Application of Perinatal Derivatives on Oncological Preclinical Models: A Review of Animal Studies
Abstract
:1. Introduction
2. Search Strategy and Data Collection Methodology
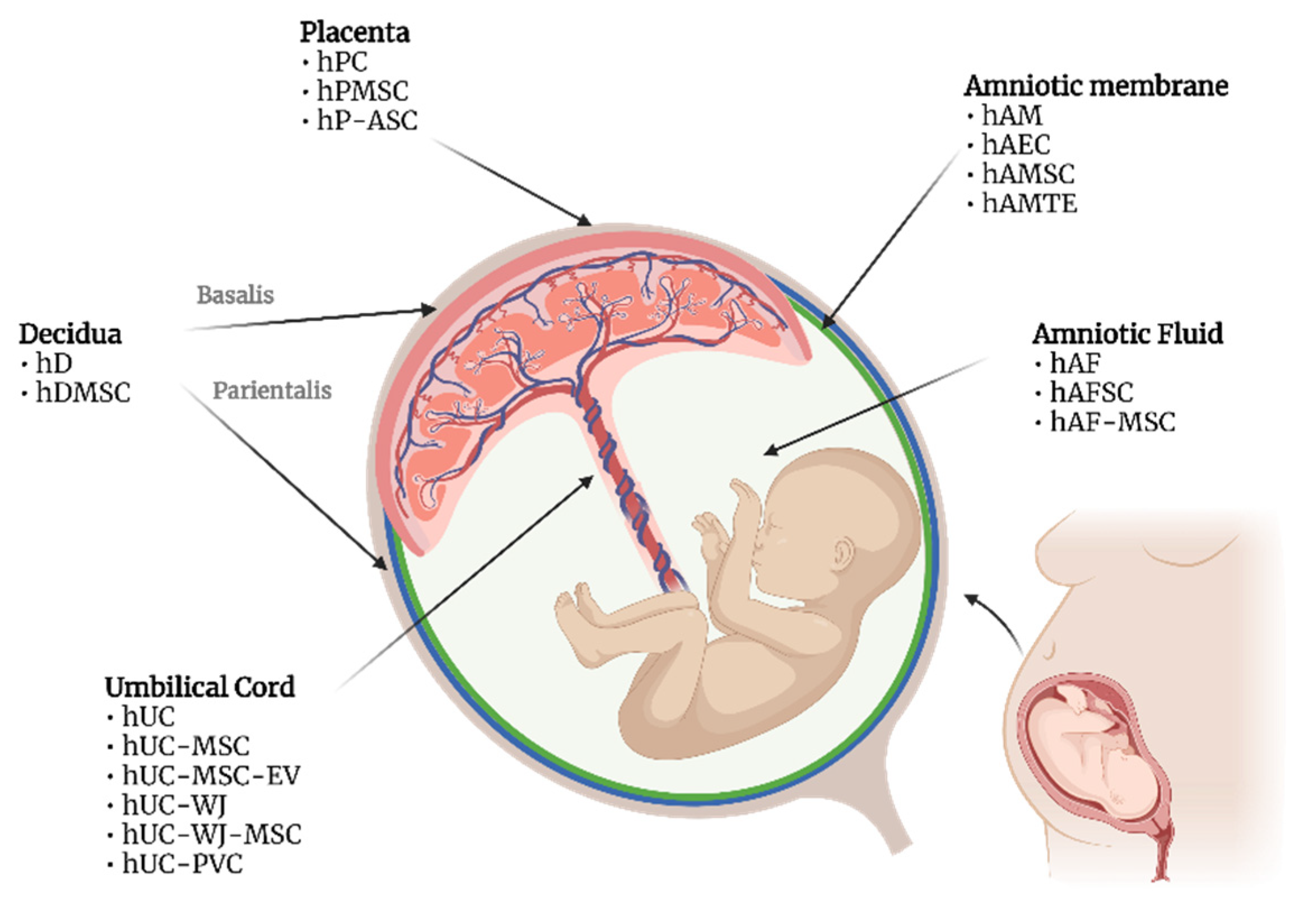
3. Placenta Cells
4. Amniotic Fluid Cells
5. Amniotic Membrane Derivatives
6. Decidua
7. Umbilical Cord Derivatives
7.1. Human Umbilical Cord Mesenchymal Stromal/Stem Cells (hUC-MSC)
7.2. Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cells (hUC-WJ-MSC)
7.3. Human Umbilical Cord Perivascular Cells (hUC-PVC)
7.4. Human Umbilical Cord Mesenchymal Stem Cells-Derived Extracellular Vesicles (hUC-MSC-EV)
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Msc, M.L.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A concise review on cancer treatment methods and delivery systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Rivera-Concepcion, J.; Uprety, D.; Adjei, A.A. Challenges in the use of targeted therapies in non–small cell lung cancer. Cancer Res. Treat. 2022, 54, 315–329. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Pichlsberger, M.; Jerman, U.D.; Obradović, H.; Tratnjek, L.; Macedo, A.S.; Mendes, F.; Fonte, P.; Hoegler, A.; Sundl, M.; Fuchs, J.; et al. Systematic review of the application of perinatal derivatives in animal models on cutaneous wound healing. Front. Bioeng. Biotechnol. 2021, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Schmiedova, I.; Ozanova, Z.; Stastna, E.; Kiselakova, L.; Lipovy, B.; Forostyak, S. Case report: Freeze-dried human amniotic membrane allograft for the treatment of chronic wounds: Results of a multicentre observational study. Front. Bioeng. Biotechnol. 2021, 9, 649446. [Google Scholar] [CrossRef]
- Lipový, B.; Hladík, M.; Štourač, P.; Forostyak, S. Case report: Wound closure acceleration in a patient with toxic epidermal necrolysis using a lyophilised amniotic membrane. Front. Bioeng. Biotechnol. 2021, 9, 649317. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cañada, C.; Bernabé-García, Á.; Liarte, S.; Rodríguez-Valiente, M.; Nicolás, F.J. Chronic wound healing by amniotic membrane: TGF-β and EGF signaling modulation in re-epithelialization. Front. Bioeng. Biotechnol. 2021, 9, 689328. [Google Scholar] [CrossRef]
- Joerger-Messerli, M.S.; Oppliger, B.; Spinelli, M.; Thomi, G.; Di Salvo, I.; Schneider, P.; Schoeberlein, A. Extracellular vesicles derived from Wharton’s jelly mesenchymal stem cells prevent and resolve programmed cell death mediated by perinatal hypoxia-ischemia in neuronal cells. Cell Transplant. 2018, 27, 168–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papait, A.; Cargnoni, A.; Sheleg, M.; Silini, A.R.; Kunis, G.; Ofir, R.; Parolini, O. Perinatal Cells: A promising COVID-19 therapy? Front. Bioeng. Biotechnol. 2021, 8, 619980. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Capra, E.; Herrera, V.; Lang-Olip, I.; Ponsaerts, P.; Cremonesi, F. Application of perinatal derivatives in ovarian diseases. Front. Bioeng. Biotechnol. 2022, 10, 811875. [Google Scholar] [CrossRef]
- Thomi, G.; Joerger-Messerli, M.; Haesler, V.; Muri, L.; Surbek, D.; Schoeberlein, A. Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells 2019, 8, 855. [Google Scholar] [CrossRef] [Green Version]
- Etchebarne, M.; Fricain, J.-C.; Kerdjoudj, H.; Di Pietro, R.; Wolbank, S.; Gindraux, F.; Fenelon, M. Use of amniotic membrane and its derived products for bone regeneration: A systematic review. Front. Bioeng. Biotechnol. 2021, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Bollini, S.; Silini, A.R.; Banerjee, A.; Wolbank, S.; Balbi, C.; Parolini, O. Cardiac restoration stemming from the placenta tree: Insights from fetal and perinatal cell biology. Front. Physiol. 2018, 9, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—a review. Cell Tissue Bank. 2017, 18, 193–204. [Google Scholar] [CrossRef]
- Costa, E.; Murta, J.N. Amniotic membrane in ophthalmology. In Amniotic Membrane: Origin, Characterization and Medical Applications; Springer: Dordrecht, The Netherlands, 2015; pp. 105–122. [Google Scholar] [CrossRef]
- Silini, A.R.; Cancelli, S.; Signoroni, P.B.; Cargnoni, A.; Magatti, M.; Parolini, O. The dichotomy of placenta-derived cells in cancer growth. Placenta 2017, 59, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Mamede, A.C.; Pires, A.S.; Brito, A.F. Amniotic membrane in cancer. In Amniotic Membrane: Origin Characterization and Medical Applications; Springer: Dordrecht, The Netherlands, 2015; pp. 139–152. [Google Scholar] [CrossRef]
- Binello, E.; Germano, I.M. Stem cells as therapeutic vehicles for the treatment of high-grade gliomas. Neuro-Oncology 2012, 14, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Mihu, C.M.; Mihu, D.; Costin, N.; Ciucă, D.R.; Suşman, S.; Ciortea, R. Isolation and characterization of stem cells from the placenta and the umbilical cord. Rom. J. Morphol. Embryol. 2008, 49, 441–446. [Google Scholar]
- Chen, Q.; Cheng, P.; Song, N.; Yin, T.; He, H.; Yang, L.; Chen, X.; Wei, Y. Antitumor activity of placenta-derived mesenchymal stem cells producing pigment epithelium-derived factor in a mouse melanoma model. Oncol. Lett. 2012, 4, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lv, K.; Sun, J.; Guan, J. Anti-tumor effects of engineered mesenchymal stem cells in colon cancer model. Cancer Manag. Res. 2019, 11, 8443–8450. [Google Scholar] [CrossRef] [Green Version]
- Ayuzawa, R.; Doi, C.; Rachakatla, R.S.; Pyle, M.M.; Maurya, D.K.; Troyer, D.; Tamura, M.; Ayuzawa, R.; Doi, C.; Rachakatla, R.S.; et al. Naïve human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Lett. 2009, 280, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, N.; Ishiguro, S.; Kawabata, A.; Uppalapati, D.; Pyle, M.; Troyer, D.; De, S.; Zhang, Y.; Becker, K.G.; Tamura, M. Human umbilical cord matrix mesenchymal stem cells suppress the growth of breast cancer by expression of tumor suppressor genes. PLoS ONE 2015, 10, e0123756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silini, A.R.; Di Pietro, R.; Lang-Olip, I.; Alviano, F.; Banerjee, A.; Basile, M.; Borutinskaite, V.; Eissner, G.; Gellhaus, A.; Giebel, B.; et al. Perinatal derivatives: Where do we stand? A roadmap of the human placenta and consensus for tissue and cell nomenclature. Front. Bioeng. Biotechnol. 2020, 8, 610544. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; De La Torre, P.; Manzano, M.; Cabañas, M.V.; Flores, A.I.; Vallet-Regí, M. Decidua-derived mesenchymal stem cells as carriers of mesoporous silica nanoparticles. In vitro and in vivo evaluation on mammary tumors. Acta Biomater. 2016, 33, 275–282. [Google Scholar] [CrossRef]
- Vegh, I.; Grau, M.; Gracia, M.; Grande, J.; De La Torre, P.; Flores, A.I. Decidua mesenchymal stem cells migrated toward mammary tumors in vitro and in vivo affecting tumor growth and tumor development. Cancer Gene Ther. 2013, 20, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Tian, C.; Zhao, M.; Sun, Y.; Huang, C. Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int. 2021, 21, 595. [Google Scholar] [CrossRef]
- Pires, S.; Bollini, S.; Botelho, M.F.; Lang-Olip, I.; Ponsaerts, P.; Balbi, C.; Lange-Consiglio, A.; Fénelon, M.; Mojsilović, S.; Cremonesi, F.; et al. Guidelines to analyse preclinical studies using perinatal derivatives. IRIS Inst. Res. Inf. Syst. 2022, 1–32. [Google Scholar] [CrossRef]
- Park, J.M.; Han, Y.M.; Hahm, K.B. Rejuvenation of Helicobacter pylori–Associated atrophic gastritis through concerted actions of placenta-derived mesenchymal stem cells prevented gastric cancer. Front. Pharmacol. 2021, 12, 675443. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Liu, J.; Xu, B.; Liang, X.; Yang, X.; Feng, Y.; Liu, J. IL-8/CXCR2 mediates tropism of human bone marrow-derived mesenchymal stem cells toward CD133+ /CD44+ colon cancer stem cells. J. Cell. Physiol. 2021, 236, 3114–3128. [Google Scholar] [CrossRef]
- Hajighasemlou, S.; Pakzad, S.; Ai, J.; Muhammadnejad, S.; Mirmoghtadaei, M.; Hosseinzadeh, F.; Gharibzadeh, S.; Kamali, A.; Ahmadi, A.; Verdi, J. Characterization and validation of hepatocellular carcinoma (HCC) xenograft tumor as a suitable liver cancer model for preclinical mesenchymal stem cell studies. Asian Pac. J. Cancer Prev. 2018, 19, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-T.; Wei, Z.-H.; Hsuan, Y.C.-Y.; Lin, W.; Su, Y.-C.; Liao, C.-H.; Hsieh, C.-L. MRI tracking of polyethylene glycol-coated superparamagnetic iron oxide-labelled placenta-derived mesenchymal stem cells toward glioblastoma stem-like cells in a mouse model. Artif. Cells Nanomed. Biotechnol. 2018, 46, S448–S459. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, D.; Chen, X.; Yang, L.; Wei, Y.; Zhao, X. Antitumor activities of human placenta-derived mesenchymal stem cells expressing endostatin on ovarian cancer. PLoS ONE 2012, 7, e39119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Zheng, L.; Shi, H.; Chen, X.; Wan, Y.; Zhang, H.; Li, M.; Lu, L.; Luo, S.; Yin, T.; et al. Suppression of peritoneal tumorigenesis by placenta-derived mesenchymal stem cells expressing endostatin on colorectal cancer. Int. J. Med. Sci. 2014, 11, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyhoun, I.; Hajighasemlou, S.; Muhammadnejad, S.; Ai, J.; Nikbakht, M.; Alizadeh, A.A.; Hosseinzadeh, F.; Mirmoghtadaei, M.; Seyhoun, S.M.; Verdi, J. Combination therapy of sorafenib with mesenchymal stem cells as a novel cancer treatment regimen in xenograft models of hepatocellular carcinoma. J. Cell. Physiol. 2019, 234, 9495–9503. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.K.; Vasei, M.; Ahmadbeigi, N.; Ebrahimibarough, S.; Soleimani, M.; Roozafzoon, R. Anti-tumour effects of TRAIL-expressing human placental derived mesenchymal stem cells with curcumin-loaded chitosan nanoparticles in a mice model of triple negative breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1011–S1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, H.; Shraga-Heled, N.; Blumenfeld, M.; Dego-Ashto, T.; Fuchs-Telem, D.; Gilert, A.; Aberman, Z.; Ofir, R. Human placental-derived adherent stromal cells co-induced with TNF-α and IFN-γ inhibit triple-negative breast cancer in nude mouse xenograft models. Sci. Rep. 2018, 8, 670. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, S.; Cai, T.; Wang, H.; Xie, X.; Liu, Z.; Zhang, Y. The targeted inhibitory effects of human amniotic fluid stem cells carrying CXCR4 promoter and DAL-1 on non-small cell lung carcinoma growth. Gene Ther. 2016, 23, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.-H.; Hwang, K.-A.; Yi, B.-R.; Lee, H.J.; Jeung, E.-B.; Kim, S.U.; Choi, K.-C. Human amniotic fluid-derived stem cells expressing cytosine deaminase and thymidine kinase inhibits the growth of breast cancer cells in cellular and xenograft mouse models. Cancer Gene Ther. 2012, 19, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Kim, J.-K.; Moon, J.-H.; Beck, S.; Piao, D.; Jin, X.; Kim, S.-H.; Lim, Y.C.; Nam, D.-H.; You, S.; et al. hMSC-mediated concurrent delivery of endostatin and carboxylesterase to mouse xenografts suppresses glioma initiation and recurrence. Mol. Ther. 2011, 19, 1161–1169. [Google Scholar] [CrossRef]
- Du, J.; Liu, A.; Zhu, R.; Zhou, C.; Su, H.; Xie, G.; Deng, Y.; Xu, X. The different effects of IFN-β and IFN-γ on the tumor-suppressive activity of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Int. 2019, 2019, 4592701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitsika, V.; Roubelakis, M.G.; Zagoura, D.; Trohatou, O.; Makridakis, M.; Pappa, K.I.; Marini, F.C.; Vlahou, A.; Anagnou, N.P. Human amniotic fluid-derived mesenchymal stem cells as therapeutic vehicles: A novel approach for the treatment of bladder cancer. Stem Cells Dev. 2012, 21, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, D.; Zhou, J.; Cheng, Y.; Liang, T.; Zhang, G. Characteristics of human amniotic fluid mesenchymal stem cells and their tropism to human ovarian cancer. PLoS ONE 2015, 10, e0123350. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Yao, Y.; Zhang, Y.; Fu, S.; DU, M.; Zhang, G. Effect of targeted ovarian cancer therapy using amniotic fluid mesenchymal stem cells transfected with enhanced green fluorescent protein-human interleukin-2 in vivo. Mol. Med. Rep. 2015, 12, 4859–4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Liang, T.; Wang, D.; Li, L.; Cheng, Y.; Guo, Q.; Zhang, G. IFNα-expressing amniotic fluid-derived mesenchymal stem cells migrate to and suppress HeLa cell-derived tumors in a mouse model. Stem Cells Int. 2018, 2018, 1241323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, S.; Zhang, Q.; Wang, Q.; Lai, D. Human amniotic epithelial cells inhibit growth of epithelial ovarian cancer cells via TGF-β1-mediated cell cycle arrest. Int. J. Oncol. 2017, 51, 1405–1414. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei, M.; Mosaffa, N.; Ghods, R.; Nikoo, S.; Kazemnejad, S.; Khanmohammadi, M.; Mirzadeghan, E.; Mahmoudi, A.R.; Bolouri, M.R.; Falak, R.; et al. Vaccination with human amniotic epithelial cells confer effective protection in a murine model of Colon adenocarcinoma. Int. J. Cancer 2018, 142, 1453–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, N.-H.; Yi, B.O.-R.; Lim, S.Y.; Hwang, K.-A.; Baek, Y.S.; Kang, K.S.; Choi, K.C. Human amniotic membrane-derived epithelial stem cells display anticancer activity in BALB/c female nude mice bearing disseminated breast cancer xenografts. Int. J. Oncol. 2012, 40, 2022–2028. [Google Scholar] [CrossRef] [Green Version]
- Di Germanio, C.; Bernier, M.; Petr, M.; Mattioli, M.; Barboni, B.; de Cabo, R. Conditioned medium derived from rat amniotic epithelial cells confers protection against inflammation, cancer, and senescence. Oncotarget 2016, 7, 39051–39064. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.; Guan, F.; Yang, B.; Li, J.; Song, L.; Hu, X.; Du, Y. Human amniotic membrane derived-mesenchymal stem cells induce C6 glioma apoptosis in vivo through the Bcl-2/caspase pathways. Mol. Biol. Rep. 2012, 39, 467–473. [Google Scholar] [CrossRef]
- Mamede, A.C.; Guerra, S.; Laranjo, M.; Carvalho, M.J.; Oliveira, R.C.; Gonçalves, A.C.; Alves, R.; Castro, L.P.; Sarmento-Ribeiro, A.B.; Moura, P.; et al. Selective cytotoxicity and cell death induced by human amniotic membrane in hepatocellular carcinoma. Med. Oncol. 2015, 32, 257. [Google Scholar] [CrossRef]
- Yun, J.W.; Ahn, J.H.; Kwon, E.; Kim, S.H.; Kim, H.; Jang, J.J.; Kim, W.H.; Kim, J.H.; Han, S.Y.; Kim, J.T.; et al. Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Liver Injury: Hepatoprotective Efficacy, Subchronic Toxicity, Tumorigenicity, and Biodistribution; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 81. [Google Scholar] [CrossRef]
- Fan, C.G.; Wang, D.L.; Zhang, Q.J.; Zhou, J.R. Migration capacity of human umbilical cord mesenchymal stem cells towards glioma in vivo. Neural Regen. Res. 2013, 8, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wang, Y.; He, N.; Wang, D.; Zhao, Q.; Feng, G.; Su, W.; Xu, Y.; Han, Z.; Kong, D.; et al. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials 2014, 35, 5162–5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciavarella, S.; Caselli, A.; Tamma, A.V.; Savonarola, A.; Loverro, G.; Paganelli, R.; Tucci, M.; Silvestris, F. A peculiar molecular profile of umbilical cord-mesenchymal stromal cells drives their inhibitory effects on multiple myeloma cell growth and tumor progression. Stem Cells Dev. 2015, 24, 1457–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachakatla, R.S.; Marini, F.; Weiss, M.L.; Tamura, M.; Troyer, D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007, 14, 828–835. [Google Scholar] [CrossRef]
- Ma, F.; Chen, D.; Chen, F.; Chi, Y.; Han, Z.; Feng, X.; Li, X.; Han, Z. Human umbilical cord mesenchymal stem cells promote breast cancer metastasis by interleukin-8- and interleukin-6-dependent induction of CD44+/CD24− cells. Cell Transplant. 2015, 24, 2585–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Hao, X.; Zhang, S.; Zhang, J. The in vitro and in vivo effects of human umbilical cord mesenchymal stem cells on the growth of breast cancer cells. Breast Cancer Res. Treat. 2012, 133, 473–485. [Google Scholar] [CrossRef]
- Cao, S.; Guo, J.; He, Y.; Alahdal, M.; Tang, S.; Zhao, Y.; Yang, Z.; Gao, H.; Hu, W.; Jiang, H.; et al. Nano-loaded human umbilical cord mesenchymal stem cells as targeted carriers of doxorubicin for breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Di, G.-H.; Liu, Y.; Lu, Y.; Liu, J.; Wu, C.; Duan, H.-F. IL-6 Secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PLoS ONE 2014, 9, e113572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Cai, J.; Huang, F.; Zhu, M.; Zhang, Q.; Yang, T.; Zhang, X.; Qian, H.; Xu, W. Pre-treatment of human umbilical cord-derived mesenchymal stem cells with interleukin-6 abolishes their growth-promoting effect on gastric cancer cells. Int. J. Mol. Med. 2015, 35, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Wang, Y.; Fan, H.; Zhou, B.; Ju, Z.; Yu, L.; Guo, L.; Han, J. Fusion of human umbilical cord mesenchymal stem cells with esophageal carcinoma cells inhibits the tumorigenicity of esophageal carcinoma cells. Int. J. Oncol. 2012, 40, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Zhu, Y.; Sun, Z.; Ji, R.; Zhang, X.; Xu, W.; Yuan, X.; Zhang, B.; Yan, Y.; Yin, L.; et al. Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness. BMC Cancer 2015, 15, 793. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Han, G.; Liu, H.; Qin, C. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: A possible role of Wnt and Akt signaling. PLoS ONE 2013, 8, e62844. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhong, W.; Yuan, J.; Yan, C.; Hu, S.; Tong, Y.; Mao, Y.; Hu, T.; Zhang, B.; Song, G. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 2015, 6, 42276–42289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Yang, M.; Li, Z.; Li, S.; Hu, X.; Fan, D.; Zhang, Y.; Wang, J.; Xiong, D. Suppression of orthotopically implanted hepatocarcinoma in mice by umbilical cord-derived mesenchymal stem cells with sTRAIL gene expression driven by AFP promoter. Biomaterials 2014, 35, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Li, S.; Li, Z.; Peng, H.; Yuan, X.; Jiang, L.; Zhang, Y.; Fan, D.; Hu, X.; Yang, M.; et al. Human umbilical cord mesenchymal stem cells as vehicles of CD20-specific TRAIL fusion protein delivery: A double-target therapy against non-Hodgkin’s lymphoma. Mol. Pharm. 2013, 10, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Cafforio, P.; Viggiano, L.; Mannavola, F.; Pellè, E.; Caporusso, C.; Maiorano, E.; Felici, C.; Silvestris, F. pIL6-TRAIL-engineered umbilical cord mesenchymal/stromal stem cells are highly cytotoxic for myeloma cells both in vitro and in vivo. Stem Cell Res. Ther. 2017, 8, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, J.; Wu, D.; Li, M.; Zhao, F.; Ren, M.; Cai, Y.; Dou, J. IL-21-secreting hUCMSCs combined with miR-200c inhibit tumor growth and metastasis via repression of Wnt/β-catenin signaling and epithelial-mesenchymal transition in epithelial ovarian cancer. OncoTargets Ther. 2018, 11, 2037–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, S.; Gao, H.; Ji, W.; Zhu, F.; Sun, L.; Liu, Y.; Zhang, S.; Xu, Y.; Yan, Y.; Gao, Y. Umbilical cord-derived mesenchymal stromal/stem cells expressing IL-24 induce apoptosis in gliomas. J. Cell. Physiol. 2020, 235, 1769–1779. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhang, L.; Lu, Y.; Zhang, Q.; Fan, D.; Zhang, Y.; Zhang, Y.; Ye, Z.; Xiong, D. Mesenchymal stromal cells as vehicles of tetravalent bispecific Tandab (CD3/CD19) for the treatment of B cell lymphoma combined with IDO pathway inhibitor d-1-methyl-tryptophan. J. Hematol. Oncol. 2017, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Matsuzuka, T.; Rachakatla, R.S.; Doi, C.; Maurya, D.K.; Ohta, N.; Kawabata, A.; Pyle, M.M.; Pickel, L.; Reischman, J.; Marini, F.; et al. Human umbilical cord matrix-derived stem cells expressing interferon-β gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer 2010, 70, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Meng, M.-Y.; Li, L.; Wang, W.-J.; Liu, F.-F.; Song, J.; Yang, S.-L.; Tan, J.; Gao, H.; Zhao, Y.-Y.; Tang, W.-W.; et al. Assessment of tumor promoting effects of amniotic and umbilical cord mesenchymal stem cells in vitro and in vivo. J. Cancer Res. Clin. Oncol. 2019, 145, 1133–1146. [Google Scholar] [CrossRef] [Green Version]
- Di, G.-H.; Jiang, S.; Li, F.-Q.; Sun, J.-Z.; Wu, C.-T.; Hu, X.; Duan, H.-F. Human umbilical cord mesenchymal stromal cells mitigate chemotherapy-associated tissue injury in a pre-clinical mouse model. Cytotherapy 2012, 14, 412–422. [Google Scholar] [CrossRef]
- Dong, L.; Pu, Y.; Zhang, L.; Qi, Q.; Xu, L.; Li, W.; Wei, C.; Wang, X.; Zhou, S.; Zhu, J.; et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018, 9, 218. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Zhang, J.; Zhang, N.; Zeng, Y.; Tang, S.; Tao, Z.; Qu, X.; Jia, J.; Zhu, W.; et al. Nicotine-enhanced stemness and epithelial-mesenchymal transition of human umbilical cord mesenchymal stem cells promote tumor formation and growth in nude mice. Oncotarget 2018, 9, 591–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Song, X.; Yu, W.; Wei, F.; Li, H.; Lv, M.; Zhang, X.; Ren, X. Human umbilical cord mesenchymal stem cells delivering sTRAIL home to lung cancer mediated by MCP-1/CCR2 axis and exhibit antitumor effects. Tumor Biol. 2016, 37, 8425–8435. [Google Scholar] [CrossRef] [PubMed]
- Gauthaman, K.; Fong, C.-Y.; Suganya, C.-A.; Subramanian, A.; Biswas, A.; Choolani, M.; Bongso, A. Extra-embryonic human Wharton’s jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reprod. Biomed. Online 2012, 24, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthaman, K.; Fong, C.-Y.; Arularasu, S.; Subramanian, A.; Biswas, A.; Choolani, M.; Bongso, A. Human Wharton’s jelly stem cell conditioned medium and cell-free lysate inhibit human osteosarcoma and mammary carcinoma cell growth in vitro and in xenograft mice. J. Cell. Biochem. 2013, 114, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Vulcano, F.; Milazzo, L.; Ciccarelli, C.; Eramo, A.; Sette, G.; Mauro, A.; Macioce, G.; Martinelli, A.; La Torre, R.; Casalbore, P.; et al. Wharton’s jelly mesenchymal stromal cells have contrasting effects on proliferation and phenotype of cancer stem cells from different subtypes of lung cancer. Exp. Cell Res. 2016, 345, 190–198. [Google Scholar] [CrossRef]
- Wu, S.; Ju, G.-Q.; Du, T.; Zhu, Y.-J.; Liu, G.-H. Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS ONE 2013, 8, e61366. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Ju, G.; Wu, S.; Cheng, Z.; Cheng, J.; Zou, X.; Zhang, G.; Miao, S.; Liu, G.; Zhu, Y. Microvesicles derived from human Wharton’s jelly mesenchymal stem cells promote human renal cancer cell growth and aggressiveness through induction of hepatocyte growth factor. PLoS ONE 2014, 9, e96836. [Google Scholar] [CrossRef] [Green Version]
- Mirabdollahi, M.; Sadeghi-Aliabadi, H.; Javanmard, S.H. Human Wharton’s jelly mesenchymal stem cells-derived secretome could inhibit breast cancer growth in vitro and in vivo. Iran. J. Basic Med. Sci. 2020, 23, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Chetty, S.S.; Praneetha, S.; Murugan, A.V.; Govarthanan, K.; Verma, R.S. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells labeled with Mn2+ and Gd3+ co-doped CuInS2–ZnS nanocrystals for multimodality imaging in a tumor mice model. ACS Appl. Mater. Interfaces 2020, 12, 3415–3429. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.V.; Gomes, E.D.; Granja, S.; Anjo, S.I.; Baltazar, F.; Manadas, B.; Salgado, A.J.; Costa, B.M. Impact of mesenchymal stem cells’ secretome on glioblastoma pathophysiology. J. Transl. Med. 2017, 15, 353. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Ochiya, T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dong, C.; Chen, M.; Yang, T.; Wang, X.; Gao, Y.; Wang, L.; Wen, Y.; Chen, G.; Wang, X.; et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics 2020, 10, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, J. MicroRNA-320a-containing exosomes from human umbilical cord mesenchymal stem cells curtail proliferation and metastasis in lung cancer by binding to SOX4. J. Recept. Signal Transduct. 2021, 42, 268–278. [Google Scholar] [CrossRef]
- He, Z.; Li, W.; Zheng, T.; Liu, D.; Zhao, S. Human umbilical cord mesenchymal stem cells-derived exosomes deliver microRNA-375 to downregulate ENAH and thus retard esophageal squamous cell carcinoma progression. J. Exp. Clin. Cancer Res. 2020, 39, 140. [Google Scholar] [CrossRef]
- Jia, Y.; Ding, X.; Zhou, L.; Zhang, L.; Yang, X. Mesenchymal stem cells-derived exosomal microRNA-139-5p restrains tumorigenesis in bladder cancer by targeting PRC1. Oncogene 2021, 40, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, Y.; Qu, Y.; Liu, L.; Li, H. Exosomes derived from microRNA-148b-3p-overexpressing human umbilical cord mesenchymal stem cells restrain breast cancer progression. Front. Oncol. 2019, 9, 1076. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Zhou, Y.; Xu, X.; Qi, X.; Liu, J.; Pu, Y.; Zhang, S.; Gao, X.; Luo, X.; Li, M.; et al. MiR-30c-5p loss-induced PELI1 accumulation regulates cell proliferation and migration via activating PI3K/AKT pathway in papillary thyroid carcinoma. J. Transl. Med. 2022, 20, 20. [Google Scholar] [CrossRef]
- Abello, J.; Nguyen, T.D.T.; Marasini, R.; Aryal, S.; Weiss, M.L. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics 2019, 9, 2325–2345. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cao, F.; Sun, H.; Wang, Y.; Liu, S.; Wu, Y.; Cui, Q.; Mei, W.T.; Li, F. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019, 442, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Zhao, L.; Chen, X.; Lin, Z.; Zhang, L.; Li, Z. miR-224-5p carried by human umbilical cord mesenchymal stem cells-derived exosomes regulates autophagy in breast cancer cells via HOXA5. Front. Cell Dev. Biol. 2021, 9, 679185. [Google Scholar] [CrossRef]
- Ding, Y.; Mei, W.; Zheng, Z.; Cao, F.; Liang, K.; Jia, Y.; Wang, Y.; Liu, D.; Li, J.; Li, F. Exosomes secreted from human umbilical cord mesenchymal stem cells promote pancreatic ductal adenocarcinoma growth by transferring miR-100-5p. Tissue Cell 2021, 73, 101623. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, M.; Tang, Q. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-181a retards nasopharyngeal carcinoma development by mediating KDM5C. J. Cancer Res. Clin. Oncol. 2021, 147, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Oliveira, R.; Gomes, M.A.; Abrantes, A.M.; Tavares-Silva, E.; Oliveira, M.C.; Laranjo, M.; Queirós, D.B.; Casalta-Lopes, J.; Pires, S.; Carvalho, L.; et al. Revisiting colorectal cancer animal model—An improved metastatic model for distal rectosigmoid colon carcinoma. Pathophysiology Off. J. Soc. Pathophysiol. 2018, 25, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mendes, N.; Carvalho, P.D.; Martins, F.; Mendonça, S.; Malheiro, A.R.; Ribeiro, A.; Carvalho, J.; Velho, S. Animal Models to Study Cancer and Its Microenvironment; Springer: Cham, Switzerland, 2020; pp. 389–401. [Google Scholar] [CrossRef]
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer: Utility and limitations. Drug Des. Dev. Ther. 2014, 8, 1911–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, K.; Yatsyna, A.; Charbonneau, M.; Brochu-Gaudreau, K.; Perreault, A.; Jeldres, C.; McDonald, P.P.; Dubois, C.M. The chicken chorioallantoic membrane tumor assay as a relevant in vivo model to study the impact of hypoxia on tumor progression and metastasis. Cancers 2021, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
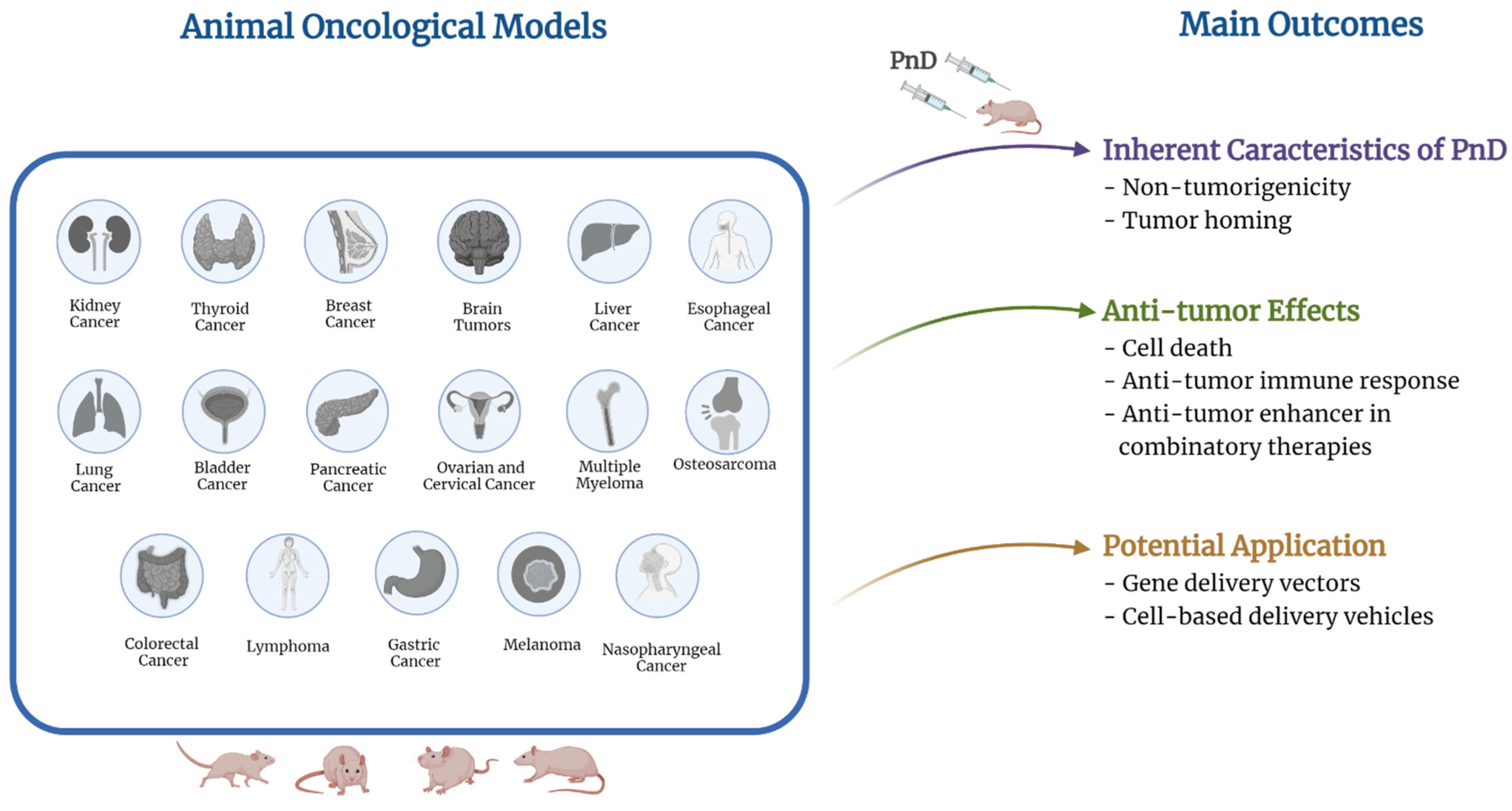
| Reference | Disease Target | Animal Model | PnD | PnD Subtype | Experimental Design | Outcome |
|---|---|---|---|---|---|---|
| Park, J. M. et al., 2021 | Gastric Cancer | C57BL/6 mice; intragastric inoculation of Helicobacter pylori | hPC | hPMSC | 22 weeks after Helicobacter pylori infection, oral administration (about ten times) of 1 × 107 hPMSC or 100 μL of concentrated conditioned medium from hPMSC | Significant reduction of inflammation and gastric atrophy, which can contribute to the prevention of the evolution of Helicobacter pylori-associated gastric precancerous lesions to gastric cancer. |
| Ma, X. et al., 2020 | Colon cancer | BALB/c nude mice; SC injection of HCT116-GFP cells | hPC | hPMSC | When tumors reached 50 mm3, administration in the tail vein of 1 × 106 hPMSC, every 4 days, four administrations | Tumor development induction CSC phenotype promotion Increase in the CD133 expression |
| Hajighasemlou, S. et al., 2018 | Hepatocellular carcinoma | C57BL/6 nude mice; SC injection of HepG2 cells | hPC | hPMSC | 15 days after tumor inoculation, IV injection in tail vein and tumor margins of 5 × 105 hPMSC | Higher ability for tumor tropism |
| Hsu, F. T. et al., 2018 | Glioblastoma | BALB/c nude mice; U87 GSCs injection 2 mm below the brain surface | hPC | hPMSC | Two weeks after tumor inoculation, IV injection into tumor-bearing mice and IV and IP injection into normal mice of hPMSC | Rapid clearance of hPMSC hPMSC tropism to glioblastoma |
| Chen, Q. et al., 2012 | Melanoma | C57BL/6 mice; SC injection of B16-F10 melanoma cells | hPC | hPMSC | When the tumor reached 3 mm diameter, two IT injections of 5 × 105 hPMSC, 4 days apart | Significant reduction of tumor volume and cell apoptosis by hPMSC expressing PEDF |
| Zheng, L. et al., 2012 | Ovarian cancer | BALB/c nude mice; IP injection of A2780 cells | hPC | hPMSC | Homing study: 16 days after tumor inoculation, IP injection of 2 × 105 hPMSC Treatment study: 5 days after tumor inoculation, IP injection of 2 × 105 hPMSC, every 3 days, six times | Inhibition of tumor development, angiogenesis Promotion of tissues apoptosis Tumor homing capacity |
| Zhang, D. et al., 2014 | Colorectal cancer | BALB/c mice; CT26 cells injection into the abdomen cavity | hPC | hPMSC | Four days after tumor implantation, IP injection of 1 × 105 of hPMSC, every 4 days, four administrations | Inhibition of tumor development and angiogenesis Induction of tumor apoptosis |
| Yang, J. et al., 2019 | Colon cancer | Nude mice; SC injection of HT29 cells (HT29-DF) transfected with DF gene (Flu-eGFP) in the right axilla | hPC | hPMSC | Ten days post tumor cells implantation, IV injection into the tail vein of 200 µL of transduced hPMSC | Engineered hPMSC-DF: tumor growth inhibition; tumor tropism capacity hPMSC-DF + GCV: tumor growth inhibition; induction of apoptosis of tumors |
| Seyhoun, I. et al., 2019 | Hepatocellular carcinoma | BALB/c nude mice; SC injection of HepG2 cells into both flanks | hPC | hPMSC | When tumors reached a volume superior to 100 mm3, IT injection of 5 × 105 hPMSC 4 times, 3 days apart | Inhibition of tumor spreading and apoptosis by hPMSC + sorafenib No effect induced by hPMSC alone |
| Kamalabadi-Farahani, M. et al., 2018 | Triple-negative breast cancer | BALB/c nude mice, syngeneic animal model; SC injection of 4T1 cells into the flank | hPC | hPMSC | Seven days after tumor implantation, injection in the tumor area of 1 × 106 of each type of hPMSC | Inhibition of tumor proliferation and apoptosis by combination therapy No effects with hPMSC alone |
| Allen, H. et al., 2018 | Triple-negative breast cancer | Foxn1nu nude mice; SC injection of MDA-MB-231 cells into the right flank and injection into the left inguinal mammary fat pad | hPC | hP-ASC | Heterotopic model: on days 9 and 28 after tumor cells implantation, IM injection of 1 × 106 and 5 × 106 induced hP-ASC; on day 28, IM injection of 5 × 106 non-induced hP-ASC. The control group was untreated mice. Orthotopic model: from day 48 to 83, weekly injection of 1 × 106 induced hP-ASC. The induced hP-ASC group from day 6 to 41 received PlasmaLyte weekly prior to administration of hP-ASC. The control group received PlasmaLyte weekly from day 6 to 83 | Slower tumor progression in orthotopic animals after treatment with induced hP-ASC Complete response in 30% of treated animals |
| Li, L. et al., 2015 | Lung cancer | BALB/c nude mice; SC injection of A549 cells | hAF | hAFSC | After tumors reach a volume of 150 mm3, IV injection of 5 × 106 cells per day for two consecutive days | Tumor homing capacity Impaired tumor growth with increased necrosis by hAFSC overexpressing DAL-1 No hAFSC mRNA in liver, lung, spleen, heart, kidney, small intestine, and testis |
| Kang, N. H. et al., 2012 | Breast cancer | BALB/c nude mice; SC injection of MDA-MB-231 cells on mammary fat pads | hAF | hAFSC | Seven weeks after tumor inoculation, circumtumoral injection of 4 × 106 cells two weeks apart | Migration of hAFSC to IT region Inhibition of tumor growth after treatment with hAFSC expressing suicide genes + 5-FC + GCV No toxicity in breast tissues induced by hAFSC High toxicity in breast tissues induced by 5-FU |
| Li, L. et al., 2015 | Ovarian cancer | Nude mice; (1) SC injection of SKOV3 cells or hAFMSC, (2) SC injection of SKOV3 cells | hAF | hAF-MSC | (1) At the beginning of the study, SC injection of 6 × 106 cells (2) A week after ovarian cancer cell inoculation, IV injection of 6 × 106 cells, two weekly injections | (1) No tumor formation (2) Tumor tropism capacity, with cells detected in the liver and spleen |
| You, Q. et al., 2015 | Ovarian cancer | (1) SCID mice; IM injection of hAF-MSC (2) BALB/c nude mice SC injection of SKOV3 cells | hAF | hAF-MSC | (1) At the beginning of the study, IM injection of 3 × 106 cells (2) When tumors reached 1 cm in diameter, IV injection of 4 × 106 cells | (1) No tumor formation (2) Tumor tropism capacity |
| Bitsika, V. et al., 2012 | Bladder cancer | NOD-SCID mice; SC injection of T24M cells near the tail base | hAF | hAF-MSC | Ten days after tumor inoculation, an IV injection of 1 × 106 cells was administered in three weekly doses | hAF-MSC migration to tumors No changes in tumor growth by hAF.MSC Inhibition of tumor growth by hAF-MSC carriers of IFNβ Small number of hAF-MSC was found in the lungs |
| Zhou, J. et al., 2018 | Cervical cancer | BALB/c nude mice; SC injection of eGFP-HeLa cells | hAF | hAF-MSC | Ten days after tumor inoculation, IV injection of 5 × 106 hAF-MSC cells. Three doses of hAF-MSC (naif vs. overexpressing IFNα) doses every five days or a single administration (biodistribution). | Tumor homing capacity of hAF-MSC Few cells were found in the liver and spleen Inhibition of tumor growth after three administrations of IFNα overexpressing hAF-MSC Increased tumor growth induced by naive hAF-MSC |
| Yin, J. et al., 2011 | Glioma | BALB/c nude mice; (1) Stereotactic injection into the brain, (2) SC injection of U87MG-EGFRvIII cells | hAF | hAF-MSC | (1) Stereotactic injection of 5 × 104 cells, simultaneously with glioma cells. (2) Injection in the resection cavity of the tumor of 2.5 × 105, when tumors reached 2 cm3, after removal of 90% of the tumor mass | No tumor formation after hAF-MSC (alone) orthotopic injection Reduction of tumor burden after treatment with hAF-MSC carrying endostatin and carboxylesterases Inhibition of tumor regrowth after treatment with hAF-MSC expressing endostatin and carboxylesterase |
| Du, J. et al., 2019 | Lung cancer | BALB/c and NOD-SCID mice; SC injection of H460 cells | hAF | hAF-MSC | SC injection of 2 × 106 cells for BALB/c animals and 1 × 106 or 2 × 106 for NOD-SCID mice, simultaneously with cancer cells | Promotion of the earlier onset and delayed the disappearance of tumor mass by hAF-MSC or IFN-γ-primed hAF-MSC Delayed onset and promotion of the disappearance of tumor mass by IFN-β- or IFN-β + IFN-γ-primed hAF-MSC |
| Tabatabaei, M. et al., 2018 | Colorectal, melanoma, and breast cancer | BALB/c and C57BL/6 mice; SC injection of CT26 (colon), Renca (kidney), 4T1 (breast) or B16F10 (melanoma) cells | hAM | hAEC | At the beginning of the study, SC injection of 1 × 106 hAEC, three weakly administrations | Complete inhibition of colorectal tumor development No alteration induced in breast cancer Delayed melanoma tumor development and reduced tumor weight Increased percentage of peripheral blood and splenic T cells (CD3+) in hAEC and CT26 vaccine groups Increased frequency of peripheral and splenic CD4+ T and CD8+ cells Increased activity of splenocytes activity against CT26 cells from hAECs-vaccinated mice Increased level of IFN-γ after stimulation with hAECs or CT26. |
| Bu, S. et al., 2017 | Ovarian cancer | BALB/c nude mice; SC injection of SK-OV-3 cells or SK-OV-3/hAECs | hAM | hAEC | SC injection of 1 × 106, simultaneous with cancer cells | Inhibition of tumor growth, with small tumor size, weight Decreased expression of PCNA and Ki-67 |
| Kang, N. H.et al., 2012 | Breast cancer | BALB/c nude mice; SC injection of MDA-MB-231 cells | hAM | hAEC | Seven weeks after tumor implantation or when tumor volumes reached 250–300 cm3, circumtumoral injection of 4 × 106 or 8 × 106 cells | Reduced tumor volumes Tumor homing capacity Protection of breast tissues in hAEC group Destruction of breast tissues in 5-FU group Increased survival after hAEC treatment |
| DiGermano, C. et al., 2016 | Melanoma | C57BL/6J mice; SC injection of B16F10 cells | hAM | hAEC | SC injection of B16F10 melanoma cells or hAEC alone or a mix of both cells with increasing amounts of hAECs (0.25–1 × 106) | Delayed tumor growth with decreased tumor size, but not with tumor weight. |
| Jiao, H. et al., 2012 | Glioma | BALB/c nude mice; SC injection of C6 cells | hAM | hAMSC | Six and twelve days after tumor inoculation, IT injection of 2 × 106 cells in a single dose or three doses, three days | Reduced tumor size after a single administration Increased reduction of tumor volume with multiple doses Increased apoptosis and expression of caspase 3, caspase 8, and BAX/BCL-2 ratio |
| Mamede, A.C. et al., 2015 | Hepatocellular carcinoma | BALB/c nude mice; SC injection of HuH7 or HepG2 cells | hAM | hAMTE | When the tumors reached 300 mm3, an IP injection of 60 mg/kg of hAMTE was administered every two days for 12 days | Decreased tumor volume of HepG2 hepatocellular tumors No alterations in HUH7 hepatocellular tumors |
| Vegh et al., 2012 | Breast cancer | Sprague–Dawley female rats; Induction of mammary carcinomas by IP inoculation of NMU (5 mg per 100g body) | hD | hDMSC | When breast tumors were palpable, IV injection of 1.5 × 106 fluorescence-labeled cells | Specific tropism and homing to mammary tumors |
| Paris et al., 2016 | Breast cancer | Sprague–Dawley female rats; Induction of mammary carcinomas by IP inoculation of NMU (5 mg per 100g body) | hD | hDMSC | After tumor development, IV injection of 106 of hDMSC labeled with green fluorescent mesoporus silica NPs | No alterations in tumor homing capacity after NP loading |
| Yun, J. W. et al., 2016 | Hepatocellular carcinoma | A 26-week tumorigenicity study using BALB/c nude mice treated with hUC-MSC | hUC | hUC-MSC | At the beginning of the study, IV injection of 1 × 108 cells/kg, 2 × 107 cells/kg, or 4 × 106 cells/kg of body weight | No tumor formation due to injection of hUC-MSC |
| Fan, C. et al., 2013 | Glioma | Sprague–Dawley mice; Stereotactic injection of C6 cells | hUC | hUC-MSC | One week after tumor cell implantation, contralateral ventricular and IT injection of 5 × 105 cells at 1.3 mm posterior to bregma, 3 mm left to the midline, and 3.5 mm beneath the dura | Migration to glioma site through corpus callosum after contralateral ventricle injection, located at the tumor-normal brain parenchyma interface Distribution at the border zone between tumor and tumor bed after intratumor injection and migration to outgrowing glioma satellites |
| Ciavarella et al., 2015 | Multiple myeloma | NOD.CB17-Prkdcscid/J mice; SC injection of RPMI-8226 cells | hUC | hUC-MSC | 2 × 106 cells SC injected simultaneously with the tumor cells or PT injection after 7 days | Tumor inhibition by 50% after simultaneous injection of hUC-MSC Tumor size decreased by 20 times after PT inoculations, 30 days post-implantation of tumor cells |
| Rachakatla et al., 2007 | Metastatic breast cancer in the lung | CB17/SCID mice; IV injection of MDA 231 cells | hUC | hUC-MSC | SC: 2 × 106 or 1 × 107 cells, IV: 2 × 106 or 3 × 106 or 6.5 × 106 cells to evaluate the tumor formation; IV: 1 × 106 cells on days 17 and 24 or 11 and 18 after tumor inoculation to evaluate selective engraftment; IV: 0.5 × 106 cells eight days after tumor inoculation twice at 1-week intervals to evaluate the ability to reduce tumor burden | No tumor induction by hUC-MSC Tumor homing capacity Reduced tumor burden in SCID mice following systemic administration |
| Ma, F. et al., 2015 | Breast cancer | BALB/c nude mice; SC injection of MCF-7 cells | hUC | hUC-MSC | When the tumor reaches 50 mm3, IV injection of cells 4 × 104, 2 × 105, 1 × 106 of hUC-MSC | Similar tumor growth with or without hUC-MSC Induction of tumor in the lungs |
| Ma, Y. et al., 2012 | Breast cancer | Nude mice and CB17 SCID mice; Injection of MDA-MB-231 breast CSC into the mammary pad on the right side of the chest wall | hUC | hUC-MSC | When the tumor reaches 0.5 cm, SC injection near the tumor site with 0.5 × 106, 1 × 106, and 3 × 106 cells hUC-MSC, once a week for three consecutive weeks. | Decreased tumor weight dependent on the number of hUC-MSC Decreased expression of PI3K and Akt with the increasing number of hUC-MSC injected |
| Cao, S. et al., 2018 | Breast cancer | BALB/c nude mice; IM injection of 1.5 mg/kg estradiol followed by SC injection of MCF-7 cells into the mice’s armpit | hUC | hUC-MSC | When tumors reached 300 mm3, IV injection of 2.5 mg/kg and then every four days | Efficient tumor targeting. Decreased tumor growth induced by doxorubicin nanoparticles-loaded hUC-MSC compared to control and hUC-MSC alone |
| Di, G. H. et al., 2014 | Breast cancer | Female immunodeficient mice; SC injection of MDA-MB-231 alone or mixed with an equal number of hUC-MSC | hUC | hUC-MSC | SC injection of 2 × 106 of hUC-MSC or H2O2-induced hUC-MSC together with tumor cells, in the right flank region | Increased tumor formation and tumor growth after injection of senescent hUC-MSC Increased vascularization |
| Wang, M. et al., 2014 | Gastric cancer | BALB/c nude mice; SC injection with untreated SGC-7901 cells alone, SGC-7901 cells together with hUC-MSCs or IL-6-pre-treated hUC-MSCs into the backside of mice | hUC | hUC-MSC | At the beginning of the study, SC injection of hUC-MSC | Tumor growth increased in the co-injection group Decreased tumor cell apoptosis in hUC-MSC group. Increased tumor cell apoptosis in IL-6-hUC-MSC group. |
| Wang, Y. et al., 2011 | Esophageal cancer | SCID mice and BALB/c nude mice | hUC | hUC-MSC | At the beginning of the study, injection of 1 × 106 fusion cells for BALB/c nude mice and 1 × 105 for SCID animals | Decreased tumor growth in fusion groups in SCID model; No tumor formation with hUC-MSC alone or self-fused; Increased tumor latency time and decreased tumor weight and volume in BALB/c models with the injection of fused-cells |
| Xue, J. et al., 2015 | Gastric cancer | BALB/C nude mice; SC injection of HGC-27 alone or HGC-27- hUC-MSC fusion cells | hUC | hUC-MSC | At the beginning of the study, SC injection of 2 × 106 hUC-MSC | Increased tumor growth in the fusion-cell group Increased heterogeneity, abnormal nuclear/cytoplasmatic ratio, and derangement distribution in tumor regions |
| Liu, J. et al., 2013 | Cholangiocarcinoma | BALB/c nude mice; SC injection of HCCC-9810 cells or hUC-MSC, or a mixture of cancer cells with hUC-MSC or HUVEC | hUC | hUC-MSC | At the beginning of the study, SC injection of 1 × 106 hUC-MSC, alone or with tumor cells | Decreased tumor incidence with hUC-MSC Decreased tumor volume with hUC-MSC and with hUC-MSC conditioned medium |
| Wang, W. et al., 2015 | Cholangiocarcinoma | BALB/c nude mice; SC injection of QBC939 cells | hUC | hUC-MSC | At the beginning of the study, SC injection of 0.5 × 106 hUC-MSC | Increased tumor volume and weight in the mixed-cell group and hUC-MSC treated group |
| Yan, C. et al., 2014 | Hepatocellular carcinoma | BALB/c nude mice; Orthotopic injection of HepG2 cells | hUC | hUC-MSC | Seven days after tumor implantation, IV injection of 3 × 105 hUC-MSC, 5-FU injected for successive 5 days (10 mg/kg) from the next day of hUC-MSC injection | Tumor tropism capacity Decreased tumor growth by engineered hUC-MSC Synergistic antitumor effects with combination treatment 5FU + engineered hUC-MSC. |
| Yan, C. et al., 2013 | Non-Hodgkin B-cell lymphoma | NOD/SCID mice; SC injection of BJAB cells | hUC | hUC-MSC | One week after tumor cells implantation, an IV injection of 5 × 105 hUC-MSC | Efficient accumulation of fusion protein scFvCD20-sTRAIL secreted by hUC-MSC Tumor growth inhibition by fusion proteins MSC.scFvCD20-sTRAIL and MSC.ISZ-sTRAIL Increased apoptosis in tumor cells, with MSC.scFvCD20-sTRAIL exhibiting greater antitumor potential than MSC.ISZ-sTRAIL. |
| Cafforio et al., 2017 | Multiple myeloma | NOD.CB17-Prkdcscid/J; Intratibial injection of Red-Luc + U-266 cells | hUC | hUC-MSC | Three days after tumor inoculation, 2.5 × 105 cells were injected intracardially | Tumor tropism to multiple myeloma tibia lesions Reduced tumor burden by induction of apoptosis |
| Zhang, Y. et al., 2018 | Ovarian cancer | BALB/c nude mice; SC injection of SKOV3 cells at the mouse’s right flank. | hUC | hUC-MSC | 8–9 days after tumor cell implantation, IT injection of 1 × 106 hUC-MSC | Decreased tumor volumes with hUC-MSC-LV-IL-21 combined with miR-200c agomir No evidence of cancer metastasis in the lung, liver, spleen, and stomach Decreased expression of β-catenin, cyclin-D1, Gli1, Gli2, and ZEB1 in the combination group |
| Fan et al., 2020 | Glioma | BALB/c nude mice; SC injection of U251 cells into the left flank near the axillary fossa | hUC | hUC-MSC | Ten days post tumor cells inoculation, IV injection of 2 × 106 cells, every week for 3 weeks | Induction of apoptosis by hUC-MSC expressing IL-24 Reduced tumor growth |
| Zhang et al., 2017 | B cell lymphoma | BALB/c nude mice; SC injection of Raji cells into the right flank | hUC | hUC-MSC | 1 × 106 cells IV at day 0; 1 × 106 cells IV at day 0 with PBMCs; IV at day 2 every 7 days for 2 weeks; D-1MT in the drinking water for 21 days | Tumor homing capacity Decreased tumor weight by 61.2% |
| Matsuzuka et al., 2010 | Lung cancer | CB17/SCID mice; IV injection of H358 cells | hUC | hUC-MSC | One week after the second injection of tumor cells, IV injection of 3 × 105 cells, every 5 days, for 4 times | Reduction of tumor burden by IFN-β-expressing hUC-MSC |
| Ayuzawa et al., 2009 | Metastatic breast cancer in the lung | CB17/SCID mice; IV injection of MDA 231 cells | hUC | hUC-MSC | Eight days after tumor implantation, IV injection of 0.5 × 106 cells for 3 weeks | Tumor homing capacity Decreased tumor growth in the lungs |
| Ohta et al., 2015 | Metastatic breast cancer in the lung | CB17/SCID mice; IV injection of MDA 231 cells | hUC | hUC-MSC | On days 6, 13, and 20 after cancer cell inoculation, IV injection of 5 × 105 cells IV, for 4 weeks | Decreased metastatic tumor growth by FST over-expressing cells Decreased number of tumor nodules in the lung |
| Meng, M. Y. et al., 2019 | Lung and Gastric cancer | BALB/c nude mice; IV injection of cells | hUC | hUC-MSC | At the beginning of the study, SC injection of 1 × 106 hAF-MSC and hUC-MSC, mixed with tumor cells | Induction of increased tumor size by hAF-MSC No differences induced by hUC-MSC |
| Di et al., 2012 | Murine Lewis lung carcinoma and human colon carcinoma | C57BL/6 mice; SC injection of cells in the right flank region; BALB/C nude mice; SC injection of Lovo cells into the left flank | hUC | hUC-MSC | 5, 11, 17 days after tumor cells inoculation, IV injection of 1 × 106 cells or 5, 12, 19, 26, and 33 days after tumor cells inoculation, IV injection of 0.5–1 × 106 cells | Reduction of adriamycin-induced side effects Improved general quality of life of animals as adjuvant therapy |
| Li, T. et al., 2018 | Lung cancer | BALB/c nude mice; SC injection into the flank with co-cultured cells | hUC | hUC-MSC | Simultaneously with tumor cells, SC injection | Increased tumor growth by nicotine-treated hUC-MSC High heterogeneity, elevated nuclear/cytoplasmatic rations, and derangement distribution in nicotine-treated hUC-MSC tumors |
| Yan, C. et al., 2016 | Lung cancer | BALB/c nude mice; SC injection of A549 cells into the right flank | hUC | hUC-MSC | When tumor volume was 80–120 mm3, IV injection of 3 × 105 cells | Tumor homing capacity Higher survival rate induced by engineered hUC-MSC |
| Dong, L. et al., 2018 | Lung cancer | BALB/c nude mice; SC injection with H1299 cells alone, H1299 cells mixed with hUCMSCs or with hUCMSC-EVs or hUCMSCs alone | hUC | hUC-MSC | Simultaneous with the injection of tumor cells, SC injection of 1.5 × 106 or 6 × 109 cells hUC- MSC and 200 µg of hUC-MSC-EV | Increased proliferation of cancer cells in tumor tissues Decreased apoptosis |
| Gauthaman, K. et al., 2012 | General oncology (teratomas) | SCID mice | hUC | hUC-WJ | SC, IM, and IP Injection of 2 × 106 cells/site of unlabeled human embryonic stem cells (ESC) + Matrigel, 5 × 106 cells/site of fluorescence-labeled human extra-embryonic hUC-WJ or and labeled human extra-embryonic hUC-WJ + Matrigel | No tumors or inflammatory reactions induced by hUC-WJ Tumor development induced by hESCs + Matrigel Increased levels of anti-inflammatory cytokines induced by hUC-WJ |
| Gauthaman et al., 2013 | Mammary carcinoma | SCID mice; SC injection of MDA-MB-231 cells | hUC | hUC-WJ-MSC | Protocol A: 4 days after tumor induction, IT injection of 1 × 106 hUC-WJ-MSC and 100 µL of hUC-WJ-MSC -conditioned medium (50%); Protocol B: 5 weeks after tumor, IT injection of 5 × 106 hUC-WJ-MSC and 100 µL of hUC-WJ-MSC-conditioned medium (50%). | Decreased tumor sizes and weights induced by hUC-WJ-MSC and hUC-WJ-MSC-CM Increased lymphocytic infiltration and vacuolation of tumor cells |
| Vulcano et al., 2016 | Lung cancer | NOD/SCID mice | hUC | hUC-WJ-MSC | SC injection of two types of AC-LCSC or SCC-LCSC alone or co-injected with 5 × 106 of hUC-WJ-MSC or Normal Human Dermal Fibroblast (NHDF) | Increased tumor size and growth induced by AC-LCSC co-inoculated with hUC-WJ-MSC Increased percentage of CD133 and CD166 Tumors with a high proliferation index No evidence of necrotic areas or pyknosis |
| Wu et al., 2013 | Bladder cancer | BALB/c nude mice | hUC | hUC-WJ-MSC | SC injection of 1 × 107 T24 cells; 1 × 107 T24 cells mixed with 1 × 107 of hUC-WJ-MSC; 1 × 107 T24 cells mixed with 200 mg protein hUC-WJ-MSC; 200 mg protein hUC-WJ-MSC. | Decreased tumor incidence induced by hUC-WJ-MSC or MVs derived hUC-WJ-MSC co-injected with tumor cells Increased apoptosis compared to control Decreased nuclear size, extracellular matrices Decreased proliferation index |
| Du et al., 2014 | Renal cell carcinoma | BALB/c nude mice; SC injection of 786-0 cells | hUC | hUC-WJ-MSC | Simultaneous SC injection of 1 × 107 of 786-0 cells with the addition of MVs (200 µg/mL), RNase-MVs, or M199 (control). | Compared to control, increased tumor incidence and volume for animals treated with MVs derived from hUC-WJ-MSC. Enhanced expression of cyclin D1, MMP-2, and MMP-9 in tumor tissues. High proliferation index in the presence of MVs, associated with the activation of AKT and ERK1/2 signaling pathways |
| Mirabdollahi et al. (2020) | Breast cancer | BALB/c mice; Injection of 4T1 cells | hUC | hUC-WJ-MSC | (1) Three IV injections of hUC-WJ-MSC-derived secretome (20 mg), cisplatin (three injections, 10 mg/kg), and PBS were made for 10 days (on days 5, 15, and 15). On day 30, mice were inoculated with 3.5 × 106 4T1 cells; (2) SC injection of 3.5 × 106 4T1 cells, and when tumor appears, they received the same injections used in approach 1 | Higher latency period in treatment groups Decreased tumor incidence in treatment groups Decreased tumor size and weight in secretome and cisplatin-treated groups |
| Chetty et al. (2020) | Melanoma | C57BL/6 mice; SC injection of B16F10 cells on the shoulder and left hind limb towards skeletal muscle | hUC | hUC-WJ-MSC | When the tumor attained the desired growth, injection of 106 of hUC-WJ-MSC labeled with Mn2+ and Gd3+ co-doped CuInS2-ZnS (CIS-ZMGS) Nanocrystals (NCs) | Tumor tropism capacity No alterations in heart, kidney, or lung cells, nor liver metabolism |
| Vieira de Castro et al., 2017 | Glioblastoma | Chicken chorioallantoic membrane assay; U251 or SNB-19 cells injected into a window made into the eggshell after puncturing the air chamber | hUC | hUC-PVC | Tumor cells were previously exposed to hUC-PVC conditioned media for 4 days, and on days 11, 13, and 15 of incubation, 100 µL of new conditioned media was added | Increased tumor growth Increased vessel density |
| Xie, H. et al., 2021 | Hepatocellular carcinoma | BALB/c nude mice; SC injection of H1299 cells in the right flank | hUC | hUC-MSC-EV | Exosomes administered daily | Decreased tumor growth after treatment with exosomes |
| He, Z. et al., 2020 | Esophageal cancer | BALB/c nude mice; SC injection of KYSE70 cells or EC9706 cells | hUC | hUC-MSC-EV | On days 5, 10, 15, 20, and 25, IV injection | Decreased tumor growth Increased expression of BAX and E-cadherin Decreased expression of ENAH, Bcl-2, Bcl-xl, N-cadherin, Snail stemness-related mRNAs |
| Jia, Y. et al., 2021 | Bladder cancer | BALB/c nude mice; SC injection of cells | hUC | hUC-MSC-EV | On days 5, 10, 15, 20, and 25, an IV injection of 100 µg of hUC-MSC-EV | Decreased tumor volume and weight; decreased expression of N-cadherin, vimentin, SNAIL, Bcl-2, and PCNA; increased expression of E-cadherin and Bax |
| Yuan, L. et al., 2019 | Breast cancer | Athymia nude mice; injection of MDA-MB- 231 cells through the mammary fat pad | hUC | hUC-MSC-EV | At days 5, 10, 15, 20, and 25, IV injection of 100 µL of hUC-MSC-EV | Decreased tumor volume Decreased expression of TRIM59, N-cadherin, vimentin, Bcl-2, BCL-xl Increased expression of Bax and E-cadherin. |
| Zheng, T. et al., 2022 | Papillary thyroid cancer | BALB/c nude mice; SC injection of W3 cells | hUC | hUC-MSC-EV | Seven days after tumor cell inoculation, IT injection of 2 × 1010 hUC-MSC-EV, weekly, until day 28 | Decreased tumor volume and weight after miR-30c-5p-EV treatment Decreased tumor volume and weight after injection of hUC- MSC-EV |
| Abello et al., 2019 | Osteosarcoma | 088/NUDE homozygous mice; SC injection of K7M2 cells in the lower flank | hUC | hUC-MSC | Ten days post-implantation of tumor cells, IV injection of 0.015 mmol/kg | Increased accumulation at tumor compared to Magnevist® |
| Ding et al., 2019 | Pancreatic ductal adenocarcinoma | BALB/c nude mouse; SC injection of Panc-1 cells on both flanks of the mice | hUC | hUC-MSC-EV | After 7 days of tumor growth, IT injection, 3 days per week for 35 days | Decreased tumor growth Downregulation of Smad3, N-cadherin, and Bax expression; upregulation of E-cadherin, and Bcl-2 expression |
| Wang, Y. et al., 2021 | Breast cancer | Nude mice; SC injection of MCF-7 cells | hUC | hUC-MSC-EV | 10 days after tumor cell inoculation, injection of 200 µL of hUC-MSC-EV | Decreased tumor volume and weight after treatment with the inhibitor of mir-224-5p carrying exosomes |
| Deng, Y. et al., 2021 | Pancreatic cancer | BALB/c nude mice; SC injection of Panc-1 cells | hUC | hUC-MSC-EV | Seven days after tumor growth, IT injection of 400 µL of hUC-MSC-EV every day, for three days each week | Increased tumor growth, volume, and weight with hUC-MSC-EV treatment Increased tumor growth after miR-100-5p agomir treatment |
| Liu, L. et al., 2021 | Nasopharyngeal cancer | BALB/c nude mice; SC injection of cancer cells | hUC | hUC-MSC-EV | One week after tumor cells inoculation, SC injection | Increased tumor volume |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixo, R.; Pires, A.S.; Pereira, E.; Serambeque, B.; Marques, I.A.; Laranjo, M.; Mojsilović, S.; Gramignoli, R.; Ponsaerts, P.; Schoeberlein, A.; et al. Application of Perinatal Derivatives on Oncological Preclinical Models: A Review of Animal Studies. Int. J. Mol. Sci. 2022, 23, 8570. https://doi.org/10.3390/ijms23158570
Teixo R, Pires AS, Pereira E, Serambeque B, Marques IA, Laranjo M, Mojsilović S, Gramignoli R, Ponsaerts P, Schoeberlein A, et al. Application of Perinatal Derivatives on Oncological Preclinical Models: A Review of Animal Studies. International Journal of Molecular Sciences. 2022; 23(15):8570. https://doi.org/10.3390/ijms23158570
Chicago/Turabian StyleTeixo, Ricardo, Ana Salomé Pires, Eurico Pereira, Beatriz Serambeque, Inês Alexandra Marques, Mafalda Laranjo, Slavko Mojsilović, Roberto Gramignoli, Peter Ponsaerts, Andreina Schoeberlein, and et al. 2022. "Application of Perinatal Derivatives on Oncological Preclinical Models: A Review of Animal Studies" International Journal of Molecular Sciences 23, no. 15: 8570. https://doi.org/10.3390/ijms23158570
APA StyleTeixo, R., Pires, A. S., Pereira, E., Serambeque, B., Marques, I. A., Laranjo, M., Mojsilović, S., Gramignoli, R., Ponsaerts, P., Schoeberlein, A., & Botelho, M. F. (2022). Application of Perinatal Derivatives on Oncological Preclinical Models: A Review of Animal Studies. International Journal of Molecular Sciences, 23(15), 8570. https://doi.org/10.3390/ijms23158570













