Further Evidence of Neuroprotective Effects of Recombinant Human Erythropoietin and Growth Hormone in Hypoxic Brain Injury in Neonatal Mice
Abstract
1. Introduction
2. Results
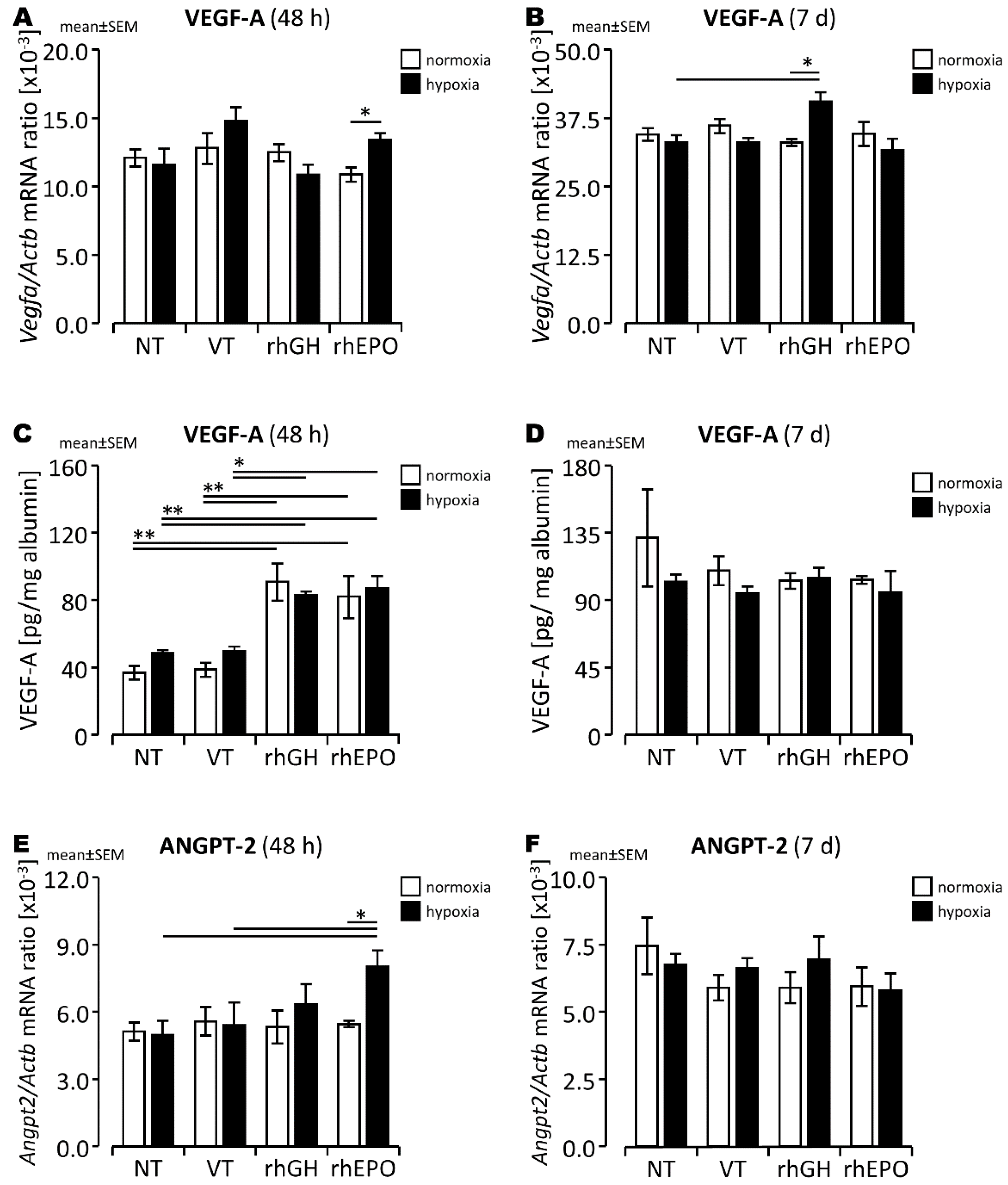
2.1. Differential Changes of Angiogenesis-Associated Gene Expression and Vascular Structures in Response to rhGH in the Hypoxic Developing Mouse Brain
2.2. Differential Changes of Angiogenesis-Associated Gene Expression and Vascular Structures in Response to rhEPO in the Hypoxic Developing Mouse Brain
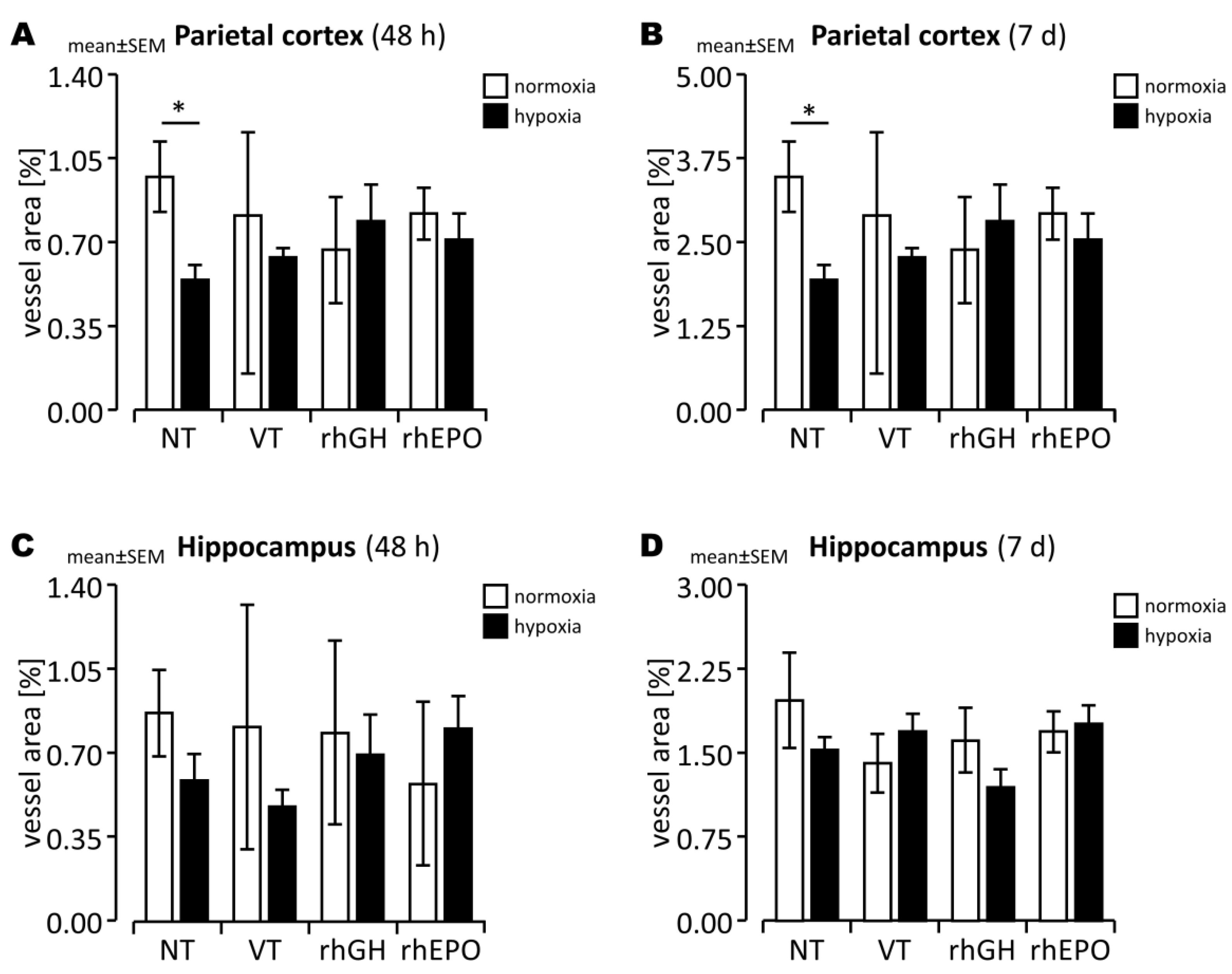
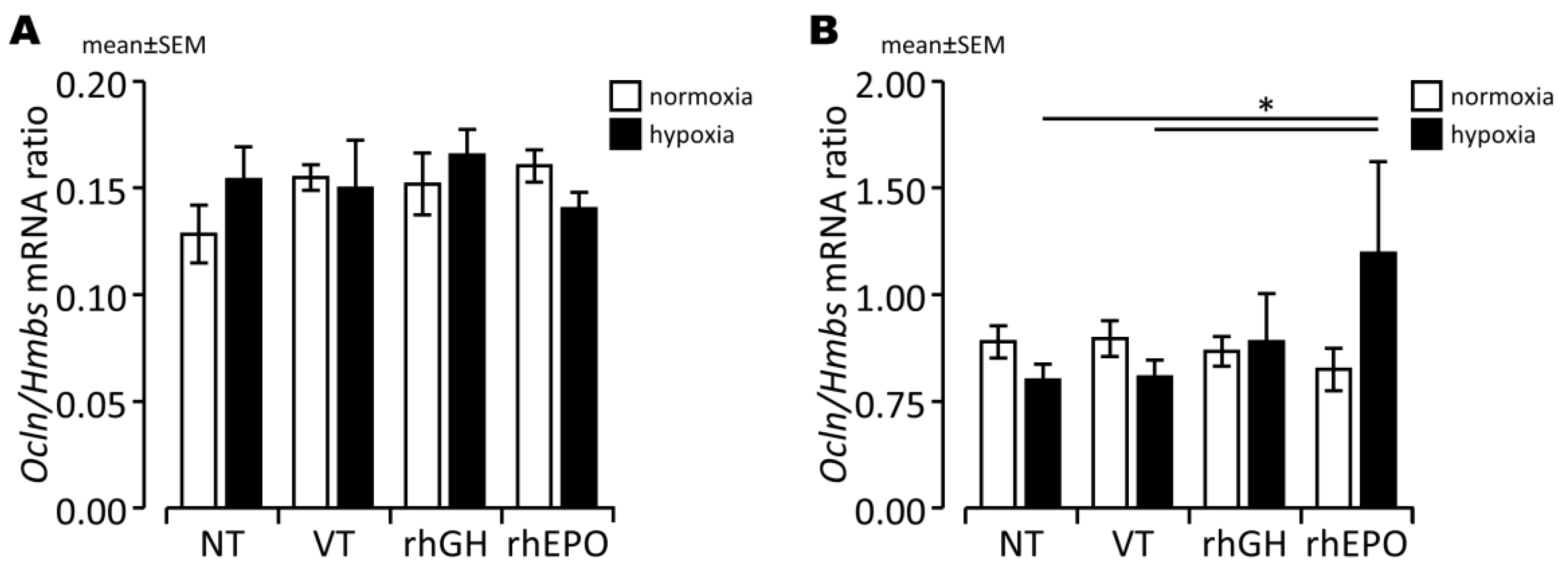
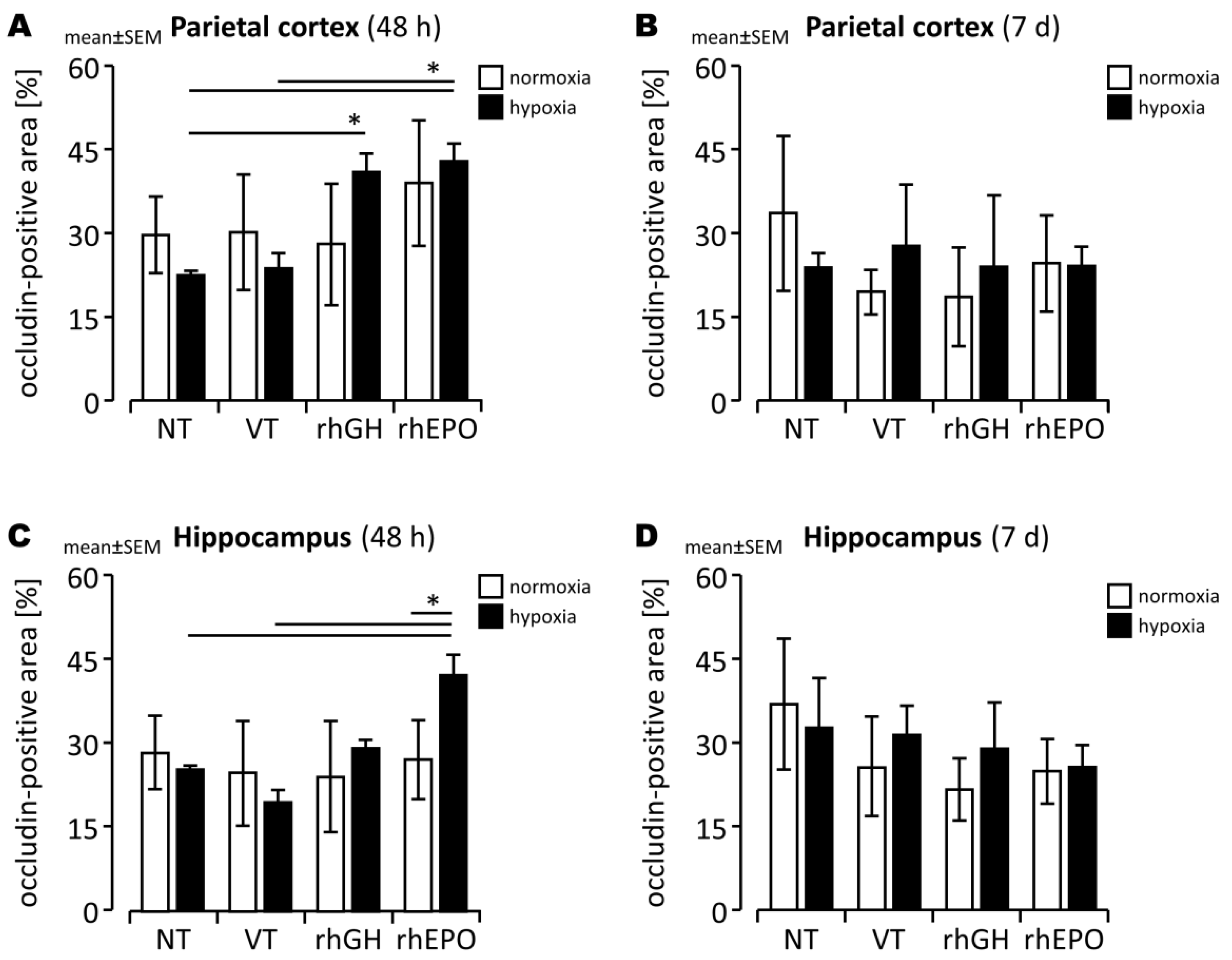
2.3. Effects of rhGH on Hypoxia-Induced Disruption of Developing BBB
2.4. Effects of rhEPO on Hypoxia-Induced Disruption of Developing BBB
2.5. Anti-Inflammatory Effects of rhGH and rhEPO Attenuate Hypoxia-Induced Disruption of the Developing BBB
3. Discussion
3.1. RhGH Has Pro-Angiogenic Effects in the Hypoxic Developing Mouse Brain
3.2. RhEPO Has Pro-Angiogenic Effects in the Hypoxic Developing Mouse Brain
3.3. Prevention of BBB Damage in the Hypoxic Developing Mouse Brain
4. Materials and Methods
4.1. Animal Experiments
4.2. Exposure to Acute Hypoxia
4.3. Drug Treatment Regimens
4.4. Real-Time PCR
4.5. Protein Extraction and Quantification
4.6. ELISA
4.7. Immunohistochemistry
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cainelli, E.; Vedovelli, L.; Mastretta, E.; Gregori, D.; Suppiej, A.; Bisiacchi, P.S. Long-Term Outcomes after Neonatal Hypoxic-Ischemic Encephalopathy in the Era of Therapeutic Hypothermia: A Longitudinal, Prospective, Multicenter Case-Control Study in Children without Overt Brain Damage. Children 2021, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.-Y.; Chen, H.-R.; Gao, N.; Kuo, Y.-M.; Chen, C.-W.; Yang, D.; Kinkaid, M.M.; Hu, E.; Sun, Y.-Y. Brain-targeted hypoxia-inducible factor stabilization reduces neonatal hypoxic-ischemic brain injury. Neurobiol. Dis. 2020, 148, 105200. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, M.; Meng, Y.; Li, H.; Yu, L.; Fu, X.; Tang, Y.; Jiang, C. Erythropoietin improves hypoxic-ischemic encephalopathy in neonatal rats after short-term anoxia by enhancing angiogenesis. Brain Res. 2016, 1651, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Yang, X.; Qu, Y.; Chen, H.; Yue, Y.; Wang, H.; Zhao, F.; Li, S.; Zou, R.; Zhang, L.; et al. Erythropoietin induces synaptogenesis and neurite repair after hypoxia ischemia-mediated brain injury in neonatal rats. Neuro Rep. 2019, 30, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Trollmann, R.; Mühlberger, T.; Richter, M.; Boie, G.; Feigenspan, A.; Brackmann, F.; Jung, S. Differential regulation of angiogenesis in the developing mouse brain in response to exogenous activation of the hypoxia-inducible transcription factor system. Brain Res. 2018, 1688, 91–102. [Google Scholar] [CrossRef]
- Jung, S.; Boie, G.; Doerr, H.-G.; Trollmann, R. Oxygen-sensitive regulation and neuroprotective effects of growth hormone-dependent growth factors during early postnatal development. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R539–R548. [Google Scholar] [CrossRef]
- Cengiz, P.; Zafer, D.; Chandrashekhar, J.H.; Chanana, V.; Bogost, J.; Waldman, A.; Novak, B.; Kintner, D.B.; Ferrazzano, P.A. Developmental differences in microglia morphology and gene expression during normal brain development and in response to hypoxia-ischemia. Neurochem. Int. 2019, 127, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Cao, G.; Yin, W.; Stetler, R.A.; Liu, J.; Chen, J. Erythropoietin Promotes Neuronal Replacement Through Revascularization and Neurogenesis After Neonatal Hypoxia/Ischemia in Rats. Stroke 2007, 38, 2795–2803. [Google Scholar] [CrossRef]
- Vaes, J.E.G.; Kosmeijer, C.M.; Kaal, M.; Van Vliet, R.; Brandt, M.J.V.; Benders, M.J.N.L.; Nijboer, C.H. Regenerative Therapies to Restore Interneuron Disturbances in Experimental Models of Encephalopathy of Prematurity. Int. J. Mol. Sci. 2020, 22, 211. [Google Scholar] [CrossRef]
- Jain, A.; Kratimenos, P.; Koutroulis, I.; Jain, A.; Buddhavarapu, A.; Ara, J. Effect of Intranasally Delivered rh-VEGF165 on Angiogenesis Following Cerebral Hypoxia-Ischemia in the Cerebral Cortex of Newborn Piglets. Int. J. Mol. Sci. 2017, 18, 2356. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Chang, Y.-C.; Lin, Y.-C.; Sze, C.-I.; Huang, C.-C.; Ho, C.-J. Cerebral Microvascular Damage Occurs Early after Hypoxia–Ischemia via nNOS Activation in the Neonatal Brain. J. Cereb. Blood Flow Metab. 2014, 34, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Dasgupta, C.; Li, Y.; Huang, L.; Zhang, L. MicroRNA-210 Suppresses Junction Proteins and Disrupts Blood-Brain Barrier Integrity in Neonatal Rat Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2017, 18, 1356. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ma, Y.; Liu, J.; Zhang, D.; Ren, J.; Zhao, R.; Chang, J.; Guo, Z.-N.; Yang, Y. Biological Functions and Regulatory Mechanisms of Hypoxia-Inducible Factor-1α in Ischemic Stroke. Front. Immunol. 2021, 12, 801985. [Google Scholar] [CrossRef]
- Jin, M.-L.; Zou, Z.-H.; Tao, T.; Li, J.; Xu, J.; Luo, K.-J.; Liu, Z. Effect of the Recombinant Adenovirus-Mediated HIF-1 Alpha on the Expression of VEGF in the Hypoxic Brain Microvascular Endothelial Cells of Rats. Neuropsychiatr. Dis. Treat. 2020, 16, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kestner, R.-I.; Mayser, F.; Vutukuri, R.; Hansen, L.; Günther, S.; Brunkhorst, R.; Devraj, K.; Pfeilschifter, W. Gene Expression Dynamics at the Neurovascular Unit During Early Regeneration After Cerebral Ischemia/Reperfusion Injury in Mice. Front. Neurosci. 2020, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Baltazar-Lara, R.; Ávila-Mendoza, J.; Martínez-Moreno, C.G.; Carranza, M.; Pech-Pool, S.; Vázquez-Martínez, O.; Díaz-Muñoz, M.; Luna, M.; Arámburo, C. Neuroprotective Effects of Growth Hormone (GH) and Insulin-Like Growth Factor Type 1 (IGF-1) after Hypoxic-Ischemic Injury in Chicken Cerebellar Cell Cultures. Int. J. Mol. Sci. 2020, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; König, A.; Lang, M.; Fiedler, J.; Oerter, S.; Roewer, N.; Bohnert, M.; Thal, S.C.; Blecharz-Lang, K.G.; Woitzik, J.; et al. Hypoxia-Induced MicroRNA-212/132 Alter Blood-Brain Barrier Integrity Through Inhibition of Tight Junction-Associated Proteins in Human and Mouse Brain Microvascular Endothelial Cells. Transl. Stroke Res. 2019, 10, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Jiang, H.; Ye, L.; Cai, C.; Hu, Y.; Pan, S.; Li, P.; Xiao, J.; Lin, Z. Metformin treatment after the hypoxia-ischemia attenuates brain injury in newborn rats. Oncotarget 2017, 8, 75308–75325. [Google Scholar] [CrossRef]
- Choi, J.I.; Choi, J.-W.; Shim, K.-H.; Choung, J.S.; Kim, H.-J.; Sim, H.R.; Suh, M.R.; Jung, J.E.; Kim, M. Synergistic Effect in Neurological Recovery via Anti-Apoptotic Akt Signaling in Umbilical Cord Blood and Erythropoietin Combination Therapy for Neonatal Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2021, 22, 11995. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, J.; Ren, C.; Zhang, X.; Gu, L.; Dong, Y.; Zhang, J. Effect of erythropoietin on Fas/FasL expression in brain tissues of neonatal rats with hypoxic-ischemic brain damage. NeuroReport 2019, 30, 262–268. [Google Scholar] [CrossRef]
- Yu, D.-F.; Zhu, L.-H.; Jiang, L. Recombinant Human Erythropoietin Augments Neovascularization Responses in a Neonatal Rat Model of Premature Brain Damage by Phosphatidylinositol 3 Kinase/Akt Pathway. Chin. Med. J. 2017, 130, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Bai, X.; Wang, S.; Hu, Y.; Wang, T.; Qian, L.; Jiang, L. Recombinant Human Erythropoietin Augments Angiogenic Responses in a Neonatal Rat Model of Cerebral Unilateral Hypoxia-Ischemia. Neonatology 2014, 106, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, X.; Liang, J.; Qi, Z.; Liu, X.; Min, L.; Ji, X.; Luo, Y.; Zhao, H. Intra-artery infusion of recombinant human erythropoietin reduces blood–brain barrier disruption in rats following cerebral ischemia and reperfusion. Int. J. Neurosci. 2014, 125, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; LaRosa, D.A.; Zahra, V.A.; Barton, S.K.; Tolcos, M.; Li, A.; Polglase, G.R. Optimizing the Dose of Erythropoietin Required to Prevent Acute Ventilation-Induced Cerebral White Matter Injury in Preterm Lambs. Dev. Neurosci. 2017, 39, 298–309. [Google Scholar] [CrossRef]
- Zhiyuan, Q.; Qingyong, L.; Shengming, H.; Hui, M. Protective effect of rhEPO on tight junctions of cerebral microvascular endothelial cells early following traumatic brain injury in rats. Brain Inj. 2016, 30, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Ajo, R.; Cacicedo, L.; Navarro, C.; Sánchez-Franco, F. Growth Hormone Action on Proliferation and Differentiation of Cerebral Cortical Cells from Fetal Rat. Endocrinology 2003, 144, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, S.; Raccurt, M.; Moudilou, E.; Morel, G.; Brittian, K.; Gozal, D. Exogenous growth hormone attenuates cognitive deficits induced by intermittent hypoxia in rats. Neuroscience 2011, 196, 237–250. [Google Scholar] [CrossRef]
- Kartal, Ö.; Aydınöz, S.; Kartal, A.T.; Kelestemur, T.; Caglayan, A.B.; Beker, M.C.; Karademir, F.; Süleymanoğlu, S.; Kul, M.; Yulug, B.; et al. Time dependent impact of perinatal hypoxia on growth hormone, insulin-like growth factor 1 and insulin-like growth factor binding protein-3. Metab. Brain Dis. 2016, 31, 827–835. [Google Scholar] [CrossRef]
- Le Grevès, M.; Zhou, Q.; Berg, M.; Le Grevès, P.; Fhölenhag, K.; Meyerson, B.; Nyberg, F. Growth hormone replacement in hypophysectomized rats affects spatial performance and hippocampal levels of NMDA receptor subunit and PSD-95 gene transcript levels. Exp. Brain Res 2006, 173, 267–273. [Google Scholar] [CrossRef]
- Kusano, K.; Tsutsumi, Y.; Dean, J.; Gavin, M.; Ma, H.; Silver, M.; Thorne, T.; Zhu, Y.; Losordo, D.W.; Aikawa, R. Long-term stable expression of human growth hormone by rAAV promotes myocardial protection post-myocardial infarction. J. Mol. Cell. Cardiol. 2007, 42, 390–399. [Google Scholar] [CrossRef]
- Rong, S.-L.; Lu, Y.-X.; Liao, Y.-H.; Wang, X.-L.; Wang, Y.-J.; Chang, C.; Wang, Y.-Q.; Liu, Q.-Y.; Gao, Y.-Z.; Mi, S.-H. Effects of transplanted myoblasts transfected with human growth hormone gene on improvement of ventricular function of rats. Chin. Med. J. 2008, 121, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Terörde, K.; Dörr, H.-G.; Trollmann, R. Recombinant Human Growth Hormone Activates Neuroprotective Growth Factors in Hypoxic Brain Injury in Neonatal Mice. Endocrinology 2021, 162, bqab008. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. F-box and WD repeat domain-containing 7 (FBXW7) mediates the hypoxia inducible factor-1α (HIF-1α)/vascular endothelial growth factor (VEGF) signaling pathway to affect hypoxic-ischemic brain damage in neonatal rats. Bioengineered 2021, 13, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Doycheva, D.; Xu, L.; Tang, J.; Yan, M.; Zhang, J.H. Sestrin2 induced by hypoxia inducible factor1 alpha protects the blood-brain barrier via inhibiting VEGF after severe hypoxic-ischemic injury in neonatal rats. Neurobiol. Dis. 2016, 95, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, K.; Zhang, X.; Zhang, J.; Xu, B. ATP Induces Disruption of Tight Junction Proteins via IL-1 Beta-Dependent MMP-9 Activation of Human Blood-Brain Barrier In Vitro. Neural Plast. 2016, 2016, 8928530. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, J.; Liu, Z.; Xu, C.; Qian, S.; Zhang, X.; Zhou, B.; Guan, Q.; Sun, Y.; Wang, Y.; et al. Inhibition of HIF-1α Reduced Blood Brain Barrier Damage by Regulating MMP-2 and VEGF During Acute Cerebral Ischemia. Front. Cell. Neurosci. 2018, 12, 288. [Google Scholar] [CrossRef]
- Abdel-Latif, R.G.; Rifaai, R.A.; Amin, E.F. Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1α/VEGF signaling pathway. Arch. Pharmacal Res. 2020, 43, 514–525. [Google Scholar] [CrossRef]
- Liang, X.; Liu, X.; Lu, F.; Zhang, Y.; Jiang, X.; Ferriero, D.M. HIF1α Signaling in the Endogenous Protective Responses after Neonatal Brain Hypoxia-Ischemia. Dev. Neurosci. 2018, 40, 617–626. [Google Scholar] [CrossRef]
- Chang, D.; Brown, Q.; Tsui, G.; He, Y.; Liu, J.; Shi, L.; Rodríguez-Contreras, A. Distinct Cellular Profiles of Hif1a and Vegf mRNA Localization in Microglia, Astrocytes and Neurons during a Period of Vascular Maturation in the Auditory Brainstem of Neonate Rats. Brain Sci. 2021, 11, 944. [Google Scholar] [CrossRef]
- Wang, M.-J.; Li, Z.-H.; Gao, R.-W.; Chen, Q.-F.; Lin, J.; Xiao, M.-L.; Zhang, K.; Chen, C. Effects of delayed HIF-1α expression in astrocytes on myelination following hypoxia-ischaemia white matter injury in immature rats. Transl. Pediatr. 2022, 11, 20–32. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Chen, H.; Yu, D.; Zhao, J.; Chen, J. Neuroprotective effects of SOX5 against ischemic stroke by regulating VEGF/PI3K/AKT pathway. Gene 2020, 767, 145148. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, T.; Wang, P.; Liu, Y.-H.; Zhang, L.-W.; Xue, Y.-X. Effects of Erythropoietin on Blood–Brain Barrier Tight Junctions in Ischemia–Reperfusion Rats. J. Mol. Neurosci. 2012, 49, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Benderro, G.F.; LaManna, J.C. HIF-1α/COX-2 expression and mouse brain capillary remodeling during prolonged moderate hypoxia and subsequent re-oxygenation. Brain Res. 2014, 1569, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Baburamani, A.A.; Castillo-Melendez, M.; Walker, D.W. VEGF expression and microvascular responses to severe transient hypoxia in the fetal sheep brain. Pediatr. Res. 2012, 73, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Hernández, J.D.; Balderas-Márquez, J.E.; Carranza, M.; Luna, M.; Martínez-Moreno, C.G.; Arámburo, C. Growth Hormone (GH) Enhances Endogenous Mechanisms of Neuroprotection and Neuroplasticity after Oxygen and Glucose Deprivation Injury (OGD) and Reoxygenation (OGD/R) in Chicken Hippocampal Cell Cultures. Neural Plast. 2021, 2021, 9990166. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, E.; Kim, J.-W.; Kwon, B.-S.; Jung, M.K.; Jee, Y.H.; Kim, J.; Bae, S.-R.; Chang, Y.P. Protective effect of growth hormone on neuronal apoptosis after hypoxia–ischemia in the neonatal rat brain. Neurosci. Lett. 2003, 354, 64–68. [Google Scholar] [CrossRef]
- Ong, L.K.; Chow, W.Z.; Tebay, C.; Kluge, M.; Pietrogrande, G.; Zalewska, K.; Crock, P.; Åberg, N.D.; Bivard, A.; Johnson, S.J.; et al. Growth Hormone Improves Cognitive Function After Experimental Stroke. Stroke 2018, 49, 1257–1266. [Google Scholar] [CrossRef]
- Sanchez-Bezanilla, S.; Åberg, N.D.; Crock, P.; Walker, F.R.; Nilsson, M.; Isgaard, J.; Ong, L.K. Growth Hormone Promotes Motor Function after Experimental Stroke and Enhances Recovery-Promoting Mechanisms within the Peri-Infarct Area. Int. J. Mol. Sci. 2020, 21, 606. [Google Scholar] [CrossRef]
- Devesa, P.; Agasse, F.; Xapelli, S.; Almengló, C.; Devesa, J.; Malva, J.O.; Arce, V.M. Growth hormone pathways signaling for cell proliferation and survival in hippocampal neural precursors from postnatal mice. BMC Neurosci. 2014, 15, 100. [Google Scholar] [CrossRef]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef]
- Nylander, E.; Zelleroth, S.; Stam, F.; Nyberg, F.; Grönbladh, A.; Hallberg, M. Growth hormone increases dendritic spine density in primary hippocampal cell cultures. Growth Horm. IGF Res. 2019, 50, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Wang, J.; Li, M.; Qu, H.; Gu, R.; Liu, R.; Wang, L.; Li, S.; Hu, X. Erythropoietin protects neurons from apoptosis via activating PI3K/AKT and inhibiting Erk1/2 signaling pathway. 3 Biotech 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Larpthaveesarp, A.; Georgevits, M.; Ferriero, D.M.; Gonzalez, F.F. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol. Dis. 2016, 93, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chopp, M.; Gregg, S.R.; Zhang, R.L.; Teng, H.; Jiang, A.; Feng, Y.; Zhang, Z.G. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J. Cereb. Blood Flow Metab. 2008, 28, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Ning, R.; Xiong, Y.; Mahmood, A.; Zhang, Y.; Meng, Y.; Qu, C.; Chopp, M. Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury. Brain Res. 2011, 1384, 140–150. [Google Scholar] [CrossRef][Green Version]
- Meng, Y.; Xiong, Y.; Mahmood, A.; Zhang, Y.; Qu, C.; Chopp, M. Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J. Neurosurg. 2011, 115, 550–560. [Google Scholar] [CrossRef]
- Gurnik, S.; Devraj, K.; Macas, J.; Yamaji, M.; Starke, J.; Scholz, A.; Sommer, K.; Di Tacchio, M.; Vutukuri, R.; Beck, H.; et al. Angiopoietin-2-induced blood–brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol. 2016, 131, 753–773. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, K.J.; Qi, Z. Occludin regulation of blood-brain barrier and potential therapeutic target in ischemic stroke. Brain Circ. 2020, 6, 152–162. [Google Scholar] [CrossRef]
- Murakami, T.; Felinski, E.A.; Antonetti, D.A. Occludin Phosphorylation and Ubiquitination Regulate Tight Junction Trafficking and Vascular Endothelial Growth Factor-induced Permeability. J. Biol. Chem. 2009, 284, 21036–21046. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, Z.; Liu, Y.; Wang, P.; Xue, Y. Specific Role of Tight Junction Proteins Claudin-5, Occludin, and ZO-1 of the Blood–Brain Barrier in a Focal Cerebral Ischemic Insult. J. Mol. Neurosci. 2011, 44, 130–139. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Marriott, I. The Interleukin-10 Family of Cytokines and Their Role in the CNS. Front. Cell. Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Meola, D.; Huang, Z.; Petitto, J.M. Selective Neuronal and Brain Regional Expession of IL-2 in IL2P 8-GFP Transgenic Mice: Relation to Sensorimotor Gating. J. Alzheimers Dis Parkinsonism. 2013, 3, 1000127. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K.; Quirion, R. Interleukin-2 as a neuroregulatory cytokine. Brain Res. Brain Res. Rev. 1995, 21, 246–284. [Google Scholar] [CrossRef]
- Girard, S.; Larouche, A.; Kadhim, H.; Rola-Pleszczynski, M.; Gobeil, F.; Sébire, G. Lipopolysaccharide and hypoxia/ischemia induced IL-2 expression by microglia in neonatal brain. NeuroReport 2008, 19, 997–1002. [Google Scholar] [CrossRef]
- Wylezinski, L.S.; Hawiger, J. Interleukin 2 Activates Brain Microvascular Endothelial Cells Resulting in Destabilization of Adherens Junctions. J. Biol. Chem. 2016, 291, 22913–22923. [Google Scholar] [CrossRef]
- Strle, K.; Zhou, J.H.; Shen, W.H.; Broussard, S.R.; Johnson, R.W.; Freund, G.G.; Dantzer, R.; Kelley, K.W. Interleukin-10 in the brain. Crit Rev. Immunol. 2001, 21, 427–449. [Google Scholar] [CrossRef]
- Garcia, J.M.; Stillings, S.A.; Leclerc, J.L.; Phillips, H.; Edwards, N.J.; Robicsek, S.A.; Hoh, B.L.; Blackburn, S.; Doré, S. Role of Interleukin-10 in Acute Brain Injuries. Front. Neurol. 2017, 8, 244. [Google Scholar] [CrossRef]
- Martinez-Moreno, C.; Fleming, T.; Carranza, M.; Ávila-Mendoza, J.; Luna, M.; Harvey, S.; Arámburo, C. Growth hormone protects against kainate excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal cells. Exp. Eye Res. 2018, 166, 1–12. [Google Scholar] [CrossRef]
- Fleming, T.; Balderas-Márquez, J.E.; Epardo, D.; Ávila-Mendoza, J.; Carranza, M.; Luna, M.; Harvey, S.; Arámburo, C.; Martínez-Moreno, C.G. Growth Hormone Neuroprotection Against Kainate Excitotoxicity in the Retina is Mediated by Notch/PTEN/Akt Signaling. Investig. Opthalmology Vis. Sci. 2019, 60, 4532–4547. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, T.; He, J.; Liao, Q.; Wang, J. Influence of miR-1 on Nerve Cell Apoptosis in Rats with Cerebral Stroke via Regulating ERK Signaling Pathway. BioMed Res. Int. 2021, 2021, 9988534. [Google Scholar] [CrossRef]
- Thei, L.; Rocha-Ferreira, E.; Peebles, N.; Raivich, G.; Hristova, M. Extracellular signal-regulated kinase 2 has duality in function between neuronal and astrocyte expression following neonatal hypoxic-ischaemic cerebral injury. J. Physiol. 2018, 596, 6043–6062. [Google Scholar] [CrossRef] [PubMed]
- Kellert, B.A.; McPherson, R.J.; Juul, S.E. A Comparison of High-Dose Recombinant Erythropoietin Treatment Regimens in Brain-Injured Neonatal Rats. Pediatr. Res. 2007, 61, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Statler, P.A.; McPherson, R.J.; Bauer, L.A.; Kellert, B.A.; Juul, S.E. Pharmacokinetics of High-Dose Recombinant Erythropoietin in Plasma and Brain of Neonatal Rats. Pediatr. Res. 2007, 61, 671–675. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Baghirova, S.; Hughes, B.G.; Hendzel, M.J.; Schulz, R. Sequential fractionation and isolation of subcellular proteins from tissue or cultured cells. MethodsX 2015, 2, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Elsevier Inc.: New York, NY, USA, 2007. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Elfarnawany Mai, H. Signal Processing Methods for Quantitative Power Doppler Microvascular Angiography. Electron. Thesis Diss. Repos. 2015, 3106. Available online: https://ir.lib.uwo.ca/etd/3106 (accessed on 19 April 2022).
- Rust, R.; Kirabali, T.; Grönnert, L.; Dogancay, B.; Limasale, Y.D.P.; Meinhardt, A.; Werner, C.; Laviña, B.; Kulic, L.; Nitsch, R.M.; et al. A Practical Guide to the Automated Analysis of Vascular Growth, Maturation and Injury in the Brain. Front. Neurosci. 2020, 14, 244. [Google Scholar] [CrossRef]


| Target | 5′-3′ Sequence | |
|---|---|---|
| ß-actin | forward | 5′-ATGCTCCCCGGGCTGTAT-3′ |
| (Actb) | reverse | 5′-TCACCCACATAGGAGTCCTTCTG-3′ |
| probe | 5′(Fam)-ATCACACCCTGGTGCCTAGGGCG-(BMN-Q535)-3′ | |
| PBGD | forward | 5′-ACAAGATTCTTGATACTGCACTCTCTAAG-3′ |
| (Hmbs) | reverse | 5′-CCTTCAGGGAGTGAACGACCA-3′ |
| probe | 5′(Fam)-TCTAGCTCCTTGGTAAACAGGCTCTTCTCTCCA-(BMN-Q535)-3′ | |
| VEGF-A | forward | 5′-GCACTGGACCCTGGCTTTACT-3′ |
| (Vegfa) | reverse | 5′-ACTTGATCACTTCATGGGACTTCTG-3′ |
| probe | 5′(Fam)-CCATGCCAAGTGGTCCCAGGCTG-(BMN-Q535)-3′ | |
| VEGFR-1 | forward | 5′-TAAGACGGTTAGCACATTGGTGG-3′ |
| (Flt1) | reverse | 5′-AGTTTCAGGTCCTCTCCTTCGG-3′ |
| probe | 5′(Fam)-TGTCACAGATGTGCCGAATGGCTTTC-3′ | |
| VEGFR-2 | forward | 5′-ACTAGGAAAACCTCTTGGCCG-3′ |
| (Kdr) | reverse | 5′-TCTTGAGTTCAGACATGAGGGCT-3′ |
| probe | 5′(Fam)-AGATGTTGAAAGAAGGAGCAACACACAGCG-3′ | |
| TIE-2 | forward | 5′-TCAAGAGGATGAAAGAGTATGCC-3′ |
| (Tek) | reverse | 5′-TAGGTACAAATAGCCTCGGTG-3′ |
| probe | 5′(Fam)-ATCACAGGGACTTCGCAGGAGAACTGGAG-3′ | |
| ANGPT-1 | forward | 5′-TCCACATAGGAAATGAAAAGCAGAAC-3′ |
| (Angpt1) | reverse | 5′-ACACCAACCTCCTGTTAGCAT-3′ |
| probe | 5′(Fam)-AGGTCACACAGGGACAGCAGGCAAACAGAG-3′ | |
| ANGPT-2 | forward | 5′-CAACTACAGGATTCACCTTACAG-3′ |
| (Angpt2) | reverse | 5′-CAAACCACCAGCCTCCTG-3′ |
| probe | 5′(Fam)-CAAAATAAGTAGCATCAGCCAACCAGGAAGTG-3′ | |
| OCLN | forward | 5′-TCTAGATAAAGAGCTGGATGAC-3′ |
| (Ocln) | reverse | 5′-TCTTACTTTTATAATCTGCAGATCCC-3′ |
| probe | 5′(Fam)-AGCAGCCATGTACTCTTCACTCTCCTCTCTG-3′ | |
| Claudin-1 | forward | 5′-CCCATCAATGCCAGGTATG-3′ |
| (Cldn1) | reverse | 5′-AAAGTAGGACACCTCCCAG-3′ |
| probe | 5′(Fam)-TCTTTACTGGCTGGGCCGCTGCCTC-3′ | |
| Claudin-5 | forward | 5′-AGTTAAGGCACGGGTAGCAC-3′ |
| (Cldn5) | reverse | 5′-ATGTTGGCGAACCAGCAGAG-3′ |
| probe | 5′(Fam)-ACGGGAGGAGCGCTTTACGCGGTGT-3′ | |
| ZO-1 | forward | 5′-TTGCCCTCACAGTACAGC-3′ |
| (Tjp1) | reverse | 5′-TGATACTGAGTTGCCTTCACC-3′ |
| probe | 5′(Fam)-ACCTCTGTCCAGCTCTTCTCTCCACATAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klepper, S.; Jung, S.; Dittmann, L.; Geppert, C.I.; Hartmann, A.; Beier, N.; Trollmann, R. Further Evidence of Neuroprotective Effects of Recombinant Human Erythropoietin and Growth Hormone in Hypoxic Brain Injury in Neonatal Mice. Int. J. Mol. Sci. 2022, 23, 8693. https://doi.org/10.3390/ijms23158693
Klepper S, Jung S, Dittmann L, Geppert CI, Hartmann A, Beier N, Trollmann R. Further Evidence of Neuroprotective Effects of Recombinant Human Erythropoietin and Growth Hormone in Hypoxic Brain Injury in Neonatal Mice. International Journal of Molecular Sciences. 2022; 23(15):8693. https://doi.org/10.3390/ijms23158693
Chicago/Turabian StyleKlepper, Simon, Susan Jung, Lara Dittmann, Carol I. Geppert, Arnd Hartmann, Nicole Beier, and Regina Trollmann. 2022. "Further Evidence of Neuroprotective Effects of Recombinant Human Erythropoietin and Growth Hormone in Hypoxic Brain Injury in Neonatal Mice" International Journal of Molecular Sciences 23, no. 15: 8693. https://doi.org/10.3390/ijms23158693
APA StyleKlepper, S., Jung, S., Dittmann, L., Geppert, C. I., Hartmann, A., Beier, N., & Trollmann, R. (2022). Further Evidence of Neuroprotective Effects of Recombinant Human Erythropoietin and Growth Hormone in Hypoxic Brain Injury in Neonatal Mice. International Journal of Molecular Sciences, 23(15), 8693. https://doi.org/10.3390/ijms23158693







