The Significance of Cell Surface N-Glycosylation for Internalization and Potency of Cytotoxic Conjugates Targeting Receptor Tyrosine Kinases
Abstract
:1. Introduction
2. Results
2.1. The Contribution of Cell Surface N-Glycosylation to the Uptake of CCs Targeting RTKs
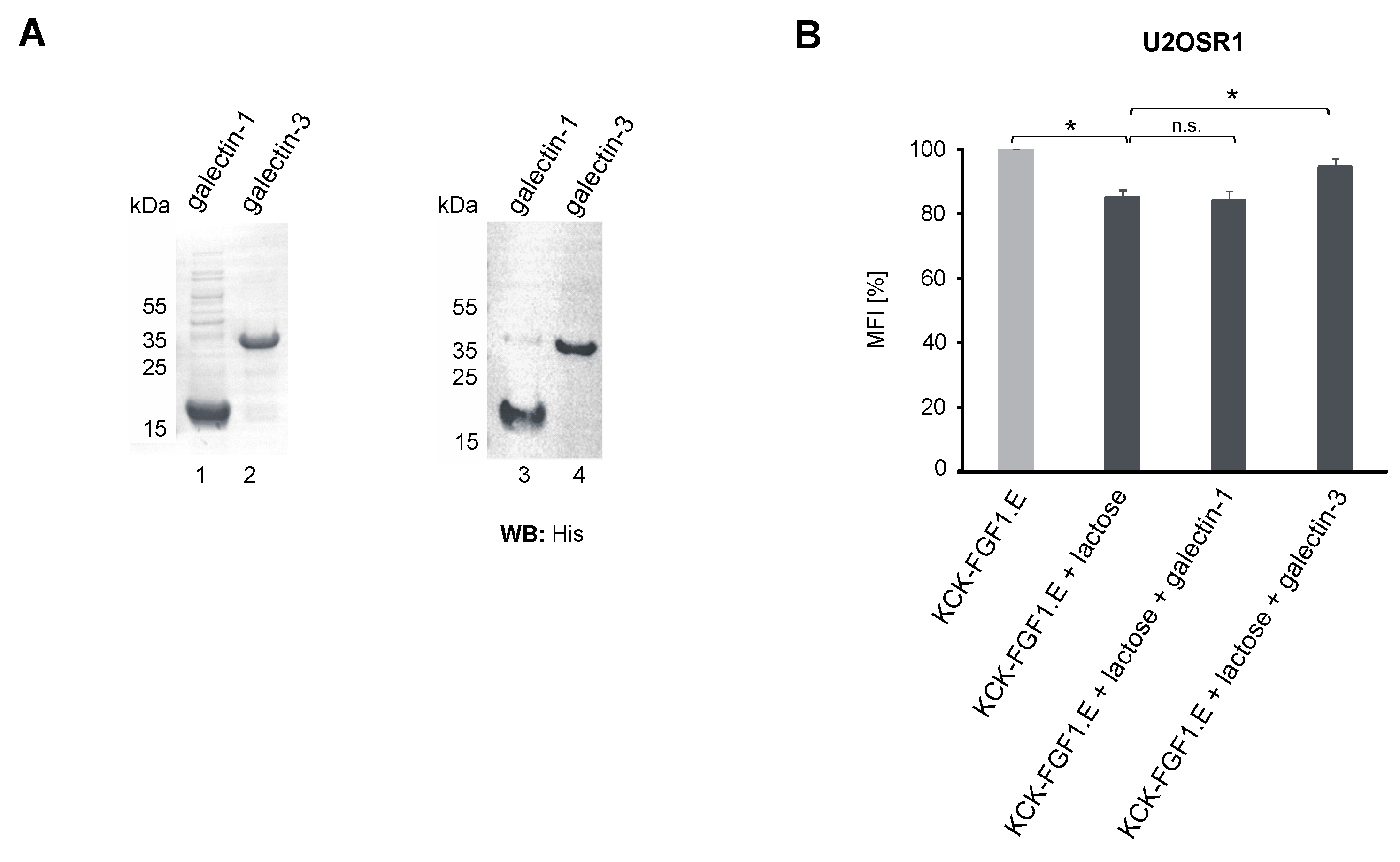
2.2. Role of the Galectin Network in the Internalization of Conjugates
2.3. Differential Effects of N-Glycosylation of RTKs on Their Recognition by the Conjugates
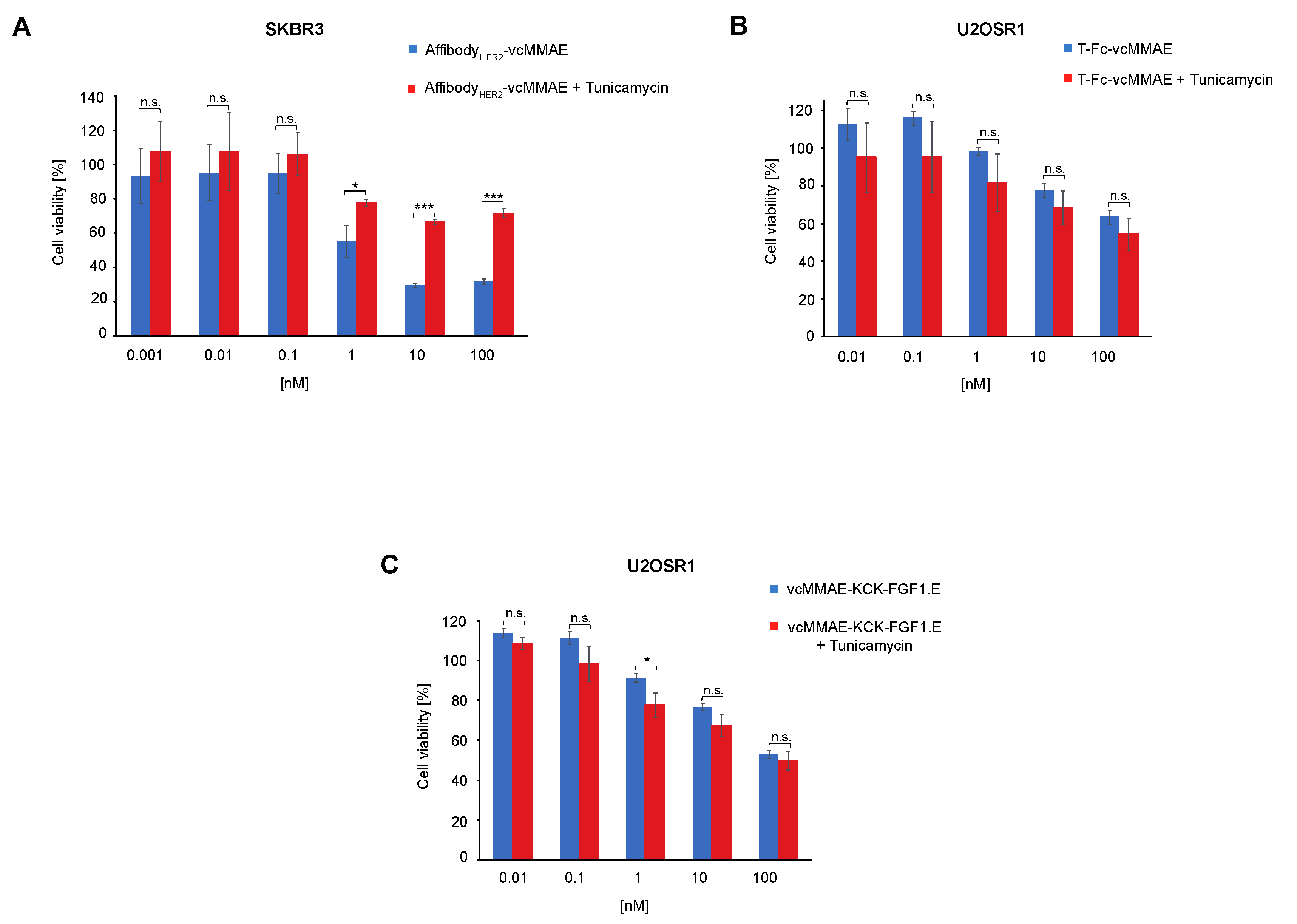
2.4. Significance of Cell Surface N-Glycosylation for the Potency of CCs Targeting RTKs
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Recombinant Proteins
4.3. Cell Culture
4.4. BLI Measurements
4.5. Flow Cytometry
4.6. Cytotoxicity Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Do Pazo, C.; Nawaz, K.; Webster, R.M. The oncology market for antibody-drug conjugates. Nat. Rev. Drug Discov. 2021, 20, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.; Tchistiakova, L.; Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 2013, 5, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody–drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef]
- Saraon, P.; Pathmanathan, S.; Snider, J.; Lyakisheva, A.; Wong, V.; Stagljar, I. Receptor tyrosine kinases and cancer: Oncogenic mechanisms and therapeutic approaches. Oncogene 2021, 40, 4079–4093. [Google Scholar] [CrossRef]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [Green Version]
- Porębska, N.; Poźniak, M.; Matynia, A.; Żukowska, D.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Galectins as modulators of receptor tyrosine kinases signaling in health and disease. Cytokine Growth Factor Rev. 2021, 60, 89–106. [Google Scholar] [CrossRef]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Krook, M.A.; Reeser, J.W.; Ernst, G.; Barker, H.; Wilberding, M.; Li, G.; Chen, H.Z.; Roychowdhury, S. Fibroblast growth factor receptors in cancer: Genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br. J. Cancer 2021, 124, 880–892. [Google Scholar] [CrossRef]
- Guo, H.B.; Johnson, H.; Randolph, M.; Nagy, T.; Blalock, R.; Pierce, M. Specific posttranslational modification regulates early events in mammary carcinoma formation. Proc. Natl. Acad. Sci. USA 2010, 107, 21116–21121. [Google Scholar] [CrossRef] [Green Version]
- Poźniak, M.; Porębska, N.; Krzyścik, M.A.; Sokołowska-Wędzina, A.; Jastrzębski, K.; Sochacka, M.; Szymczyk, J.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. The cytotoxic conjugate of highly internalizing tetravalent antibody for targeting FGFR1-overproducing cancer cells. BioMed Central 2021, 27, 46. [Google Scholar] [CrossRef]
- Sochaj-Gregorczyk, A.M.; Serwotka-Suszczak, A.M.; Otlewski, J. A Novel Affibody-Auristatin E Conjugate With a Potent and Selective Activity Against HER2+ Cell Lines. J. Immunother. 2016, 39, 223–232. [Google Scholar] [CrossRef]
- Lobocki, M.; Zakrzewska, M.; Szlachcic, A.; Krzyscik, M.A.; Sokolowska-Wedzina, A.; Otlewski, J. High-Yield Site-Specific Conjugation of Fibroblast Growth Factor 1 with Monomethylauristatin e via Cysteine Flanked by Basic Residues. Bioconjug. Chem. 2017, 28, 1850–1858. [Google Scholar] [CrossRef]
- Pozniak, M.; Sokolowska-Wedzina, A.; Jastrzebski, K.; Szymczyk, J.; Porebska, N.; Krzyscik, M.A.; Zakrzewska, M.; Miaczynska, M.; Otlewski, J.; Opalinski, L. FGFR1 clustering with engineered tetravalent antibody improves the efficiency and modifies the mechanism of receptor internalization. Mol. Oncol. 2020, 14, 1998–2021. [Google Scholar] [CrossRef]
- Johannes, L.; Billet, A. Glycosylation and raft endocytosis in cancer. Cancer Metastasis Rev. 2020, 39, 375–396. [Google Scholar] [CrossRef]
- Kucińska, M.; Porębska, N.; Lampart, A.; Latko, M.; Knapik, A.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Differential regulation of fibroblast growth factor receptor 1 trafficking and function by extracellular galectins. Cell Commun. Signal. 2019, 17, 65. [Google Scholar] [CrossRef] [Green Version]
- Lakshminarayan, R.; Wunder, C.; Becken, U.; Howes, M.T.; Benzing, C.; Arumugam, S.; Sales, S.; Ariotti, N.; Chambon, V.; Lamaze, C.; et al. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat. Cell Biol. 2014, 16, 592–603. [Google Scholar] [CrossRef]
- Poźniak, M.; Porębska, N.; Jastrzębski, K.; Krzyścik, M.A.; Kucińska, M.; Zarzycka, W.; Barbach, A.; Zakrzewska, M.; Otlewski, J.; Miączyńska, M.; et al. Modular self-assembly system for development of oligomeric, highly internalizing and potent cytotoxic conjugates targeting fibroblast growth factor receptors. J. Biomed. Sci. 2021, 28, 69. [Google Scholar] [CrossRef]
- Porębska, N.; Latko, M.; Kucińska, M.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Targeting Cellular Trafficking of Fibroblast Growth Factor Receptors as a Strategy for Selective Cancer Treatment. J. Clin. Med. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammood, M.; Craig, A.W.; Leyton, J.V. Impact of endocytosis mechanisms for the receptors targeted by the currently approved adcs—A necessity for future adc research and development. Pharmaceuticals 2021, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.; Campos, D.; Reis, C.A.; Gomes, C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer 2020, 6, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska-Wedzina, A.; Borek, A.; Chudzian, J.; Jakimowicz, P.; Zakrzewska, M.; Otlewski, J. Efficient production and purification of extracellular domain of human FGFR-Fc fusion proteins from Chinese hamster ovary cells. Protein Expr. Purif. 2014, 99, 50–57. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poźniak, M.; Żukowska, D.; Gędaj, A.; Krzyścik, M.A.; Porębska, N.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. The Significance of Cell Surface N-Glycosylation for Internalization and Potency of Cytotoxic Conjugates Targeting Receptor Tyrosine Kinases. Int. J. Mol. Sci. 2022, 23, 8514. https://doi.org/10.3390/ijms23158514
Poźniak M, Żukowska D, Gędaj A, Krzyścik MA, Porębska N, Zakrzewska M, Otlewski J, Opaliński Ł. The Significance of Cell Surface N-Glycosylation for Internalization and Potency of Cytotoxic Conjugates Targeting Receptor Tyrosine Kinases. International Journal of Molecular Sciences. 2022; 23(15):8514. https://doi.org/10.3390/ijms23158514
Chicago/Turabian StylePoźniak, Marta, Dominika Żukowska, Aleksandra Gędaj, Mateusz Adam Krzyścik, Natalia Porębska, Małgorzata Zakrzewska, Jacek Otlewski, and Łukasz Opaliński. 2022. "The Significance of Cell Surface N-Glycosylation for Internalization and Potency of Cytotoxic Conjugates Targeting Receptor Tyrosine Kinases" International Journal of Molecular Sciences 23, no. 15: 8514. https://doi.org/10.3390/ijms23158514
APA StylePoźniak, M., Żukowska, D., Gędaj, A., Krzyścik, M. A., Porębska, N., Zakrzewska, M., Otlewski, J., & Opaliński, Ł. (2022). The Significance of Cell Surface N-Glycosylation for Internalization and Potency of Cytotoxic Conjugates Targeting Receptor Tyrosine Kinases. International Journal of Molecular Sciences, 23(15), 8514. https://doi.org/10.3390/ijms23158514





