Effects of Combining Biofactors on Bioenergetic Parameters, Aβ Levels and Survival in Alzheimer Model Organisms
Abstract
:1. Introduction
2. Results
2.1. General Overview of Tests and Results
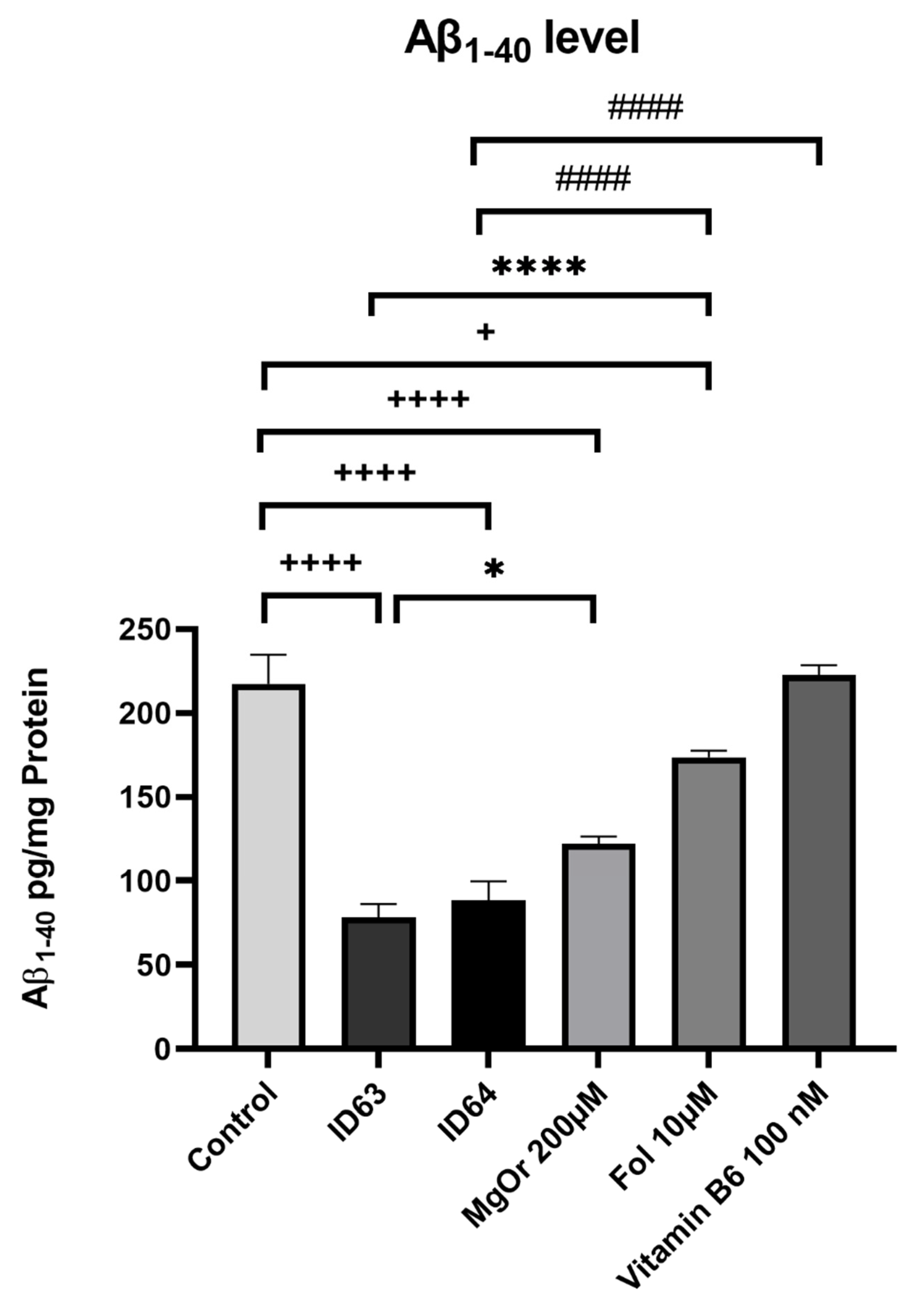
2.2. Aβ1–40 Production
2.3. Aβ1–42 Production
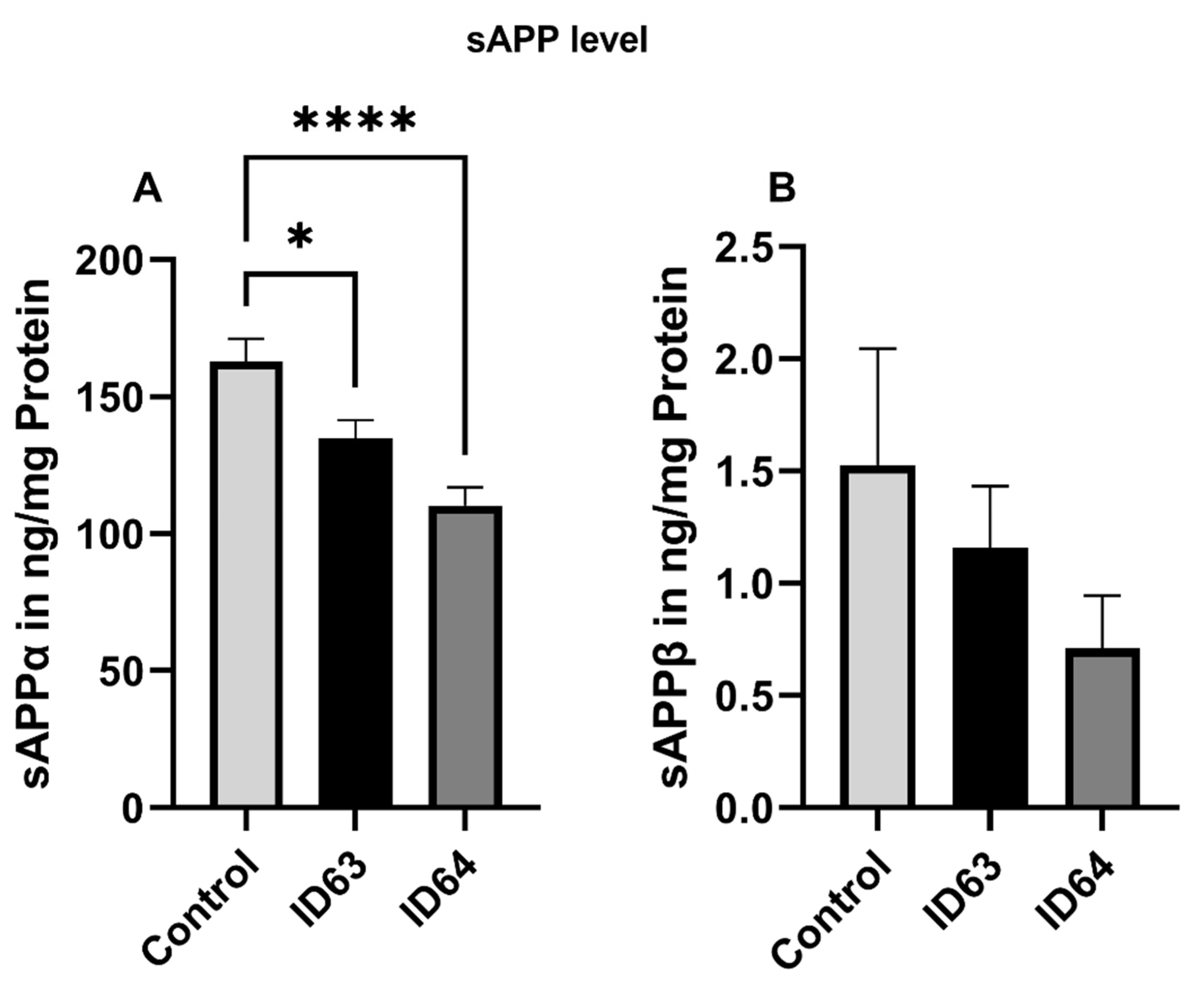
2.4. sAPPα and sAPPβ Level
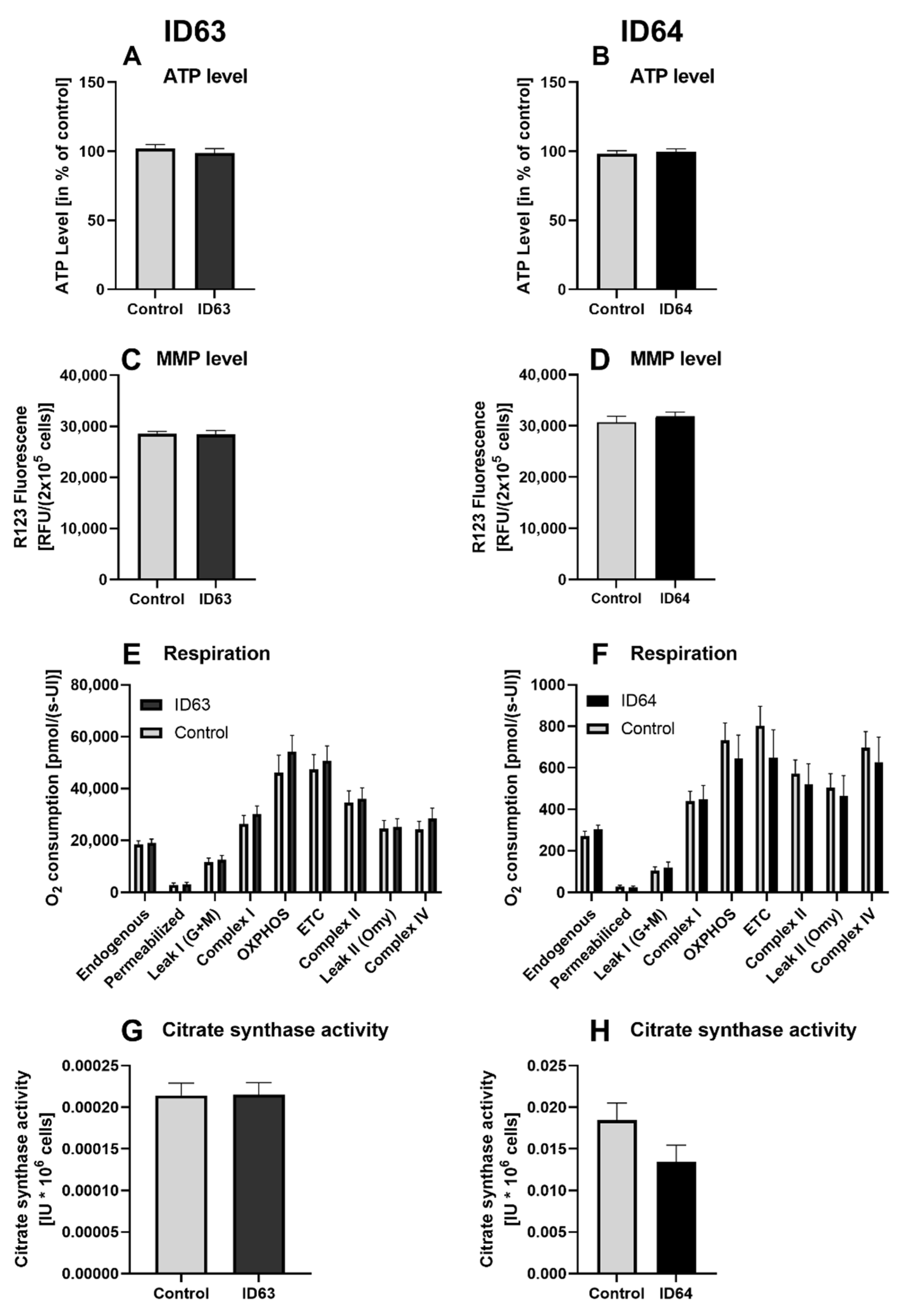
2.5. Effect on the Mitochondrial Function
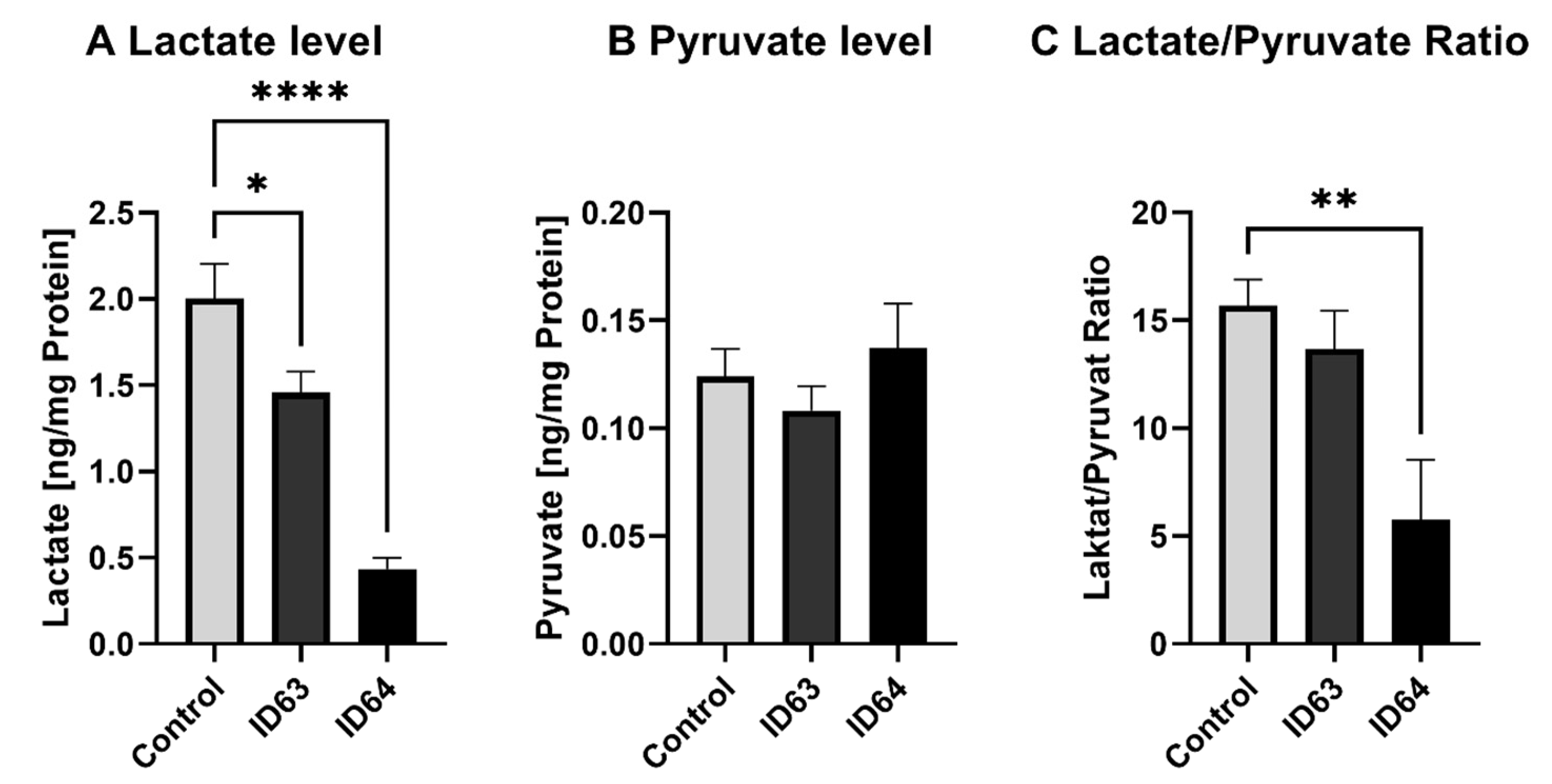
2.6. Lactate and Pyruvate Level
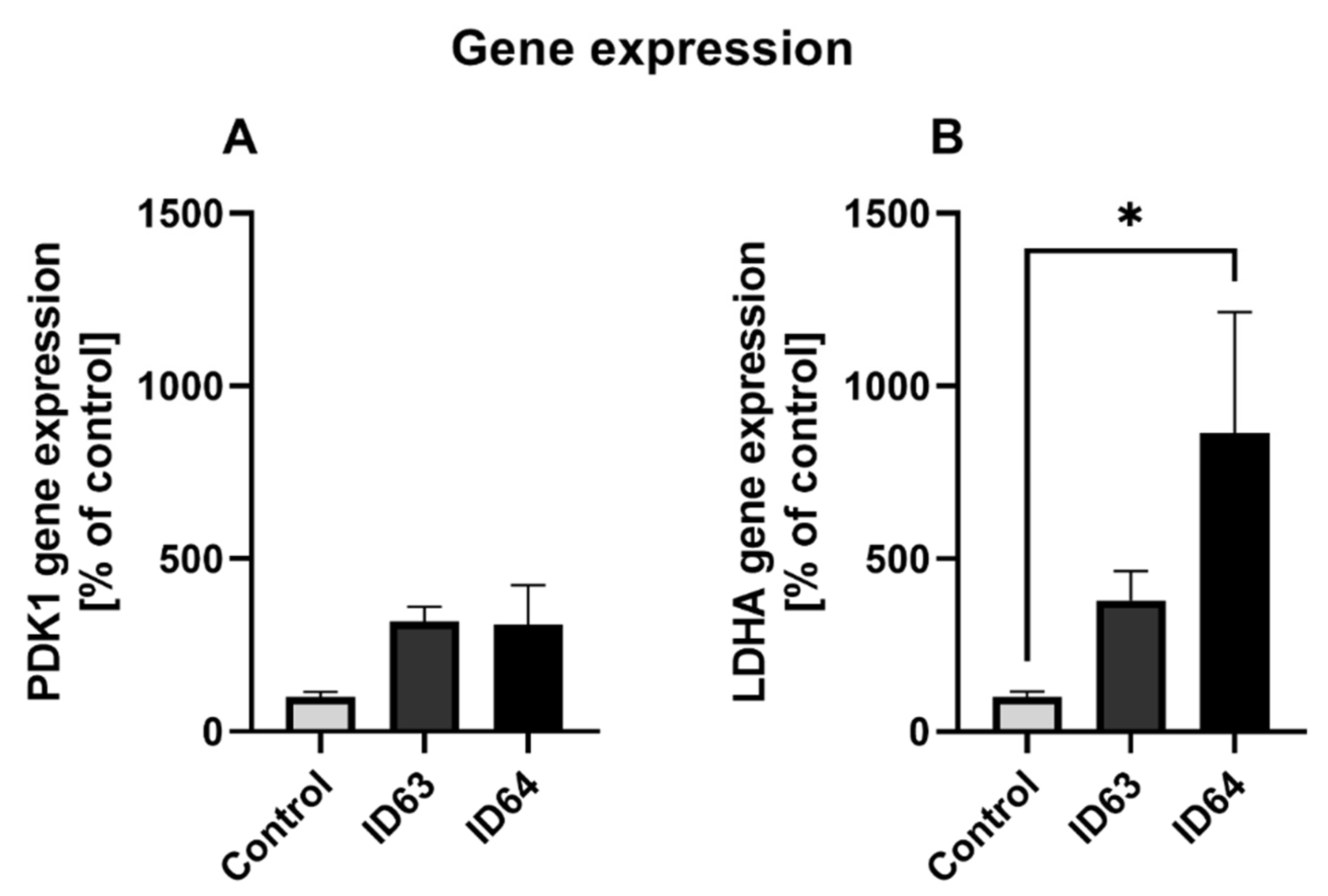
2.7. qPCR
2.8. Effect on the Lifespan of C. elegans in Heat Stress Survival Assay
2.9. Effect on the Paralysis
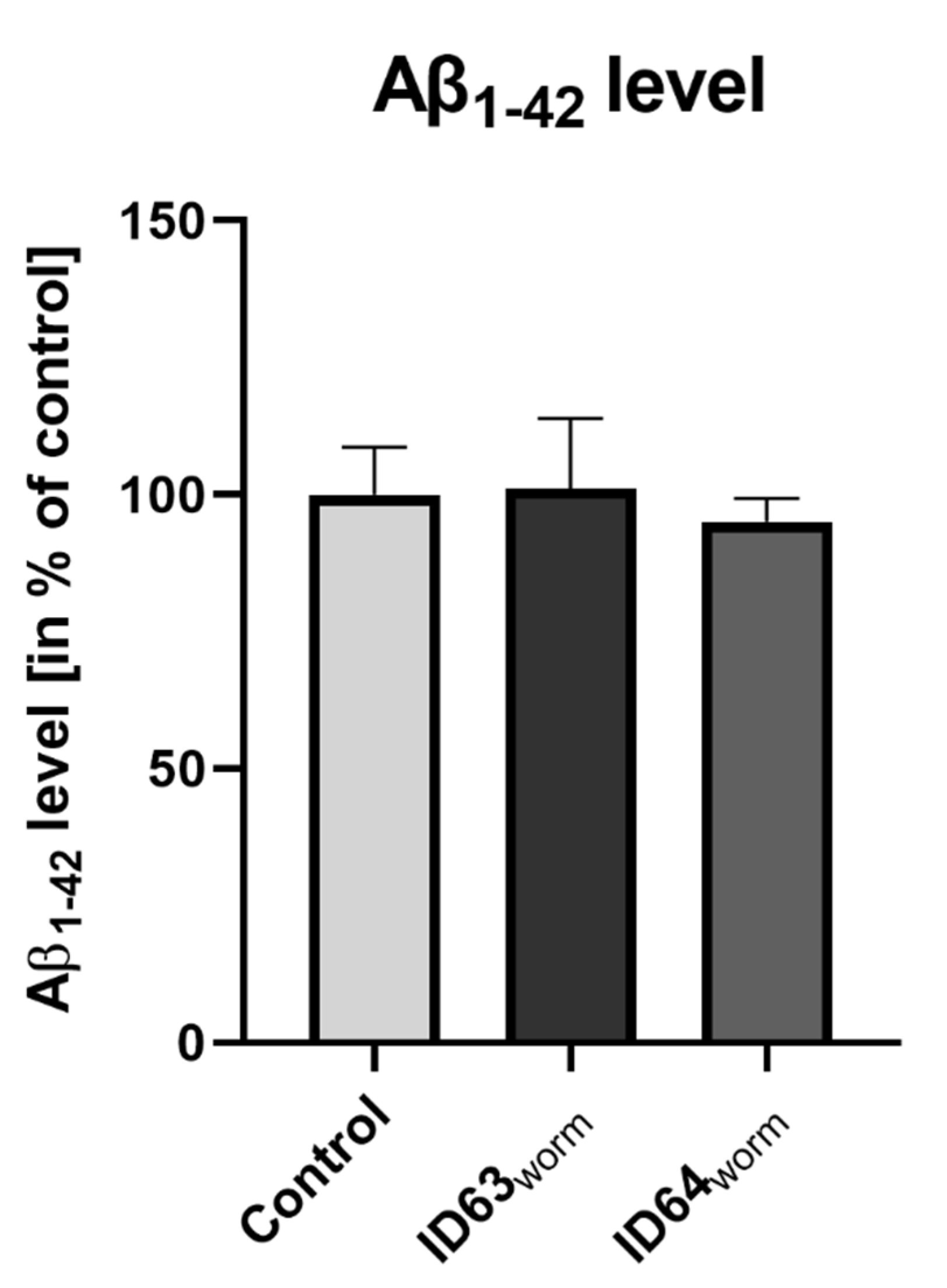
2.10. Aβ1–42 Production in GMC101
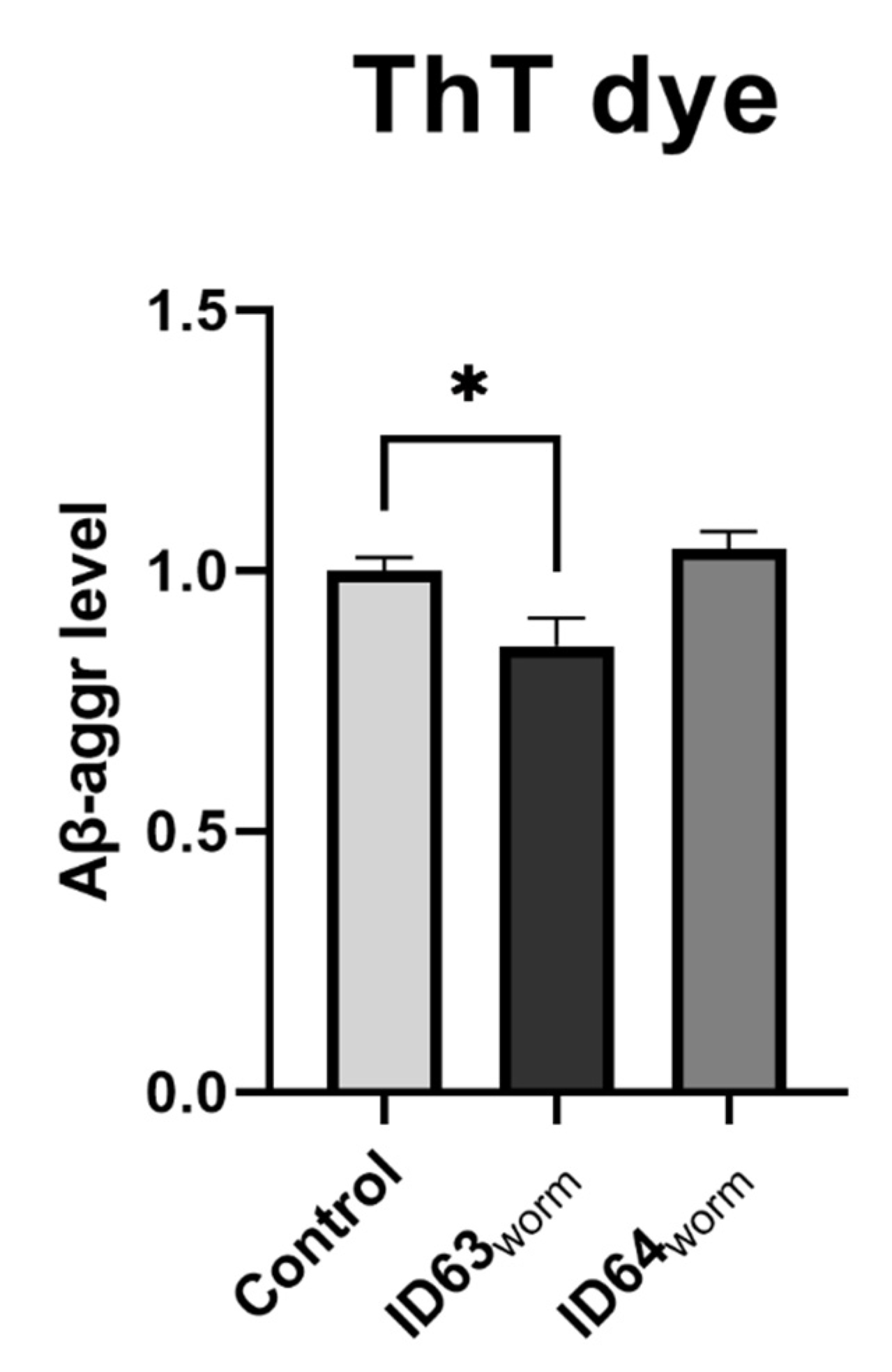
2.11. Aβ1–42 Aggregation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Treatment
4.3. ATP Measurement
4.4. MMP Measurement
4.5. Cellular Respiration
4.6. Citrate Synthase Activity
4.7. Aβ1–40 Measurement
4.8. Aβ1–42 Measurement
4.9. Protein Quantification
4.10. Quantification of Human Soluble Amyloid Precursor Protein α (sAPPα)
4.11. Quantification of Human Soluble Amyloid Precursor Protein β (sAPPβ)
4.12. Pyruvate and Lactate Content
4.13. Real-Time qRT-PCR
4.14. Nematode and Bacterial Strain
4.15. Cultivation and Treatment
4.16. Paralyze Assay
4.17. Heat-Stress Survival Assay
4.18. ThT Dying of Aβ Aggregates
4.19. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.M.P.; Swerdlow, R.H. Mitochondrial dysfunction in Alzheimer’s disease: Role in pathogenesis and novel therapeutic opportunities. J. Cereb. Blood Flow Metab. 2019, 176, 3489–3507. [Google Scholar] [CrossRef]
- Gallardo, G.; Holtzman, D.M. Amyloid-β and Tau at the Crossroads of Alzheimer’s Disease. Tau Biol. 2019, 1184, 187–203. [Google Scholar] [CrossRef]
- Landau, S.M.; Harvey, D.; Madison, C.M.; Koeppe, R.A.; Reiman, E.M.; Foster, N.L.; Weiner, M.W.; Jagust, W.J.; The Alzheimer’s Disease Neuroimaging Initiative. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging 2011, 32, 1207–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, K.; Giordano, T.; Brady, D.R.; Stoll, J.; Martin, L.J.; Rapoport, S.I. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Mol. Brain Res. 1994, 24, 336–340. [Google Scholar] [CrossRef]
- Parker, W.D., Jr.; Parks, J.; Filley, C.M.; Kleinschmidt-DeMasters, B.K. Electron transport chain defects in Alzheimer’s disease brain. Neurology 1994, 44, 1090–1096. [Google Scholar] [CrossRef]
- Müller, W.E.; Eckert, A.; Kurz, C.; Eckert, G.P.; Leuner, K. Mitochondrial Dysfunction: Common Final Pathway in Brain Aging and Alzheimer’s Disease—Therapeutic Aspects. Mol. Neurobiol. 2010, 41, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, B.; Zheng, L.; Perry, G.; Smith, M.A.; Zhu, X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2009, 109, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Rhein, V.; Song, X.; Wiesner, A.; Ittner, L.M.; Baysang, G.; Meier, F.; Ozmen, L.; Bluethmann, H.; Dröse, S.; Brandt, U.; et al. Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20057–20062. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Nunan, J.; Small, D.H. Regulation of APP cleavage by α-, β- and γ-secretases. FEBS Lett. 2000, 483, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef]
- Kuperstein, I.; Broersen, K.; Benilova, I.; Rozenski, J.; Jonckheere, W.; Debulpaep, M.; Vandersteen, A.; Segers-Nolten, G.M.; Van Der Werf, K.; Subramaniam, V.; et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010, 29, 3408–3420. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, K.; Williams, T.L.; Morris, K.L.; Jonckheere, W.; Vandersteen, A.; Kelly, G.; Schymkowitz, J.; Rousseau, F.; Pastore, A.; Serpell, L.C.; et al. Structural Basis for Increased Toxicity of Pathological Aβ42:Aβ40 Ratios in Alzheimer Disease. J. Biol. Chem. 2012, 287, 5650–5660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chasseigneaux, S.; Allinquant, B. Functions of Aβ, sAPPα and sAPPβ: Similarities and differences. J. Neurochem. 2011, 120, 99–108. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Q.; Zhang, Y.-W.; Xu, H. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. J. Neurochem. 2011, 120, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Blass, J.P.; Sheu, R.K.-F.; Gibson, G.E. Inherent Abnormalities in Energy Metabolism in Alzheimer Disease: Interaction with Cerebrovascular Compromise. Ann. N. Y. Acad. Sci. 2000, 903, 204–221. [Google Scholar] [CrossRef]
- Harris, R.A.; Tindale, L.; Lone, A.; Singh, O.; Macauley, S.L.; Stanley, M.; Holtzman, D.M.; Bartha, R.; Cumming, R.C. Aerobic Glycolysis in the Frontal Cortex Correlates with Memory Performance in Wild-Type Mice But Not the APP/PS1 Mouse Model of Cerebral Amyloidosis. J. Neurosci. 2016, 36, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Soucek, T.; Cumming, R.; Dargusch, R.; Maher, P.; Schubert, D. The Regulation of Glucose Metabolism by HIF-1 Mediates a Neuroprotective Response to Amyloid Beta Peptide. Neuron 2003, 39, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Cheng, X.; Dang, R.; Zhang, W.; Zhang, J.; Yao, Z. Lactate Deficit in an Alzheimer Disease Mouse Model: The Relationship with Neuronal Damage. J. Neuropathol. Exp. Neurol. 2018, 77, 1163–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newington, J.T.; Rappon, T.; Albers, S.; Wong, D.Y.; Rylett, R.J.; Cumming, R.C. Overexpression of Pyruvate Dehydrogenase Kinase 1 and Lactate Dehydrogenase A in Nerve Cells Confers Resistance to Amyloid β and Other Toxins by Decreasing Mitochondrial Respiration and Reactive Oxygen Species Production. J. Biol. Chem. 2012, 287, 37245–37258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, J.; Kisters, K.; Stirban, O.A.; Obeid, R.; Lorkowski, S.; Wallert, M.; Egert, S.; Podszun, M.C.; Eckert, G.P.; Pettersen, J.A.; et al. The role of biofactors in the prevention and treatment of age-related diseases. BioFactors 2021, 47, 522–550. [Google Scholar] [CrossRef]
- Botchway, B.O.; Moore, M.K.; Akinleye, F.O.; Iyer, I.C.; Fang, M. Nutrition: Review on the Possible Treatment for Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Dangour, A.D.; Whitehouse, P.J.; Rafferty, K.; Mitchell, S.A.; Smith, L.; Hawkesworth, S.; Vellas, B. B-Vitamins and Fatty Acids in the Prevention and Treatment of Alzheimer’s Disease and Dementia: A Systematic Review. J. Alzheimer’s Dis. 2010, 22, 205–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielech, A.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Vitamins in Alzheimer’s Disease—Review of the Latest Reports. Nutrients 2020, 12, 3458. [Google Scholar] [CrossRef]
- Viel, C.; Brandtner, A.T.; Weißhaar, A.; Lehto, A.; Fuchs, M.; Klein, J. Effects of Magnesium Orotate, Benfotiamine and a Combination of Vitamins on Mitochondrial and Cholinergic Function in the TgF344-AD Rat Model of Alzheimer’s Disease. Pharmaceuticals 2021, 14, 1218. [Google Scholar] [CrossRef]
- Ebara, S. Nutritional role of folate. Congenit. Anomalies 2017, 57, 138–141. [Google Scholar] [CrossRef]
- Gressner, A.M.; Arndt, T. Lexikon der Medizinischen Laboratoriumsdiagnostik; Living reference work, continuously updated ed., Springer Reference Medizin; Springer: Berlin, Germany, 2017. [Google Scholar]
- Hinterberger, M.; Fischer, P. Folate and Alzheimer: When time matters. J. Neural Transm. 2012, 120, 211–224. [Google Scholar] [CrossRef]
- Robinson, N.; Grabowski, P.; Rehman, I. Alzheimer’s disease pathogenesis: Is there a role for folate? Mech. Ageing Dev. 2018, 174, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Classen, H.G. Magnesium orotate—Experimental and clinical evidence. Rom. J. Intern. Med. 2004, 42, 491–501. [Google Scholar] [PubMed]
- Toffa, D.H.; Magnerou, M.A.; Kassab, A.; Djibo, F.H.; Sow, A.D. Can magnesium reduce central neurodegeneration in Alzheimer’s disease? Basic evidences and research needs. Neurochem. Int. 2019, 126, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. Int. Rev. J. 2013, 4, 378S–383S. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Sun, M.; Chen, Z.; Lu, J.; Liu, Y.; Zhou, L.; Xu, X.; Fan, D.; Chui, D. Magnesium Modulates Amyloid-β Protein Precursor Trafficking and Processing. J. Alzheimer’s Dis. 2010, 20, 1091–1106. [Google Scholar] [CrossRef]
- Du, K.; Zheng, X.; Ma, Z.-T.; Lv, J.-Y.; Jiang, W.-J.; Liu, M.-Y. Association of Circulating Magnesium Levels in Patients with Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 13. [Google Scholar] [CrossRef]
- Hellmann, H.; Mooney, S. Vitamin B6: A Molecule for Human Health? Molecules 2010, 15, 442–459. [Google Scholar] [CrossRef] [Green Version]
- Hashim, A.; Wang, L.; Juneja, K.; Ye, Y.; Zhao, Y.; Ming, L.-J. Vitamin B6s inhibit oxidative stress caused by Alzheimer’s disease-related CuII-β-amyloid complexes—cooperative action of phospho-moiety. Bioorganic Med. Chem. Lett. 2011, 21, 6430–6432. [Google Scholar] [CrossRef]
- Flicker, L.; Martins, R.; Thomas, J.; Acres, J.; Taddei, K.; Vasikaran, S.D.; Norman, P.; Jamrozik, K.; Almeida, O.P. B-vitamins reduce plasma levels of beta amyloid. Neurobiol. Aging 2008, 29, 303–305. [Google Scholar] [CrossRef]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, G.F.G.; Neergheen, V.; Oppenheim, M.; Fitzgerald, J.C.; Footitt, E.; Hyland, K.; Clayton, P.T.; Land, J.M.; Heales, S.J.R. Pyridoxal 5′-phosphate deficiency causes a loss of aromatic l-amino acid decarboxylase in patients and human neuroblastoma cells, implications for aromatic l-amino acid decarboxylase and vitamin B6 deficiency states. J. Neurochem. 2010, 114, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Romero, A.; Molz, S.; Martín-De-Saavedra, M.; Egea, J.; Del Barrio, L.; Tasca, C.; Rodrigues, A.; López, M. Neurotoxicity induced by dexamethasone in the human neuroblastoma SH-SY5Y cell line can be prevented by folic acid. Neuroscience 2011, 190, 346–353. [Google Scholar] [CrossRef]
- Li, W.; Jiang, M.; Xiao, Y.; Zhang, X.; Cui, S.; Huang, G. Folic acid inhibits tau phosphorylation through regulation of PP2A methylation in SH-SY5Y cells. J. Nutr. Health Aging 2014, 19, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ke, Z.; Lv, M.; Lin, R.; Wu, B.; Zheng, Z. Effects of MgSO 4 and magnesium transporters on 6-hydroxydopamine-induced SH-SY5Y cells. Life Sci. 2017, 172, 48–54. [Google Scholar] [CrossRef]
- Vrolijk, M.F.; Opperhuizen, A.; Jansen, E.H.; Hageman, G.J.; Bast, A.; Haenen, G.R. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol. Vitr. 2017, 44, 206–212. [Google Scholar] [CrossRef]
- Li, W.; Jiang, M.; Zhao, S.; Liu, H.; Zhang, X.; Wilson, J.X.; Huang, G. Folic Acid Inhibits Amyloid β-Peptide Production through Modulating DNA Methyltransferase Activity in N2a-APP Cells. Int. J. Mol. Sci. 2015, 16, 25002–25013. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic Acid Supplementation Mitigates Alzheimer’s Disease by Reducing Inflammation: A Randomized Controlled Trial. Mediat. Inflamm. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, S.; Fuso, A.; D’Anselmi, F.; Cavallaro, R.A. Presenilin 1 gene silencing by S-adenosylmethionine: A treatment for Alzheimer disease? FEBS Lett. 2003, 541, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Das, N.; Ren, J.; Spence, J.; Chapman, S.B. Phosphate Brain Energy Metabolism and Cognition in Alzheimer’s Disease: A Spectroscopy Study Using Whole-Brain Volume-Coil 31Phosphorus Magnetic Resonance Spectroscopy at 7Tesla. Front. Neurosci. 2021, 15, 641739. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Elgharabawy, R.; Al-Najjar, A.H. Ameliorating effect of anti-Alzheimer’s drugs on the bidirectional association between type 2 diabetes mellitus and Alzheimer’s disease. Exp. Biol. Med. 2017, 242, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Bai, D.; Li, W.; Huang, G.-W.; Liu, H. Effects of Folic Acid on Secretases Involved in Aβ Deposition in APP/PS1 Mice. Nutrients 2016, 8, 556. [Google Scholar] [CrossRef] [Green Version]
- Kamenetz, F.; Tomita, T.; Hsieh, H.; Seabrook, G.; Borchelt, D.; Iwatsubo, T.; Sisodia, S.; Malinow, R. APP Processing and Synaptic Function. Neuron 2003, 37, 925–937. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Ruan, Y.-T.; Zhao, J.; Yang, Y.-W.; Chen, L.-P.; Mai, Y.-R.; Yu, Q.; Cao, Z.-Y.; Liu, F.-F.; Liao, W.; et al. Magnesium-L-threonate exhibited a neuroprotective effect against oxidative stress damage in HT22 cells and Alzheimer’s disease mouse model. World J. Psychiatry 2022, 12, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Andreasson, U.; Hansson, O.; Wu, G.; Sankaranarayanan, S.; Andersson, M.E.; Buchhave, P.; Londos, E.; Umek, R.M.; Minthon, L.; et al. Elevated Cerebrospinal Fluid BACE1 Activity in Incipient Alzheimer Disease. Arch. Neurol. 2008, 65, 1102–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, A.; Höglund, K.; Sjögren, M.; Andreasen, N.; Minthon, L.; Lannfelt, L.; Buerger, K.; Möller, H.-J.; Hampel, H.; Davidsson, P.; et al. Measurement of α- and β-secretase cleaved amyloid precursor protein in cerebrospinal fluid from Alzheimer patients. Exp. Neurol. 2003, 183, 74–80. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Yu, M.; Zhang, X.; Zhang, M.; Wilson, J.X.; Huang, G. Folic acid administration inhibits amyloid β-peptide accumulation in APP/PS1 transgenic mice. J. Nutr. Biochem. 2015, 26, 883–891. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Zhao, S.; Zhang, X.; Zhang, M.; Xiao, Y.; Wilson, J.X.; Huang, G. Folic acid attenuates the effects of amyloid β oligomers on DNA methylation in neuronal cells. Eur. J. Nutr. 2015, 55, 1849–1862. [Google Scholar] [CrossRef]
- Babylon, L.; Grewal, R.; Stahr, P.-L.; Eckert, R.; Keck, C.; Eckert, G. Hesperetin Nanocrystals Improve Mitochondrial Function in a Cell Model of Early Alzheimer Disease. Antioxidants 2021, 10, 1003. [Google Scholar] [CrossRef]
- Ryu, W.-I.; Bormann, M.K.; Shen, M.; Kim, D.; Forester, B.; Park, Y.; So, J.; Seo, H.; Sonntag, K.-C.; Cohen, B.M. Brain cells derived from Alzheimer’s disease patients have multiple specific innate abnormalities in energy metabolism. Mol. Psychiatry 2021, 26, 5702–5714. [Google Scholar] [CrossRef]
- Ross, J.M.; Öberg, J.; Brené, S.; Coppotelli, G.; Terzioglu, M.; Pernold, K.; Goiny, M.; Sitnikov, R.; Kehr, J.; Trifunovic, A.; et al. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc. Natl. Acad. Sci. USA 2010, 107, 20087–20092. [Google Scholar] [CrossRef] [Green Version]
- Parnetti, L.; Gaiti, A.; Polidori, M.; Brunetti, M.; Palumbo, B.; Chionne, F.; Cadini, D.; Cecchetti, R.; Senin, U. Increased cerebrospinal fluid pyruvate levels in Alzheimer’s disease. Neurosci. Lett. 1995, 199, 231–233. [Google Scholar] [CrossRef]
- Liguori, C.; Stefani, A.; Sancesario, G.; Sancesario, G.; Marciani, M.; Pierantozzi, M. CSF lactate levels, τ proteins, cognitive decline: A dynamic relationship in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 655–659. [Google Scholar] [CrossRef]
- Parnetti, L.; Reboldi, G.; Gallai, V. Cerebrospinal fluid pyruvate levels in Alzheimer’s disease and vascular dementia. Neurology 2000, 54, 735. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Reshkin, S.J.; Harguindey, S. Pharmacological interventions part IV: Metabolic modifiers. In An Innovative Approach to Understanding and Treating Cancer: Targeting pH—From Etiopatho-Genesis to New Therapeutic Avenues; Koltai, T., Ed.; Elsevier Science & Technology: San Diego, CA, USA, 2020; pp. 361–416. [Google Scholar]
- Uyeda, K. Pyruvate Kinase. In Encyclopedia of Biological Chemistry, 2nd ed.; Elsevier: London, UK, 2013; pp. 719–721. [Google Scholar]
- Newington, J.T.; Pitts, A.; Chien, A.; Arseneault, R.; Schubert, D.; Cumming, R.C. Amyloid Beta Resistance in Nerve Cell Lines Is Mediated by the Warburg Effect. PLoS ONE 2011, 6, e19191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Link, C.D. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1995, 92, 9368–9372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Zhao, X.; Zhang, Y.; Xie, J.; Zhou, A. 6‴-Feruloylspinosin alleviated beta-amyloid induced toxicity by promoting mitophagy in Caenorhabditis elegans (GMC101) and PC12 cells. Sci. Total Environ. 2020, 715, 136953. [Google Scholar] [CrossRef]
- McColl, G.; Roberts, B.R.; Pukala, T.L.; Kenche, V.B.; Roberts, C.M.; Link, C.D.; Ryan, T.M.; Masters, C.L.; Barnham, K.J.; Bush, A.I.; et al. Utility of an improved model of amyloid-beta (Aβ1-42) toxicity in Caenorhabditis elegans for drug screening for Alzheimer’s disease. Mol. Neurodegener. 2012, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Leiteritz, A.; Dilberger, B.; Wenzel, U.; Fitzenberger, E. Betaine reduces β-amyloid-induced paralysis through activation of cystathionine-β-synthase in an Alzheimer model of Caenorhabditis elegans. Genes Nutr. 2018, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, B.; Park, S. Selenocysteine mimics the effect of dietary restriction on lifespan via SKN-1 and retards age-associated pathophysiological changes in Caenorhabditis elegans. Mol. Med. Rep. 2018, 18, 5389–5398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, S.T.; Johnson, T.E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Dorman, J.B.; Rodan, A.; Kenyon, C. daf-16: An HNF-3/forkhead Family Member That Can Function to Double the Life-Span of Caenorhabditis elegans. Science 1997, 278, 1319–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesp, K.; Smant, G.; Kammenga, J.E. Caenorhabditis elegans DAF-16/FOXO transcription factor and its mammalian homologs associate with age-related disease. Exp. Gerontol. 2015, 72, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathor, L.; Akhoon, B.A.; Pandey, S.; Srivastava, S.; Pandey, R. Folic acid supplementation at lower doses increases oxidative stress resistance and longevity in Caenorhabditis elegans. AGE 2015, 37, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Wang, W.; Zhang, Z.; Li, M.; Chen, Z.; Wang, J.; Zhao, Y.; An, L.; Wang, Y.; Xing, S.; et al. Ethanol Alleviates Amyloid-β-Induced Toxicity in an Alzheimer’s Disease Model of Caenorhabiditis elegans. Front. Aging Neurosci. 2021, 13. [Google Scholar] [CrossRef]
- Regitz, C.; Dußling, L.M.; Wenzel, U. Amyloid-beta (Aβ1-42)-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol. Nutr. Food Res. 2014, 58, 1931–1940. [Google Scholar] [CrossRef]
- Regitz, C.; Fitzenberger, E.; Mahn, F.L.; Dußling, L.M.; Wenzel, U. Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. Eur. J. Nutr. 2015, 55, 741–747. [Google Scholar] [CrossRef]
- Yu, X.; Guan, P.; Guo, J.; Wang, Y.; Cao, L.; Xu, G.; Konstantopoulos, K.; Wang, Z.; Wang, P. By suppressing the expression of anterior pharynx-defective-1α and -1β and inhibiting the aggregation of β-amyloid protein, magnesium ions inhibit the cognitive decline of amyloid precursor protein/presenilin 1 transgenic mice. FASEB J. 2015, 29, 5044–5058. [Google Scholar] [CrossRef]
- Grewal, R.; Reutzel, M.; Dilberger, B.; Hein, H.; Zotzel, J.; Marx, S.; Tretzel, J.; Sarafeddinov, A.; Fuchs, C.; Eckert, G.P. Purified oleocanthal and ligstroside protect against mitochondrial dysfunction in models of early Alzheimer’s disease and brain ageing. Exp. Neurol. 2020, 328, 113248. [Google Scholar] [CrossRef]
- Hagl, S.; Grewal, R.; Ciobanu, I.; Helal, A.; Khayyal, M.T.; Muller, W.E.; Eckert, G.P. Rice Bran Extract Compensates Mitochondrial Dysfunction in a Cellular Model of Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 43, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, S.; Renner, K.; Pollheimer, J.; Moser, P.L.; Zeimet, A.G.; Offner, F.A.; Gnaiger, E. Preserved Coupling of Oxidative Phosphorylation but Decreased Mitochondrial Respiratory Capacity in IL-1β-Treated Human Peritoneal Mesothelial Cells. Cell Biophys. 2006, 44, 179–186. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans; WormBook: Pasadena, CA, USA, 2006; pp. 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fitzenberger, E.; Deusing, D.J.; Marx, C.; Boll, M.; Lüersen, K.; Wenzel, U. The polyphenol quercetin protects themev-1mutant of Caenorhabditis elegans from glucose-induced reduction of survival under heat-stress depending on SIR-2.1, DAF-12, and proteasomal activity. Mol. Nutr. Food Res. 2014, 58, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Babylon, L.; Dieter, F.; Eckert, G.P. Effects of Pesticides on Longevity and Bioenergetics in Invertebrates—The Impact of Polyphenolic Metabolites. Int. J. Mol. Sci. 2021, 22, 13478. [Google Scholar] [CrossRef]
- Huang, J.; Chen, S.; Hu, L.; Niu, H.; Sun, Q.; Li, W.; Tan, G.; Li, J.; Jin, L.; Lyu, J.; et al. Mitoferrin-1 is Involved in the Progression of Alzheimer’s Disease Through Targeting Mitochondrial Iron Metabolism in a Caenorhabditis elegans Model of Alzheimer’s Disease. Neuroscience 2018, 385, 90–101. [Google Scholar] [CrossRef] [PubMed]



| ID63 vs. CTR | ID64 vs. CTR | MgOr vs. CTR | Fol vs. CTR | Vit B6 vs. CTR | |
|---|---|---|---|---|---|
| Aβ1–40 | Significant lower | Significant lower | Significant lower | Significant lower | No significant change |
| Aβ1–42 | No significant change | Significant lower | Significant lower | Significant lower | Significant lower |
| sAPPα | Significant lower | Significant lower | Not tested | Not tested | Not tested |
| sAPPβ | No significant change | No significant change | Not tested | Not tested | Not tested |
| ATP level | No significant change | No significant change | Not tested | Not tested | Not tested |
| MMP level | No significant change | No significant change | Not tested | Not tested | Not tested |
| Respiration | No significant change | No significant change | Not tested | Not tested | Not tested |
| Citrate synthase activity | No significant change | No significant change | Not tested | Not tested | Not tested |
| Lactate level | Significant lower | Significant lower | Not tested | Not tested | Not tested |
| Pyruvate level | No significant change | No significant change | Not tested | Not tested | Not tested |
| Lactate/Pyruvate Ratio | No significant change | Significant lower | Not tested | Not tested | Not tested |
| Gen expression PDK1 | No significant change | No significant change | Not tested | Not tested | Not tested |
| Gen expression LDHA | No significant change | Significant higher | Not tested | Not tested | Not tested |
| Lifespan C. elegans in % | No significant change | Significant higher | Significant higher | Significant higher | Significant higher |
| Mean survival C. elegans | Significant higher | Significant higher | Significant higher | Significant higher | Significant higher |
| Paralysis C. elegans | Significant lower | Significant lower | Not tested | Not tested | Not tested |
| Aβ1–42 C. elegans | No significant change | No significant change | Not tested | Not tested | Not tested |
| Aβ1–42 aggreation C. elegans | Significant lower | No significant change | Not tested | Not tested | Not tested |
| Primer | Sequence | Manufacturer | Product Size | Concentration (nM) |
|---|---|---|---|---|
| ß-Actin (ACTB) NM_001101.2 | 5′-ggacttcgagcaagagatgg-3′ 5′-agcactgtgttggcgtacag-3′ | Biomol, Hamburg, Germany | 234 | 200 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) NM_002046.2 | 5′-gagtcaacggatttggtcgt-3′ 5′-ttgattttggagggatctcg-3′ | Biomol, Hamburg, Germany | 238 | 200 |
| Phosphoglycerate kinase 1 (PGK1) NM_000291.2 | 5′-ctgtgggggtatttgaatgg-3′ 5′-cttccaggagctccaaa-3′ | Biomol, Hamburg, Germany | 198 | 200 |
| Pyruvate dehydrogenase kinase, isozyme 1 (PDK1) NM_002610 | 5′-atacggatcagaaaccgaca-3′ 5′-cagacgcctagcattttcat-3′ | Biomol, Hamburg, Germany | 291 | 100 |
| Human lactate dehydrogenase A like 6B (LDHA) NM_033195 | 5′-ggtgtccctttgaaggatct-3′ 5′-tgcagtcacttctttgtgga-3′ | Biomol, Hamburg, Germany | 87 | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babylon, L.; Schmitt, F.; Franke, Y.; Hubert, T.; Eckert, G.P. Effects of Combining Biofactors on Bioenergetic Parameters, Aβ Levels and Survival in Alzheimer Model Organisms. Int. J. Mol. Sci. 2022, 23, 8670. https://doi.org/10.3390/ijms23158670
Babylon L, Schmitt F, Franke Y, Hubert T, Eckert GP. Effects of Combining Biofactors on Bioenergetic Parameters, Aβ Levels and Survival in Alzheimer Model Organisms. International Journal of Molecular Sciences. 2022; 23(15):8670. https://doi.org/10.3390/ijms23158670
Chicago/Turabian StyleBabylon, Lukas, Fabian Schmitt, Yannik Franke, Tim Hubert, and Gunter P. Eckert. 2022. "Effects of Combining Biofactors on Bioenergetic Parameters, Aβ Levels and Survival in Alzheimer Model Organisms" International Journal of Molecular Sciences 23, no. 15: 8670. https://doi.org/10.3390/ijms23158670
APA StyleBabylon, L., Schmitt, F., Franke, Y., Hubert, T., & Eckert, G. P. (2022). Effects of Combining Biofactors on Bioenergetic Parameters, Aβ Levels and Survival in Alzheimer Model Organisms. International Journal of Molecular Sciences, 23(15), 8670. https://doi.org/10.3390/ijms23158670






