Abstract
Psoriasis is a systemic, chronic, immune-mediated disease that affects approximately 2–3% of the world’s population. The etiology and pathophysiology of psoriasis are still unknown, but the activation of the adaptive immune system with the main role of T-cells is key in psoriasis pathogenesis. The modulation of the local neuroendocrine system with the downregulation of pro-inflammatory and the upregulation of anti-inflammatory messengers represent a promising adjuvant treatment in psoriasis therapies. Vitamin D receptors and vitamin D-mediated signaling pathways function in the skin and are essential in maintaining the skin homeostasis. The active forms of vitamin D act as powerful immunomodulators of clinical response in psoriatic patients and represent the effective and safe adjuvant treatments for psoriasis, even when high doses of vitamin D are administered. The phototherapy of psoriasis, especially UVB-based, changes the serum level of 25(OH)D, but the correlation of 25(OH)D changes and psoriasis improvement need more clinical trials, since contradictory data have been published. Vitamin D derivatives can improve the efficacy of psoriasis phototherapy without inducing adverse side effects. The anti-psoriatic treatment could include non-calcemic CYP11A1-derived vitamin D hydroxyderivatives that would act on the VDR or as inverse agonists on RORs or activate alternative nuclear receptors including AhR and LXRs. In conclusion, vitamin D signaling can play an important role in the natural history of psoriasis. Selective targeting of proper nuclear receptors could represent potential treatment options in psoriasis.
1. Psoriasis: An Overview of the Clinical Problem
Psoriasis is a systemic, chronic, immune-mediated disease that is characterized by raised patches on the skin that affects approximately 2–3% of the world’s population [1]. The most common type of psoriasis is plaque psoriasis, which accounts for about 80–90% of cases. The other types include pustular psoriasis, which is more common in adults; guttate psoriasis, which is common in children; inverse psoriasis; and erythrodermic psoriasis. Psoriatic lesions are usually found on the scalp, skin folds, hands, feet, nails, and genitals [1]. Psoriasis usually manifests with cutaneous symptoms such as red, dry skin with raised, inflamed patches, silver scales or plaques, itch, thick, pitted nails, and swelling [1,2]. The psoriatic plaques are formed as an effect of epidermal hyperplasia resulting from enhanced proliferation and disturbed differentiation of keratinocytes [3]. These manifestations are related to the inflammatory process since psoriasis is an immune-mediated disease that is caused by the dysfunction of the immune system that resulted in inflammation [4,5,6]. The etiology and pathophysiology of psoriasis are still unknown, but the activation of the adaptive immune system with the main role of T-cells is key in psoriasis pathogenesis [2]. It is suggested that the impaired balance between T helper Type 1 (Th1) and Type 2 (Th2) cells, as well as cytokine production are the top causative factors of psoriasis [2,4,7,8,9]. In psoriatic patients, there is a shift towards the Th1 phenotype, which is characterized by the increased expression of IL-2, IFN-gamma, IL-12, T-bet [7,8], and the attenuation of the Th2 phenotype, with decreased expression of GATA3 and IL-4 [8] was found. In addition, the increased expression of IL-23 resulted in the increased level of Th17 and Th22 lymphocytes and their cytokines (IL-6, IL-20, IL-17, and IL-22) [2,9,10,11]. The production of Th17 cytokines in psoriasis is also related to the impairment function of regulatory T-cells (Tregs) [9,12,13,14,15]. Apart from T-cells, the other cells that are linked to psoriasis pathogenesis are the following: innate lymphoid cells, dendritic cells, mast cells, monocytes and macrophages, neutrophils, natural killers, keratinocytes, and many others (reviewed in [3,16,17]. Some data indicate dendritic cells activation that is mediated by peptide LL-37 and self-DNA, resulting in interferon production as a trigger for psoriasis pathogenesis [18,19]. Dendritic cells also promote the Th1 phenotype and the production of Th1 cytokines [19]. Figure 1 presents the major effector cells and signaling pathways in the immunopathogenesis of psoriasis.
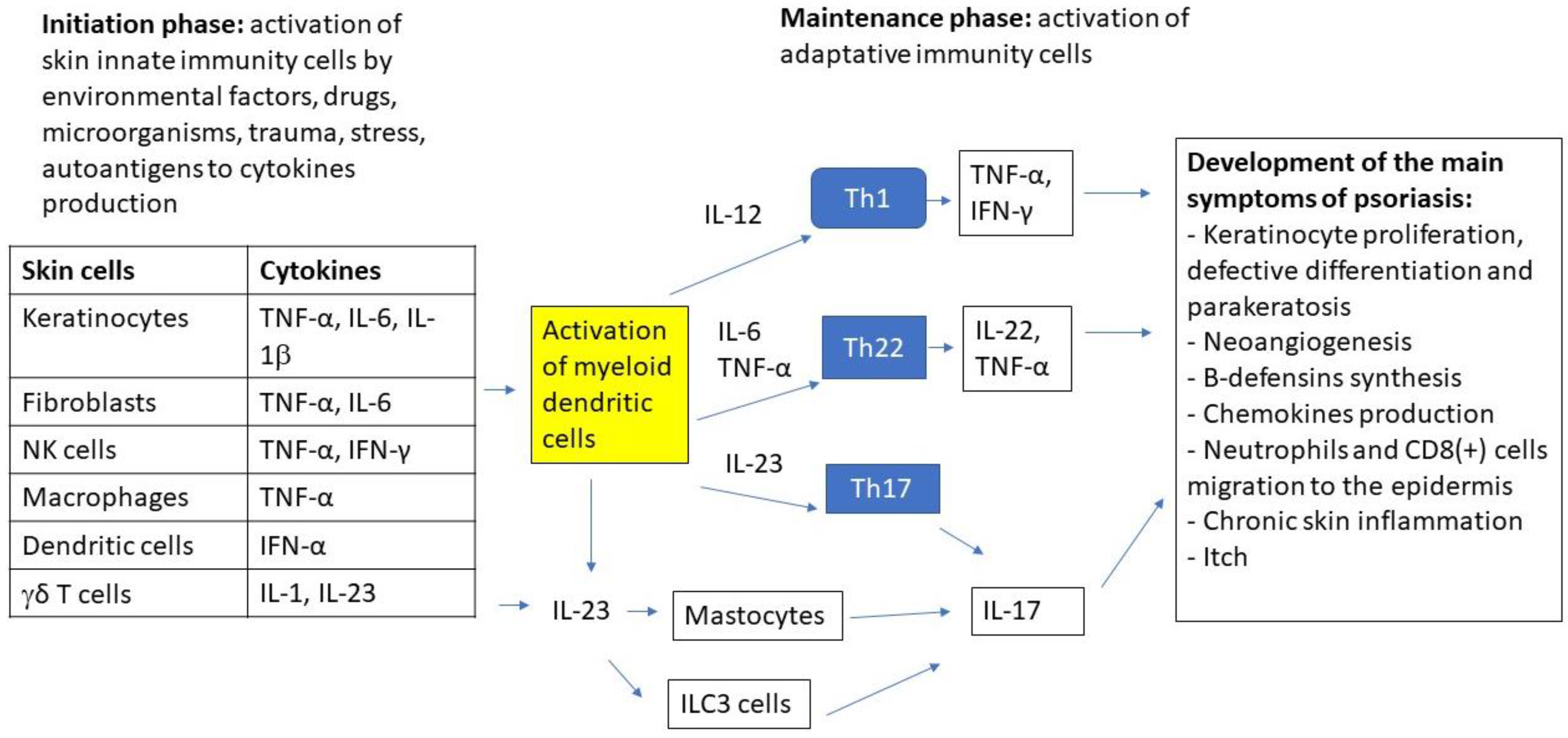
Figure 1.
The major effector cells and signaling pathways in the immunopathogenesis of psoriasis.
The immunopathogenesis of psoriasis involves a complex inflammatory cascade, which is initially triggered by innate immune cells (keratinocytes, dendritic cells, NKT cells, macrophages, fibroblasts, γδ T-cells) that are activated by external (trauma, UV, microorganisms, drugs, smoking, diet and obesity, etc.) or internal factors (stress, autoantigens, DNA/RNA AMP complex, etc.) in genetically predisposed individuals. Cytokines that are produced by innate cells activate myeloid dendritic cells to increase the production of cytokines that are involved in the differentiation of lymphocytes to main adaptive immune cells: Th1, Th22, and Th17 which play the central role in the disease pathogenesis. Cytokines that are produced by these cells, which include: TNFα, IL-22, and IL-17A/F lead to keratinocyte proliferation, neoangiogenesis, chemokines production, neutrophils and CD8+ cells migration to the epidermis, and chronic inflammatory process. For this reason, biologic drugs targeting ILs such as IL 17, 23, and TNFα are the mainstay in the management of severe psoriasis [20,21]
2. Psoriasis and the Local Endocrine Regulators
The skin acts as a barrier and the organ that is involved in the coordination of the responses to environmental stimuli producing neurotransmitters, neuropeptides, and hormones [22], including the hypothalamic-pituitary-adrenal (HPA) axis [23,24,25,26]. The disturbances in steroidogenesis and the feedback of cutaneous neurohormones contributes to inflammation, as well as psoriasis development [27,28,29]. The corticotropin-releasing hormone (CRH) is overexpressed in areas of inflammation, as well as in peripheral tissues including the skin [30], thus its dysregulation is an important factor in psoriasis pathogenesis [31,32,33,34,35]. Correspondingly, aberrant CRH-receptor 1 is linked to psoriasis [28,36,37,38]. The increase of CRH in psoriasis can be accompanied by the up-regulation of proopiomelanocortin (POMC) [39], melanin-concentrating hormone receptor (MCHR1), and melanocortin receptors 2, 3 and 4 [40].
The glucocorticoids maintain skin barrier integrity and controls the inflammatory response by inhibiting the expression of IL-4 and IL-5 [41,42,43]. Glucocorticoid receptor level is decreased, and its activity is impaired in psoriasis [43]. The steroidogenic acute regulatory protein (StAR) that is expressed among other by keratinocytes [44,45], is involved in steroidogenesis and acute steroidogenic response [46]. In psoriasis, the expression of StAR is lowered or undetected [47,48], underlying the role of glucocorticoids in inflammatory skin diseases. In psoriasis several enzymes that are active in steroid synthesis are decreased: CYP11A1 (involved in cholesterol to pregnenolone conversion), CYP17 (involved in pregnenolone to cortisol conversion), 11βHSD1 (activator and inactivator of glucocorticoids), and 11βHSD2 (inactivator of cortisol by converting to its ketone form) [43,45,49]. It must be noted that CYP11A1 is expressed in the skin [44] and immune cells [36] and cortisol and corticosterone are produced by skin cells under tight regulatory mechanisms [26,50,51,52]. The deregulation of this process can lead to inflammatory diseases including psoriasis [28,33].
Since inflammation is the main process in the pathogenesis and development of psoriasis, it is recognized that other immune-mediated inflammatory diseases are common psoriasis comorbidities, e.g., psoriatic arthritis, cardiovascular disease, metabolic syndrome, inflammatory bowel diseases [2,4]. It should be noted that psoriatic patients are more likely to develop severe vascular events such as myocardial infarction and stroke (up to 50%) than the general population [53,54]. In addition, among psoriatic patients, different endotypes with different risks, severity, and treatment options are distinguished based on hypertension, red cell distribution width, and the mean platelet volume [55,56]. Thus, the modulation of the local neuroendocrine system with the downregulation of pro-inflammatory and upregulation of anti-inflammatory messengers represent a promising adjuvant treatment in psoriasis therapies [28].
3. Vitamin D Endocrine System
Classical and Non-Classical Activation Pathways
The exposure of keratinocytes to ultraviolet B radiation initiates the 7-dehydrocholesterol (7DHC) photochemical transformation to a pro-hormone vitamin D3 [57,58,59]. Its transformation activation requires two-steps: hydroxylations at C25 (by CYP2R1 and CYP27A1) and C1α (by CYP27B1) to produce 1,25(OH)2D3. The cutaneous synthesis supplies more than 90% of its body’s requirement [57,59,60]. 1,25(OH)2D3, in addition to regulating calcium homeostasis, has important pleiotropic effects affecting almost all body functions. This action is mediated through interactions with vitamin D receptor (VDR), belonging to a subfamily of nuclear receptors [57,58,59,61,62,63]. VDR heterodimerizes with the retinoid X receptor (RXR) and functions as a ligand-activated transcription factor, after binding to the promoter regions of VDR responsive element (VDRE) to influence the expression of responsive genes [58,63,64]. Not only the expression of the VDR receptor determines the responsiveness of the cells to vitamin D, but also its polymorphisms. It was shown that the F and T alleles of Fok1 and Taq1 have been associated with increased VDR activity [65]. There is growing evidence that induction of transcriptional activity of VDR by 1,25(OH)2D3 does not fully explain the complexity and variety of cellular responses to this multipotent hormone. Thus, so called alternative, non-genomic response has been described. It was suggested that this rapid response requires a membrane receptor for 1,25(OH)2D3 and the subsequent activation of secondary messengers such as cAMP or calcium (recently reviewed in: [66]). Protein disulfide isomerase (PDIA3), also known as pER57 or 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) is the most studied candidate for membrane vitamin D receptor, although a detailed mechanism of interaction between 1,25(OH)2D3 and PDIA3 is not fully understood [66,67,68]. Recent studies also provided evidence that mitochondria could be a direct target of 1,25(OH)2D3 [69,70]. This observation may support previous studies showing the protection of mitochondria by 1,25(OH)2D3 by the modulation of the levels of oxidative stress (e.g., mitochondrial membrane potential) and the expression of genes thata re involved in response to reactive oxygen species [71,72,73]. Interestingly, it seems that mitochondrial localization of VDR protect mitochondrial from oxidative and nitrosative stress [72]. On the other hand, in vitro results suggested that the mitoprotective effects may depend on the concentration of active analogs of vitamin D, the time of incubation, or are cell-type-specific [71,74]. The mitoprotective effect of vitamin D and its analogs was also observed in skin cells that were subjected to ultraviolet light [70,73,75,76,77,78,79]. Interestingly, the mitochondria could also be the targets for anticancer and/or inflammatory activities of vitamin D and its analogs as well as derivatives of lumisterol or other related steroidal analogs [76]. The malfunction of mitochondria and the excessive production of ROS contributes to inflammation that is characteristic for psoriasis [80]. Furthermore, the impairment of the mitochondrial-induced apoptotic pathway may also result in hyperproliferation of keratinocytes [81]. Thus, it seems that the direct, nongenomic impact of 1,25(OH)2D3 on cellular processes including mitochondrial function may contribute to its anti- psoriatic activities of this powerful hormone [66].
Our previous study showed that vitamin D in addition to activation by 25 and 1-hydroxylations, can also be activated by the rate limiting enzyme of steroidogenesis, CYP11A1 [82,83,84,85], with the generation of 20(OH)D3, as the first and main product of this pathway. 20(OHD)3 can be further hydroxylated by other enzymes together with downstream metabolites [83,86,87,88]. This pathway functions in vivo in humans and animals and can act on a local and systemic level [82,83,86,89]. 20(OH)D3 and its metabolites without OH at C1α can act as a biased agonist on VDR as indicated by the lack of calcemic effects and the poor activation of CYP24A1 [86,90,91], and by studies on ligand-induced VDR translocation to nucleus and molecular modeling [86,92,93,94] and crystallography using the ligand binding domain of the VDR [95,96]. In addition, novel pathways of lumisterol [97,98] and tachysterol [99] activation have been discovered.
Recently, we also showed that 20(OH)D3, 20,23(OH)2D3 and their metabolites, that were generated by alternative pathway, can act as inverse agonists on retinoic acid-related orphan receptors, RORα and RORγ [100,101,102], which belong to the ROR subfamily of nuclear receptors, that play a crucial role in the variety of physiological processes, including immune functions [103,104,105]. In addition, lumisterol hydroxyderivatives act as inverse agonists on RORs [97]. Importantly, aryl hydrocarbon (AhR) was identified as an alternative receptor for vitamin D hydroxyderivatives [102,106,107]. In addition, hydroxyderivatives of vitamin D and lumisterol compounds act as ligands on liver X receptors (LXR)α and β [102,108]. Thus, there is more than one bioactive form of vitamin D and several additional to the VDR nuclear receptors that are activated by these compounds [36]. In addition, it has been documented that lumisterol, a photoderivative of vitamin D3, can be activated to the biologically active hydroxyderivatives that would act on LXRs and RORs to exert their phenotypic effect [97,105,108,109]. Vitamin D and lumisterol hydroxyderivatives can also interact with SARS-CoV-2 replication machinery enzymes [110] and angiotensin-converting enzyme 2 (ACE2) and TMPRSS2 [111] and their protective role in COVID-19 has been discussed [112].
4. Vitamin D and Epidermal Keratinocytes
Keratinocytes express VDR and RORs and can produce and metabolize 1,25(OH)2D3 [101,113]. Skin cells also express CYP enzymes that metabolize vitamin D3 pro-hormone to its biologically active form [45,75,114,115,116,117] (Figure 2). VDR expression and VDR-mediated signaling pathways in the skin are essential in maintaining the skin homeostasis [118,119,120,121]. The expression of markers of differentiation (involucrin, profilaggrin, and loricrin) are decreased the interfollicular epidermis and the hair follicle in VDR knockout mice model [121].
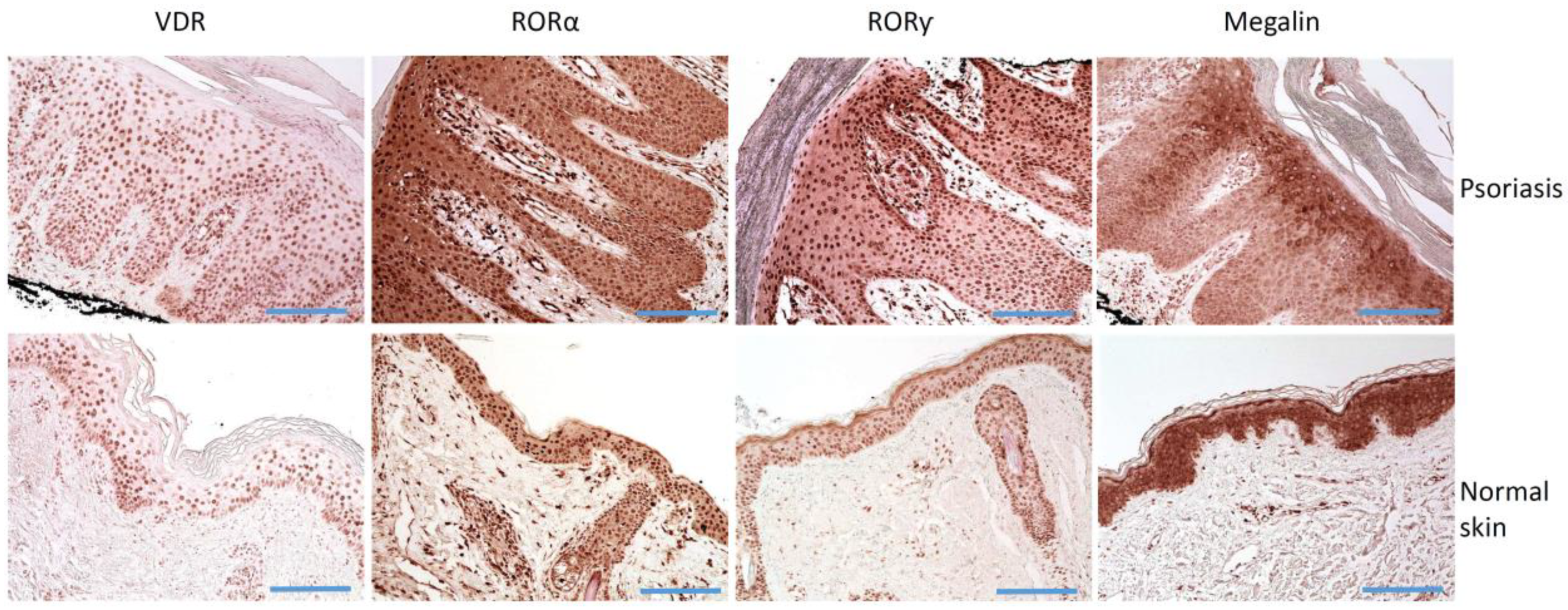
Figure 2.
Immunostaining of VDR, RORα and RORγ, and LRP2/megalin in psoriasis and normal skin. Histology of lesional skin shows skin with regular psoriasiform epidermal hyperplasia, prominent dilated vessels with an edematous dermal papillae, mounds of parakeratosis containing neutrophils, and a superficial perivascular lymphocytic infiltrate. Scale bars = 100 μm.
Hosomi et al. [122] firstly reported the stimulatory effects of 1,25(OH)2D3 on keratinocyte differentiation by inhibiting DNA synthesis, thus decreasing the number of cells, increasing the density and the size, and differentiation into squamous and enucleated cells, and the stimulation of the formation of a cornified envelope. These findings were confirmed by others [123,124,125]. The effects of VDR-mediated pathways depend on coactivators and corepressors [126,127]. Vitamin D, acting through VDR, and DRIP205, SRC2, and SRC3 coactivators can stimulate the keratinocytes differentiation markers: expression of involucrin, loricrin, filaggrin, keratins, and transglutaminase activity [128]. Bikle et al. [127] reported that the main coactivators are the vitamin D interacting protein (DRIP) complex that is involved mainly in the proliferation of keratinocyte and the steroid receptor coactivator (SRC) complexes that are involved in keratinocytes differentiation. They found also that DRIP205 plays a role in the regulation of β-catenin pathways, including cyclin D1 and Gli1 expression, and SRC3 can regulate lipid synthesis, permeability barrier formation that is related to differentiation. The effects of vitamin D on keratinocytes could be dependent on other culture conditions. Gniadecki et al. reported that in cultures of 1,25(OH)2D3 at the concentration from 10−11 to 10−6 M and 0.15 mM calcium in the absence or with low levels (0.1 ng/ml) of epidermal growth factor, the keratinocytes cell cycle was blocked in the late G1 phase, while the culture of keratinocytes with 1,25(OH)2D3 at the concentration of 10−11 to 10−9 M and high extracellular calcium concentration (1.8 mM) stimulated cell growth was observed (increasing the proportion of cells entering the S phase) [129].
20(OH)D3 inhibits proliferation, causes G1/0 and G2/M arrest, and stimulates differentiation of keratinocytes [125] and inhibits NFκβ [130]. Correspondingly, 20S(OH)D3, acts through VDR (stimulates its expression), inhibits growth, inhibits DNA synthesis. It also stimulates the expression of involucrin—differentiation marker for keratinocytes [125,131]. Other CYP11A1 vitamin D derivatives show similar anti-proliferative and pro-differentiation properties [88,93,102,132,133,134,135]. Similarly, vitamin D derivatives (resulted from CYP11A1 activity) protect from UV-induced DNA damages by the activation of Nrf2 and p53 defense mechanisms [73,77,134,136] and have shown anti-tumor activity against epidermal cancers [75,137]. Vitamin D derivatives have also shown antiproliferative activity on melanocytes [138,139] and fibroblasts with anti-fibrogenic actions [140,141,142,143], being dependent on RORγ [144]. In addition, vitamin D derivatives increased the expression of hypothalamic-pituitary-adrenal axis neuropeptides: CRF, urocortins and POMC, and their receptors, CRFR1, CRFR2, MC1R, MC2R, MC3R, and MC4R human epidermal keratinocytes [145]. Thus, vitamin D derivatives, VDR, and its coactivators are important for the epidermis differentiation and maintenance. Finally, 20(OH)D3 has recently been shown to have therapeutic effects in in vivo models of rheumatoid arthritis (RA) [146,147].
5. The Active Forms of Vitamin D Act as Powerful Immunomodulators
The active forms of vitamin D, acting through VDR, modulates the maturation, activity, and functions of monocytes, macrophages, T- and B-cells, and dendritic cells (DCs) [148]. In general, vitamin D promotes the innate immune responses by enhancing phagocytic functions of immune cells, while inhibiting the adaptive immune system. The activation of monocytes and macrophages by biologically active vitamin D derivatives resulted in the increased production of cathelicidin antimicrobial peptide (CAMP) with its processing to LL 37. In dendritic cells presenting antigens, vitamin D decreases the maturation, expression of MHC Class II molecules, co-stimulatory molecules (CD40, CD80, and CD86) and IL-12, and increases IL-10 production (reviewed in [149]). Vitamin D decreases the development of human natural killer (NK) cells and inhibits the cytotoxicity and cytokine production by developed NK cells, while in hematopoietic stem cells stimulated the expression of monocytes markers (C/EBPα and CD14) [150]. Vitamin D acts as an immunosuppressive molecule decreasing the proliferation and functions of T lymphocytes that is mediated by inhibiting IL-2 and IFNγ production [151,152]. Vitamin D inhibits polarization towards Th1 cells [153,154] and pro-inflammatory Th1-related cytokines by T lymphocytes, as IL-2, IFNγ, and TNFα [153,155,156,157,158,159]. The development of Th2 cells of Th lymphocytes, strong polarization toward a Th2 profile is also enhanced by vitamin D in the IL-4-depended pathways. The neutralization of IL-4 abolishes vitamin D3-induced polarization of Th2 independently of IFN-γ [160]. In addition, vitamin D3 enhances the expression of Th2-specific transcription factors, GATA-3 and c-maf, in developing Th cells [160]. Vitamin D promotes the immunosuppressive response by enhancing the activity of CD4+ CD25+, mostly expressing FoxP3, without the changing the number of CD4CD25Foxp3 cells [161]. Similarly, in a colitis mouse model, vitamin D suppressed the immune response (downregulates IFNƴ, TNFα, MPO activity, and IL-1β), downregulated the expression of T-bet (Th1 transcription factor), and up-regulated the expression of GATA3 and IL-4 [162].
Not only does the classical vitamin D pathway show anti-inflammatory properties, but also vitamin D derivatives suppress inflammatory responses [101,133,134,146,147]. Chaiprasongsuk and co-workers reported that CYP11A1-derived vitamin D3-hydroxyderivatives, as 20(OH)D3, 1, 20(OH)2D3, 20,23(OH)2D3, and 1, 20,23(OH)3D3 modulated the expression of inflammation-related genes, downregulating 11 out of 16 genes, including NFkB p65 (Rel A), and up-regulating 5 out of 16 genes, including NFkB p50, and IkB-α [133,134]. The key factor that is involved in the anti-inflammatory action of vitamin D derivatives is NFkB. Janjetovic at al revealed that in keratinocytes 20(OH)D3 decreases NF-κB activity by increasing IκBα levels [130], similarly to 20,23(OH)2D3 [132]. 20(OH)D3 also inhibits the production of pro-inflammatory cytokines TNFα and IL-6 [133]. Furthermore, in DBA/1 Lac J CIA model of rheumatoid arthritis, the inhibition of Th1 and Th17-related cytokines production by CYP11A1-derived secosteroids was found [133,147].
The inverse correlation between vitamin D and the pathogenesis of autoimmune diseases, including psoriasis has been published [163]. Vitamin D regulates the function of the innate and adaptive immune response, thus representing a potential protectant and therapeutic for psoriasis. The effects of vitamin D on the immune system in psoriasis are complex. For example, Vitamin D promotes differentiation of naïve T-cell differentiation into T regulatory cells, thus enhancing the production of anti-inflammatory cytokines (TGF-β, IL-4, and IL-10), and suppressing the production of pro-inflammatory cytokines (TNFα, INFƴ, IL-2, IL-17A, and IL-21) (reviewed in [148]). Calcipotriol decreased the frequency of CD8+ IL-17+ T-cells in psoriatic lesions [164]. The activity of DCs, DCs-mediated induction of T-cell proliferation, Th1 cytokine IFNγ production is suppressed by vitamin D [165]. Vitamin D inhibited the IL_17-induced expression of IL-1Ra, IL-36α, IL-36β, and IL-36γ, and the TNF-α-induced expression of IL-1Ra, IL-36Ra, IL-36α, IL-36γ, and beta-defensin 2 (HBD2) in human keratinocytes [166]. Besides, in psoriasis calcipotriol decreased the Th17 cytokine-mediated pro-inflammatory S100 psoriasin (S100A7) and koebnerisin (S100A15) [167] and HBD2 and HBD3, IL-17A, IL-17F, and IL-8 production. The inhibition of IL-17A induced-HBD2 expression was mediated by increasing IkappaB-α protein and the inhibition of NF-κB signaling, while VDR and MEK/ERK signaling pathways were activated and involved in the induction of cathelicidin [168]. Interestingly, vitamin D also stimulates the expression of IL-33 and its receptor ST2 [169] and IL-33 was show to alleviate Th17-mediated psoriatic inflammation [170]. Thus, the anti-inflammatory activity of vitamin D is an important factor that is useful in the pathogenesis and management of psoriasis.
6. Vitamin D and Psoriasis
6.1. Vitamin D Serum Level in Psoriatic Patients
The results of several epidemiologic studies have identified the correlation between the vitamin D serum level and the likelihood of some diseases development or progression in patients with low or deficient levels of vitamin D [112,171,172,173,174]. Similar studies have been published for psoriasis [175,176,177,178]. Morimoto and co-workers showed that the 1,25-dihydroxyvitamin D (1,25(OH)2D, but not 25-dihydroxyvitamin D (25(OH)D), was inversely correlated to area-severity index and the severity index in psoriasis patients. In these patients, the level of vitamin D was in the normal range [179]. Tajjour et al. reported decreased 25(OH)D levels in psoriasis patients and a negative correlation with the severity of the disease [180]. Bergler-Czop and Brzezinska-Wcislo reported a lower level of 25(OH)D in the psoriasis group than in the control group, with a deficient level in psoriasis and insufficient level in the control group [181]. Furthermore, the level of 25(OH)D was negatively correlated to PASI score and the duration of psoriasis [181,182]. The lower level of 25(OH)D was also found in case-control studies [183,184,185]. In addition, Filoni et al. also found a reduced level than in the control group and the correlation between vitamin D levels and psoriasis duration [175]. Similarly, Grassi and co-workers in a cross-sectional study observed a lower free and total vitamin D serum level in chronic plaque psoriasis patients than in the controls [176]. The relationship between vitamin D and psoriasis was also confirmed in a meta-analysis [177]. However, still the nature of this correlation is unclear and further studies are needed to elucidate its role in the pathogenesis of psoriasis, and still it is not known if low vitamin D level is the causative factor for psoriasis or the effect of the disease. On the contrary, some reports showed no differences in the 25(OH)D serum level between psoriasis and the control group [186]. Mattozzi et al. found the positive correlation between the vitamin D serum level and Tregs, and suggested that a decreased level of vitamin D may promote the activity of Th1, Th17, and Th22 [14]. They also found a negative correlation between PASI score and vitamin D level, but only Tregs were significantly related with vitamin D in multiple regression analysis [14]. The decreased level of vitamin D is negatively correlated to inflammatory activation marker—C-reactive protein [183]. A meta-analysis of 10 published reports and 571 psoriatic patients 496 controls confirmed the reduced 25(OH)D level in the disease group and negative correlation between circulating 25(OH)D levels and PASI score [187]. Vitamin D deficiency has been suggested as one of the environmental factors that is involved in psoriasis as immune-mediated disorder. Some studies have confirmed that vitamin D deficiency can be found in psoriatic patients being associated with the severity of a disease.
6.2. On the Link between UVB Phototherapy, Serum 25(OH)D Levels and Psoriasis Natural History
The synthesis of vitamin D in the skin starts with the conversion of 7-dehydrocholesterol to previtamin D3 after the absorption of UVB. The phototherapies of psoriasis are based on UVB (290–320 nm), narrowband UVB (NB-UVB) (311 nm), excimer laser (308 nm), UVA1 (340–400 nm), psoralen and UVA (PUVA, 320–400 nm), and others (reviewed in [188]. The studies revealed that NB-UVB therapy has an impact on the systemic serum level of vitamin D in psoriasis patients. In psoriasis, patients that were treated with NB-UVB, the increase of the vitamin D serum level from insufficient to normal range was found, but no relationship with PASI score and/or SCORAD improvement [189,190,191]. Similarly, Ryan et al. noted that the serum 25(OH)D level increase from median of 23 ng/mL at to 51 ng/mL at the end of NB-UVB, with no correlation with treatment response [192]. Ala-Houhala et al. published the data that were related to NB-UVB and oral supplementation of cholecalciferol, 20 μg daily. The psoriasis patients and healthy controls increased 25(OH)D level found similar results [193]. NB-UVB exposure did not change the expression of CYP27A1 and CYP27B1 in psoriasis patients, while in the healthy controls its expression decreased. In healthy controls the expression cathelidicin decreased, HBD2 increased slightly, while in psoriasis patients cathelidicin expression did not changed, while HBD2 expression decreased [193]. The increase of 25(OH)D after UVB-based psoriasis therapy is also observed after UVA/NB-UVB treatment [194,195]. A similar trend was found also for 25(OH)D and for broadband UVB (BB-UVB) therapy, with BB-UVB showing strongest effect [196]. It should be noted that UVA1 therapy decreased the 25(OH)D serum level from 21.9 to 19.0 ng/mL [194]. The increase of the vitamin D serum level after NB-UVB treatment is accompanied by changes in athe ntimicrobial peptide and cytokine expression (increasing expression of cathelicidin and decreasing levels of human beta-defensin 2) [190], but these changes are related to the season of the irradiation [197]. Thus, the balance between vitamin D and the expression of antimicrobial peptides could be involved in the therapeutic effects of NB-UVB. On the contrary, Vandicas et al. [198] noticed a higher level of 25(OH)D and vitamin D binding protein (VDBP), acting as transporter and reservoir for vitamin D and its metabolites [199,200] in psoriasis patients. In addition, as in other studies, 25(OH)D increased after UVB treatment, while VDBPs were not changed and did not correlate with 25(OH)D levels. Thus, the authors suggested that VDBPs could be a marker of systemic inflammation. However, immunosuppressive effects of the UVB can also be secondary to the activation of the central [201,202,203,204] or local neuroendocrine networks [26,28,35,205,206].
6.3. Local Vitamin D Endocrine System in Psoriasis
Vitamin D acts mainly through VDR [101,119,207], and the disturbances of its expression have been observed in several diseases (for example: [208,209,210,211]). The expression of vitamin D receptors have been found in psoriatic cells (Figure 2) [212]. Since vitamin D can regulate the proliferation and growth of keratinocytes, the impairment of VDR expression in epidermal skin cells could be involved in the pathogenesis of psoriasis [119,213,214,215]. The first reports did not show the changes in vitamin D system and reported comparable VDR levels and receptor binding to DNA in psoriatic and normal skin [216]. Milde et al. reported no differences in VDR expression between normal and non-lesional skin, but found stronger VDR expression in psoriasis when compared to non-lesional skin [217]. A very recent study showed that VDR is expressed in psoriatic skin and shows mainly strong expression, especially in the basal layer (Chandra, Roesyanto-Mahadi et al., 2020). On the other hand, Kim et al. [218] found reduced VDR expression in psoriasis and perilesional skin than in normal skin. They also reported the negative correlation between Toll-like receptor 2 (TLR2) and VDR expression in psoriasis, a negative correlation between TLR2 and VDR expression in the psoriasis skin of vitamin D-deficient groups, but a positive correlation in psoriasis skin of a vitamin D-sufficient group [218]. The authors concluded that psoriasis patients, according to vitamin D serum level, could be treated differently with therapies that modulated the TLR-VDR pathways. Similarly, Visconti et al. also observed the reduced VDR expression in psoriasis, with the preserved expression in deeper layers of the epidermis [219]. Contradictory data on VDR expression could result from methodological issues and using of different anti-VDR antibodies. The pathogenic effects of the potential disturbed expression of VDR in keratinocytes could result in keratinocytes adhesion. Visconti et al. found significantly reduced expression of occludin and claudin 1 (proteins forming tight junctions) than in normal skin. Furthermore, the percentage of claudin-1- and zonulin-1-positive cells (proteins forming tight junctions) correlated to the percentage of VDR-positive cells [219]. The authors suggested that VDR expression in the skin is essential to the preservation of skin integrity and homeostasis by forming tight junctions [219].
Not only the changes of the expression of VDR are probably linked to psoriasis but also VDR polymorphism. A meta-analysis of 11 studies revealed that FokI and ApaI VDR polymorphisms are not linked to psoriasis risk, but the BsmI B variant shows borderline association [220]. For Caucasians, the TaqI t variants were allied with reduced psoriasis risk [220]. Similarly, a meta-analysis of 16 studies showed that VDR TaqI TT variant in Caucasians but not in Asians is related to a higher risk of psoriasis, but VDR ApaI, BsmI,t or FokI polymorphisms have no association with the disease [221]. On the other hand, A-1012G VDR promoter polymorphism and Fok1 were identified as related to the susceptibility to non-familial psoriasis [65]. A recent study showed that the TaaI/Cdx-2 GG genotype, related to regulation of IL-17 and IL-23 expression, are more frequent in psoriasis patients [222].
The novel vitamin D derivatives, 20(OH)D3 and 20,23(OH)2D3, can act as the inverse agonists on RORα and RORγ, which are expressed in human skin, keratinocytes, fibroblasts, melanocytes, and others [101,135]. Previously, we reported the elevated expression of RORγ in lymphocytes in psoriasis skin [212]. RORγ is a key factor that is involved in the differentiation of lymphocytes into IL-17-producing Th17 cells [223]. In addition, RORα and RORγ can regulate IL-17 expression [103,224]. 1,25(OH)2D3, 20(OH)D3 and 20,23(OH)2D3 inhibited the RORα and RORγ-mediated activation of the IL17 promoter in a dose-dependent manner and production of IL-17 protein [101]. Since IL-17 and TH17 are the crucial factors in psoriasis pathogenesis, they represent a molecular target [225,226]. A-9758, selective RORγt inverse agonist, efficiently inhibited IL-17A release in in vitro and in vivo models [226]. A selective RORγt inhibitor Cpd A inhibited the transcriptional activity of human RORγt, differentiation of Th17 cells and the production of pro-inflammatory cytokines by T-cells, IL17F, IL22, IL26, IL23R, and CCR6 [227]. Another molecule, SR1001, synthetic RORα/γ inverse agonist in mouse models of atopic dermatitis and acute irritant dermatitis showed the anti-inflammatory effects (decreased the expression of IL-13, IL-5, IL-17A) and restored keratinocytes differentiation [228]. RORγt is also an important transcription factor regulating IL-22 in Th22 cells, which are related to inflammation and linked to the pathophysiology of psoriasis. 1,25(OH)2D3 can regulate the expression of IL-22, since vitamin D response elements have been identified in the IL22 promoter [229].
7. Vitamin D Paradigm in the Psoriasis Treatment
7.1. Introduction to the Problem
Since vitamin D regulates the immune system functions and the proliferation and differentiation of the keratinocytes, as well as other cell types, it has been effectively incorporated as an adjuvant treatment for psoriasis [230,231,232], although the precise mechanism of therapeutic action of vitamin D is not fully known. The first reports showing the therapeutic potential of vitamin D were published almost 90 years ago [233,234], but the use of high doses of vitamin D was limited due to potential toxic effects. The next important step was done by Morimoto S, who published several papers confirming the therapeutic effects of orally or topically administrated 1α(OH)D3 or 1,25(OH)2D3 [235,236,237,238,239]. The significant response of the lesional psoriatic skin to the treatment was observed in up to 85% of psoriasis cases [235,236,237,238,239]. The authors also showed the inhibitory effects of analogues of 1,25(OH)2D3 with no effects of 1,25(OH)2D3 on cultured psoriatic fibroblasts [240]. MacLaughlin et al. also observed the partial response of psoriatic fibroblasts to 1,25(OH)2D3, but only at higher tested doses (10 and 100 µM) [241]. The studies also confirmed the safety of long-term psoriasis treatment that was based on vitamin D [242,243,244]. The further studies allowed to develop the effective anti-psoriatic treatment that was based on vitamin D derivatives, including 1,25(OH)2D3, calcipotriol, maxacalcitol, tacalcitol, hexafluoro-1,25(OH)2D, calcipotriene, and others (reviewed in [230,245].
It is accepted that the therapeutic effects of vitamin D and its derivatives require VDR expression since above mentioned processes are mediated by this receptor and that VDR polymorphisms could modulate the responsiveness to the treatment. Recently, it has been reported that psoriasis patients with a PASI score that is lower than 3 and the rs7975232 CC genotypes were much more susceptible to calcipotriol treatment [246]. Lesiak et al. did not find the correlation of the rs7975232 variant and the treatment, but they observed the relationship between different variants of TaaI/Cdx-2 and the effects of UVB-based treatment, as assessed by the analysis of cytokines expression (IL-17, IL_23, TNF alpha) [222]. Taq1 VDR polymorphism (rs731236) was found to be a predictor of the duration of remission with C allele homozygotes that were related to the decreased VDR activity, showing a shorter remission duration than heterozygotes and T allele homozygotes [247]. A-1012G promoter polymorphism, and F and T alleles of Fok1 and Taq1 polymorphisms of VDR have been also identified as positively with calcipotriol response: AA and TT genotypes and AAFF, AATT, and FFTT genotype combinations [65]. On the contrary, BsmI and ApaI VDR polymorphisms are correlated to responsiveness to calcipotriol in Korean psoriasis patients [248].
It is well accepted that the topical application of vitamin D and/or its derivatives can improve psoriasis. However, psoriasis is systemic disease, thus systemic treatment should also be applied.
7.2. Oral Treatment with Vitamin D and Its Derivatives
The first vitamin D derivative that was used for psoriasis treatment by oral administration was 1α(OH)D (1.0 µg/day for 6 months) [235,237,238]. Its therapeutic action could be result from the inducing the changes in keratins expression. Holland and co-workers observed lowering the expression of keratin 16 and keratin 2 overexpression after 1α(OH)D treatment [249]. Thus, 1α(OH)D is able to inhibit the keratinocytes proliferation and promote its differentiation and shows anti-psoriatic properties. The oral administration of 2 µg/day of 1,25(OH)2D3 in the pilot study of Huckins et al. also resulted in a significant improvement of psoriatic arthritis [250]. Supplementation with vitamin D 5000 IU/day for three months significantly increased the vitamin D serum level, and the expression of anti-inflammatory cytokines (IL-10, IL-5) and decreased the PASI score and homocysteine plasma level, the expression of pro-inflammatory cytokines (IFN-7, TNF-α, IL-1β, IL-6, IL-8, and IL-17) and high-sensitivity C-reactive protein [251]. Similarly, a double-blind, randomized, placebo-controlled study with oral administration of vitamin D2 60,000 IU once every 2 weeks for 6 months resulted in an improved PASI score and an increase of 25(OH)D serum level with no signs of adverse effects. In addition, the serum level of 25(OH)D was negatively correlated to the PASI score [231].
The earlier studies used relatively low doses of vitamin D or its derivatives. But Finamor et al. in their study used 35,000 IU of vitamin D3/day for six month and observed a significant 25(OH)D3 increase and improvement of PASI score, with no signs of toxicity: no change of serum urea, creatinine, and calcium and a change of urinary calcium excretion within the normal range [252]. McCullough et al. studied the effects of long-term high doses of vitamin D3 administration (up to 50,000 IU) in patients that were affected by psoriasis with a significant improvement observed with no toxicity and adverse effects related to vitamin D treatment [253]. These results indicated that high doses of vitamin D could be efficient and safe in psoriasis treatment.
Some results are inconclusive or present contradictory data. Studies of Ingram et al. concerned the effects of vitamin D3 at the doses of 100,000 IU/month for 12 months (200,000 IU at baseline) (Australian New Zealand Clinical Trials Registry #12611000648921 [254]). They observed no changes of the PASI score, but the level of 25(OH)D increased. Additionally, an inverse correlation between a slight decrease of PASI score and 25(OH)D (up to 125 nmol/L) was also found. Similarly, Jarrett et al. [255] did not recommend the administration of vitamin D3 (100,000 IU per month) to treat the psoriasis, since no significant differences were observed between the supplemented and the placebo groups. In study of Prystowsky et al., no additive effect of orally administrated calcitriol (0.5–2.0 µg/day) on UVB phototherapy of psoriasis was found [256].
In summary, the meta-analysis of randomized controlled trials of oral vitamin D supplementation in psoriasis patients confirmed the effectiveness of the improvement the PASI score, however, Hartung-Knapp adjustment these effects were not significant [257]. Thus, more randomized controlled trials, especially with the use of vitamin D derivatives are needed. Furthermore, more effective treatment is related with topical administration. A study by Gumowski-Sunek and co-workers found changes in calcium metabolism after the oral administration of calcitriol (1.5 µg/day), while the topical administration of calcipotriol with an equivalent dose 150 µg/day, with 1% absorption) did not change calcium metabolism [258].
7.3. Topical Treatment with Vitamin D and Its Derivatives
The topical treatment is a first-line therapy in patients with mild or moderate psoriasis [259]. Early reports of Morimoto et al. showed that the topical administration of 0.1 and 0.5 µg/day of 1,25(OH)2D3 resulted in a significant improvement of psoriatic lesions with no toxicity symptoms [239]. For the topical psoriasis treatment, the calcipotriol has been introduced as an efficient and safe adjuvant already in late 1980s [260,261]. The treatment with calcipotriol resulted in the reduced expression of IL-6, with no changes in TNF alpha expression [262]. The efficacy of UVB treatment of psoriasis with ointment containing of calcipotriol (50 µg/g ointment twice a day) was greater than UVB alone [263]. Calcipotriol-containing ointment (50 µg/g twice a day) treatment also improved the psoriasis therapy with fumaric acid ester in multicenter, randomized, double-blind, vehicle-controlled study [264], psoriasis treatment with cyclosporine [265], and psoriasis treatment with acitretin [266]. Similarly, calcipotriene (0.005% and betamethasone dipropionate 0.064%) ointment improved the presentation of psoriasis [267]. Pinter et al. data showed the high effectiveness of calcipotriene and betamethasone PADTM Technology cream with no adverse drug reaction in two Pahase 3, multicenter, randomized, investigator-blind studies [268]. Different clinical studies report that the combination of calcipotriene and betamethasone dipropionate in foam solution to be the most effective treatment for mild psoriasis [269].
Equally, a multicenter study concerning the use of tacalcitol (4 µg/g) ointment once daily for 18 months showed its high effectiveness, safety, and good tolerance during the long-term topical treatment, with no changes in calcitriol, calcium, and parathyroid hormone serum level [270]. The multicenter prospective study with tacalcitol (20 µg/g) ointment applied once daily revealed the decrease of PASI score, with local adverse effects, a decrease of parathyroid hormone and 1,25(OH)2D3, but a maintained serum calcium homeostasis was observed [243]. Tacalcitol also increased the effectiveness of NB-UVB treatment [271]. Thus, tacalcitol is effective and safe during long-term treatment.
Maxacalcitol, another vitamin D derivative, is very effective in inducing of differentiation and inhibiting of keratinocyte proliferation, without inducing hypercalcemia [272]. These effects were stronger than that of either calcipotriol or tacalcitol in in vitro models. A placebo-controlled, double-blind study showed that maxacalcitol ointment (6, 12.5, 25, and 50 mg/g) was very effective in the improvement of psoriasis presentation, with 25 mg/g ointment being more efficient than calcipotriol [273], especially in combined treatment that included maxacalcitol and NB-UB [274]. However, use of maxalcitol is related to a higher risk of development of hypercalcemia, and psoriasis treatment with calcipotriol is safer in comparison to maxalcitol [275].
The topical application vitamin D and its derivatives constitutes an important element of psoriasis management and can also be used in combination with other modalities. The topical treatment offers a safer therapy option, since the active compounds can directly target the lesional areas, without excessive systemic entry.
8. Conclusions and Future Directions
Vitamin D and its derivatives have resulted in impressive clinical responses in psoriatic patients. They represent effective and safe adjuvant treatments for psoriasis, even when high doses of vitamin D are administered. The phototherapy of psoriasis, especially UVB-based, changes the serum level of 25(OH)D. However, such a correlation of 25(OH)D levels and psoriasis improvement requires future clinical trials since contradictory data have been published in this area. Vitamin D derivatives can improve the efficacy of psoriasis UVB phototherapy without inducing adverse side effects. Excellent candidates for anti-psoriatic treatment are new, none, or low calcemic CYP11A1-derived hydroxyderivatives of vitamin D3 or lumisterol compounds. These would act as agonists on VDR, LXR, and AhR receptors and as inverse agonists on RORs. In conclusion, targeting of local vitamin D signaling systems represents a promising future in therapy or prevention of psoriasis.
Author Contributions
A.A.B.—conceptualization, formal analysis, writing, figure preparation, review, and editing; R.M.S.—writing and editing; B.N.—figure preparation, writing, and editing; M.A.Z.—writing (vitamin D endocrine system section), review, and editing; A.T.S.—conceptualization, writing, review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Polish Ministry of Science and Higher Education grant (02-0066/07/253) to AB and NIH (grants nos. 1R01AR073004, R01AR071189 and R21AI149267-01A1) and VA merit (grant no. 1I01BX004293-01A1) to ATS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights From the Global Burden of Disease 2019 Study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Del Marmol, V. Psoriasis: Keratinocytes or Immune Cells—Which Is the Trigger? Dermatology 2019, 235, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gondo, G.C.; Koons, S.; Metcalf, C.; Bell, S.J.; Mehta, N.N. Viewing Psoriasis as a Systemic Disease for Better Health Outcomes. JID Innov. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Grozdev, I.; Korman, N.; Tsankov, N. Psoriasis as a systemic disease. Clin. Dermatol. 2014, 32, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Puig-Sanz, L. Psoriasis, a systemic disease? Actas Dermosifiliogr. 2007, 98, 396–402. [Google Scholar] [CrossRef]
- Jadali, Z.; Eslami, M.; Bayatian, P.; Mirshafiei, A.; Salehie, N.A.; Izad, M.; Mansouri, P.; Safari, R. Th1/Th2 cytokines in psoriasis. Iranian J. Publ. Health 2007, 36, 87–91. [Google Scholar]
- Yousefzadeh, H.; Jabbari Azad, F.; Rastin, M.; Banihashemi, M.; Mahmoudi, M. Expression of Th1 and Th2 Cytokine and Associated Transcription Factors in Peripheral Blood Mononuclear Cells and Correlation with Disease Severity. Rep. Biochem. Mol. Biol. 2017, 6, 102–111. [Google Scholar]
- Priyadarssini, M.; Divya Priya, D.; Indhumathi, S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M. Immunophenotyping of T cells in the peripheral circulation in psoriasis. Br. J. Biomed. Sci. 2016, 73, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kagami, S.; Rizzo, H.L.; Lee, J.J.; Koguchi, Y.; Blauvelt, A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Invest. Dermatol. 2010, 130, 1373–1383. [Google Scholar] [CrossRef]
- Michalak-Stoma, A.; Bartosinska, J.; Kowal, M.; Juszkiewicz-Borowiec, M.; Gerkowicz, A.; Chodorowska, G. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis. Markers 2013, 35, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, L.; Chen, Y.L.; Ogg, G.S. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br. J. Dermatol. 2021, 184, 14–24. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Lange, M.; Sokolowska-Wojdylo, M.; Renke, J.; Trzonkowski, P.; Sobjanek, M.; Szczerkowska-Dobosz, A.; Niedoszytko, M.; Gorska, A.; Romantowski, J.; et al. The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases. Part II: The Treg role in skin diseases pathogenesis. Postepy Dermatol. Alergol. 2017, 34, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Mattozzi, C.; Paolino, G.; Salvi, M.; Macaluso, L.; Luci, C.; Morrone, S.; Calvieri, S.; Richetta, A.G. Peripheral blood regulatory T cell measurements correlate with serum vitamin D level in patients with psoriasis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1675–1679. [Google Scholar] [PubMed]
- Sugiyama, H.; Gyulai, R.; Toichi, E.; Garaczi, E.; Shimada, S.; Stevens, S.R.; McCormick, T.S.; Cooper, K.D. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: Mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2005, 174, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Samotij, D.; Nedoszytko, B.; Bartosinska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.T.; Dobrucki, L.W.; Gorecka-Sokolowska, M.; Janaszak-Jasienicka, A.; Krasowska, D.; et al. Pathogenesis of psoriasis in the “omic” era. Part I. Epidemiology, clinical manifestation, immunological and neuroendocrine disturbances. Postepy Dermatol. Alergol. 2020, 37, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Vicic, M.; Kastelan, M.; Brajac, I.; Sotosek, V.; Massari, L.P. Current Concepts of Psoriasis Immunopathogenesis. Int. J. Mol. Sci. 2021, 22, 11574. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Dastoli, S.; Nistico, S.P.; Morrone, P.; Patruno, C.; Leo, A.; Citraro, R.; Gallelli, L.; Russo, E.; De Sarro, G.; Bennardo, L. Colchicine in Managing Skin Conditions: A Systematic Review. Pharmaceutics 2022, 14, 294. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Tamburi, F.; Amoruso, G.F.; Bennardo, L.; Nistico, S.P. Emerging role of anti-IL23 in the treatment of psoriasis: When humanized is very promising. Dermatol. Ther. 2020, 33, e14504. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin’s Neuroendocrine System; Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 212, pp. 1–115. [Google Scholar] [CrossRef]
- Slominski, A.; Mihm, M.C. Potential mechanism of skin response to stress. Int. J. Dermatol. 1996, 35, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Tuckey, R.C.; Paus, R. Differential expression of HPA axis homolog in the skin. Mol. Cell Endocrinol. 2007, 265, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Dowdy, J.C.; Sayre, R.M.; Tuckey, R.C.; Slominski, A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: Regulation by ultraviolet radiation. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E484–E493. [Google Scholar] [CrossRef]
- Nikolakis, G.; Stratakis, C.A.; Kanaki, T.; Slominski, A.; Zouboulis, C.C. Skin steroidogenesis in health and disease. Rev. Endocr. Metab. Disord. 2016, 17, 247–258. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brozyna, A.A.; Tuckey, R.C. Cutaneous Glucocorticoidogenesis and Cortisol Signaling Are Defective in Psoriasis. J. Invest. Dermatol. 2017, 137, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Pisarchik, A.; Zbytek, B.; Linton, E.A.; Mazurkiewicz, J.E.; Wei, E.T. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001, 15, 1678–1693. [Google Scholar] [CrossRef]
- Karalis, K.; Sano, H.; Redwine, J.; Listwak, S.; Wilder, R.L.; Chrousos, G.P. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science 1991, 254, 421–423. [Google Scholar] [CrossRef]
- Crofford, L.J.; Sano, H.; Karalis, K.; Friedman, T.C.; Epps, H.R.; Remmers, E.F.; Mathern, P.; Chrousos, G.P.; Wilder, R.L. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J. Immunol. 1993, 151, 1587–1596. [Google Scholar] [PubMed]
- Slominski, A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br. J. Dermatol. 2009, 160, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Zbytek, B.; Slominski, A.T. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J. Invest. Dermatol. 2007, 127, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Zmijewski, M.; Slominski, R.M.; Kauser, S.; Wortsman, J.; Tobin, D.J. Corticotropin releasing hormone and the skin. Front. Biosci. 2006, 11, 2230–2248. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Tuckey, R.C.; Manna, P.R.; Jetten, A.M.; Postlethwaite, A.; Raman, C.; Slominski, A.T. Extra-adrenal glucocorticoid biosynthesis: Implications for autoimmune and inflammatory disorders. Genes Immun. 2020, 21, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yu, X.; Cai, D.; Liu, C.; Li, C. Role of corticotropin-releasing hormone and receptor in the pathogenesis of psoriasis. Med. Hypotheses 2009, 73, 513–515. [Google Scholar] [CrossRef]
- Antoniewicz, J.; Nedoszytko, B.; Lange, M.; Wierzbicka, J.; Górska-Ponikowska, M.; Niedoszytko, M.; Zabłotna, M.; Nowicki, R.J.; Żmijewski, M.A. Modulation of dermal equivalent of hypothalamus-pituitary-adrenal axis in mastocytosis. Postepy Dermatol. Alergol. 2021, 38, 461–472. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [PubMed]
- Loite, U.; Kingo, K.; Reimann, E.; Reemann, P.; Vasar, E.; Silm, H.; Koks, S. Gene expression analysis of the corticotrophin-releasing hormone-proopiomelanocortin system in psoriasis skin biopsies. Acta Derm. Venereol. 2013, 93, 400–405. [Google Scholar] [CrossRef]
- Sewell, W.A.; Scurr, L.L.; Orphanides, H.; Kinder, S.; Ludowyke, R.I. Induction of interleukin-4 and interleukin-5 expression in mast cells is inhibited by glucocorticoids. Clin. Diagn. Lab. Immunol. 1998, 5, 18–23. [Google Scholar] [CrossRef]
- Sevilla, L.M.; Latorre, V.; Sanchis, A.; Perez, P. Epidermal inactivation of the glucocorticoid receptor triggers skin barrier defects and cutaneous inflammation. J. Invest. Dermatol. 2013, 133, 361–370. [Google Scholar] [CrossRef]
- Hannen, R.; Udeh-Momoh, C.; Upton, J.; Wright, M.; Michael, A.; Gulati, A.; Rajpopat, S.; Clayton, N.; Halsall, D.; Burrin, J.; et al. Dysfunctional Skin-Derived Glucocorticoid Synthesis Is a Pathogenic Mechanism of Psoriasis. J. Invest. Dermatol. 2017, 137, 1630–1637. [Google Scholar] [CrossRef]
- Slominski, A.; Zjawiony, J.; Wortsman, J.; Semak, I.; Stewart, J.; Pisarchik, A.; Sweatman, T.; Marcos, J.; Dunbar, C.; Tuckey, R.C. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur. J. Biochem. 2004, 271, 4178–4188. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Elmets, C.; Jetten, A.M.; Slominski, A.; Tuckey, R.C. The significance of CYP11A1 expression in skin physiology and pathology. Mol. Cell Endocrinol. 2021, 530, 111238. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. StAR search-what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol. Endocrinol. 2007, 21, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Hannen, R.F.; Michael, A.E.; Jaulim, A.; Bhogal, R.; Burrin, J.M.; Philpott, M.P. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem. Biophys. Res. Commun. 2011, 404, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Ermak, G.; Mihm, M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J. Clin. Endocrinol. Metab. 1996, 81, 2746–2749. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Semak, I.; Sweatman, T.; Wortsman, J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J. Neuroimmunol. 2005, 162, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Szczesniewski, A.; Semak, I.; Kaminski, J.; Sweatman, T.; Wortsman, J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E701–E706. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Szczesniewski, A.; Wortsman, J. Cultured human dermal fibroblasts do produce cortisol. J. Invest. Dermatol. 2006, 126, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Garshick, M.S.; Ward, N.L.; Krueger, J.G.; Berger, J.S. Cardiovascular Risk in Patients With Psoriasis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Purzycka-Bohdan, D.; Kisielnicka, A.; Bohdan, M.; Szczerkowska-Dobosz, A.; Sobalska-Kwapis, M.; Nedoszytko, B.; Nowicki, R.J. Analysis of the Potential Genetic Links between Psoriasis and Cardiovascular Risk Factors. Int. J. Mol. Sci. 2021, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Conic, R.R.; Damiani, G.; Schrom, K.P.; Ramser, A.E.; Zheng, C.; Xu, R.; McCormick, T.S.; Cooper, K.D. Psoriasis and Psoriatic Arthritis Cardiovascular Disease Endotypes Identified by Red Blood Cell Distribution Width and Mean Platelet Volume. J. Clin. Med. 2020, 9, 186. [Google Scholar] [CrossRef]
- Seth, D.; Ehlert, A.N.; Golden, J.B.; Damiani, G.; McCormick, T.S.; Cameron, M.J.; Cooper, K.D. Interaction of Resistin and Systolic Blood Pressure in Psoriasis Severity. J. Invest. Dermatol. 2020, 140, 1279–1282.e1. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D: Newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol. Metab. 2010, 21, 375–384. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism and function in the skin. Mol. Cell Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the skin. J. Bone Miner. Metab. 2010, 28, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D receptor, UVR, and skin cancer: A potential protective mechanism. J. Invest. Dermatol. 2008, 128, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Molnar, F. Current status of vitamin D signaling and its therapeutic applications. Curr. Top. Med. Chem. 2012, 12, 528–547. [Google Scholar] [CrossRef] [PubMed]
- Halsall, J.A.; Osborne, J.E.; Pringle, J.H.; Hutchinson, P.E. Vitamin D receptor gene polymorphisms, particularly the novel A-1012G promoter polymorphism, are associated with vitamin D3 responsiveness and non-familial susceptibility in psoriasis. Pharmacogenet. Genom. 2005, 15, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Garbi, N.; Hammerling, G.; Hintze, K.J. Role of the 1,25D3-MARRS receptor in the 1,25(OH)2D3-stimulated uptake of calcium and phosphate in intestinal cells. Steroids 2012, 77, 897–902. [Google Scholar] [CrossRef]
- Khanal, R.C.; Nemere, I. The ERp57/GRp58/1,25D3-MARRS receptor: Multiple functional roles in diverse cell systems. Curr. Med. Chem. 2007, 14, 1087–1093. [Google Scholar] [CrossRef]
- Olszewska, A.M.; Sieradzan, A.K.; Bednarczyk, P.; Szewczyk, A.; Żmijewski, M.A. Mitochondrial potassium channels: A novel calcitriol target. Cell Mol. Biol. Lett. 2022, 27, 3. [Google Scholar] [CrossRef] [PubMed]
- Rybchyn, M.S.; De Silva, W.G.M.; Sequeira, V.B.; McCarthy, B.Y.; Dilley, A.V.; Dixon, K.M.; Halliday, G.M.; Mason, R.S. Enhanced Repair of UV-Induced DNA Damage by 1,25-Dihydroxyvitamin D3 in Skin Is Linked to Pathways that Control Cellular Energy. J. Invest. Dermatol. 2018, 138, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Wierzbicka, J.; Ślebioda, T.; Woźniak, M.; Tuckey, R.C.; Slominski, A.T.; Żmijewski, M.A. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids 2016, 110, 49–61. [Google Scholar] [CrossRef]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.Y.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brożyna, A.A.; Zmijewski, M.A.; Janjetovic, Z.; Kim, T.K.; Slominski, R.M.; Tuckey, R.C.; Mason, R.S.; Jetten, A.M.; Guroji, P.; et al. The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers. Adv. Exp. Med. Biol. 2020, 1268, 257–283. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.K.; Wasilewski, P.; Rosas, S.; Hanna, S.; Sayre, R.M.; Dowdy, J.C.; Li, W.; Tuckey, R.C. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J. Steroid Biochem. Mol. Biol. 2015, 148, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Schwartz, C.J.; Tuckey, R.C.; Tang, E.K.Y.; Raman, C.; Panich, U.; Slominski, A.T. Hydroxylumisterols, photoproducts of pre-vitamin D3, protect human keratinocytes against UVB-induced damage. Int. J. Mol. Sci. 2020, 21, 9374. [Google Scholar] [CrossRef] [PubMed]
- Tongkao-On, W.; Carter, S.; Reeve, V.E.; Dixon, K.M.; Gordon-Thomson, C.; Halliday, G.M.; Tuckey, R.C.; Mason, R.S. CYP11A1 in skin: An alternative route to photoprotection by vitamin D compounds. J. Steroid Biochem. Mol. Biol. 2015, 148, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, S.; Gotoh, K.; Nakashima, Y.; Setoyama, D.; Takata, Y.; Ohga, S.; Kang, D. Mitochondrial Reactive Oxygen Species Are Essential for the Development of Psoriatic Inflammation. Front. Immunol. 2021, 12, 714897. [Google Scholar] [CrossRef] [PubMed]
- Therianou, A.; Vasiadi, M.; Delivanis, D.A.; Petrakopoulou, T.; Katsarou-Katsari, A.; Antoniou, C.; Stratigos, A.; Tsilioni, I.; Katsambas, A.; Rigopoulos, D.; et al. Mitochondrial dysfunction in affected skin and increased mitochondrial DNA in serum from patients with psoriasis. Exp. Dermatol. 2019, 28, 72–75. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Wortsman, J.; Zjawiony, J.; Li, W.; Zbytek, B.; Tuckey, R.C. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006, 273, 2891–2901. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Li, W.; Szczesniewski, A.; Tuckey, R.C. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005, 272, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Tang, E.K.; Benson, H.A.; Semak, I.; Lin, Z.; Yates, C.R.; Wang, J.; Li, W.; et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol. Cell Endocrinol. 2014, 383, 181–192. [Google Scholar] [CrossRef]
- Tang, E.K.; Chen, J.; Janjetovic, Z.; Tieu, E.W.; Slominski, A.T.; Li, W.; Tuckey, R.C. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab. Dispos. 2013, 41, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Li, W.; Shehabi, H.Z.; Janjetovic, Z.; Nguyen, M.N.; Kim, T.K.; Chen, J.; Howell, D.E.; Benson, H.A.; Sweatman, T.; et al. Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab. Dispos. 2011, 39, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Daugherty, C.; Shimizu, I.; Syapin, P.J.; Garrel, G.; Cohen-Tannoudji, J.; Huhtaniemi, I.; Slominski, A.T.; Pruitt, K.; et al. Up-regulation of steroid biosynthesis by retinoid signaling: Implications for aging. Mech. Ageing Dev. 2015, 150, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brozyna, A.A.; Skobowiat, C.; Zmijewski, M.A.; Kim, T.K.; Janjetovic, Z.; Oak, A.S.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J. Steroid Biochem. Mol. Biol. 2018, 177, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Chen, J.; Nguyen, M.N.; Li, W.; Yates, C.R.; Sweatman, T.; Janjetovic, Z.; Tuckey, R.C. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int. J. Biochem. Cell Biol. 2012, 44, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Tuckey, R.C.; Bieniek, R.; Yue, J.; Li, W.; Chen, J.; Nguyen, M.N.; Tang, E.K.; et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol. Cell Physiol. 2011, 300, C526–C541. [Google Scholar] [CrossRef]
- Kim, T.K.; Wang, J.; Janjetovic, Z.; Chen, J.; Tuckey, R.C.; Nguyen, M.N.; Tang, E.K.; Miller, D.; Li, W.; Slominski, A.T. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol. Cell Endocrinol. 2012, 361, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, H.; Belorusova, A.Y.; Bollinger, J.C.; Tang, E.K.Y.; Janjetovic, Z.; Kim, T.K.; Wu, Z.; Miller, D.D.; Slominski, A.T.; et al. 1α,20S-dihydroxyvitamin D3 interacts with vitamin D receptor: Crystal structure and route of chemical synthesis. Sci. Rep. 2017, 7, 10193. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Marepally, S.R.; Goh, E.S.Y.; Cheng, C.Y.S.; Janjetovic, Z.; Kim, T.K.; Miller, D.D.; Postlethwaite, A.E.; Slominski, A.T.; Tuckey, R.C.; et al. Investigation of 20S-hydroxyvitamin D3 analogs and their 1α-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities. Sci. Rep. 2018, 8, 1478. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Janjetovic, Z.; Oak, A.S.W.; Postlethwaite, A.; Lin, Z.; Li, W.; Takeda, Y.; Jetten, A.M.; et al. Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci. Rep. 2017, 7, 11434. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Slominski, A.T.; Cheng, C.Y.; Chen, J.; Kim, T.K.; Xiao, M.; Li, W. Lumisterol is metabolized by CYP11A1: Discovery of a new pathway. Int. J. Biochem. Cell Biol. 2014, 55, 24–34. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Slominski, R.M.; Song, Y.; Janjetovic, Z.; Podgorska, E.; Reddy, G.S.; Song, Y.; Raman, C.; Tang, E.K.; et al. Metabolic activation of tachysterol3 to biologically active hydroxyderivatives that act on VDR, AhR, LXRs, and PPARγ receptors. FASEB J. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORα and RORγ. J. Steroid Biochem. Mol. Biol. 2017, 173, 42–56. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J 2014, 28, 2775–2789. [Google Scholar] [CrossRef] [PubMed]
- Brzeminski, P.; Fabisiak, A.; Slominski, R.M.; Kim, T.K.; Janjetovic, Z.; Podgorska, E.; Song, Y.; Saleem, M.; Reddy, S.B.; Qayyum, S.; et al. Chemical synthesis, biological activities and action on nuclear receptors of 20S(OH)D3, 20S,25(OH)2D3, 20S,23S(OH)2D3 and 20S,23R(OH)2D3. Bioorg. Chem. 2022, 121, 105660. [Google Scholar] [CrossRef] [PubMed]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal 2009, 7, e003. [Google Scholar] [CrossRef]
- Jetten, A.M.; Takeda, Y.; Slominski, A.; Kang, H.S. Retinoic acid-related Orphan Receptor γ (RORγ): Connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr. Opin. Toxicol. 2018, 8, 66–80. [Google Scholar] [CrossRef]
- Jetten, A.M.; Beak, J.Y.; Slominski, A.T.; Jensen, B. Retinoic acid-related orphan receptor (ROR) inverse agonists: Potential therapeutic strategies for multiple inflammatory diseases? In Nuclear Receptors: The Art and Science of Modulator Design and Discovery; Badr, M.Z., Ed.; Springer I Nature Switzerland AG: Cham, Switzerland, 2021; pp. 349–377. [Google Scholar]
- Song, Y.; Slominski, R.M.; Qayyum, S.; Kim, T.K.; Janjetovic, Z.; Raman, C.; Tuckey, R.C.; Song, Y.; Slominski, A.T. Molecular and structural basis of interactions of vitamin D3 hydroxyderivatives with aryl hydrocarbon receptor (AhR): An integrated experimental and computational study. Int. J. Biol. Macromol. 2022, 209, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Brozyna, A.A.; Zmijewski, M.A.; Xu, H.; Sutter, T.R.; Tuckey, R.C.; Jetten, A.M.; Crossman, D.K. Differential and overlapping effects of 20,23(OH)2D3 and 1,25(OH)2D3 on gene expression in human epidermal keratinocytes: Identification of AhR as an alternative receptor for 20,23(OH)2D3. Int. J. Mol. Sci. 2018, 19, 72. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Qayyum, S.; Song, Y.; Janjetovic, Z.; Oak, A.S.W.; Slominski, R.M.; Raman, C.; Stefan, J.; Mier-Aguilar, C.A.; et al. Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs). Sci. Rep. 2021, 11, 8002. [Google Scholar] [CrossRef]
- Torezan, L.; Grinblat, B.; Haedersdal, M.; Valente, N.; Festa-Neto, C.; Szeimies, R.M. A randomized split-scalp study comparing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br. J. Dermatol. 2018, 179, 829–835. [Google Scholar] [CrossRef]
- Qayyum, S.; Mohammad, T.; Slominski, R.M.; Hassan, M.I.; Tuckey, R.C.; Raman, C.; Slominski, A.T. Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E246–E251. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qayyum, S.; Greer, R.A.; Slominski, R.M.; Raman, C.; Slominski, A.T.; Song, Y. Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: A computational study. J. Biomol. Struct. Dyn. 2021, 1–17. [Google Scholar] [CrossRef]
- Slominski, R.M.; Stefan, J.; Athar, M.; Holick, M.F.; Jetten, A.M.; Raman, C.; Slominski, A.T. COVID-19 and Vitamin D: A lesson from the skin. Exp. Dermatol. 2020, 29, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Nemanic, M.K.; Gee, E.; Elias, P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Investig. 1986, 78, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Tiebel, O.; Meurer, M. Expression of vitamin D3 25-hydroxylase (CYP27) mRNA after induction by vitamin D3 or UVB radiation in keratinocytes of human skin equivalents-a preliminary study. Arch. Dermatol. Res. 1999, 291, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jozwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) decreases during melanoma progression. Hum. Pathol. 2013, 44, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Zbytek, B.; Pisarchik, A.; Li, W.; Zjawiony, J.; Tuckey, R.C. Cytochromes p450 and skin cancer: Role of local endocrine pathways. Anticancer Agents Med. Chem. 2014, 14, 77–96. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Tuckey, R.C. Classical and non-classical metabolic transformation of vitamin D in dermal fibroblasts. Exp. Dermatol. 2016, 25, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, S.K.; Ona-Vu, K.; Teichert, A.E.; Cleaver, J.E.; Bikle, D.D.; Oh, D.H. Vitamin D receptor mediates DNA repair and is UV inducible in intact epidermis but not in cultured keratinocytes. J. Investig. Dermatol. 2012, 132, 2097–2100. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Bikle, D.D.; Elalieh, H.; Welsh, J.; Oh, D.; Cleaver, J.; Teichert, A. Protective role of vitamin D signaling in skin cancer formation. J. Steroid Biochem. Mol. Biol. 2013, 136, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Komuves, L.; Yu, Q.C.; Elalieh, H.; Ng, D.C.; Leary, C.; Chang, S.; Crumrine, D.; Yoshizawa, T.; Kato, S.; et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J. Investig. Dermatol. 2002, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, J.; Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 α,25-dihydroxyvitamin D3. Endocrinology 1983, 113, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- McLane, J.A.; Katz, M.; Abdelkader, N. Effect of 1,25-dihydroxyvitamin D3 on human keratinocytes grown under different culture conditions. In Vitro Cell. Dev. Biol. 1990, 26, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Walworth, N.C.; Holick, M.F. Effect of 1 α,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J. Investig. Dermatol. 1986, 86, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Zbytek, B.; Janjetovic, Z.; Tuckey, R.C.; Zmijewski, M.A.; Sweatman, T.W.; Jones, E.; Nguyen, M.N.; Slominski, A.T. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J. Investig. Dermatol. 2008, 128, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Ng, D.; Tu, C.L.; Oda, Y.; Xie, Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol. Cell Endocrinol. 2001, 177, 161–171. [Google Scholar] [CrossRef]
- Bikle, D.D.; Teichert, A.; Arnold, L.A.; Uchida, Y.; Elias, P.M.; Oda, Y. Differential regulation of epidermal function by VDR coactivators. J. Steroid Biochem. Mol. Biol. 2010, 121, 308–313. [Google Scholar] [CrossRef]
- Hawker, N.P.; Pennypacker, S.D.; Chang, S.M.; Bikle, D.D. Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J. Investig. Dermatol. 2007, 127, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Gniadecki, R. Stimulation versus inhibition of keratinocyte growth by 1,25-Dihydroxyvitamin D3: Dependence on cell culture conditions. J. Investig. Dermatol. 1996, 106, 510–516. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Zmijewski, M.A.; Tuckey, R.C.; DeLeon, D.A.; Nguyen, M.N.; Pfeffer, L.M.; Slominski, A.T. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-κB activity by increasing IκBα levels in human keratinocytes. PLoS ONE 2009, 4, e5988. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Janjetovic, Z.; Kim, T.K.; Sweatman, T.; Lu, Y.; Zjawiony, J.; Tuckey, R.C.; Miller, D.; Slominski, A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids 2010, 75, 926–935. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Thorpe, E.M., Jr.; Slominski, A.T. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-κB activity in human keratinocytes. J. Cell Physiol. 2010, 223, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Li, W.; Yi, A.K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 28–39. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Tuckey, R.C.; Li, W.; Raman, C.; Panich, U.; Slominski, A.T. CYP11A1-derived vitamin D3 products protect against UVB-induced inflammation and promote keratinocytes differentiation. Free Radic. Biol. Med. 2020, 155, 87–98. [Google Scholar] [CrossRef]
- Slominski, A.; Kim, T.K.; Zmijewski, M.A.; Janjetovic, Z.; Li, W.; Chen, J.; Kusniatsova, E.I.; Semak, I.; Postlethwaite, A.; Miller, D.D.; et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinology 2013, 5, 7–19. [Google Scholar] [CrossRef]
- De Silva, W.G.M.; Han, J.Z.R.; Yang, C.; Tongkao-On, W.; McCarthy, B.Y.; Ince, F.A.; Holland, A.J.A.; Tuckey, R.C.; Slominski, A.T.; Abboud, M.; et al. Evidence for Involvement of Nonclassical Pathways in the Protection From UV-Induced DNA Damage by Vitamin D-Related Compounds. JBMR Plus 2021, 5, e10555. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Brozyna, A.A.; Elsayed, M.; Janjetovic, Z.; Qayyum, S.; Slominski, R.M.; Oak, A.S.; Li, C.; Podgorska, E.; Li, W.; et al. CYP11A1-derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas. Int. J. Oncol. 2022, in press. [CrossRef] [PubMed]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.K.; Wright, A.C.; Grese, L.N.; Riney, S.J.; Nguyen, M.N.; Tuckey, R.C. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012, 32, 3733–3742. [Google Scholar]
- Slominski, A.T.; Janjetovic, Z.; Fuller, B.E.; Zmijewski, M.A.; Tuckey, R.C.; Nguyen, M.N.; Sweatman, T.; Li, W.; Zjawiony, J.; Miller, D.; et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS ONE 2010, 5, e9907. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Li, W.; Bhattacharya, S.K.; Smith, R.A.; Johnson, P.L.; Chen, J.; Nelson, K.E.; Tuckey, R.C.; Miller, D.; Jiao, Y.; et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J. Investig. Dermatol. 2011, 131, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Bhattacharya, K.G.; Wang, J.; Li, W.; Jiao, Y.; Gu, W.; Brown, M.; et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J. Clin. Endocrinol. Metab. 2013, 98, E298–E303. [Google Scholar] [CrossRef] [PubMed]
- Brown Lobbins, M.L.; Slominski, A.T.; Hasty, K.A.; Zhang, S.; Miller, D.D.; Li, W.; Kim, T.K.; Janjetovic, Z.; Tuckey, R.C.; Scott, I.O.; et al. Modulation by 17,20S(OH)2pD of Fibrosis-Related Mediators in Dermal Fibroblast Lines from Healthy Donors and from Patients with Systemic Sclerosis. Int. J. Mol. Sci. 2021, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Brown Lobbins, M.L.; Scott, I.O.; Slominski, A.T.; Hasty, K.A.; Zhang, S.; Miller, D.D.; Li, W.; Kim, T.K.; Janjetovic, Z.; Patel, T.S.; et al. 17,20S(OH)2pD Can Prevent the Development of Skin Fibrosis in the Bleomycin-Induced Scleroderma Mouse Model. Int. J. Mol. Sci. 2021, 22, 8926. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Postlethwaite, A.; Kang, H.S.; Kim, T.K.; Tuckey, R.C.; Crossman, D.K.; Qayyum, S.; Jetten, A.M.; Slominski, A.T. Antifibrogenic Activities of CYP11A1-derived Vitamin D3-hydroxyderivatives Are Dependent on RORγ. Endocrinology 2021, 162, bqaa198. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.M.; Zmijewski, M.A.; Piotrowska, A.; Nedoszytko, B.; Lange, M.; Tuckey, R.C.; Slominski, A.T. Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes. Mol. Cell Endocrinol. 2016, 437, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.K.; Winstead, M.; Kee, J.D.; Park, J.J.; Zhang, S.; Li, W.; Yi, A.K.; Stuart, J.M.; Rosloniec, E.F.; Brand, D.D.; et al. 1,25-Dihydroxyvitamin D3 and 20-Hydroxyvitamin D3 Upregulate LAIR-1 and Attenuate Collagen Induced Arthritis. Int. J. Mol. Sci. 2021, 22, 13342. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Tuckey, R.C.; Kim, T.K.; Li, W.; Bhattacharya, S.K.; Myers, L.K.; Brand, D.D.; Slominski, A.T. 20S-Hydroxyvitamin D3, a secosteroid produced in humans, is anti-inflammatory and inhibits murine autoimmune arthritis. Front. Immunol. 2021, 12, 678487. [Google Scholar] [CrossRef]
- Yamamoto, E.; Jorgensen, T.N. Immunological effects of vitamin D and their relations to autoimmunity. J. Autoimmun. 2019, 100, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Weeres, M.A.; Robien, K.; Ahn, Y.O.; Neulen, M.L.; Bergerson, R.; Miller, J.S.; Verneris, M.R. The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J. Immunol. 2014, 193, 3456–3462. [Google Scholar] [CrossRef]
- Cantorna, M.T. Mechanisms underlying the effect of vitamin D on the immune system. Proc. Nutr. Soc. 2010, 69, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J. Nutr. 1995, 125, 1704S–1708S. [Google Scholar] [CrossRef] [PubMed]
- Dzopalic, T.; Bozic-Nedeljkovic, B.; Jurisic, V. The role of vitamin A and vitamin D in modulation of the immune response with a focus on innate lymphoid cells. Cent. Eur. J. Immunol. 2021, 46, 264–269. [Google Scholar] [CrossRef]
- Alroy, I.; Towers, T.L.; Freedman, L.P. Transcriptional repression of the interleukin-2 gene by vitamin D3: Direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol. Cell Biol. 1995, 15, 5789–5799. [Google Scholar] [CrossRef] [PubMed]
- Cippitelli, M.; Santoni, A. Vitamin D3: A transcriptional modulator of the interferon-γ gene. Eur. J. Immunol. 1998, 28, 3017–3030. [Google Scholar] [CrossRef]
- Rigby, W.F.; Stacy, T.; Fanger, M.W. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J. Clin. Investig. 1984, 74, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.M.; Adams, J.S.; Kermani-Arab, V.; Bakke, A.C.; Sakai, R.; Jordan, S.C. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J. Immunol. 1985, 134, 3032–3035. [Google Scholar]
- Reichel, H.; Koeffler, H.P.; Tobler, A.; Norman, A.W. 1 α,25-Dihydroxyvitamin D3 inhibits γ-interferon synthesis by normal human peripheral blood lymphocytes. Proc. Natl. Acad. Sci. USA 1987, 84, 3385–3389. [Google Scholar] [CrossRef]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1α,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.; Kuritzky, L.A.; Judge, M.A.; Dixon, K.M.; McGlade, J.P.; Mason, R.S.; Finlay-Jones, J.J.; Hart, P.H. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J. Immunol. 2007, 179, 6273–6283. [Google Scholar] [CrossRef]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Dyring-Andersen, B.; Bonefeld, C.M.; Bzorek, M.; Lovendorf, M.B.; Lauritsen, J.P.; Skov, L.; Geisler, C. The Vitamin D Analogue Calcipotriol Reduces the Frequency of CD8+ IL-17+ T Cells in Psoriasis Lesions. Scand. J. Immunol. 2015, 82, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Karthaus, N.; van Spriel, A.B.; Looman, M.W.G.; Chen, S.; Spilgies, L.M.; Lieben, L.; Carmeliet, G.; Ansems, M.; Adema, G.J. Vitamin D controls murine and human plasmacytoid dendritic cell function. J. Investig. Dermatol. 2014, 134, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Schiattarella, M.; Lembo, S.; Mattii, M.; Prevete, N.; Balato, N.; Ayala, F. Interleukin-1 family members are enhanced in psoriasis and suppressed by vitamin D and retinoic acid. Arch. Dermatol. Res. 2013, 305, 255–262. [Google Scholar] [CrossRef]
- Hegyi, Z.; Zwicker, S.; Bureik, D.; Peric, M.; Koglin, S.; Batycka-Baran, A.; Prinz, J.C.; Ruzicka, T.; Schauber, J.; Wolf, R. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 “alarmins” psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J. Investig. Dermatol. 2012, 132, 1416–1424. [Google Scholar] [CrossRef]
- Peric, M.; Koglin, S.; Dombrowski, Y.; Gross, K.; Bradac, E.; Buchau, A.; Steinmeyer, A.; Zugel, U.; Ruzicka, T.; Schauber, J. Vitamin D analogs differentially control antimicrobial peptide/”alarmin” expression in psoriasis. PLoS ONE 2009, 4, e6340. [Google Scholar] [CrossRef]
- Wierzbicka, J.M.; Piotrowska, A.; Purzycka-Bohdan, D.; Olszewska, A.; Nowak, J.I.; Szczerkowska-Dobosz, A.; Nedoszytko, B.; Nowicki, R.J.; Żmijewski, M.A. The Effects of Vitamin D on the Expression of IL-33 and Its Receptor ST2 in Skin Cells; Potential Implication for Psoriasis. Int. J. Mol. Sci. 2021, 22, 12907. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Gong, Y.; Zhang, X.; Cui, L.; Chen, R.; Yu, Y.; Yu, Q.; Chen, Y.; Diao, H.; et al. Interleukin-33 alleviates psoriatic inflammation by suppressing the T helper type 17 immune response. Immunology 2020, 160, 382–392. [Google Scholar] [CrossRef]
- Grant, W.B. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog. Biophys. Mol. Biol. 2006, 92, 65–79. [Google Scholar] [CrossRef]
- AlSafar, H.; Grant, W.B.; Hijazi, R.; Uddin, M.; Alkaabi, N.; Tay, G.; Mahboub, B.; Al Anouti, F. COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents. Nutrients 2021, 13, 1714. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, Y.; Li, X.; Qiu, J.; Fang, P. Low serum 25-hydroxyvitamin D levels are associated with perennial allergic rhinitis but not disease severity. J. Clin. Lab. Anal. 2020, 34, e23516. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Filoni, A.; Vestita, M.; Congedo, M.; Giudice, G.; Tafuri, S.; Bonamonte, D. Association between psoriasis and vitamin D: Duration of disease correlates with decreased vitamin D serum levels: An observational case-control study. Medicine 2018, 97, e11185. [Google Scholar] [CrossRef] [PubMed]
- Grassi, T.; Panico, A.; Bagordo, F.; Imbriani, G.; Gambino, I.; Lobreglio, D.; Lobreglio, G.; Congedo, M.; De Donno, A. Direct detection of free vitamin D as a tool to assess risk conditions associated with chronic plaque psoriasis. J. Prev. Med. Hyg. 2020, 61, E489–E495. [Google Scholar] [CrossRef] [PubMed]
- Pitukweerakul, S.; Thavaraputta, S.; Prachuapthunyachart, S.; Karnchanasorn, R. Hypovitaminosis D is Associated with Psoriasis: A Systematic Review and Meta-Analysis. Kans. J. Med. 2019, 12, 103–108. [Google Scholar] [CrossRef]
- Kmieć, P.; Żmijewski, M.; Waszak, P.; Sworczak, K.; Lizakowska-Kmieć, M. Vitamin D deficiency during winter months among an adult, predominantly urban, population in Northern Poland. Endokrynol. Pol. 2014, 65, 105–113. [Google Scholar] [CrossRef]
- Morimoto, S.; Yoshikawa, K.; Fukuo, K.; Shiraishi, T.; Koh, E.; Imanaka, S.; Kitano, S.; Ogihara, T. Inverse relation between severity of psoriasis and serum 1, 25-dihydroxyvitamin D level. J. Dermatol. Sci. 1990, 1, 277–282. [Google Scholar] [CrossRef]
- Tajjour, R.; Baddour, R.; Redwan, F.; Hassan, F. The relationship between psoriasis and serum levels of vitamin D. J. Adv. Med. Med. Res. 2018, 26, 1–12. [Google Scholar] [CrossRef]
- Bergler-Czop, B.; Brzezinska-Wcislo, L. Serum vitamin D level—The effect on the clinical course of psoriasis. Postepy Dermatol. Alergol. 2016, 33, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Ricceri, F.; Pescitelli, L.; Tripo, L.; Prignano, F. Deficiency of serum concentration of 25-hydroxyvitamin D correlates with severity of disease in chronic plaque psoriasis. J. Am. Acad. Dermatol. 2013, 68, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Orgaz-Molina, J.; Buendia-Eisman, A.; Arrabal-Polo, M.A.; Ruiz, J.C.; Arias-Santiago, S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: A case-control study. J. Am. Acad. Dermatol. 2012, 67, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Srirama, L. Serum concentration of 25-hydroxy vitamin D in psoriatic patients in a tertiary care hospital: A case–control study. Egypt. J. Dermatol. Venerol. 2016, 36, 29. [Google Scholar] [CrossRef]
- Abd Mallick, Y.; Jiwani, A. Serum Concentration of 25-Hydroxy Vitamin D in Patients with Chronic Plaque Psoriasis: A case control study. J. Dow Univ. Health Sci. 2020, 14, 47–53. [Google Scholar]
- Maleki, M.; Nahidi, Y.; Azizahari, S.; Meibodi, N.T.; Hadianfar, A. Serum 25-OH Vitamin D Level in Psoriatic Patients and Comparison With Control Subjects. J. Cutan. Med. Surg. 2016, 20, 207–210. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: A meta-analysis. Clin. Exp. Dermatol. 2018, 43, 529–535. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef]
- Romani, J.; Caixas, A.; Carrascosa, J.M.; Ribera, M.; Rigla, M.; Luelmo, J. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br. J. Dermatol. 2012, 166, 1237–1244. [Google Scholar] [CrossRef]
- Vahavihu, K.; Ala-Houhala, M.; Peric, M.; Karisola, P.; Kautiainen, H.; Hasan, T.; Snellman, E.; Alenius, H.; Schauber, J.; Reunala, T. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br. J. Dermatol. 2010, 163, 321–328. [Google Scholar] [CrossRef]
- Saleky, S.; Bulur, I.; Saracoglu, Z.N. Narrowband UVB treatment increases serum 25-hydroxyvitamin D levels in patients with chronic plaque psoriasis. Cutis 2017, 99, 431–435. [Google Scholar]
- Ryan, C.; Moran, B.; McKenna, M.J.; Murray, B.F.; Brady, J.; Collins, P.; Rogers, S.; Kirby, B. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch. Dermatol. 2010, 146, 836–842. [Google Scholar] [CrossRef]
- Ala-Houhala, M.J.; Karppinen, T.; Vahavihu, K.; Kautiainen, H.; Dombrowski, Y.; Snellman, E.; Schauber, J.; Reunala, T. Narrow-band ultraviolet B treatment boosts serum 25-hydroxyvitamin D in patients with psoriasis on oral vitamin D supplementation. Acta Derm. Venereol. 2014, 94, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Shojaati, G.; Spanaus, K.S.; Navarini, A.; Theler, B.; Donghi, D.; Urosevic-Maiwald, M.; Glatz, M.; Imhof, L.; Barysch, M.J.; et al. Phototherapy with UVB narrowband, UVA/UVBnb, and UVA1 differentially impacts serum 25-hydroxyvitamin-D3. J. Am. Acad. Dermatol. 2013, 69, 530–536. [Google Scholar] [CrossRef]
- Le, P.; Tu, J.; Gebauer, K.; Brown, S. Serum 25-hydroxyvitamin D increases with NB-UVB and UVA/UVB phototherapy in patients with psoriasis and atopic dermatitis in Western Australia. Australas. J. Dermatol. 2016, 57, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Osmancevic, A.; Landin-Wilhelmsen, K.; Larko, O.; Krogstad, A.L. Vitamin D status in psoriasis patients during different treatments with phototherapy. J. Photochem. Photobiol. B 2010, 101, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, A.; Narbutt, J.; Pawlaczyk, M.; Sysa-Jedrzejowska, A.; Krzyscin, J. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol. Photoimmunol. Photomed. 2011, 27, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Vandikas, M.S.; Landin-Wilhelmsen, K.; Holmang, A.; Gillstedt, M.; Osmancevic, A. High levels of serum vitamin D-binding protein in patients with psoriasis: A case-control study and effects of ultraviolet B phototherapy. J. Steroid Biochem. Mol. Biol. 2021, 211, 105895. [Google Scholar] [CrossRef]
- Bouillon, R.; Van Assche, F.A.; Van Baelen, H.; Heyns, W.; De Moor, P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J. Clin. Investig. 1981, 67, 589–596. [Google Scholar] [CrossRef]
- Bouillon, R.; Van Baelen, H. Transport of vitamin D: Significance of free and total concentrations of the vitamin D metabolites. Calcif. Tissue Int. 1981, 33, 451–453. [Google Scholar] [CrossRef]
- Skobowiat, C.; Slominski, A.T. UVB Activates Hypothalamic-Pituitary-Adrenal Axis in C57BL/6 Mice. J. Investig. Dermatol. 2015, 135, 1638–1648. [Google Scholar] [CrossRef]
- Skobowiat, C.; Slominski, A.T. Ultraviolet B stimulates proopiomelanocortin signalling in the arcuate nucleus of the hypothalamus in mice. Exp. Dermatol. 2016, 25, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Postlethwaite, A.E.; Slominski, A.T. Skin Exposure to Ultraviolet B Rapidly Activates Systemic Neuroendocrine and Immunosuppressive Responses. Photochem. Photobiol. 2017, 93, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Sayre, R.M.; Dowdy, J.C.; Slominski, A.T. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br. J. Dermatol. 2013, 168, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Nejati, R.; Lu, L.; Williams, R.W.; Slominski, A.T. Genetic variation of the cutaneous HPA axis: An analysis of UVB-induced differential responses. Gene 2013, 530, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jozwicki, W.; Slominski, A.T. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: New data and analyses. Anticancer Res. 2014, 34, 2735–2743. [Google Scholar]
- Knabl, J.; Vattai, A.; Ye, Y.; Jueckstock, J.; Hutter, S.; Kainer, F.; Mahner, S.; Jeschke, U. Role of Placental VDR Expression and Function in Common Late Pregnancy Disorders. Int. J. Mol. Sci. 2017, 18, 2340. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Castillo, D.; Morcillo, S.; Clemente-Postigo, M.; Crujeiras, A.B.; Fernandez-Garcia, J.C.; Torres, E.; Tinahones, F.J.; Macias-Gonzalez, M. Adipose tissue inflammation and VDR expression and methylation in colorectal cancer. Clin. Epigenetics 2018, 10, 60. [Google Scholar] [CrossRef]
- Garg, M.; Royce, S.G.; Tikellis, C.; Shallue, C.; Sluka, P.; Wardan, H.; Hosking, P.; Monagle, S.; Thomas, M.; Lubel, J.S.; et al. The intestinal vitamin D receptor in inflammatory bowel disease: Inverse correlation with inflammation but no relationship with circulating vitamin D status. Therap. Adv. Gastroenterol. 2019, 12, 1756284818822566. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.; Żmijewski, M.A.; Linowiecka, K.; Kim, T.-K.; Slominski, R.M.; Slominski, A.T. The disturbed expression of vitamin D and retinoic acid-related orphan receptors α and γ and of megalin in inflammatory skin diseases. Exp. Dermatol. 2022, 31, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Fu, T.; Lau, C.; Oh, D.H.; Bikle, D.D.; Asgari, M.M. Vitamin D in cutaneous carcinogenesis: Part II. J. Am. Acad. Dermatol. 2012, 67, 817.e1–817.e11. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Fu, T.; Lau, C.; Oh, D.H.; Bikle, D.D.; Asgari, M.M. Vitamin D in cutaneous carcinogenesis: Part I. J. Am. Acad. Dermatol. 2012, 67, 803.e1–803.e12. [Google Scholar] [CrossRef] [PubMed]
- Gniadecki, R.; Gajkowska, B.; Hansen, M. 1,25-dihydroxyvitamin D3 stimulates the assembly of adherens junctions in keratinocytes: Involvement of protein kinase C. Endocrinology 1997, 138, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Sorensen, S.; Solvsten, H.; Kragballe, K. The vitamin D3 receptor and retinoid X receptors in psoriatic skin: The receptor levels correlate with the receptor binding to DNA. Br. J. Dermatol. 1998, 138, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Milde, P.; Hauser, U.; Simon, T.; Mall, G.; Ernst, V.; Haussler, M.R.; Frosch, P.; Rauterberg, E.W. Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J. Investig. Dermatol. 1991, 97, 230–239. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, S.; Lee, E.S. Toll-like receptors and antimicrobial peptides expressions of psoriasis: Correlation with serum vitamin D level. J. Korean Med. Sci. 2010, 25, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Visconti, B.; Paolino, G.; Carotti, S.; Pendolino, A.L.; Morini, S.; Richetta, A.G.; Calvieri, S. Immunohistochemical expression of VDR is associated with reduced integrity of tight junction complex in psoriatic skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Stefanic, M.; Rucevic, I.; Barisic-Drusko, V. Meta-analysis of vitamin D receptor polymorphisms and psoriasis risk. Int. J. Dermatol. 2013, 52, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H. Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: An updated meta-analysis. Clin. Exp. Dermatol. 2019, 44, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, A.; Wodz, K.; Ciazynska, M.; Skibinska, M.; Waszczykowski, M.; Ciazynski, K.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J. TaaI/Cdx-2 AA Variant of VDR Defines the Response to Phototherapy amongst Patients with Psoriasis. Life 2021, 11, 567. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Fili, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Peiser, M. Role of Th17 cells in skin inflammation of allergic contact dermatitis. Clin. Dev. Immunol. 2013, 2013, 261037. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, X.; Liang, Y.; Xie, H.; Dai, Z.; Zheng, G. Transcription Factor Retinoid-Related Orphan Receptor γt: A Promising Target for the Treatment of Psoriasis. Front. Immunol. 2018, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- Gauld, S.B.; Jacquet, S.; Gauvin, D.; Wallace, C.; Wang, Y.; McCarthy, R.; Goess, C.; Leys, L.; Huang, S.; Su, Z.; et al. Inhibition of Interleukin-23-Mediated Inflammation with a Novel Small Molecule Inverse Agonist of RORγt. J. Pharmacol. Exp. Ther. 2019, 371, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Ecoeur, F.; Weiss, J.; Kaupmann, K.; Hintermann, S.; Orain, D.; Guntermann, C. Antagonizing Retinoic Acid-Related-Orphan Receptor Gamma Activity Blocks the T Helper 17/Interleukin-17 Pathway Leading to Attenuated Pro-inflammatory Human Keratinocyte and Skin Responses. Front. Immunol. 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Choo, M.K.; Park, J.M.; Fisher, D.E. Topical ROR Inverse Agonists Suppress Inflammation in Mouse Models of Atopic Dermatitis and Acute Irritant Dermatitis. J. Investig. Dermatol. 2017, 137, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.V.; Al-Jaberi, F.A.H.; Damas, N.D.; Weinert, B.T.; Pus, U.; Torres-Rusillo, S.; Woetmann, A.; Odum, N.; Bonefeld, C.M.; Kongsbak-Wismann, M.; et al. Vitamin D Inhibits IL-22 Production Through a Repressive Vitamin D Response Element in the il22 Promoter. Front. Immunol. 2021, 12, 715059. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Disphanurat, W.; Viarasilpa, W.; Chakkavittumrong, P.; Pongcharoen, P. The Clinical Effect of Oral Vitamin D2 Supplementation on Psoriasis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol. Res. Pract. 2019, 2019, 5237642. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Zouboulis, C.C.; Vogt, T.; Holick, M.F. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: An historical view and outlook. Rev. Endocr. Metab. Disord. 2016, 17, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Krafka, J., Jr. A simple treatment for psoriasis. J. Lab. Clin. Med. 1936, 21, 1147–1148. [Google Scholar]
- Ceder, E.T.; Zon, L. Treatment of psoriasis with massive doses of crystalline vitamin D and irradiated ergosterol, A preliminary report. Public Health Rep. 1937, 52, 1580–1584. [Google Scholar] [CrossRef]
- Morimoto, S.; Kumahara, Y. A patient with psoriasis cured by 1 α-hydroxyvitamin D3. Med. J. Osaka Univ. 1985, 35, 51–54. [Google Scholar] [PubMed]
- Morimoto, S.; Yoshikawa, K. Psoriasis and vitamin D3: A review of our experience. Arch. Dermatol. 1989, 125, 231–234. [Google Scholar] [CrossRef]
- Morimoto, S.; Yoshikawa, K.; Kozuka, T.; Kitano, Y.; Imanaka, S.; Fukuo, K.; Koh, E.; Kumahara, Y. An open study of vitamin D3 treatment in psoriasis vulgaris. Br. J. Dermatol. 1986, 115, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, S.; Onishi, T.; Morimoto, S.; Imanaka, S.; Yukawa, S.; Kozuka, T.; Kitano, Y.; Seino, Y.; Kumahara, Y. Effect of 1 α-hydroxycholecalciferol on psoriasis vulgaris: A pilot study. Calcif. Tissue Int. 1986, 39, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Onishi, T.; Imanaka, S.; Yukawa, H.; Kozuka, T.; Kitano, Y.; Yoshikawa, K.; Kumahara, Y. Topical administration of 1,25-dihydroxyvitamin D3 for psoriasis: Report of five cases. Calcif. Tissue Int. 1986, 38, 119–122. [Google Scholar] [CrossRef]
- Morimoto, S.; Imanaka, S.; Koh, E.; Shiraishi, T.; Nabata, T.; Kitano, S.; Miyashita, Y.; Nishii, Y.; Ogihara, T. Comparison of the inhibitions of proliferation of normal and psoriatic fibroblasts by 1 α,25-dihydroxyvitamin D3 and synthetic analogues of vitamin D3 with an oxygen atom in their side chain. Biochem. Int. 1989, 19, 1143–1149. [Google Scholar] [PubMed]
- MacLaughlin, J.A.; Gange, W.; Taylor, D.; Smith, E.; Holick, M.F. Cultured psoriatic fibroblasts from involved and uninvolved sites have a partial but not absolute resistance to the proliferation-inhibition activity of 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1985, 82, 5409–5412. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Pincus, S.H.; Donovan, L.; Holick, M.F. A novel approach for the evaluation and treatment of psoriasis. Oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J. Am. Acad. Dermatol. 1988, 19, 516–528. [Google Scholar] [CrossRef]
- Miyachi, Y.; Ohkawara, A.; Ohkido, M.; Harada, S.; Tamaki, K.; Nakagawa, H.; Hori, Y.; Nishiyama, S. Long-term safety and efficacy of high-concentration (20 microg/g) tacalcitol ointment in psoriasis vulgaris. Eur. J. Dermatol. 2002, 12, 463–468. [Google Scholar] [PubMed]
- Syuto, T.; Ishibuchi, H.; Sogabe, Y.; Yokoyama, Y.; Ishikawa, O. Efficacy of high-concentration tacalcitol ointment in psoriasis vulgaris after changing from other high-concentration vitamin D3 ointments. Dermatol. Online J. 2008, 14, 2. [Google Scholar] [PubMed]
- Soleymani, T.; Hung, T.; Soung, J. The role of vitamin D in psoriasis: A review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Liu, K.; Wan, D.; Wu, Z.; Cao, Z.; Luo, Y.; Xiao, C.; Yin, M. Vitamin D receptor gene polymorphisms are associated with psoriasis susceptibility and the clinical response to calcipotriol in psoriatic patients. Exp. Dermatol. 2020, 29, 1186–1190. [Google Scholar] [CrossRef]
- Ryan, C.; Renfro, L.; Collins, P.; Kirby, B.; Rogers, S. Clinical and genetic predictors of response to narrowband ultraviolet B for the treatment of chronic plaque psoriasis. Br. J. Dermatol. 2010, 163, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, B.S.; Choi, K.H.; Jeon, J.H.; Cho, K.H.; Song, K.Y.; Kim, I.G.; Youn, J.I. Vitamin D receptor genotypes are not associated with clinical response to calcipotriol in Korean psoriasis patients. Arch. Dermatol. Res. 2002, 294, 1–5. [Google Scholar] [CrossRef]
- Holland, D.B.; Wood, E.J.; Roberts, S.G.; West, M.R.; Cunliffe, W.J. Epidermal keratin levels during oral 1-α-hydroxyvitamin D3 treatment for psoriasis. Skin Pharmacol. 1989, 2, 68–76. [Google Scholar] [CrossRef]
- Huckins, D.; Felson, D.T.; Holick, M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: A pilot study. Arthritis Rheum. 1990, 33, 1723–1727. [Google Scholar] [CrossRef]
- Prtina, A.; Raseta Simovic, N.; Milivojac, T.; Vujnic, M.; Grabez, M.; Djuric, D.; Stojiljkovic, M.P.; Soldat Stankovic, V.; Colic, M.J.; Skrbic, R. The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients. Biomolecules 2021, 11, 1865. [Google Scholar] [CrossRef]
- Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinology 2013, 5, 222–234. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef]
- Ingram, M.A.; Jones, M.B.; Stonehouse, W.; Jarrett, P.; Scragg, R.; Mugridge, O.; von Hurst, P.R. Oral vitamin D3 supplementation for chronic plaque psoriasis: A randomized, double-blind, placebo-controlled trial. J. Dermatolog. Treat 2018, 29, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, P.; Camargo, C.A., Jr.; Coomarasamy, C.; Scragg, R. A randomized, double-blind, placebo-controlled trial of the effect of monthly vitamin D supplementation in mild psoriasis. J. Dermatolog. Treat. 2018, 29, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Prystowsky, J.H.; Muzio, P.J.; Sevran, S.; Clemens, T.L. Effect of UVB phototherapy and oral calcitriol (1,25-dihydroxyvitamin D3) on vitamin D photosynthesis in patients with psoriasis. J. Am. Acad. Dermatol. 1996, 35, 690–695. [Google Scholar] [CrossRef]
- Theodoridis, X.; Grammatikopoulou, M.G.; Stamouli, E.M.; Talimtzi, P.; Pagkalidou, E.; Zafiriou, E.; Haidich, A.B.; Bogdanos, D.P. Effectiveness of oral vitamin D supplementation in lessening disease severity among patients with psoriasis: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2021, 82, 111024. [Google Scholar] [CrossRef]
- Gumowski-Sunek, D.; Rizzoli, R.; Saurat, J.H. Effects of topical calcipotriol on calcium metabolism in psoriatic patients: Comparison with oral calcitriol. Dermatologica 1991, 183, 275–279. [Google Scholar] [CrossRef]
- Shah, K.N. Diagnosis and treatment of pediatric psoriasis: Current and future. Am. J. Clin. Dermatol. 2013, 14, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Kragballe, K. Treatment of psoriasis by the topical application of the novel cholecalciferol analogue calcipotriol (MC 903). Arch. Dermatol. 1989, 125, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Staberg, B.; Roed-Petersen, J.; Menne, T. Efficacy of topical treatment in psoriasis with MC903, a new vitamin D analogue. Acta Derm. Venereol. 1989, 69, 147–150. [Google Scholar] [PubMed]
- Oxholm, A.; Oxholm, P.; Staberg, B.; Bendtzen, K. Expression of interleukin-6-like molecules and tumour necrosis factor after topical treatment of psoriasis with a new vitamin D analogue (MC 903). Acta Derm. Venereol. 1989, 69, 385–390. [Google Scholar] [PubMed]
- Kokelj, F.; Lavaroni, G.; Guadagnini, A. UVB versus UVB plus calcipotriol (MC 903) therapy for psoriasis vulgaris. Acta Derm. Venereol. 1995, 75, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.; Altmeyer, P.; Kaufmann, R.; Ring, J.; Christophers, E.; Pavel, S.; Ziegler, J. Topical calcipotriol plus oral fumaric acid is more effective and faster acting than oral fumaric acid monotherapy in the treatment of severe chronic plaque psoriasis vulgaris. Dermatology 2002, 205, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kokelj, F.; Torsello, P.; Plozzer, C. Calcipotriol improves the efficacy of cyclosporine in the treatment of psoriasis vulgaris. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 143–146. [Google Scholar] [CrossRef]
- Rim, J.H.; Park, J.Y.; Choe, Y.B.; Youn, J.I. The efficacy of calcipotriol + acitretin combination therapy for psoriasis: Comparison with acitretin monotherapy. Am. J. Clin. Dermatol. 2003, 4, 507–510. [Google Scholar] [CrossRef]
- Kircik, L.H. Topical calcipotriene 0.005% and betamethasone dipropionate 0.064% maintains efficacy of etanercept after step-down dose in patients with moderate-to-severe plaque psoriasis: Results of an open label trial. J. Drugs Dermatol. 2011, 10, 878–882. [Google Scholar] [PubMed]
- Pinter, A.; Green, L.J.; Selmer, J.; Praestegaard, M.; Gold, L.S.; Augustin, M.; Trial Investigator Group. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2021, 36, 228–236. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Rizzuto, F.; Dastoli, S.; Patruno, C.; Bianchi, L.; Nistico, S.P. A novel vehicle for the treatment of psoriasis. Dermatol. Ther. 2020, 33, e13185. [Google Scholar] [CrossRef] [PubMed]
- Van de Kerkhof, P.C.; Berth-Jones, J.; Griffiths, C.E.; Harrison, P.V.; Honigsmann, H.; Marks, R.; Roelandts, R.; Schopf, E.; Trompke, C. Long-term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. Br. J. Dermatol. 2002, 146, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Aggarwal, K.; Jain, V.K. Tacalcitol: A useful adjunct to narrow band ultraviolet B phototherapy in psoriasis. J. Dermatolog. Treat. 2016, 27, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Morikawa, M.; Miyamoto, K.; Kaiho, S.; Fukushima, M.; Miyaura, C.; Abe, E.; Suda, T.; Nishii, Y. Synthetic analogues of vitamin D3 with an oxygen atom in the side chain skeleton. A trial of the development of vitamin D compounds which exhibit potent differentiation-inducing activity without inducing hypercalcemia. FEBS Lett. 1987, 226, 58–62. [Google Scholar] [CrossRef]
- Barker, J.N.; Ashton, R.E.; Marks, R.; Harris, R.I.; Berth-Jones, J. Topical maxacalcitol for the treatment of psoriasis vulgaris: A placebo-controlled, double-blind, dose-finding study with active comparator. Br. J. Dermatol. 1999, 141, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, T.; Asahina, A.; Komine, M.; Tamaki, K. Treatment of psoriasis vulgaris with narrow-band UVB and topical Maxacalcitol. Acta Derm. Venereol. 2006, 86, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Furuhashi, T.; Matsumoto, K.; Morita, A. Safety profiles of topical vitamin D3 in psoriasis patients: A retrospective large-scale study. Psoriasis Targets Ther. 2012, 2, 81. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).