Examination of Intracellular GPCR-Mediated Signaling with High Temporal Resolution
Abstract
1. Introduction
2. Results
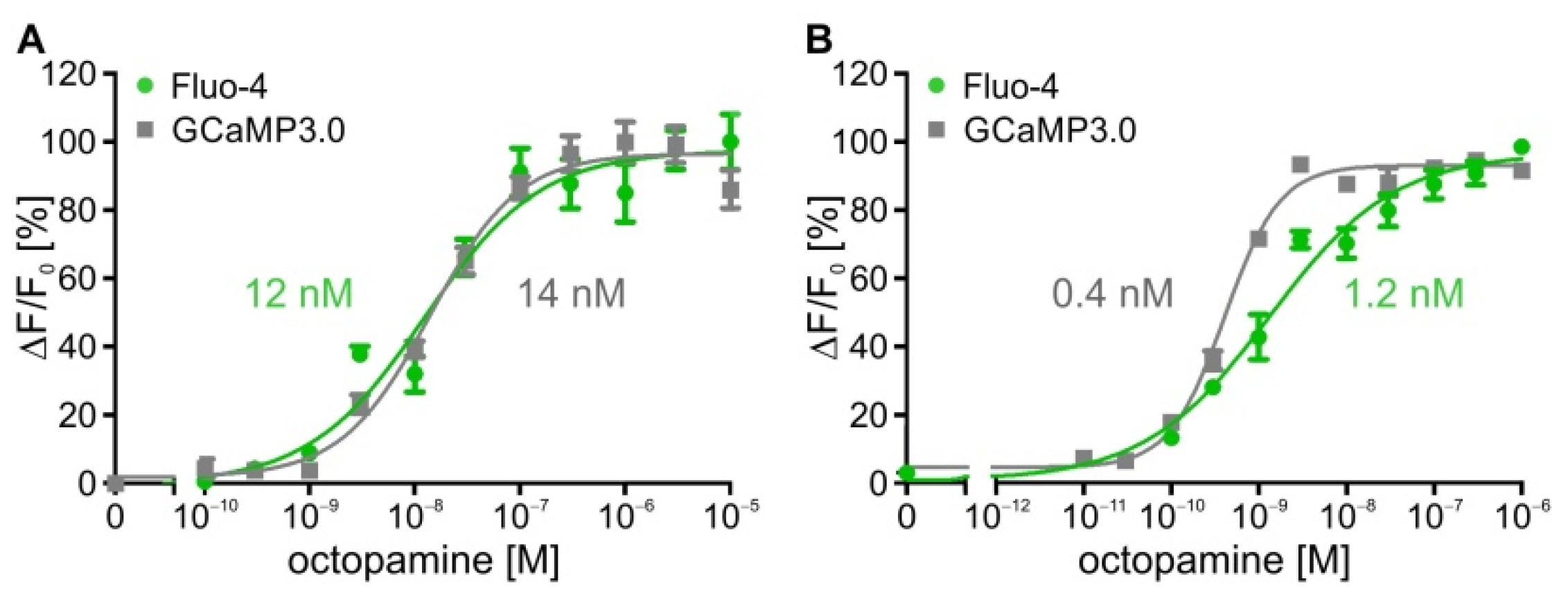
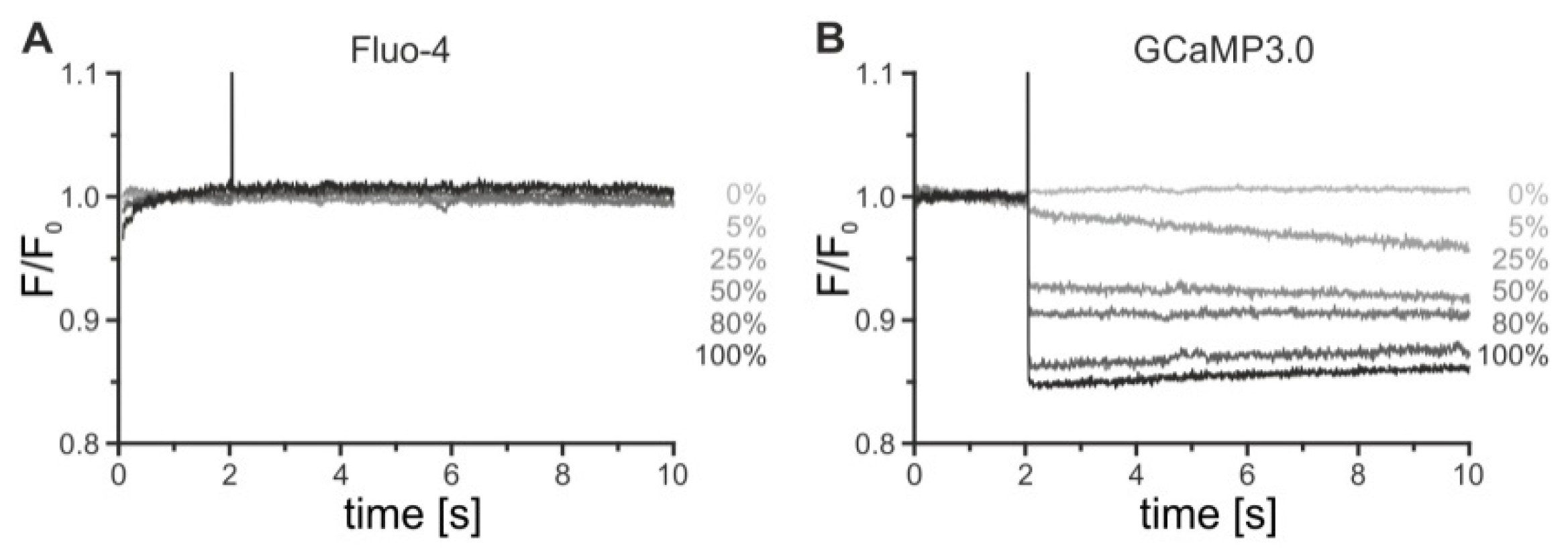
2.1. Establishing Stably Transfected Cell Lines for Kinetic Measurements
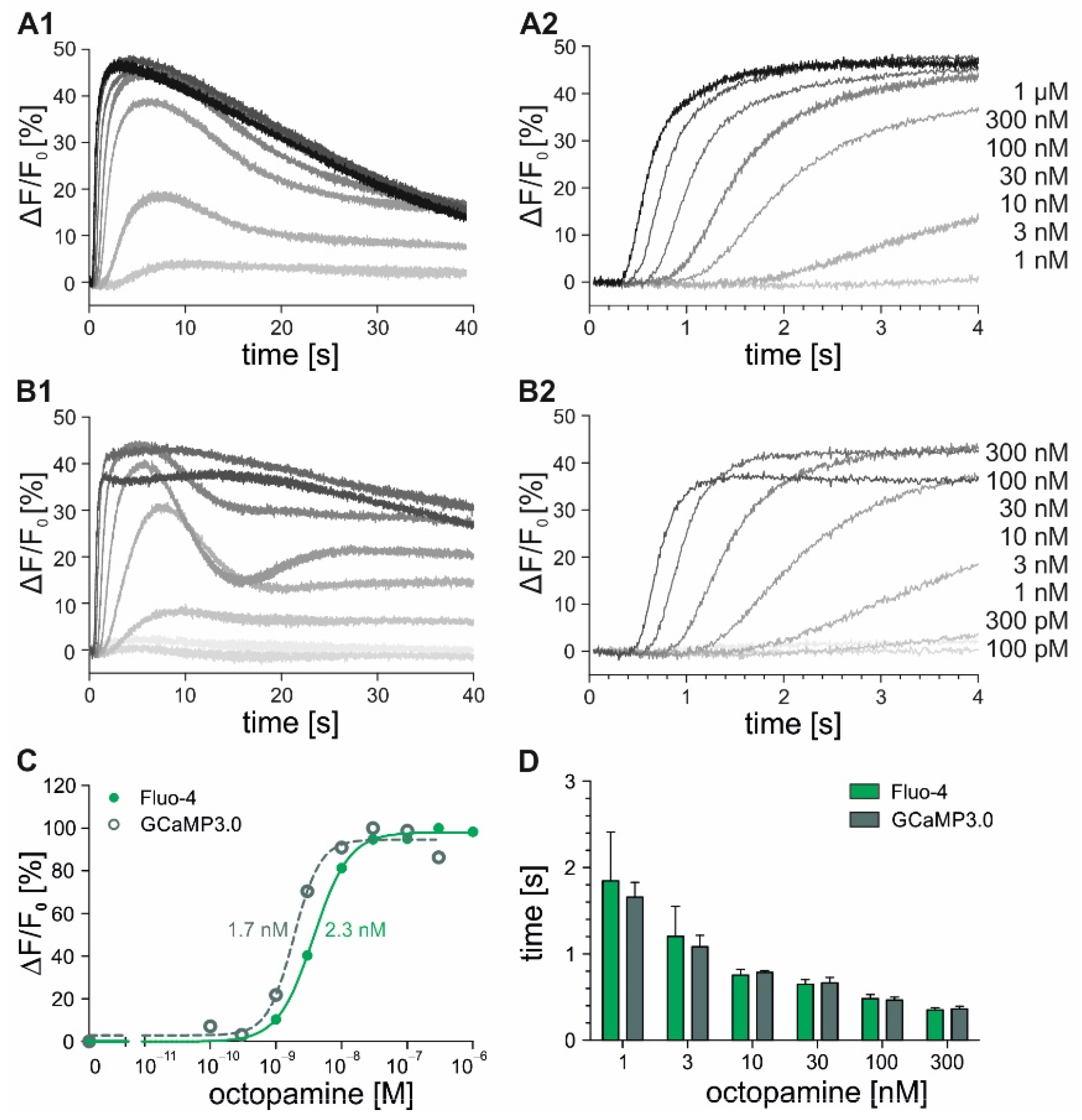
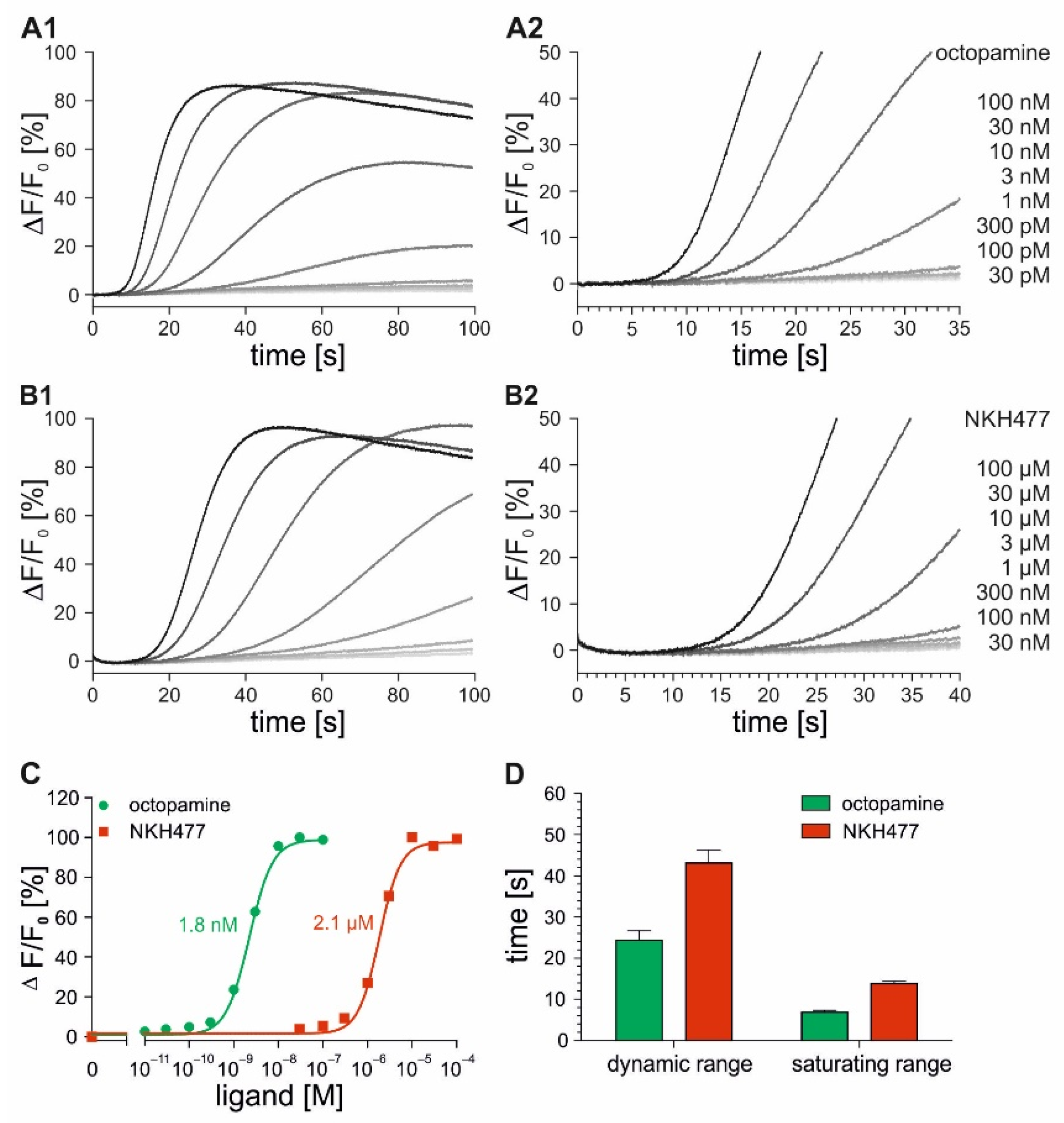
2.2. Time-Resolved Measurements with DmOctα1B-Expressing Cells
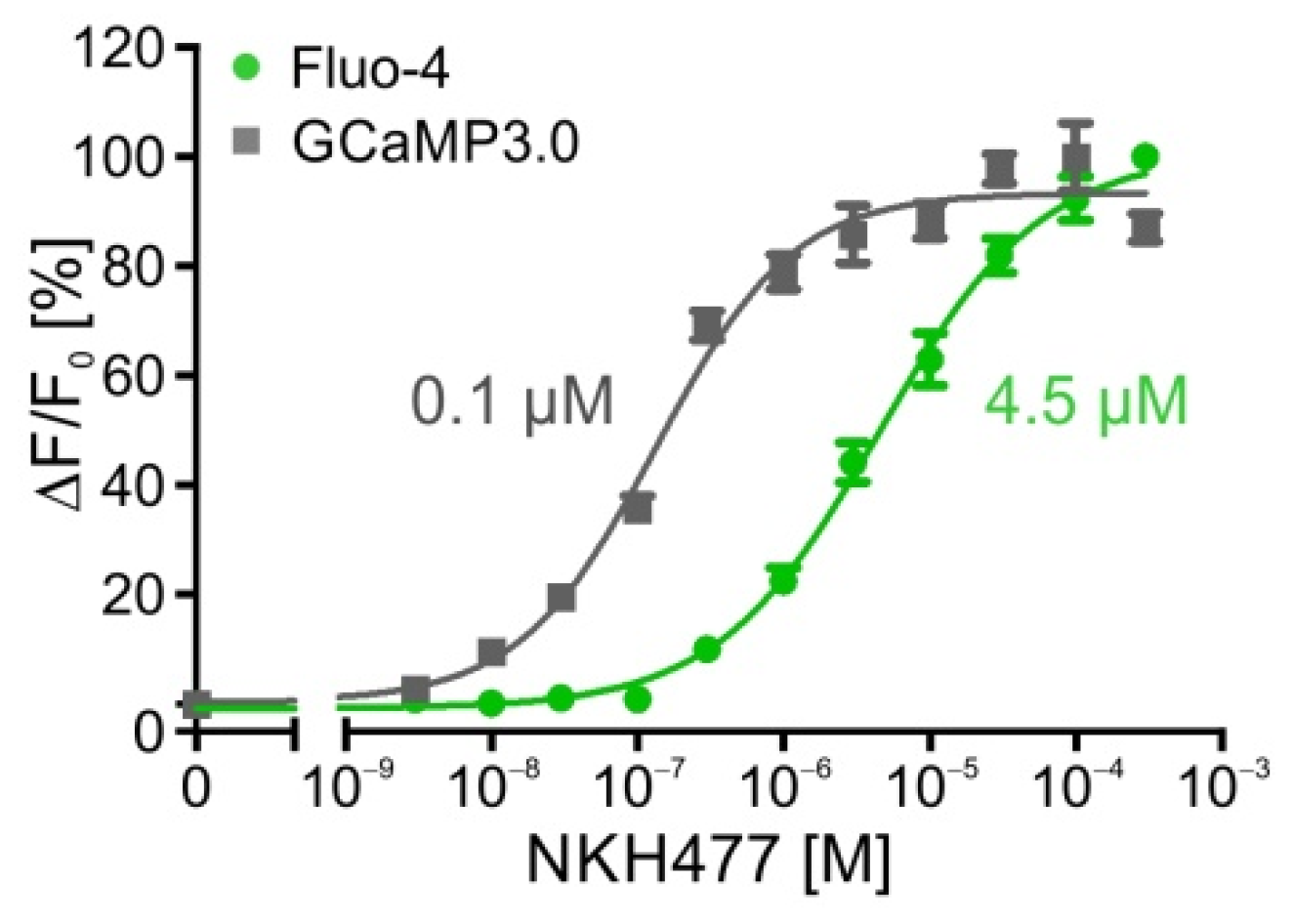
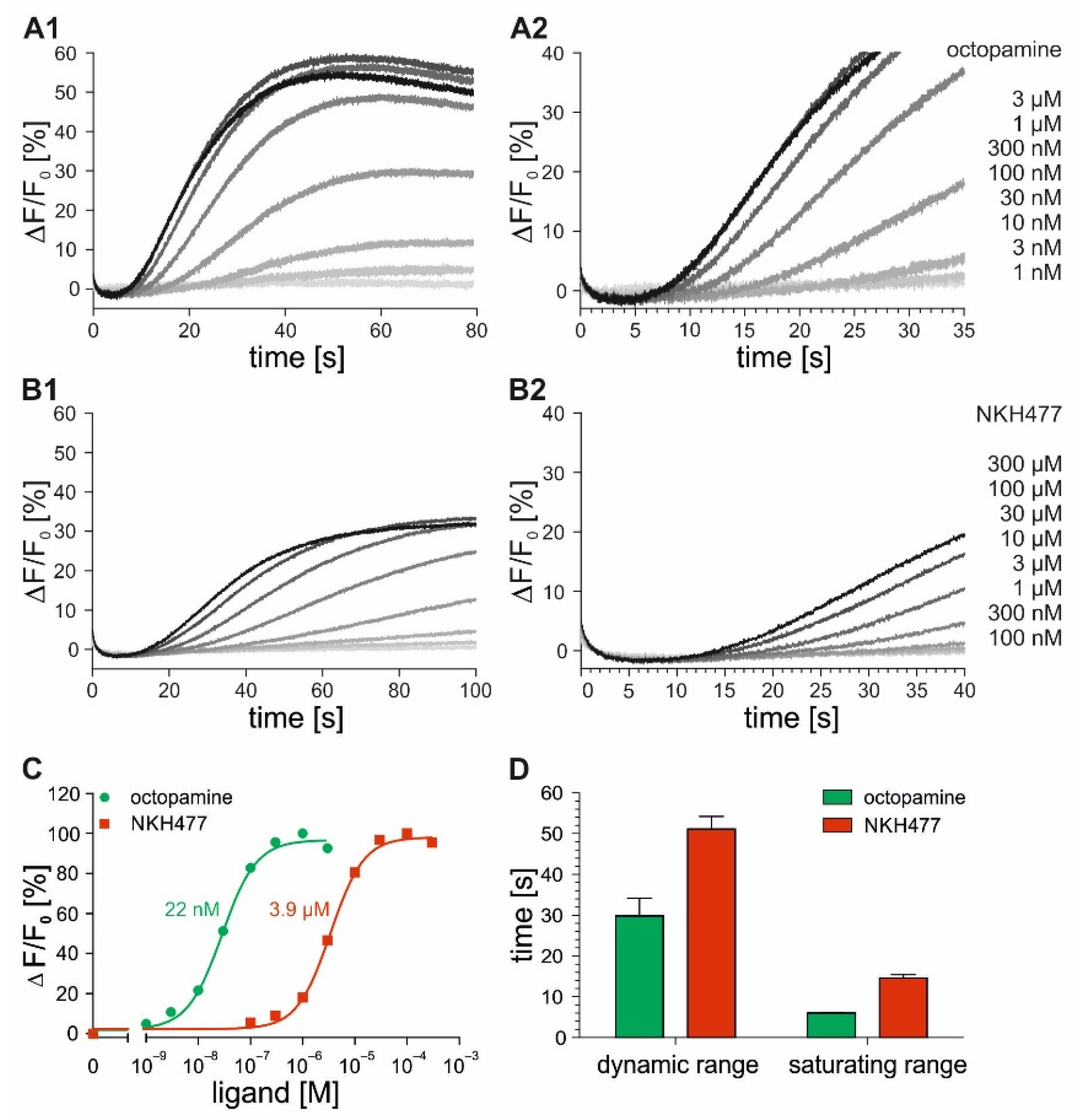
2.3. Time-Resolved Measurements with DmOctβ1-Expressing Cells
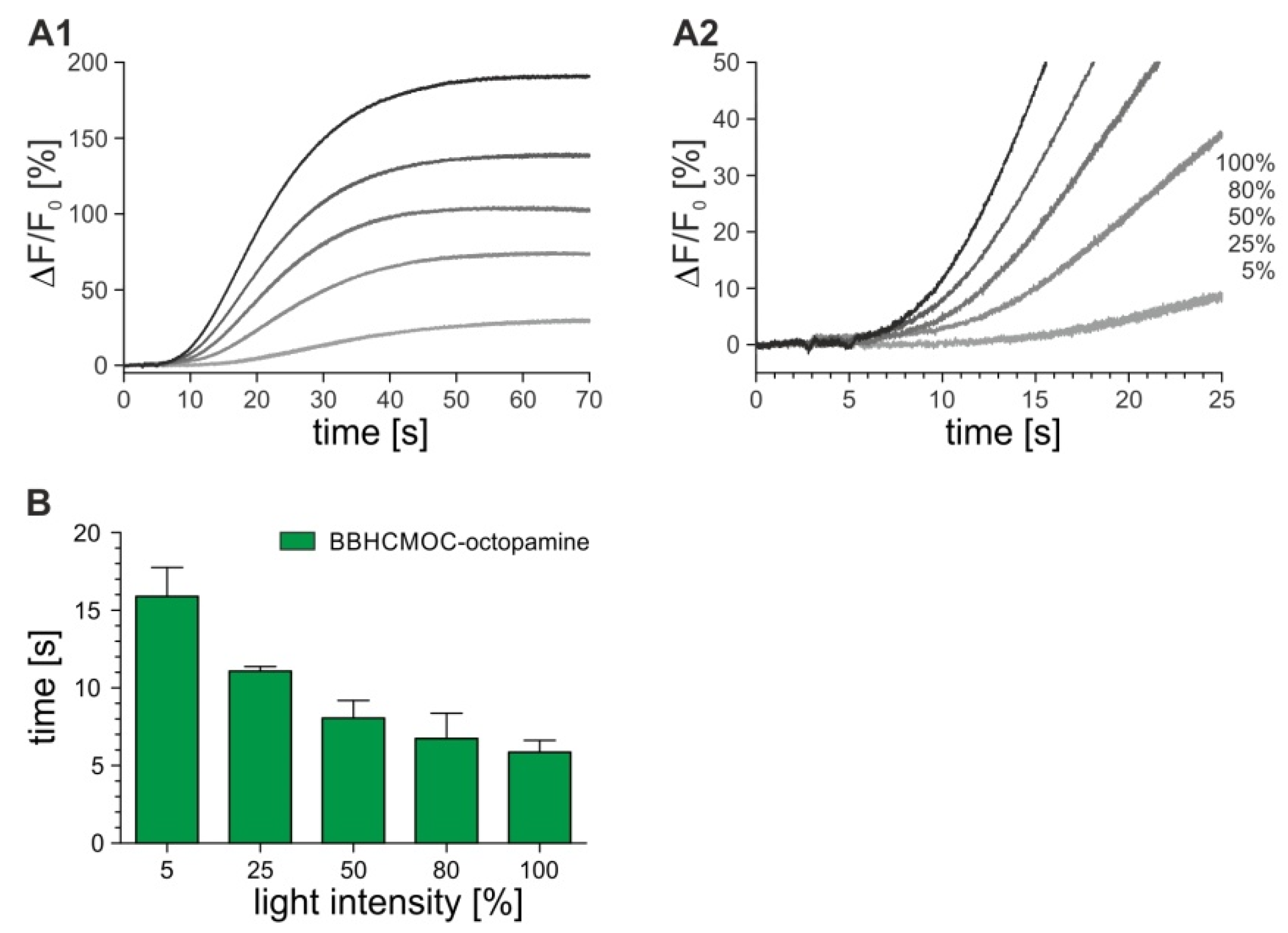
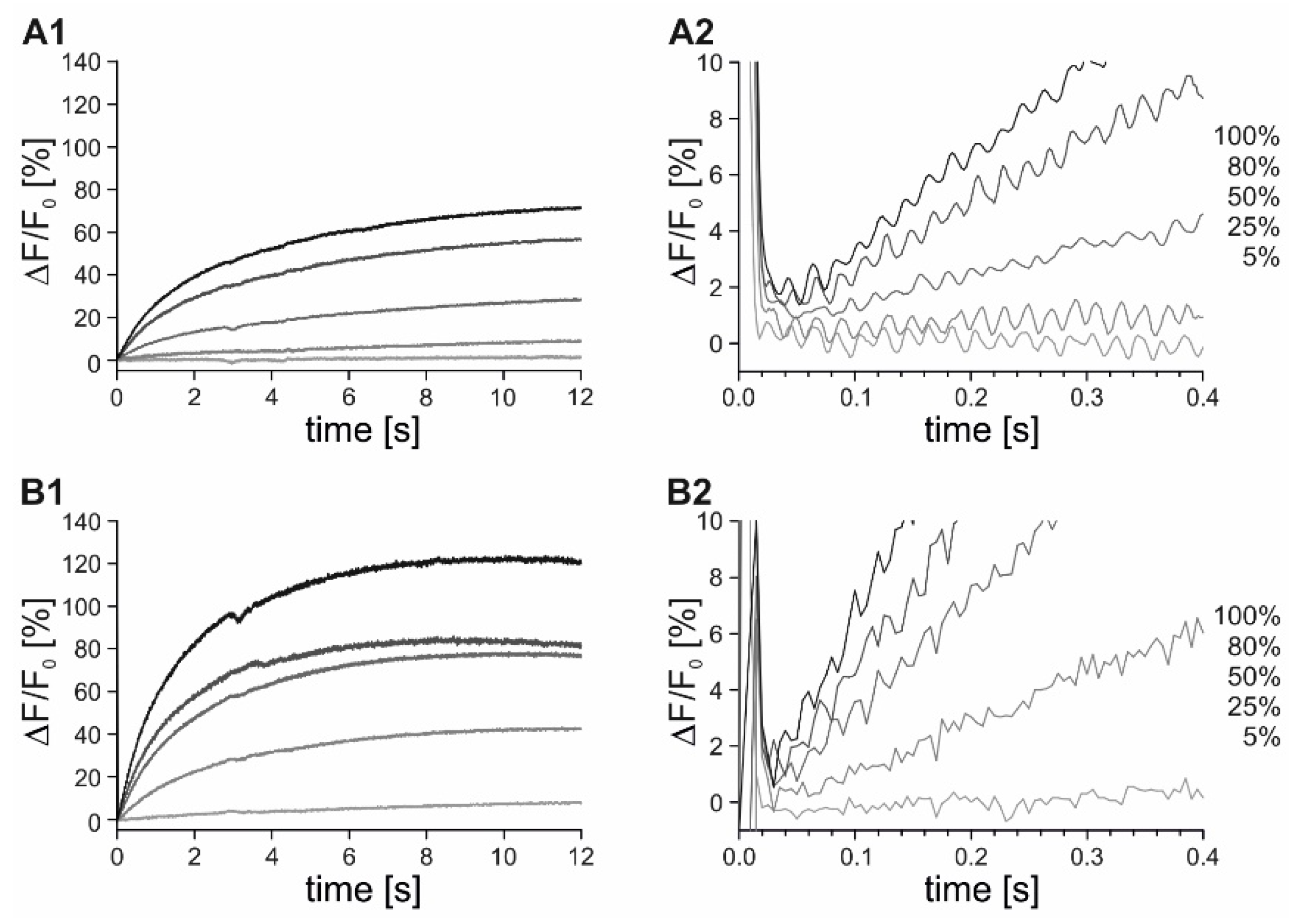
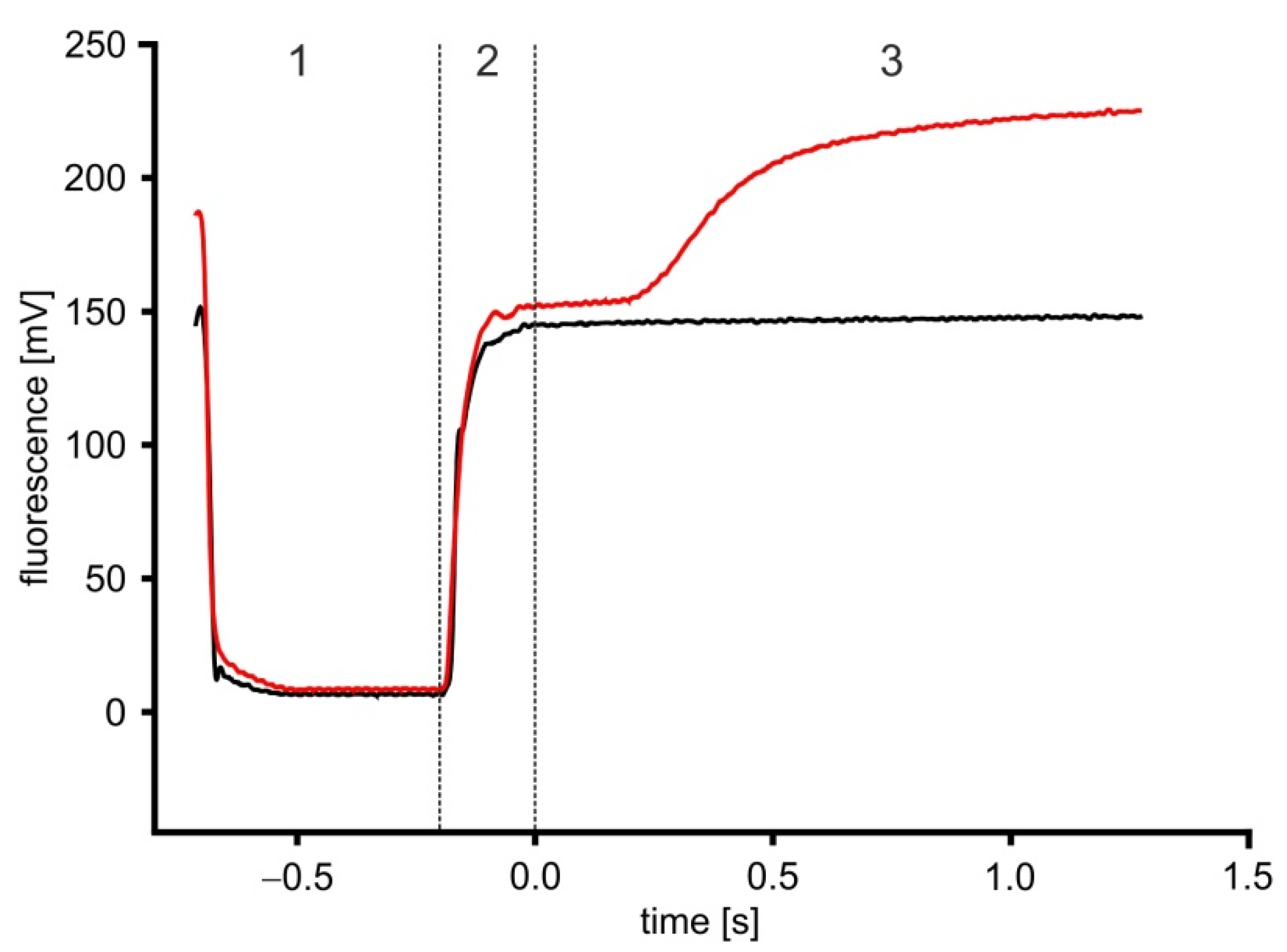
2.4. Time-Resolved Measurements with Caged Compounds
3. Discussion
3.1. Functional and Pharmacological Characterization of Transgenic Cell Lines
3.2. Time-Resolved Measurements of Signaling Cascades
4. Materials and Methods
4.1. Generation of Stably Transfected HEK293 Cell Lines
4.2. Ca2+ Fluorimetry in Stably Transfected Cell Lines
4.3. Stopped-Flow Measurements with Live Cells
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Böhme, I.; Beck-Sickinger, A.G. Illuminating the life of GPCRs. Cell Commun. Signal. 2009, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Thathia, A. Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett. 2015, 589, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–563. [Google Scholar] [CrossRef] [PubMed]
- Siram, K.; Insel, P.A. G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Alhosaini, K.; Azhar, A.; Alonazi, A.; Al-Zoghibi, F. GPCRs: The most promiscuous druggable receptor of the mankind. Saudi Pharm. J. 2021, 29, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.-G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef]
- Katritch, V.; Cherezov, V.; Stevens, R.C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531–556. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194. [Google Scholar] [CrossRef]
- Malik, R.U.; Ritt, M.; DeVree, B.T.; Neubig, R.R.; Sunahara, R.K.; Sivaramakrishnan, S. Detection of G protein selective G protein-coupled receptor (GPCR) conformations in live cells. J. Biol. Chem. 2013, 288, 17167–17178. [Google Scholar] [CrossRef]
- Milligan, G.; Kostenis, E. Heterotrimeric G-proteins: A short history. Br. J. Pharmacol. 2006, 147, S46–S55. [Google Scholar] [CrossRef]
- Barak, L.R.; Gilchrist, J.; Becker, J.M.; Kim, K.-M. Relationship between the G protein signaling and homologous desensitization of G protein-coupled receptors. Biochem. Biophys. Res. Commun. 2006, 339, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.S.G. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol. Rev. 2001, 53, 1–24. [Google Scholar]
- Kelly, E.; Bailey, C.P.; Henderson, G. Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol. 2008, 153, S379–S388. [Google Scholar] [CrossRef]
- Vernier, P.; Cardinaud, B.; Valdenaire, O.; Philippe, H.; Vincent, J.D. An evolutionary view of drug-receptor interaction: The bioamine receptor family. Trends Pharmacol. Sci. 1995, 16, 375–381. [Google Scholar] [CrossRef]
- Roeder, T. Tyramine and octopamine: Ruling behavior and metabolism. Annu. Rev. Entomol. 2005, 50, 447–477. [Google Scholar] [CrossRef]
- Sloley, B.D.; Juorio, A.V. Monoamine neurotransmitters in invertebrates and vertebrates: An examination of the diverse enzymatic pathways utilized to synthesize and inactivate biogenic amines. Int. Rev. Neurobiol. 1995, 38, 253–303. [Google Scholar] [CrossRef]
- Axelrod, J.; Saavedra, J.M. Octopamine. Nature 1977, 265, 501–504. [Google Scholar] [CrossRef]
- David, J.-C.; Coulon, J.-F. Octopamine in invertebrates and vertebrates: A review. Progr. Neurobiol. 1985, 24, 141–185. [Google Scholar] [CrossRef]
- Röder, T. Octopamine in invertebrates. Progr. Neurobiol. 1999, 59, 533–561. [Google Scholar] [CrossRef]
- Han, K.A.; Millar, N.S.; Davis, R.L. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J. Neurosci. 1998, 18, 3650–3658. [Google Scholar] [CrossRef]
- Balfanz, S.; Strünker, T.; Frings, S.; Baumann, A. A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 2005, 93, 440–451. [Google Scholar] [CrossRef]
- Maqueira, B.; Chatwin, H.; Evans, P.D. Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 2005, 94, 547–560. [Google Scholar] [CrossRef]
- Qi, Y.X.; Xu, G.; Gu, G.X.; Mao, F.; Ye, G.Y.; Liu, W.; Huang, J. A new Drosophila octopamine receptor responds to serotonin. Insect Biochem. Mol. Biol. 2017, 90, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, L.; Blenau, W.; Erber, J.; Ebert, P.R.; Strünker, T.; Baumann, A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J. Neurochem. 2003, 86, 725–735. [Google Scholar] [CrossRef]
- Blenau, W.; Wilms, J.A.; Balfanz, S.; Baumann, A. AmOctα2R: Functional characterization of a honeybee octopamine receptor inhibiting adenylyl cyclase activity. Int. J. Mol. Sci. 2020, 21, 9334. [Google Scholar] [CrossRef]
- Balfanz, S.; Jordan, N.; Langenstück, T.; Breuer, J.; Bergmeier, V.; Baumann, A. Molecular, pharmacological, and signaling properties of octopamine receptors from honeybee (Apis mellifera) brain. J. Neurochem. 2014, 129, 284–296. [Google Scholar] [CrossRef]
- Bischof, L.J.; Enan, E.E. Cloning, expression and functional analysis of an octopamine receptor from Periplaneta americana. Insect Biochem. Mol. Biol. 2004, 34, 511–521. [Google Scholar] [CrossRef]
- Blenau, W.; Bremer, A.-S.; Schwietz, Y.; Friedrich, D.; Ragionieri, L.; Predel, R.; Balfanz, S.; Baumann, A. PaOctβ2R: Identification and functional characterization of an octopamine receptor activating adenylyl cyclase activity in the American cockroach Periplaneta americana. Int. J. Mol. Sci. 2022, 23, 1677. [Google Scholar] [CrossRef]
- Gee, K.R.; Brown, K.A.; Chen, W.N.U.; Bishop-Stewart, J.; Gray, D.; Johnson, I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium 2000, 27, 97–106. [Google Scholar] [CrossRef]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; McKinney, S.A.; Schreiter, E.R.; et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Meth. 2009, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Margalit, T.; Eismann, E.; Lancet, D.; Kaupp, U.B. Primary structure of cAMP-gated channel from bovine olfactory epithelium. FEBS Lett. 1990, 270, 24–29. [Google Scholar] [CrossRef]
- Wachten, S.; Schlenstedt, J.; Gauss, R.; Baumann, A. Molecular identification and functional characterization of an adenylyl cyclase from the honeybee. J. Neurochem. 2006, 96, 1580–1590. [Google Scholar] [CrossRef]
- Hoff, M.; Balfanz, S.; Ehling, P.; Gensch, T.; Baumann, A. A single amino acid residue controls Ca2+ signaling by an octopamine receptor from Drosophila melanogaster. FASEB J. 2011, 25, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Schaal, J.; Dekowski, B.; Wiesner, B.; Eichhorst, J.; Marter, K.; Vargas, C.; Keller, S.; Eremina, N.; Barth, A.; Baumann, A.; et al. Coumarin-based octopamine phototriggers and their effects on an insect octopamine receptor. Chembiochem. 2012, 13, 1458–1464. [Google Scholar] [CrossRef]
- Hagen, V.; Bendig, J.; Frings, S.; Eckardt, T.; Helm, S.; Reuter, D.; Kaupp, U.B. Highly efficient and ultrafast phototriggers for cAMP and cGMP by using long wavelength UV/VIS-activation. Angew. Chem. Int. Ed. 2001, 40, 1045–1048. [Google Scholar] [CrossRef]
- Lohse, M.J.; Nikolaev, V.O.; Hein, P.; Hoffmann, C.; Vilardaga, J.-P.; Bünemann, M. Optical techniques to analyze real-time activation and signaling of G-protein-coupled receptors. Trends Pharmacol. Sci. 2008, 29, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.J.; Maiellaro, I.; Calebiro, D. Kinetics and mechanism of G protein-coupled receptor activation. Curr. Opin. Cell Biol. 2014, 27, 87–93. [Google Scholar] [CrossRef]
- Vilardaga, J.-P.; Bünemann, M.; Feinstein, T.N.; Lambert, N.; Nikolaev, V.O.; Engelhardt, S.; Lohse, M.J.; Hoffmann, C. GPCR and G proteins: Drug efficacy and activation in live cells. Mol. Endocrinol. 2009, 23, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Gibson, Q.H. Rapid mixing: Stopped flow. Meth. Enzymol. 1969, 16, 187–228. [Google Scholar] [CrossRef]
- Johnson, K.A. Rapid kinetic analysis of mechanochemical adenosinetriphosphatases. Methods Enzymol. 1986, 134, 677–705. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, U.B.; Kashikar, N.D.; Weyand, I. Mechanisms of Sperm Chemotaxis. Annu. Rev. Physiol. 2008, 70, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, J.; Chen, T.W.; Wardill, T.J.; Tian, L.; Marvin, J.S.; Mutlu, S.; Calderon, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012, 32, 13819–138402. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Cooper, D.M. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 2007, 87, 965–1010. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Davies, G.C.R. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat. Methods 2007, 4, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Vilardaga, J.-P.; Bünemann, M.; Krasel, C.; Castro, M.; Lohse, M.J. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 2003, 21, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Gaietta, G.; Bünemann, M.; Adams, S.R.; Oberdorff-Maass, S.; Behr, B.; Vilardaga, J.-P.; Tsien, R.Y.; Ellisman, M.H.; Lohse, M.J. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat. Methods. 2005, 2, 171–176. [Google Scholar] [CrossRef]
- Hoffmann, C.; Nuber, S.; Zabel, U.; Ziegler, N.; Winkler, C.; Hein, P.; Berlot, C.H.; Bünemann, M.; Lohse, M.J. Comparison of the activation kinetics of the M3 acetylcholine receptor and a constitutively active mutant receptor in living cells. Mol. Pharmacol. 2012, 82, 236–245. [Google Scholar] [CrossRef]
- Jensen, J.B.; Lyssand, J.S.; Hague, C.; Hille, B. Fluorescence changes reveal kinetic steps of muscarinic receptor-mediated modulation of phosphoinositides and Kv7.2/7.3 K+ channels. J. Gen. Physiol. 2009, 133, 347–359. [Google Scholar] [CrossRef]
- Maier-Peuschel, M.; Frölich, N.; Dees, C.; Hommers, L.G.; Hoffmann, C.; Nikolaev, V.O.; Lohse, M.J. A fluorescence resonance energy transfer-based M2 muscarinic receptor sensor reveals rapid kinetics of allosteric modulation. J. Biol. Chem. 2010, 285, 8793–8800. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, N.; Bätz, J.; Zabel, U.; Lohse, M.J.; Hoffmann, C. FRET-based sensors for the human M1-, M3-, and M5-acetylcholine receptors. Bioorg. Med. Chem. 2011, 19, 1048–1054. [Google Scholar] [CrossRef]
- Dror, R.O.; Arlow, D.H.; Maragakis, P.; Mildorf, T.J.; Pan, A.C.; Xu, H.; Borhani, D.W.; Shaw, D.E. Activation mechanism of the β2-adrenergic receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 18684–18689. [Google Scholar] [CrossRef] [PubMed]
- Dror, R.O.; Pan, A.C.; Arlow, D.H.; Borhani, D.W.; Maragakis, P.; Shan, Y.; Xu, H.; Shaw, D.E. Pathway and mechanism of drug binding to G-protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 13118–13123. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Hu, J.; Pan, A.C.; Arlow, D.H.; Rosenbaum, D.M.; Rosemond, E.; Green, H.F.; Liu, T.; Chae, P.S.; Dror, R.O.; et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 2012, 482, 552–556. [Google Scholar] [CrossRef]
- Manglik, A.; Kim, T.H.; Masureel, M.; Altenbach, C.; Yang, Z.; Hilger, D.; Lerch, M.T.; Kobilka, T.S.; Thian, F.S.; Hubbell, W.L.; et al. Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 2015, 161, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Hein, P.; Frank, M.; Hoffmann, C.; Lohse, M.J.; Bünemann, M. Dynamics of receptor/G protein coupling in living cells. EMBO J. 2005, 24, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, M.; Frank, M.; Lohse, M.J. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. USA 2003, 100, 16077–16082. [Google Scholar] [CrossRef] [PubMed]
- Hein, P.; Rochais, F.; Hoffmann, C.; Dorsch, S.; Nikolaev, V.O.; Engelhardt, S.; Berlot, C.H.; Lohse, M.J.; Bünemann, M. GS activation is time-limiting in initiating receptor-mediated signaling. J. Biol. Chem. 2006, 281, 33345–33351. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, M.; Bücheler, M.M.; Philipp, M.; Lohse, M.J.; Hein, L. Activation and deactivation kinetics of α2A- and α2C-adrenergic receptor-activated G protein-activated inwardly rectifying K+ channel currents. J. Biol. Chem. 2001, 276, 47512–47517. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.O.; Bünemann, M.; Hein, L.; Hannawacker, A.; Lohse, M.J. Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem. 2004, 279, 37215–37218. [Google Scholar] [CrossRef] [PubMed]
- DiPilato, L.M.; Cheng, X.; Zhang, J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. USA 2004, 101, 16513–16518. [Google Scholar] [CrossRef]
- Ponsioen, B.; Zhao, J.; Riedl, J.; Zwartkruis, F.; van der Krogt, G.; Zaccolo, M.; Moolenaar, W.H.; Bos, J.L.; Jalink, K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004, 5, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Kadamur, G.; Ross, E.M. Mammalian phospholipase C. Annu. Rev. Physiol. 2013, 75, 127–154. [Google Scholar] [CrossRef] [PubMed]
- Demuro, A.; Parker, I. Picomolar sensitivity to inositol trisphosphate in Xenopus oocytes. Cell Calcium. 2015, 58, 511–517. [Google Scholar] [CrossRef][Green Version]
- Dessauer, C.W.; Gilman, A.G. The catalytic mechanism of mammalian adenylyl cyclase. J. Biol. Chem. 1997, 272, 27787–27795. [Google Scholar] [CrossRef]
- Hahn, D.K.; Tusell, J.R.; Sprang, S.R.; Chu, X. Catalytic mechanism of mammalian adenylyl cyclase: A computational investigation. Biochemistry. 2015, 54, 6252–6262. [Google Scholar] [CrossRef] [PubMed]
- Tolkovsky, A.M. Newly synthesized catalytic and regulatory components of adenylyl cyclase are expressed in neurites of cultured sympathetic neurons. J. Neurosci. 1987, 7, 110–117. [Google Scholar] [CrossRef]
- Chen, C.; Okayama, H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell Biol. 1987, 7, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
| EC50/logEC50 ± SD | ||
|---|---|---|
| Cell Line | Octopamine | NKH477 |
| DmOctα1B | 12.0 nM/−7.9 ± 0.1 | n.d. |
| DmOctα1B-GCaMP3.0 | 14.0 nM/−7.8 ± 0.1 | n.d. |
| flpTM-DmOctβ1 | 1.2 nM/−8.9 ± 0.04 | 4.5 µM/−5.34 ± 0.09 |
| flpTM-DmOctβ1-GCaMP3.0 | 0.4 nM/−9.36 ± 0.04 | 0.1 µM/−6.91 ± 0.03 |
| EC50 | Signal Response Time | |||
|---|---|---|---|---|
| Cell Line | Octopamine | NKH477 | Octopamine | NKH477 |
| DmOctα1B | 2.25 ± 0.15 nM | - | 0.35 ± 0.04 s | - |
| DmOctα1B-GCaMP3.0 | 1.70 ± 0.10 nM | - | 0.36 ± 0.05 s | - |
| flpTM-DmOctβ1 | 22.00 ± 4.49 nM | 3.92 ± 0.35 µM | 6.00 ± 0.13 s | 13.35 ± 0.82 s |
| flpTM-DmOctβ1- GCaMP3.0 | 1.78 ± 0.28 nM | 2.12 ± 0.45 µM | 6.00 ± 0.20 s | 12.70 ± 0.52 s |
| 96 MWP | Stopped-Flow | |||
|---|---|---|---|---|
| Cell Line | Octopamine | NKH477 | Octopamine | NKH477 |
| DmOctα1B | 12.0 nM | - | 2.3 nM | - |
| DmOctα1B-GCaMP3.0 | 14.0 nM | - | 1.7 nM | - |
| flpTM-DmOctβ1 | 1.2 nM | 4.5 µM | 22.0 nM | 4.0 µM |
| flpTM-DmOctβ1-GCaMP3.0 | 0.4 nM | 0.1 µM | 1.8 nM | 2.0 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruteser, N.; Baumann, A. Examination of Intracellular GPCR-Mediated Signaling with High Temporal Resolution. Int. J. Mol. Sci. 2022, 23, 8516. https://doi.org/10.3390/ijms23158516
Gruteser N, Baumann A. Examination of Intracellular GPCR-Mediated Signaling with High Temporal Resolution. International Journal of Molecular Sciences. 2022; 23(15):8516. https://doi.org/10.3390/ijms23158516
Chicago/Turabian StyleGruteser, Nadine, and Arnd Baumann. 2022. "Examination of Intracellular GPCR-Mediated Signaling with High Temporal Resolution" International Journal of Molecular Sciences 23, no. 15: 8516. https://doi.org/10.3390/ijms23158516
APA StyleGruteser, N., & Baumann, A. (2022). Examination of Intracellular GPCR-Mediated Signaling with High Temporal Resolution. International Journal of Molecular Sciences, 23(15), 8516. https://doi.org/10.3390/ijms23158516






