- Teranishi, K. Non-invasive and accurate readout of superoxide anion in biological systems by near-infrared light. Anal. Chim. Acta 2021, 1179, 338827. [Google Scholar] [CrossRef] [PubMed]
- Mizui, Y.; Eguchi, M.; Tanaka, M.; Ikeda, Y.; Yoshimura, H.; Ozawa, T.; Citterio, D.; Hiruta, Y. Long-term single cell bioluminescence imaging with C-3 position protected coelenterazine analogues. Org. Biomol. Chem. 2021, 19, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Orioka, M.; Eguchi, M.; Mizui, Y.; Ikeda, Y.; Sakarma, A.; Li, Q.; Yoshimura, H.; Ozawa, T.; Citterio, D.; Hiruta, Y. A Series of Furimazine Derivatives for Sustained Live-Cell Bioluminescence Imaging and Application to the Monitoring Myogenesis at the Single-Cell Level. Bioconjug. Chem. 2022, 33, 496–504. [Google Scholar] [CrossRef]
- Liu, S.; Su, Y.; Lin, Z.M.; Ronald, J.A. Brightening up Biology: Advances in Luciferase Systems for in Vivo Imaging. ACS Chem. Biol. 2021, 16, 2707–2718. [Google Scholar] [CrossRef]
- Bronsart, L.L.; Stokes, C.; Contag, C.H. Multimodality Imaging of Cancer Superoxide Anion Using the Small Molecule Coelenterazine. Mol. Imaging Biol. 2016, 18, 166–171. [Google Scholar] [CrossRef]
- Su, Y.; Song, H.; Lv, Y. Recent advances in chemiluminescence for reactive oxygen species sensing and imaging analysis. Microchem. J. 2019, 146, 83–97. [Google Scholar] [CrossRef]
- Braslavsky, S.E. Glossary of terms used in photochemistry, 3rd edition (IUPAC Recommendations 2006). Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Study of Coelenterazine Luminescence: Electrostatic Interactions as the Controlling Factor for Efficient Chemiexcitation. J. Lumin. 2018, 199, 339–347. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kondo, H.; Maki, S.; Niwa, H.; Ikeda, H.; Hirano, T. Chemiluminescence of 6-aryl-2-methylimidazo[1,2-a]pyrazin-3(7H)-ones in DMSO/TMG and in diglyme/acetate buffer: Support for the chemiexcitation process to generate the singlet-excited state of neutral oxyluciferin in a high quantum yield in the Cypridina (Vargula) bioluminescence mechanism. Tetrahedron Lett. 2006, 47, 6057. [Google Scholar]
- Pinto da Silva, L.; Esteves da Silva, J.C.G. Kinetics of inhibition of firefly luciferase by dehydroluciferyl-coenzyme A, dehydroluciferin and l-luciferin. Photochem. Photobiol. Sci. 2011, 10, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Esteves da Silva, J.C.G. Kinetics of inhibition of firefly luciferase by oxyluciferin and dehydroluciferyl-adenylate. Photochem. Photobiol. Sci. 2008, 7, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.A. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Pereira, R.F.J.; Magalhães, C.M.; Esteves da Silva, J.C.G. Mechanistic Insight into Cypridina Bioluminescence with a Combined Experimental and Theoretical Chemiluminescent Approach. J. Phys. Chem. B 2017, 121, 7862–7871. [Google Scholar] [CrossRef]
- Min, C.G.; Liu, Q.B.; Leng, Y.; Magalhães, C.M.; Huang, S.J.; Liu, C.X.; Yang, X.K.; Pinto da Silva, L. Mechanistic Insight into the Chemiluminescent Decomposition of Cypridina Dioxetanone and the Chemiluminescent, Fluorescent Properties of the Light Emitter of Cypridina Bioluminescence. J. Chem. Inf. Model. 2019, 59, 4398–4401. [Google Scholar] [CrossRef]
- Ding, B.W.; Liu, Y.J. Bioluminescence of Firefly Squid via Mechanism of Single Electron-Transfer Oxygenation and Charge-Transfer-Induced Luminescence. J. Am. Chem. Soc. 2017, 139, 1106–1119. [Google Scholar] [CrossRef]
- Ding, B.W.; Naumov, P.; Liu, Y.J. Mechanistic insight into marine bioluminescence: Photochemistry of the chemiexcited cypridina (sea firefly) lumophore. J. Chem. Theory Comput. 2015, 11, 591–599. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Magalhães, C.M.; Crista, D.M.A.; Esteves da Silva, J.C.G. Theoretical modulation of singlet/triplet chemiexcitation of chemiluminescent imidazopyrazinone dioxetanone via C8-substitutition. Photochem. Photobiol. Sci. 2017, 16, 897–907. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Tuning the Intramolecular Chemiexcitation of Neutral Dioxetanones by Interaction with Ionic Species. Molecules 2022, 27, 3861. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Esteves da Silva, J.C.G. Rationalizing the role of electron/charge transfer in the intramolecular chemiexcitation of dioxetanone-based chemi-/bioluminescent systems. J. Photochem. Photobiol. A 2022, 429, 113904. [Google Scholar] [CrossRef]
- Goto, T.; Takagi, T. Chemiluminescence of a Cypridina luciferin analogue, 2-methyl-6-phenyl-3,7-dihydroimidazo(1,2)pyrazin-3-one, in the presence of the xanthine-xanthine oxidase system. Bull. Chem. Soc. Jpn. 1980, 53, 833–834. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 6, 14. [Google Scholar] [CrossRef]
- Arnold, D.E.; Heimall, J.R. A review of chronic ganulomatous disease. Adv. Ther. 2017, 34, 2543–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayyan, M.; Hashim, M.A.; Al Nashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, D.T.; Valenting, J.S. How super is superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Lourenço, J.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Combined experimental and theoretical study of Coelenterazine chemiluminescence in aqueous solution. J. Lumin. 2018, 194, 139–145. [Google Scholar] [CrossRef]
- Green, O.; Eilon, T.; Hananya, N.; Gutkin, S.; Bauer, C.R.; Shabat, D. Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescence Dioxetane Probes. ACS Cent. Sci. 2017, 3, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Gnaim, S.; Green, O.; Shabat, D. The emergence of aqueous chemiluminescence: New promising class of phenoxy 1,2-dioxetane luminophores. Chem. Commun. 2018, 54, 2073–2085. [Google Scholar] [CrossRef]
| Molecules | Emax | Area | thalf | vinitial |
|---|---|---|---|---|
| Br2-Cla | 100 ± 10% | 100 ± 10% | 14 ± 1 s | 100 ± 11% |
| Clz | 33 ± 6% | 14 ± 2% | 19.2 ± 2.2 s | 55 ± 11% |
| Clz400a | 61 ± 2% | 103 ± 5% | 24.8 ± 0.5 s | 66 ± 4% |
| CypLuc | 40 ± 3% | 15 ± 1% | 5.9 ± 0.1 s | 55 ± 6% |
| Clz-e | 696 ± 45% | 272 ± 17% | 6.1 ± 0.3 s | 702 ± 84% |
| Molecules | Emax | Area | thalf | vinitial |
|---|---|---|---|---|
| Br2-Cla | 100 ± 4% | 100 ± 8% | 79.8 ± 10.2 s | 100 ± 10% |
| Clz | 54 ± 8% | 81 ± 11% | 164.0 ± 14.4 s | 51 ± 11% |
| Clz400a | 69 ± 8% | 64 ± 14% | 73.7 ± 17.6 s | 90 ± 13% |
| CypLuc | 413 ± 37% | 115 ± 5% | 9.5 ± 1.2 s | 317 ± 51% |
| Clz-e | 587 ± 71% | 156 ± 17% | 8.2 ± 1.3 s | 676 ± 126% |
| Solvent | Emax | Area | thalf | vinitial |
|---|---|---|---|---|
| DMSO—sodium acetate buffer pH 5.2 (1%) | 100 ± 10% | 100 ± 4% | 40.0 ± 4.8 s | 100 ± 24% |
| DMSO—NaOH (0.1 M) | 222 ± 11% | 88 ± 4% | 0.48 ± 0.02 s | 1517 ± 30% |
| DMF—sodium acetate buffer pH 5.2 (1%) | 100 ± 4% | 100 ± 5% | 108.5 ± 5.4 s | 100 ± 19% |
| DMF—NaOH (0.1 M) | 1253 ± 105% | 96 ± 4% | 0.48 ± 0.05 s | 1999 ± 252% |
| Molecule—KO2 Amount | Emax |
|---|---|
| Br2-Cla—5 mg | 109 ± 9% |
| Br2-Cla—10 mg | 100 ± 8% |
| Br2-Cla—15 mg | 56 ± 7% |
| Clz—5 mg | 3 ± 1% |
| Clz—10 mg | 6 ± 1% |
| Clz—15 mg | 8 ± 1% |
| Clz-e—10 mg | 35 ± 7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, J.; Magalhães, C.M.; González-Berdullas, P.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Comparative Investigation of the Chemiluminescent Properties of a Dibrominated Coelenterazine Analog. Int. J. Mol. Sci. 2022, 23, 8490. https://doi.org/10.3390/ijms23158490
Sousa J, Magalhães CM, González-Berdullas P, Esteves da Silva JCG, Pinto da Silva L. Comparative Investigation of the Chemiluminescent Properties of a Dibrominated Coelenterazine Analog. International Journal of Molecular Sciences. 2022; 23(15):8490. https://doi.org/10.3390/ijms23158490
Chicago/Turabian StyleSousa, João, Carla M. Magalhães, Patricia González-Berdullas, Joaquim C. G. Esteves da Silva, and Luís Pinto da Silva. 2022. "Comparative Investigation of the Chemiluminescent Properties of a Dibrominated Coelenterazine Analog" International Journal of Molecular Sciences 23, no. 15: 8490. https://doi.org/10.3390/ijms23158490
APA StyleSousa, J., Magalhães, C. M., González-Berdullas, P., Esteves da Silva, J. C. G., & Pinto da Silva, L. (2022). Comparative Investigation of the Chemiluminescent Properties of a Dibrominated Coelenterazine Analog. International Journal of Molecular Sciences, 23(15), 8490. https://doi.org/10.3390/ijms23158490










