Slc25a39 and Slc25a40 Expression in Mice with Bile Duct Ligation or Lipopolysaccharide Treatment
Abstract
:1. Introduction
2. Results
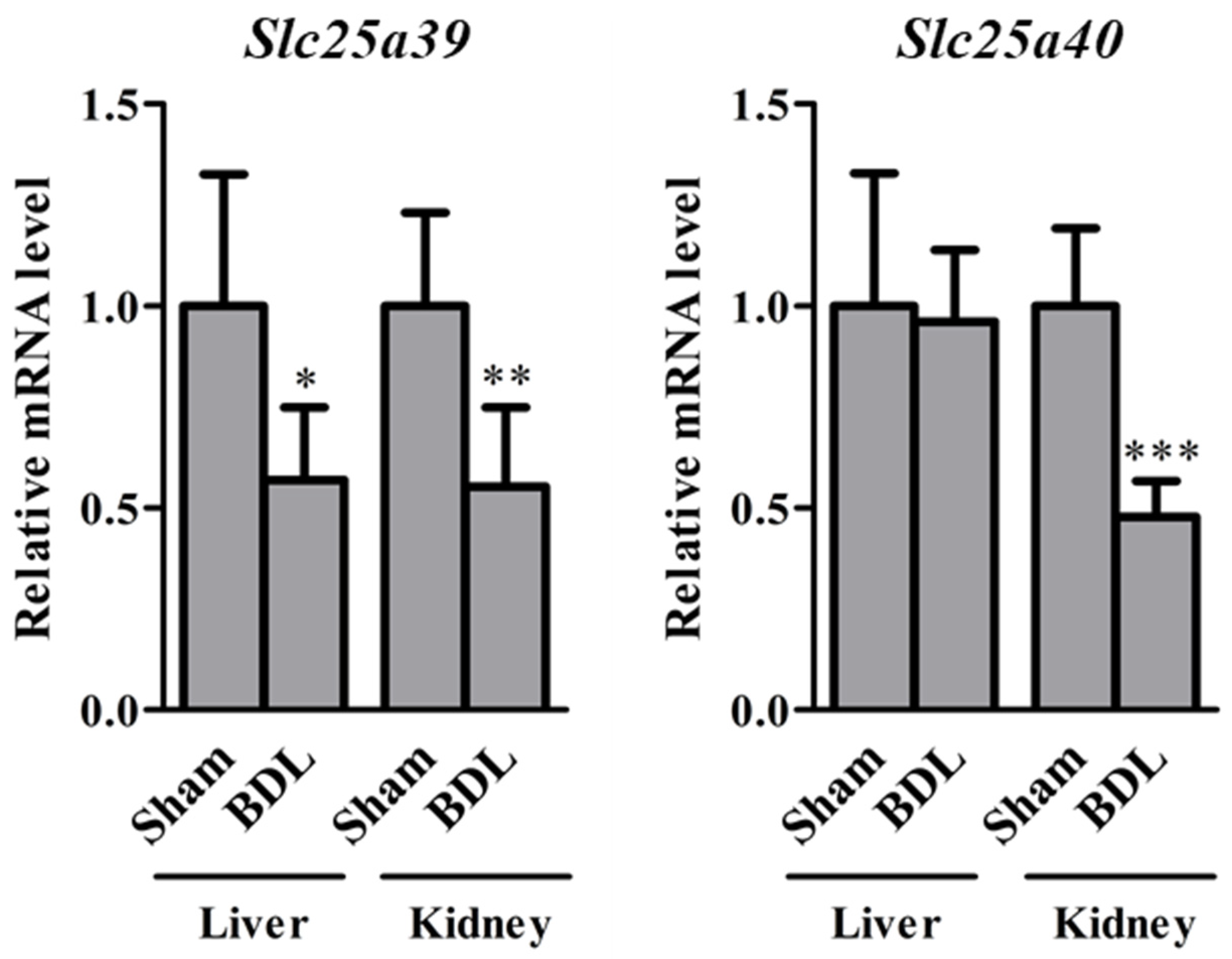
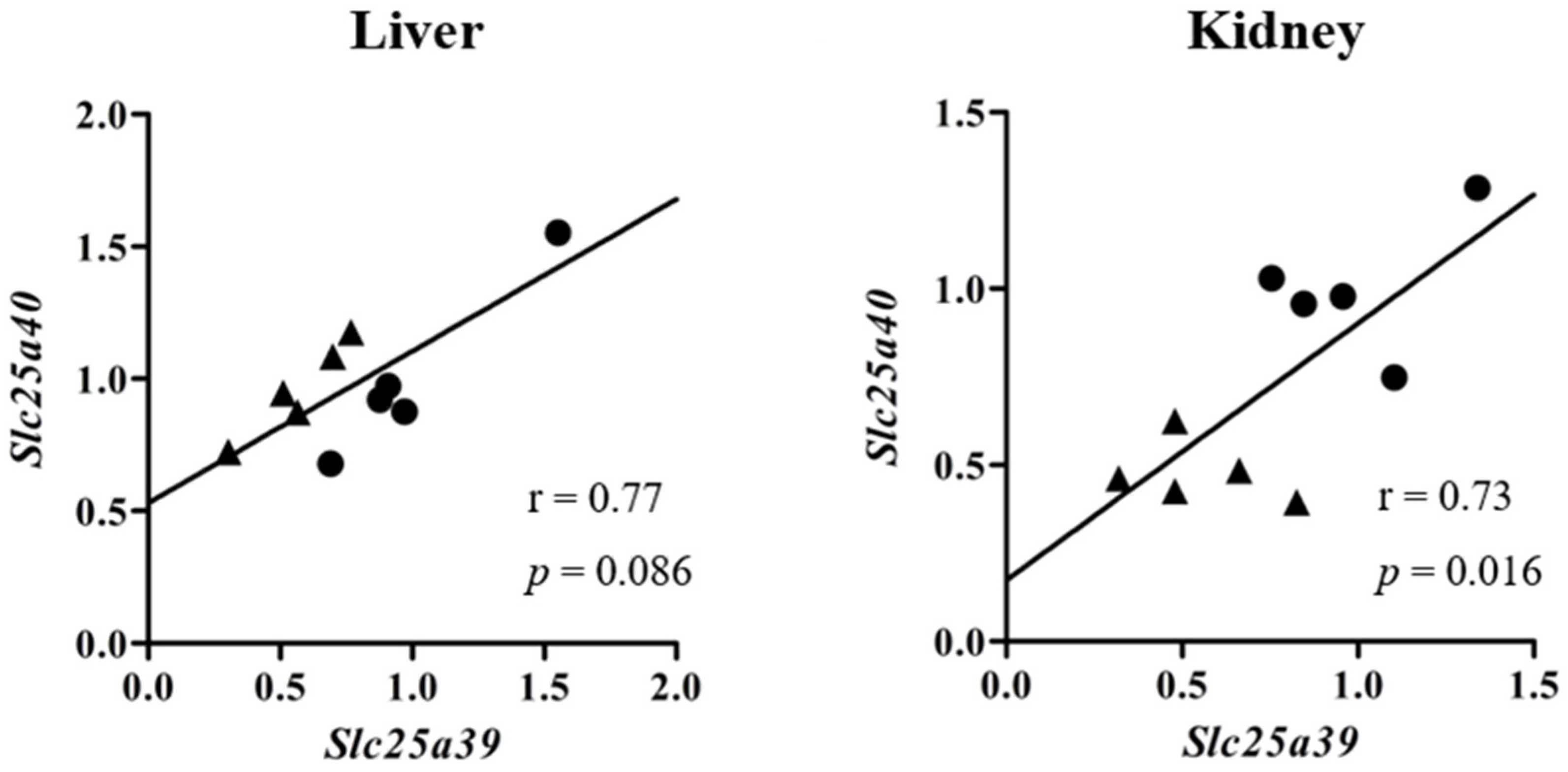
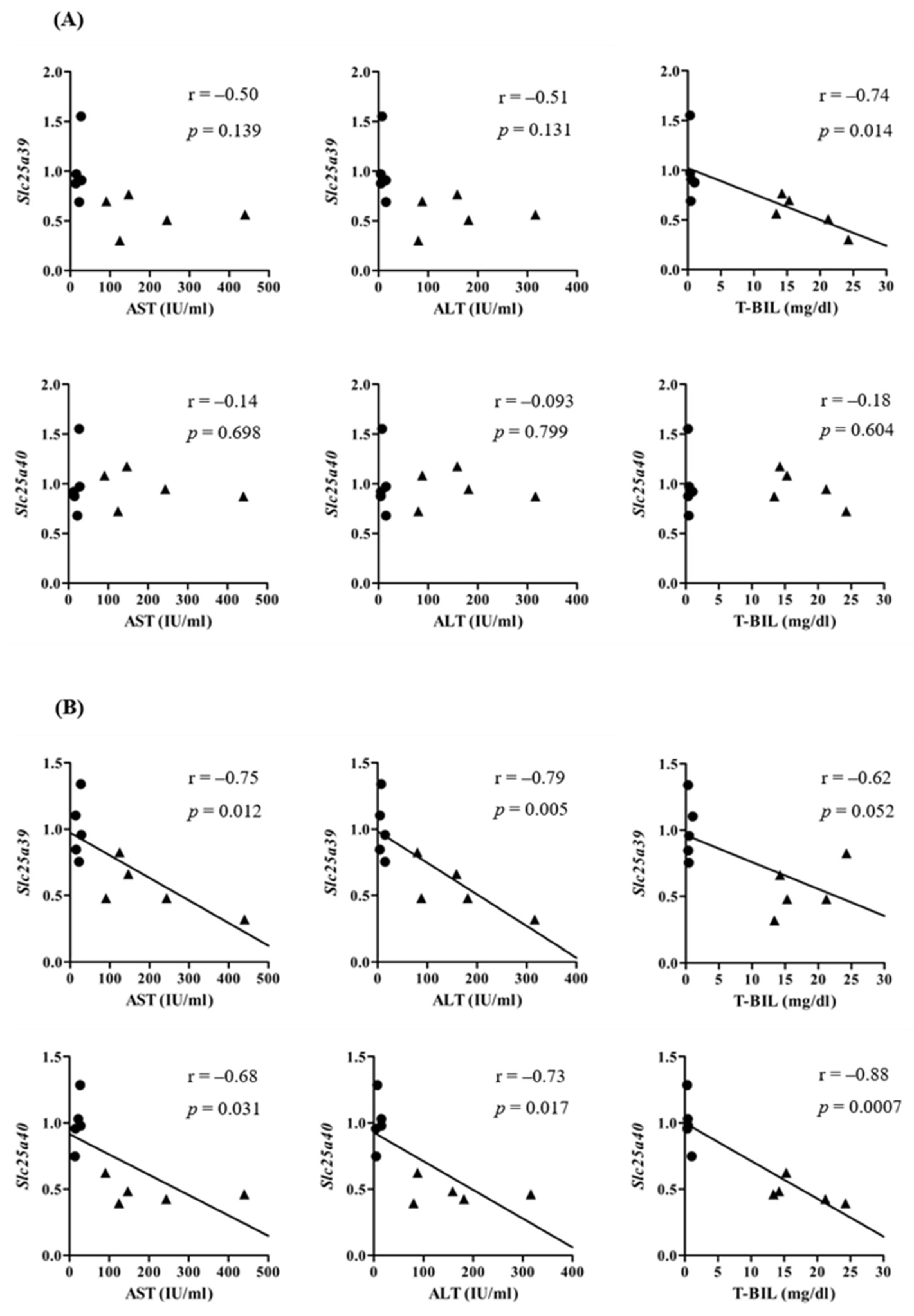
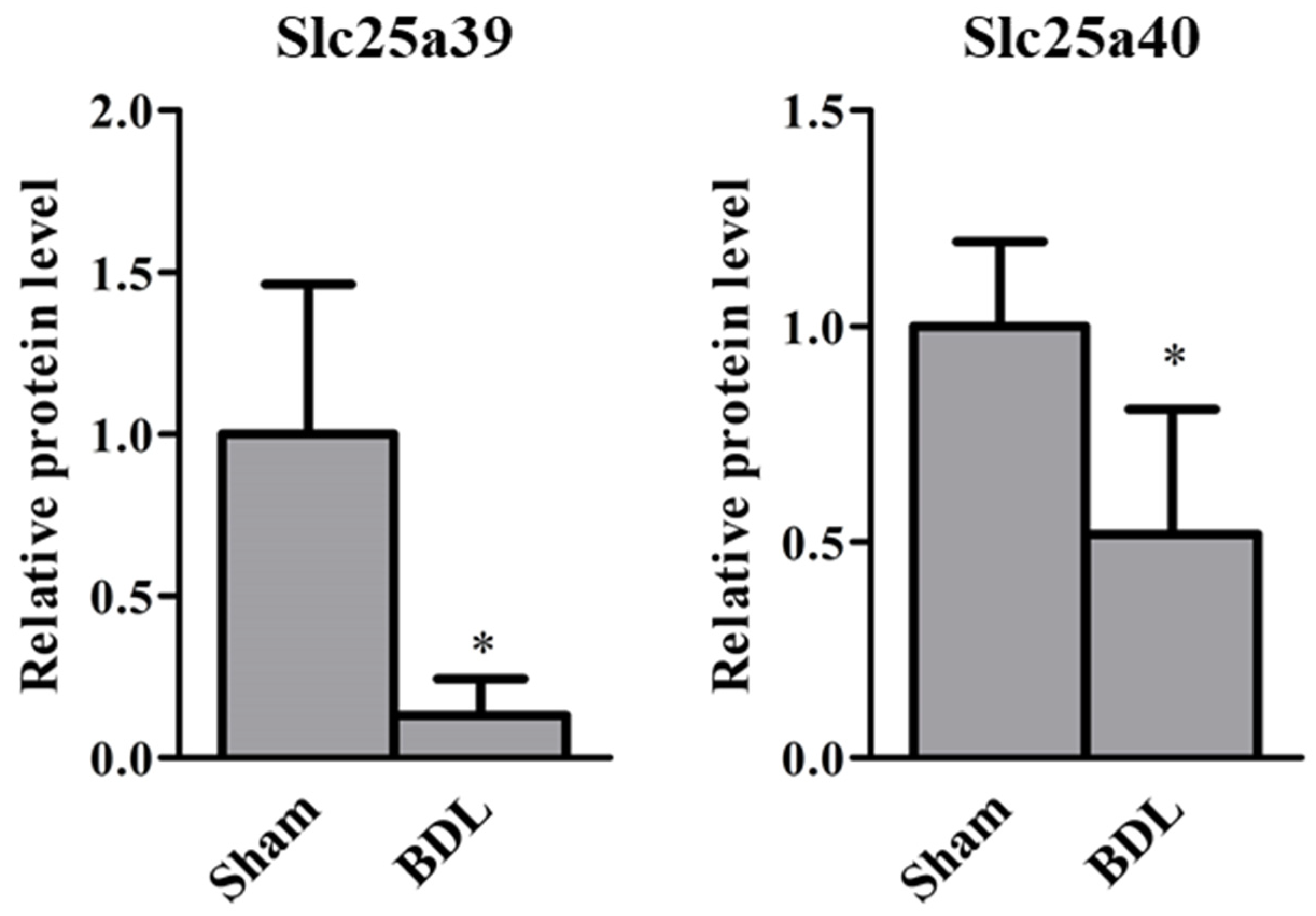
2.1. Slc25a39/40 Expression in Mice with BDL
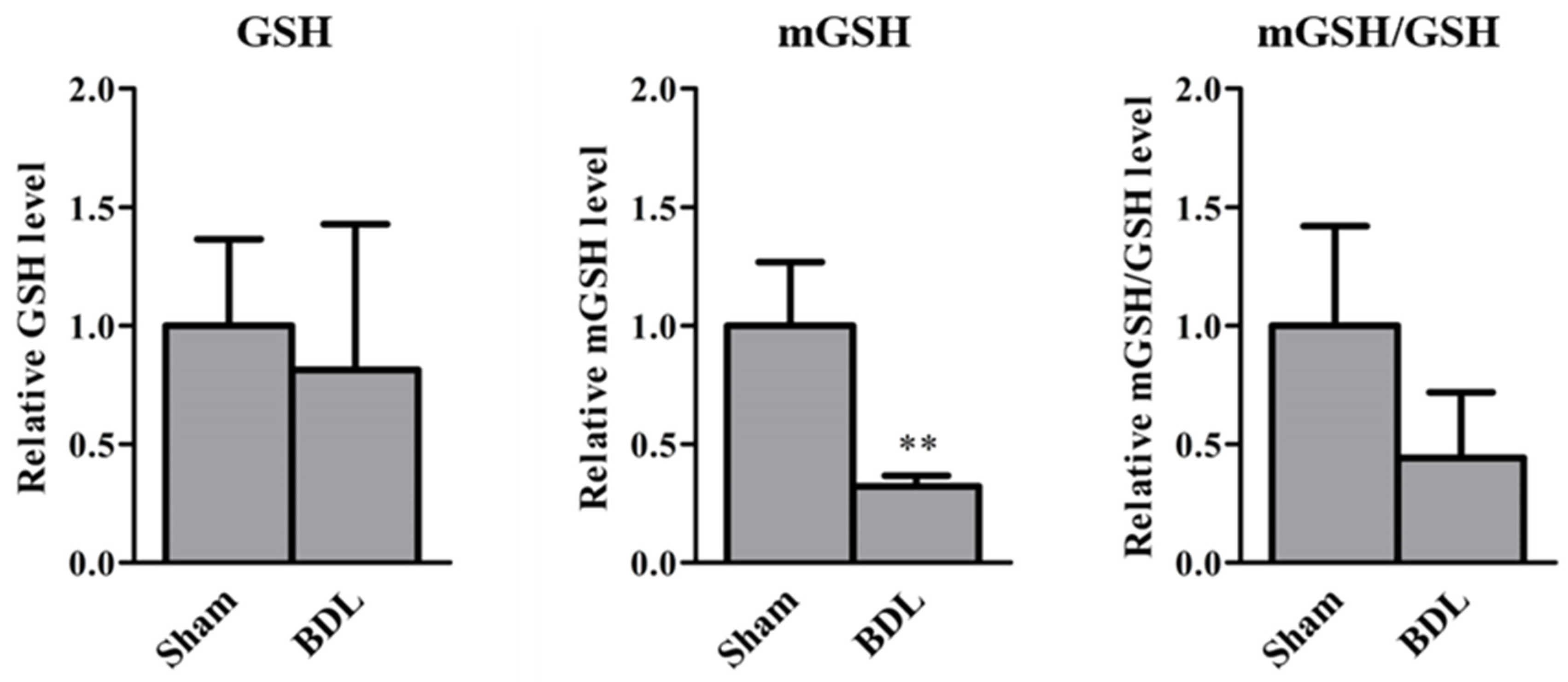
2.2. GSH Levels in Mice with BDL
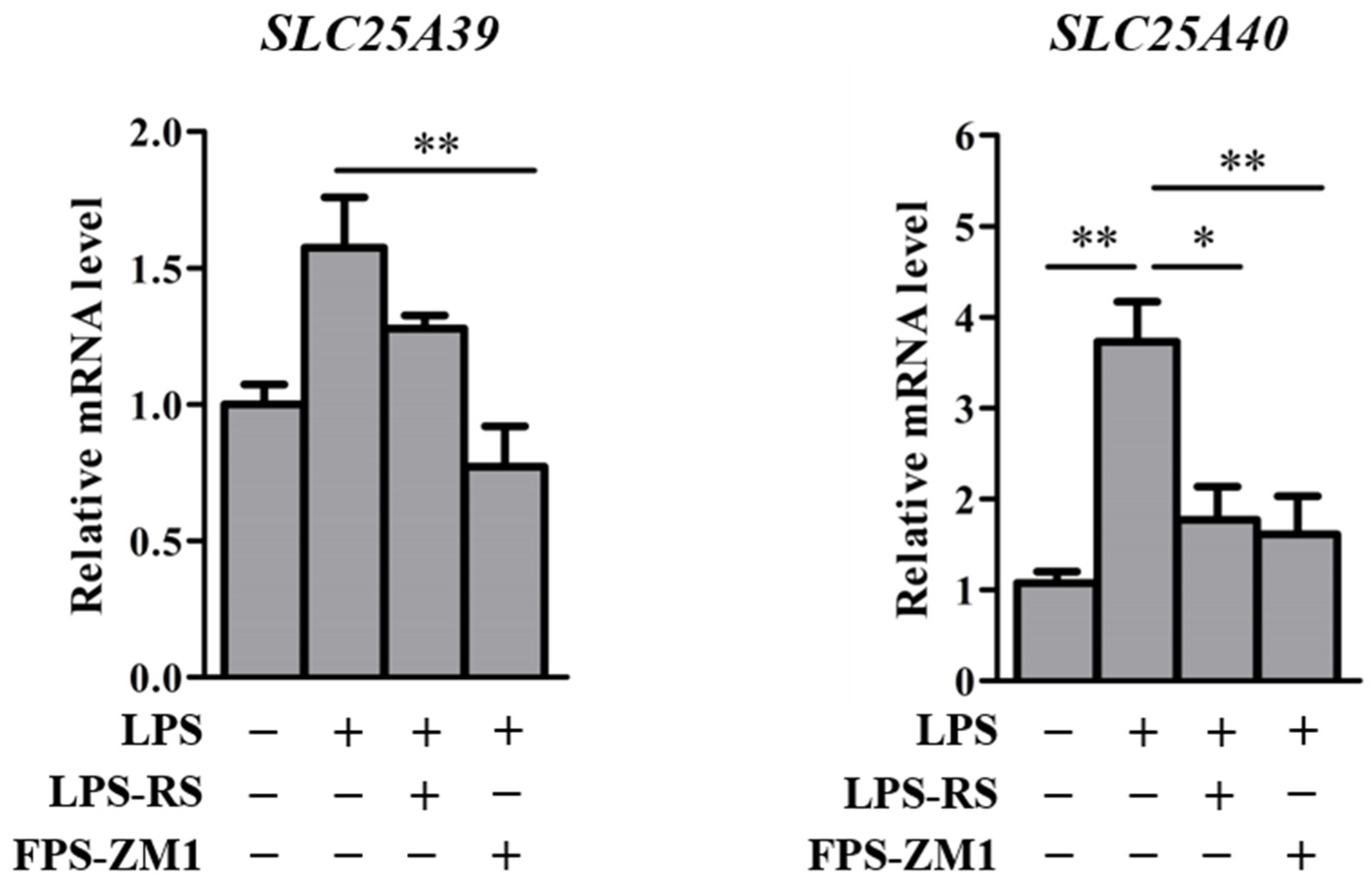
2.3. Slc25a39/40 Expression in Mice with LPS-Induced Inflammation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Treatments
4.3. Determination of mRNA Levels of Slc25a39/40 Using Real-Time Reverse Transcription-Polymerase Chain Reaction
4.4. Plasma AST, ALT, and T-BIL Levels
4.5. Isolation of Mitochondria
4.6. Estimation of Protein Levels of Slc25a39/40 by LC-MS/MS-Based Targeted Proteomics
4.7. GSH Levels
4.8. Cell Culture and Treatments
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase; |
| AST | Aspartate aminotransferase; |
| BA | Bile acid; |
| BDL | Bile duct ligation; |
| DMEM | Dulbecco’s modified Eagle’s medium; |
| EDTA | Ethylenediaminetetraacetic acid; |
| GSSG | Glutathione disulfide; |
| LC-MS/MS | Liquid chromatographic–tandem mass spectrometry; |
| LPS | Lipopolysaccharide; |
| mGSH | Mitochondrial GSH; |
| Mit | Mitochondrial fraction; |
| m/z | Mass-to-charge ratio; |
| RAGE | Receptor for advanced glycation end products; |
| T-BIL | Total bilirubin; |
| TLR | Toll-like receptor. |
References
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ruiz, C.; Morales, A.; Ballesta, A.; Rodés, J.; Kaplowitz, N.; Fernández-Checa, J.C. Effect of Chronic Ethanol Feeding on Glutathione and Functional Integrity of Mitochondria in Periportal and Perivenous Rat Hepatocytes. J. Clin. Investig. 1994, 94, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, O.W.; Meister, A. Origin and Turnover of Mitochondrial Glutathione. Proc. Natl. Acad. Sci. USA 1985, 82, 4668–4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yen, F.S.; Zhu, X.G.; Timson, R.C.; Weber, R.; Xing, C.; Liu, Y.; Allwein, B.; Luo, H.; Yeh, H.-W.; et al. SLC25A39 Is Necessary for Mitochondrial Glutathione Import in Mammalian Cells. Nature 2021, 599, 136–140. [Google Scholar] [CrossRef]
- Mitochondrial Glutathione Transport: Physiological, Pathological and Toxicological Implications—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16600197/ (accessed on 28 July 2022).
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Kaplowitz, N.; Fernández-Checa, J.C. Mitochondrial Glutathione: Features, Regulation and Role in Disease. Biochim. Biophys. Acta 2013, 1830, 3317–3328. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, G.; Tuchweber, B.; Yousef, I.M. Bile Salt Independent Flow during Bile Salt-Induced Choleresis and Cholestasis in the Rat: Role of Biliary Thiol Secretion. Liver 2000, 20, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, G.; Yousef, I.M.; Barriault, C.; Tuchweber, B. Role of Glutathione and Oxidative Stress in Phalloidin-Induced Cholestasis. J. Hepatol. 2000, 32, 550–560. [Google Scholar] [CrossRef]
- Krähenbühl, S.; Talos, C.; Lauterburg, B.H.; Reichen, J. Reduced Antioxidative Capacity in Liver Mitochondria from Bile Duct Ligated Rats. Hepatology 1995, 22, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Nicholson, C.; Wells, L.D.; Tracy, T.F. Cholestatic Liver Injury Down-Regulates Hepatic Glutathione Synthesis. J. Surg. Res. 1996, 63, 447–451. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox Status Expressed as GSH:GSSG Ratio as a Marker for Oxidative Stress in Paediatric Tumour Patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlinger, S. Bile Acids in Cholestasis: Bad for the Liver, Not so Good for the Kidney. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 392–394. [Google Scholar] [CrossRef]
- Fickert, P.; Krones, E.; Pollheimer, M.J.; Thueringer, A.; Moustafa, T.; Silbert, D.; Halilbasic, E.; Yang, M.; Jaeschke, H.; Stokman, G.; et al. Bile Acids Trigger Cholemic Nephropathy in Common Bile-Duct-Ligated Mice. Hepatology 2013, 58, 2056–2069. [Google Scholar] [CrossRef] [PubMed]
- Krones, E.; Wagner, M.; Eller, K.; Rosenkranz, A.R.; Trauner, M.; Fickert, P. Bile Acid-Induced Cholemic Nephropathy. Dig. Dis. 2015, 33, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Kim, H.; Piquette-Miller, M. Regulation of the Hepatic Multidrug Resistance Gene Expression by Endotoxin and Inflammatory Cytokines in Mice. Int. Immunopharmacol. 2001, 1, 189–199. [Google Scholar] [CrossRef]
- Hartmann, G.; Vassileva, V.; Piquette-Miller, M. Impact of Endotoxin-Induced Changes in P-Glycoprotein Expression on Disposition of Doxorubicin in Mice. Drug Metab. Dispos. 2005, 33, 820–828. [Google Scholar] [CrossRef] [Green Version]
- Kato, R.; Moriguchi, J.; Irie, T.; Nakagawa, M.; Kusukawa, Y.; Matsumura, H.; Ijiri, Y.; Tanaka, K. Effects of Lipopolysaccharide on P-Glycoprotein Expression and Activity in the Liver and Kidneys. Eur. J. Pharmacol. 2010, 636, 155–158. [Google Scholar] [CrossRef]
- Zhong, H.; Li, X.; Zhou, S.; Jiang, P.; Liu, X.; Ouyang, M.; Nie, Y.; Chen, X.; Zhang, L.; Liu, Y.; et al. Interplay between RAGE and TLR4 Regulates HMGB1-Induced Inflammation by Promoting Cell Surface Expression of RAGE and TLR4. J. Immunol. 2020, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarinejad, V.; Jamshidzadeh, A.; Khalvati, B.; Farshad, O.; Li, H.; Shi, X.; Chen, Y.; Ommati, M.M.; Heidari, R. Apoptosis-Inducing Factor Plays a Role in the Pathogenesis of Hepatic and Renal Injury during Cholestasis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hong, J.-Y.; Rockwell, C.E.; Copple, B.L.; Jaeschke, H.; Klaassen, C.D. EFFECT OF BILE DUCT LIGATION ON BILE ACID COMPOSITION IN MOUSE SERUM AND LIVER. Liver Int. 2012, 32, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleier, J.I.; Katz, S.C.; Chaudhry, U.I.; Pillarisetty, V.G.; Kingham, T.P.; Shah, A.B.; Raab, J.R.; DeMatteo, R.P. Biliary Obstruction Selectively Expands and Activates Liver Myeloid Dendritic Cells. J. Immunol. 2006, 176, 7189–7195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sener, G.; Sehirli, A.O.; Toklu, H.Z.; Yuksel, M.; Ercan, F.; Gedik, N. Erdosteine Treatment Attenuates Oxidative Stress and Fibrosis in Experimental Biliary Obstruction. Pediatr. Surg. Int. 2007, 23, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Patterson, A.M.; Cotter, P.D.; Beraza, N. Cholestasis Induced by Bile Duct Ligation Promotes Changes in the Intestinal Microbiome in Mice. Sci. Rep. 2019, 9, 12324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari, R.; Ghanbarinejad, V.; Mohammadi, H.; Ahmadi, A.; Esfandiari, A.; Azarpira, N.; Niknahad, H. Dithiothreitol Supplementation Mitigates Hepatic and Renal Injury in Bile Duct Ligated Mice: Potential Application in the Treatment of Cholestasis-Associated Complications. Biomed. Pharmacother. 2018, 99, 1022–1032. [Google Scholar] [CrossRef]
- Heidari, R.; Ghanbarinejad, V.; Mohammadi, H.; Ahmadi, A.; Ommati, M.M.; Abdoli, N.; Aghaei, F.; Esfandiari, A.; Azarpira, N.; Niknahad, H. Mitochondria Protection as a Mechanism Underlying the Hepatoprotective Effects of Glycine in Cholestatic Mice. Biomed. Pharmacother. 2018, 97, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Ommati, M.M.; Heidari, R.; Ghanbarinejad, V.; Aminian, A.; Abdoli, N.; Niknahad, H. The Neuroprotective Properties of Carnosine in a Mouse Model of Manganism Is Mediated via Mitochondria Regulating and Antioxidative Mechanisms. Nutr. Neurosci. 2020, 23, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.L. Nuclear Receptor Ligands: Rational and Effective Therapy for Chronic Cholestatic Liver Disease? Gastroenterology 2005, 129, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Gartung, C.; Ananthanarayanan, M.; Rahman, M.A.; Schuele, S.; Nundy, S.; Soroka, C.J.; Stolz, A.; Suchy, F.J.; Boyer, J.L. Down-Regulation of Expression and Function of the Rat Liver Na+/Bile Acid Cotransporter in Extrahepatic Cholestasis. Gastroenterology 1996, 110, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Azzaroli, F.; Wang, L.; Soroka, C.J.; Gigliozzi, A.; Setchell, K.D.; Kramer, W.; Boyer, J.L. Adaptive Regulation of Bile Salt Transporters in Kidney and Liver in Obstructive Cholestasis in the Rat. Gastroenterology 2001, 121, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Slitt, A.L.; Allen, K.; Morrone, J.; Aleksunes, L.M.; Chen, C.; Maher, J.M.; Manautou, J.E.; Cherrington, N.J.; Klaassen, C.D. Regulation of Transporter Expression in Mouse Liver, Kidney, and Intestine during Extrahepatic Cholestasis. Biochim. Biophys. Acta 2007, 1768, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ommati, M.M.; Farshad, O.; Azarpira, N.; Ghazanfari, E.; Niknahad, H.; Heidari, R. Silymarin Mitigates Bile Duct Obstruction-Induced Cholemic Nephropathy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Purucker, E.; Marschall, H.-U.; Geier, A.; Gartung, C.; Matern, S. Increase in Renal Glutathione in Cholestatic Liver Disease Is Due to a Direct Effect of Bile Acids. Am. J. Physiol.-Renal Physiol. 2002, 283, F1281–F1289. [Google Scholar] [CrossRef] [Green Version]
- Visintin, A.; Latz, E.; Monks, B.G.; Espevik, T.; Golenbock, D.T. Lysines 128 and 132 Enable Lipopolysaccharide Binding to MD-2, Leading to Toll-like Receptor-4 Aggregation and Signal Transduction. J. Biol. Chem. 2003, 278, 48313–48320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Harashima, A.; Saito, H.; Tsuneyama, K.; Munesue, S.; Motoyoshi, S.; Han, D.; Watanabe, T.; Asano, M.; Takasawa, S.; et al. Septic Shock Is Associated with Receptor for Advanced Glycation End Products Ligation of LPS. J. Immunol. 2011, 186, 3248–3257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawase, A.; Norikane, S.; Okada, A.; Adachi, M.; Kato, Y.; Iwaki, M. Distinct Alterations in ATP-Binding Cassette Transporter Expression in Liver, Kidney, Small Intestine, and Brain in Adjuvant-Induced Arthritic Rats. J. Pharm. Sci. 2014, 103, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.H.; Shen, S.; Wang, J.J.; He, Z.; Poon, A.; Li, J.; Qu, J.; Zhang, S.X. Comparative Proteomic Analysis of the Mitochondria-Associated ER Membrane (MAM) in a Long-Term Type 2 Diabetic Rodent Model. Sci. Rep. 2017, 7, 2062. [Google Scholar] [CrossRef] [Green Version]
- Kawase, A.; Yamashita, R.; Yoshizato, T.; Yoshikawa, M.; Shimada, H.; Iwaki, M. Stereoselective Covalent Adduct Formation of Acyl Glucuronide Metabolite of Nonsteroidal Anti-Inflammatory Drugs with UDP-Glucuronosyltransferase. Int. J. Mol. Sci. 2022, 23, 4724. [Google Scholar] [CrossRef]
- Kawase, A.; Kazaoka, A.; Yamamoto, R.; Minakata, R.; Shimada, H.; Iwaki, M. Changes in Transporters and Metabolizing Enzymes in the Livers of Rats with Bile Duct Ligation. J. Pharm. Pharm. Sci. 2019, 22, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kawase, A.; Tateishi, S.; Kazaoka, A. Profiling of Hepatic Metabolizing Enzymes and Nuclear Receptors in Rats with Adjuvant Arthritis by Targeted Proteomics. Biopharm. Drug Dispos. 2018, 39, 308–314. [Google Scholar] [CrossRef]
- Squellerio, I.; Caruso, D.; Porro, B.; Veglia, F.; Tremoli, E.; Cavalca, V. Direct Glutathione Quantification in Human Blood by LC-MS/MS: Comparison with HPLC with Electrochemical Detection. J. Pharm. Biomed. Anal. 2012, 71, 111–118. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawase, A.; Hatanaka, M.; Matsuda, N.; Shimada, H.; Iwaki, M. Slc25a39 and Slc25a40 Expression in Mice with Bile Duct Ligation or Lipopolysaccharide Treatment. Int. J. Mol. Sci. 2022, 23, 8573. https://doi.org/10.3390/ijms23158573
Kawase A, Hatanaka M, Matsuda N, Shimada H, Iwaki M. Slc25a39 and Slc25a40 Expression in Mice with Bile Duct Ligation or Lipopolysaccharide Treatment. International Journal of Molecular Sciences. 2022; 23(15):8573. https://doi.org/10.3390/ijms23158573
Chicago/Turabian StyleKawase, Atsushi, Momoko Hatanaka, Naoya Matsuda, Hiroaki Shimada, and Masahiro Iwaki. 2022. "Slc25a39 and Slc25a40 Expression in Mice with Bile Duct Ligation or Lipopolysaccharide Treatment" International Journal of Molecular Sciences 23, no. 15: 8573. https://doi.org/10.3390/ijms23158573
APA StyleKawase, A., Hatanaka, M., Matsuda, N., Shimada, H., & Iwaki, M. (2022). Slc25a39 and Slc25a40 Expression in Mice with Bile Duct Ligation or Lipopolysaccharide Treatment. International Journal of Molecular Sciences, 23(15), 8573. https://doi.org/10.3390/ijms23158573




