Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1
Abstract
:1. Introduction
2. Results
2.1. Effect of Osteostatin on Osteoclast Differentiation
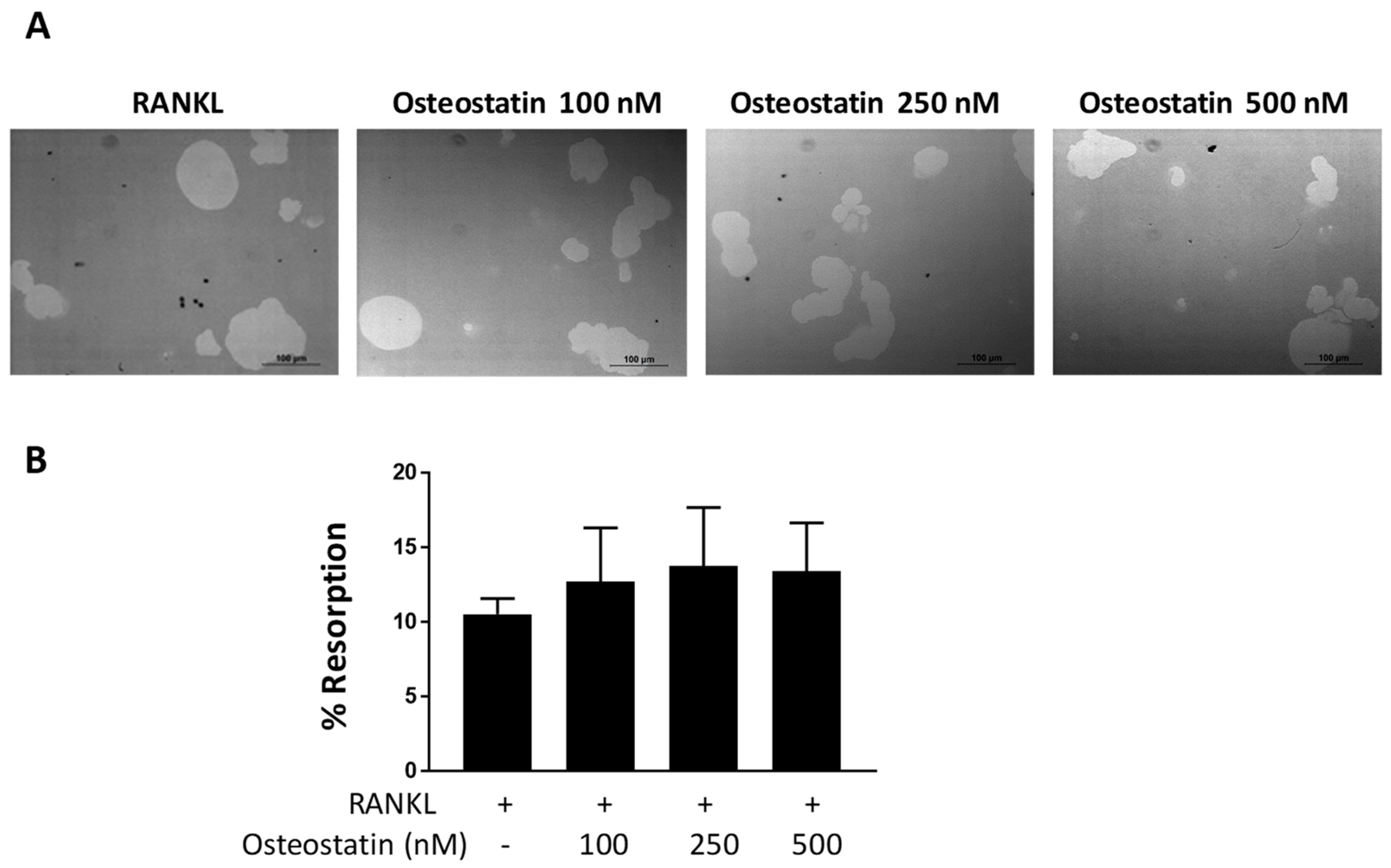
2.2. Osteoclast Functional Activity
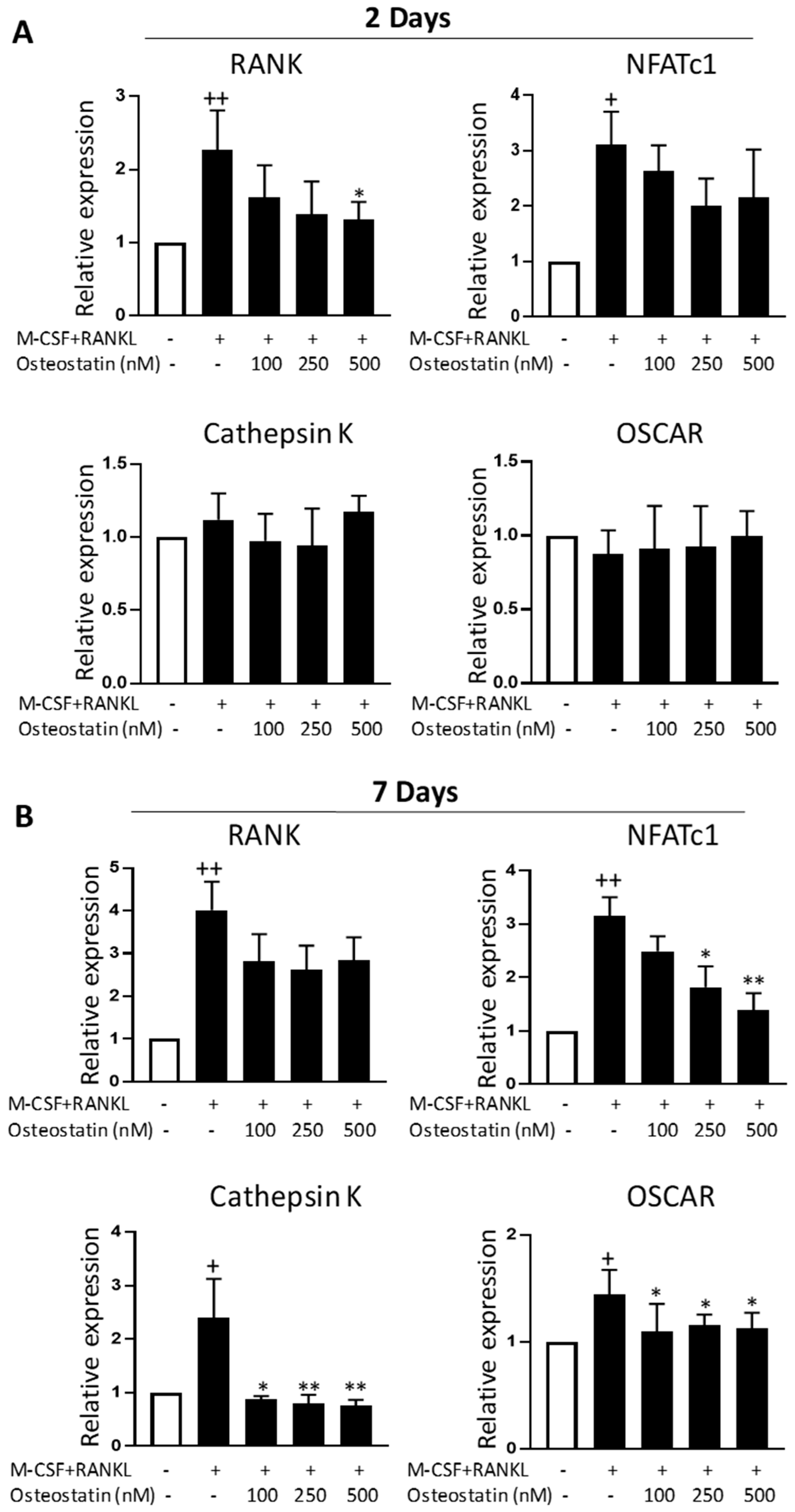
2.3. Effect on Gene Expression
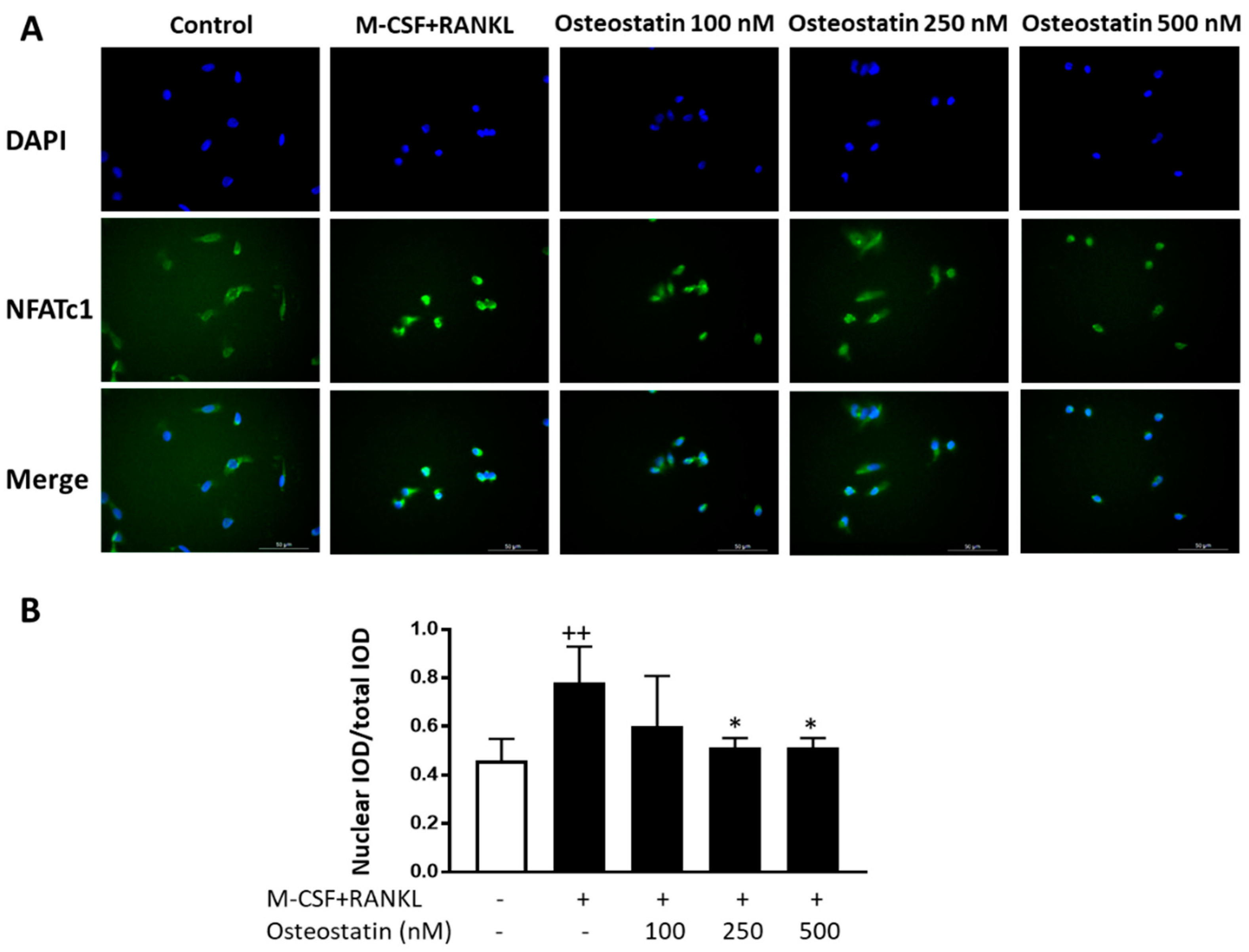
2.4. NFATc1 Nuclear Translocation
3. Discussion
4. Materials and Methods
4.1. Osteoclast Differentiation from Human PBMCs and TRAP Staining
4.2. MTT Assay
4.3. Resorption Assay
4.4. Quantitative Reverse-Transcription PCR (qRT-PCR)
4.5. Immunofluorescence Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.F. PTHrP (1–36) as a skeletal anabolic agent for the treatment of osteoporosis. Bone 1996, 19, 303–306. [Google Scholar] [CrossRef]
- de Castro, L.F.; Lozano, D.; Dapia, S.; Portal-Nuñez, S.; Caeiro, J.R.; Gomez-Barrena, E.; Esbrit, P. Role of the N- and C-terminal fragments of parathyroid-hormone-related protein as putative therapies to improve bone regeneration under high glucocorticoid treatment. Tissue Eng. Part A 2010, 16, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.F.; Lozano, D.; Portal-Nuñez, S.; Maycas, M.; De la Fuente, M.; Caeiro, J.R.; Esbrit, P. Comparison of the skeletal effects induced by daily administration of PTHrP (1–36) and PTHrP (107–139) to ovariectomized mice. J. Cell. Physiol. 2012, 227, 1752–1760. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Lin, C.; Xiao, C.; Moseley, J.M.; Reid, I.R. Stimulation of osteoblast proliferation by C-terminal fragments of parathyroid hormone-related protein. J. Bone Miner. Res. 1999, 14, 915–922. [Google Scholar] [CrossRef]
- Fenton, A.J.; Kemp, B.E.; Kent, G.N.; Moseley, J.M.; Zheng, M.H.; Rowe, D.J.; Britto, J.M.; Martin, T.J.; Nicholson, G.C. A carboxyl-terminal peptide from the parathyroid hormone-related protein inhibits bone resorption by osteoclasts. Endocrinology 1991, 129, 1762–1768. [Google Scholar] [CrossRef]
- Fenton, A.J.; Martin, T.J.; Nicholson, G.C. Long-term culture of disaggregated rat osteoclasts: Inhibition of bone resorption and reduction of osteoclast-like cell number by calcitonin and PTHrP [107–139]. J. Cell. Physiol. 1993, 155, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.J.; Kemp, B.E.; Hammonds, R.G., Jr.; Mitchelhill, K.; Moseley, J.M.; Martin, T.J.; Nicholson, G.C. A potent inhibitor of osteoclastic bone resorption within a highly conserved pentapeptide region of parathyroid hormone-related protein; PTHrP [107–111]. Endocrinology 1991, 129, 3424–3426. [Google Scholar] [CrossRef]
- Wu, D.; Liu, L.; Fu, S.; Zhang, J. Osteostatin improves the osteogenic differentiation of mesenchymal stem cells and enhances angiogenesis through HIF-1α under hypoxia conditions in vitro. Biochem. Biophys. Res. Commun. 2022, 606, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Platas, J.; Guillén, M.I.; Gomar, F.; Castejón, M.A.; Esbrit, P.; Alcaraz, M.J. Anti-senescence and anti-inflammatory effects of the C-terminal moiety of PTHrP peptides in OA osteoblasts. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 172, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-de la Rosa, L.; Lopez-Herradon, A.; Portal-Nuñez, S.; Murillo-Cuesta, S.; Lozano, D.; Cediel, R.; Varela-Nieto, I.; Esbrit, P. Treatment with N- and C-terminal peptides of parathyroid hormone-related protein partly compensate the skeletal abnormalities in IGF-1 deficient mice. PLoS ONE 2014, 9, e87536. [Google Scholar]
- Lozano, D.; Trejo, C.G.; Gómez-Barrena, E.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Vallet-Regi, M.; García-Honduvilla, N.; Esbrit, P.; Buján, J. Osteostatin-loaded onto mesoporous ceramics improves the early phase of bone regeneration in a rabbit osteopenia model. Acta Biomater. 2012, 8, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Sanchez-Salcedo, S.; Portal-Nuñez, S.; Vila, M.; Lopez-Herradon, A.; Ardura, J.A.; Mulero, F.; Gomez-Barrena, E.; Vallet-Regi, M.; Esbrit, P. Parathyroid hormone-related protein (107–111) improves the bone regeneration potential of gelatin-glutaraldehyde biopolymer-coated hydroxyapatite. Acta Biomater. 2014, 10, 3307–3316. [Google Scholar] [CrossRef] [Green Version]
- Trejo, C.G.; Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Dapía, S.; Gómez-Barrena, E.; Vallet-Regi, M.; Garcia-Honduvilla, N.; Buján, J.; et al. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010, 31, 8564–8573. [Google Scholar] [CrossRef]
- Nácher-Juan, J.; Terencio, M.C.; Alcaraz, M.J.; Ferrándiz, M.L. Osteostatin inhibits collagen-induced arthritis by regulation of immune activation, pro-inflammatory cytokines, and osteoclastogenesis. Int. J. Mol. Sci. 2019, 20, 3845. [Google Scholar] [CrossRef] [Green Version]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shavit, Z. The osteoclast: A multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J. Cell. Biochem. 2007, 102, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Feng, X. RANKing intracellular signaling in osteoclasts. IUBMB Life 2005, 57, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2005, 24, 33–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenton, A.J.; Martin, T.J.; Nicholson, G.C. Carboxyl-terminal parathyroid hormone-related protein inhibits bone resorption by isolated chicken osteoclasts. J. Bone Miner. Res. 1994, 9, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Murrills, R.J.; Stein, L.S.; Dempster, D.W. Lack of significant effect of carboxyl-terminal parathyroid hormone-related peptide fragments on isolated rat and chick osteoclasts. Calcif. Tissue Int. 1995, 57, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Zheng, M.H.; McCaughan, H.B.; Papadimitriou, J.M.; Nicholson, G.C.; Wood, D.J. Tartrate resistant acid phosphatase activity in rat cultured osteoclasts is inhibited by a carboxyl terminal peptide (osteostatin) from parathyroid hormone-related protein. J. Cell. Biochem. 1994, 54, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.; Callon, K.E.; Nicholson, G.C.; Reid, I.R. Parathyroid hormone-related protein-(107–139) inhibits bone resorption in vivo. Endocrinology 1997, 138, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Sone, T.; Kohno, H.; Kikuchi, H.; Ikeda, T.; Kasai, R.; Kikuchi, Y.; Takeuchi, R.; Konishi, J.; Shigeno, C. Human parathyroid hormone-related peptide-(107–111) does not inhibit bone resorption in neonatal mouse calvariae. Endocrinology 1992, 131, 2742–2746. [Google Scholar] [CrossRef] [PubMed]
- Rihani-Basharat, S.; Lewinson, D. PTHrP (107–111) inhibits in vivo resorption that was stimulated by PTHrP (1–34) when applied intermittently to neonatal mice. Calcif. Tissue Int. 1997, 61, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Arai, F.; Miyamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Suda, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappa B (RANK) receptors. J. Exp. Med. 1999, 190, 1741–1754. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Del Fattore, A.; Teti, A.; Rucci, N. Osteoclast receptors and signaling. Arch. Biochem. Biophys. 2008, 73, 147–160. [Google Scholar] [CrossRef]
- Lamothe, B.; Webster, W.K.; Gopinathan, A.; Besse, A.; Campos, A.D.; Darnay, B.G. Traf6 ubiquitin ligase is essential for RANKL signaling and osteoclast differentiation. Biochem. Biophys. Res. Commun. 2007, 359, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Gardella, T.J.; Vilardaga, J.P. International Union of Basic and Clinical Pharmacology. XCIII. The parathyroid hormone receptors—Family B G protein-coupled receptors. Pharmacol. Rev. 2015, 67, 310–337. [Google Scholar]
- Valin, A.; Guillen, C.; Esbrit, P. C-terminal parathyroid hormone-related protein (PTHrP) (107–139) stimulates intracellular Ca2+ through a receptor different from the type 1 PTH/PTHrP receptor in osteoblastic osteosarcoma UMR 106 cells. Endocrinology 2001, 142, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Tabacco, G.; Bilezikian, J.P. Osteoanabolic and dual action drugs. Br. J. Clin. Pharmacol. 2019, 85, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez, L.; Nácher-Juan, J.; Terencio, M.C.; Ferrándiz, M.L.; Alcaraz, M.J. Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1. Int. J. Mol. Sci. 2022, 23, 8551. https://doi.org/10.3390/ijms23158551
Ibáñez L, Nácher-Juan J, Terencio MC, Ferrándiz ML, Alcaraz MJ. Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1. International Journal of Molecular Sciences. 2022; 23(15):8551. https://doi.org/10.3390/ijms23158551
Chicago/Turabian StyleIbáñez, Lidia, Josep Nácher-Juan, María Carmen Terencio, María Luisa Ferrándiz, and María José Alcaraz. 2022. "Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1" International Journal of Molecular Sciences 23, no. 15: 8551. https://doi.org/10.3390/ijms23158551
APA StyleIbáñez, L., Nácher-Juan, J., Terencio, M. C., Ferrándiz, M. L., & Alcaraz, M. J. (2022). Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1. International Journal of Molecular Sciences, 23(15), 8551. https://doi.org/10.3390/ijms23158551






