Intracellular Biotransformation of Ultrasmall Iron Oxide Nanoparticles and Their Effect in Cultured Human Cells and in Drosophila Larvae In Vivo
Abstract
:1. Introduction
2. Results and Discussion
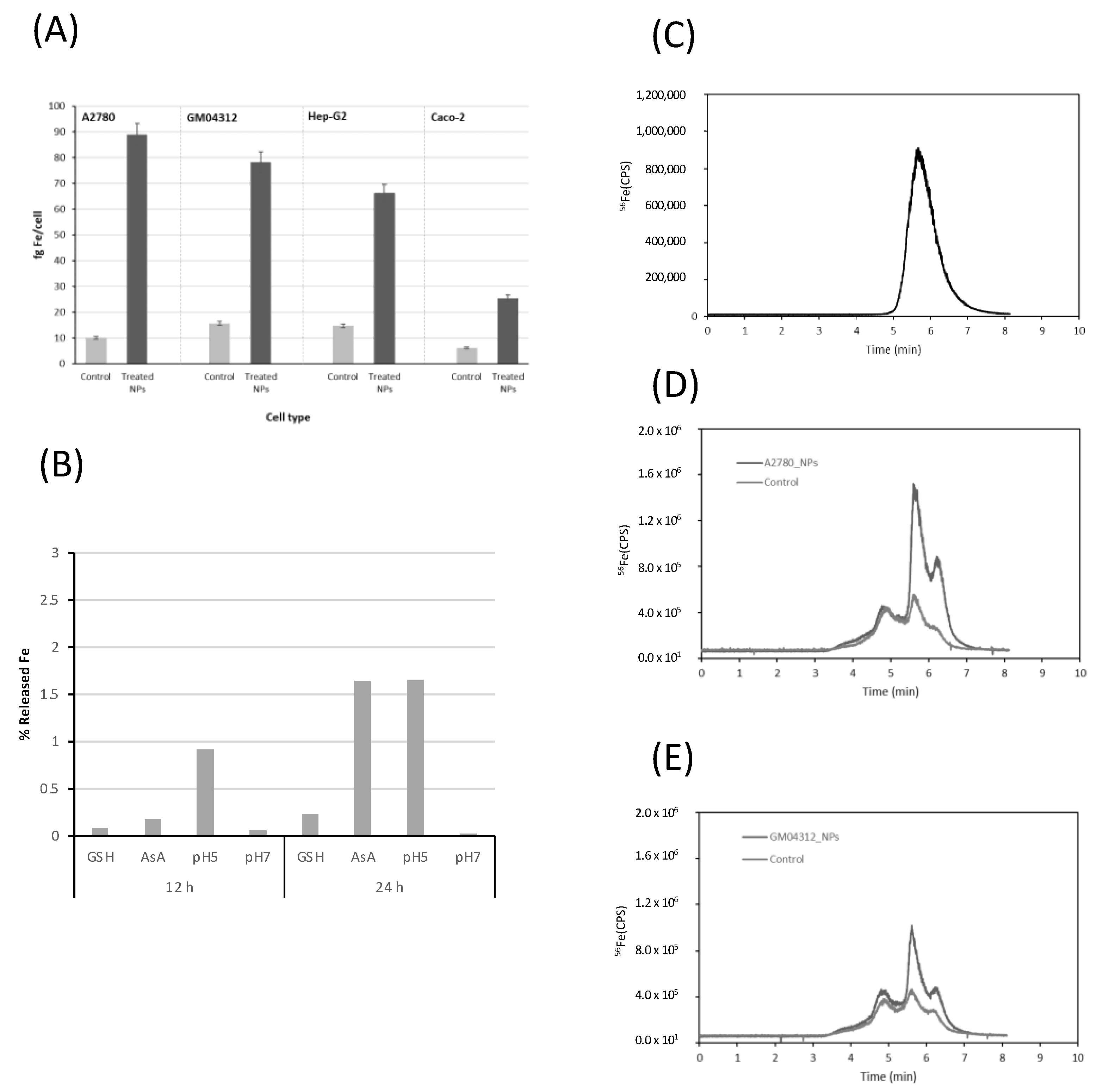
2.1. Cellular Uptake and Intracellular Dissolution of Ultra-Small FeAT-NPs
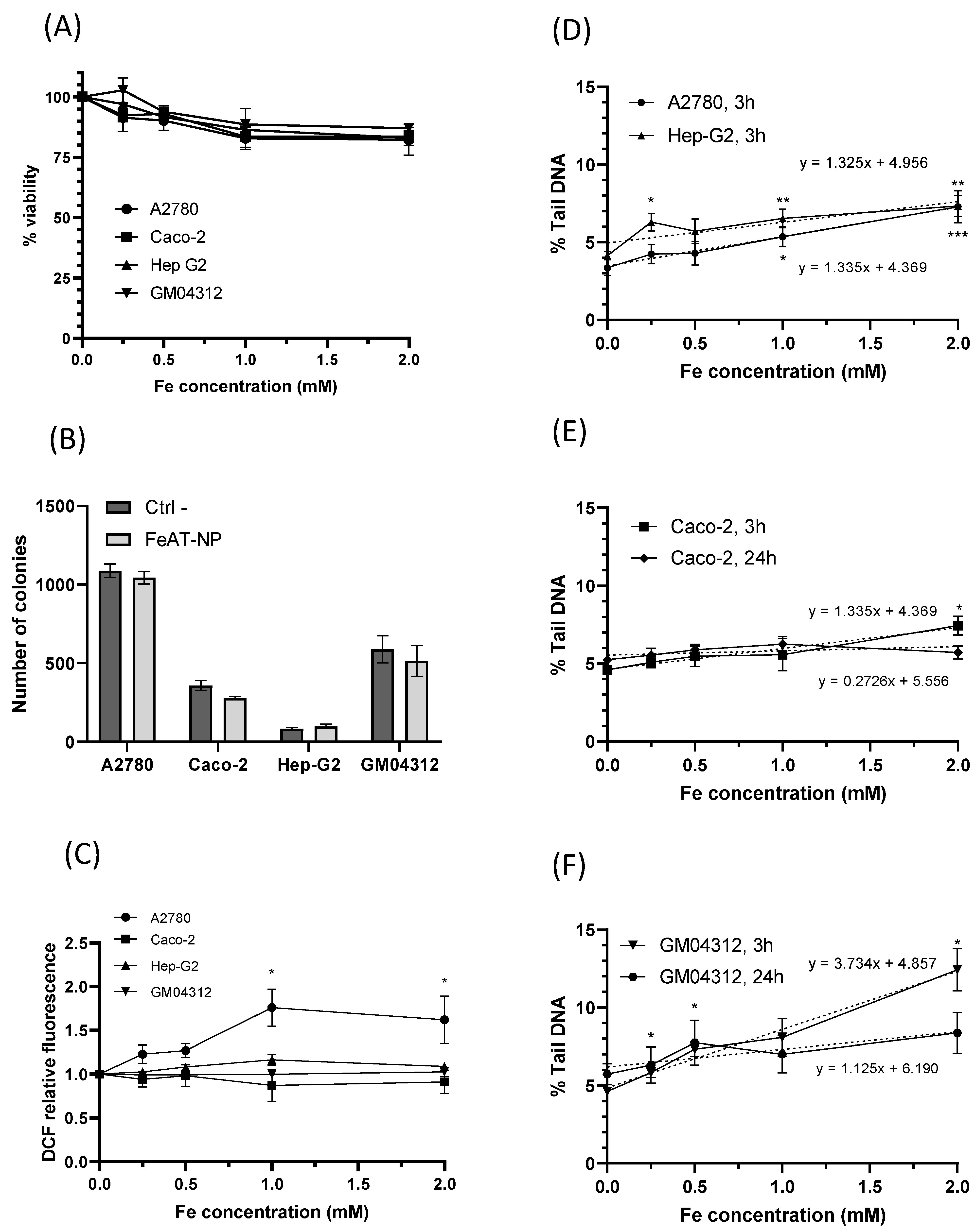
2.2. Biological Effects
2.2.1. Cell Viability and Clonogenic Activity
2.2.2. ROS Induction
2.2.3. In Vitro DNA Damage: Comet Assay
2.2.4. In Vivo Somatic Mutation and Recombination: SMART Assay
3. Materials and Methods
3.1. Instrumentation
3.2. Chemicals and Materials
3.3. Synthesis of Ultrasmall Iron Hydroxide Adipate Tartrate Nanoparticles
3.4. Cell Lines, Cell Culture and Drosophila Strains
3.5. Quantification of Iron in Cells and D. melanogaster Larvae, and Iron Speciation
3.6. Cell Viability Assay
3.7. Clonogenic Activity
3.8. Reactive Oxygen Species Measurement
3.9. Comet Assay
3.10. In Vivo SMART Assay of Drosophila
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alphandéry, E. Biodistribution and Targeting Properties of Iron Oxide Nanoparticles for Treatments of Cancer and Iron Anemia Disease. Nanotoxicology 2019, 13, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Kudasheva, D.S.; Lai, J.; Ulman, A.; Cowman, M.K. Structure of Carbohydrate-Bound Polynuclear Iron Oxyhydroxide Nanoparticles in Parenteral Formulations. J. Inorg. Biochem. 2004, 98, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Lee, J.H.; Lee, J.; Kim, H.Y.; Park, J.Y.; Cho, J.; Lee, J.; Han, D.W. Subtle Cytotoxicity and Genotoxicity Differences in Superparamagnetic Iron Oxide Nanoparticles Coated with Various Functional Groups. Int. J. Nanomed. 2011, 6, 3219–3231. [Google Scholar] [CrossRef] [Green Version]
- Latunde-Dada, G.O.; Pereira, D.I.; Tempest, B.; Ilyas, H.; Flynn, A.C.; Aslam, M.F.; Simpson, R.J.; Powell, J.J. A Nanoparticulate Ferritin-Core Mimetic Is Well Taken up by HuTu 80 Duodenal Cells and Its Absorption in Mice Is Regulated by Body Iron. J. Nutr. 2014, 144, 1896–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, D.I.A.; Bruggraber, S.F.A.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate Iron(III) Oxo-Hydroxide Delivers Safe Iron That Is Well Absorbed and Utilised in Humans. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1877–1886. [Google Scholar] [CrossRef] [Green Version]
- Powell, J.J.; Bruggraber, S.F.A.; Faria, N.; Poots, L.K.; Hondow, N.; Pennycook, T.J.; Latunde-Dada, G.O.; Simpson, R.J.; Brown, A.P.; Pereira, D.I.A. A Nano-Disperse Ferritin-Core Mimetic That Efficiently Corrects Anemia without Luminal Iron Redox Activity. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.I.A.; Mohammed, N.I.; Ofordile, O.; Camara, F.; Baldeh, B.; Mendy, T.; Sanyang, C.; Jallow, A.T.; Hossain, I.; Wason, J.; et al. A Novel Nano-Iron Supplement to Safely Combat Iron Deficiency and Anaemia in Young Children: The IHAT-GUT Double-Blind, Randomised, Placebo-Controlled Trial Protocol. Gates Open Res. 2018, 2, 48. [Google Scholar] [CrossRef]
- Fernández, J.G.; Sánchez-González, C.; Bettmer, J.; Llopis, J.; Jakubowski, N.; Panne, U.; Montes-Bayón, M. Quantitative Assessment of the Metabolic Products of Iron Oxide Nanoparticles to Be Used as Iron Supplements in Cell Cultures. Anal. Chim. Acta 2018, 1039, 24–30. [Google Scholar] [CrossRef]
- Eid, R.; Arab, N.T.T.; Greenwood, M.T. Iron Mediated Toxicity and Programmed Cell Death: A Review and a Re-Examination of Existing Paradigms. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, G.; Costa, C.; Fernández-Bertólez, N.; Pásaro, E.; Teixeira, J.P.; Laffon, B.; Valdiglesias, V. In Vitro Toxicity Evaluation of Silica-Coated Iron Oxide Nanoparticles in Human SHSY5Y Neuronal Cells. Toxicol. Res. 2015, 5, 235–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajiv, S.; Jerobin, J.; Saranya, V.; Nainawat, M.; Sharma, A.; Makwana, P.; Gayathri, C.; Bharath, L.; Singh, M.; Kumar, M.; et al. Comparative Cytotoxicity and Genotoxicity of Cobalt (II, III) Oxide, Iron (III) Oxide, Silicon Dioxide, and Aluminum Oxide Nanoparticles on Human Lymphocytes in Vitro. Hum. Exp. Toxicol. 2016, 35, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Duarte, J.A.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Evaluation of Cytotoxicity and Genotoxicity Induced by Oleic Acid-Coated Iron Oxide Nanoparticles in Human Astrocytes. Environ. Mol. Mutagen. 2019, 60, 816–829. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-Dependent Toxicity of Metal Oxide Particles-A Comparison between Nano- and Micrometer Size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Drlickova, M.; Henjum, K.; Rundén-Pran, E.; Tulinska, J.; Bilanicova, D.; Pojana, G.; Kazimirova, A.; Barancokova, M.; Kuricova, M.; et al. Coating-Dependent Induction of Cytotoxicity and Genotoxicity of Iron Oxide Nanoparticles. Nanotoxicology 2015, 9, 44–56. [Google Scholar] [CrossRef]
- Seabra, A.B.; Pasquôto, T.; Ferrarini, A.C.F.; Santos, M.D.C.; Haddad, P.S.; De Lima, R. Preparation, Characterization, Cytotoxicity, and Genotoxicity Evaluations of Thiolated- and S-Nitrosated Superparamagnetic Iron Oxide Nanoparticles: Implications for Cancer Treatment. Chem. Res. Toxicol. 2014, 27, 1207–1218. [Google Scholar] [CrossRef]
- Satokata, I.; Tanaka, K.; Miura, N.; Miyamoto, I.; Satoh, Y.; Kondo, S.; Okada, Y. Characterization of a Splicing Mutation in Group A Xeroderma Pigmentosum. Proc. Natl. Acad. Sci. USA 1990, 87, 9908–9912. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Raja, S.; Van Houten, B. The Involvement of Nucleotide Excision Repair Proteins in the Removal of Oxidative DNA Damage. Nucleic Acids Res. 2020, 48, 11227–11243. [Google Scholar] [CrossRef]
- Sassa, A.; Odagiri, M. Understanding the Sequence and Structural Context Effects in Oxidative DNA Damage Repair. DNA Repair 2020, 93, 102906. [Google Scholar] [CrossRef]
- Cowie, H.; Magdolenova, Z.; Saunders, M.; Drlickova, M.; Carreira, S.C.; Kenzaoi, B.H.; Gombau, L.; Guadagnini, R.; Lorenzo, Y.; Walker, L.; et al. Suitability of Human and Mammalian Cells of Different Origin for the Assessment of Genotoxicity of Metal and Polymeric Engineered Nanoparticles. Nanotoxicology 2015, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Abdalaziz, M.A.; Annangi, B.; Marcos, R. Testing the Genotoxic Potential of Nanomaterials Using Drosophila. In Genotoxicity and DNA Repair: A Practical Approach; Sierra, L.M., Gaivão, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 297–304. ISBN 978-1-4939-1068-7. [Google Scholar]
- Vogel, E.W.; Nivard, M.J.M. Performance of 181 Chemicals in a Drosophila Assay Predominantly Monitoring Interchromosomal Mitotic Recombination. Mutagenesis 1993, 8, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Gaivão, I.; Sierra, L.M.; Comendador, M.A. The w/W+ SMART Assay of Drosophila Melanogaster Detects the Genotoxic Effects of Reactive Oxygen Species Inducing Compounds. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 440, 139–145. [Google Scholar] [CrossRef]
- Sario, S.; Silva, A.M.; Gaivão, I. Titanium Dioxide Nanoparticles: Toxicity and Genotoxicity in Drosophila Melanogaster (SMART Eye-Spot Test and Comet Assay in Neuroblasts). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 831, 19–23. [Google Scholar] [CrossRef]
- Pereira, D.; Mergler, B.I.; Faria, N.; Bruggraber, S.F.A.; Aslam, M.F. Caco-2 Cell Acquisition of Dietary Iron(III) Invokes a Nanoparticulate Endocytic Pathway. PLoS ONE 2013, 8, 81250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Huang, J.; Feng, Q.; Zhang, T.; Chen, X.; Li, X.; Liu, X.; Li, H.; Zhong, Z.; Xiao, K. Multi-Modal Visualization of Uptake and Distribution of Iron Oxide Nanoparticles in Macrophages, Cancer Cells, and Xenograft Models. J. Biomed. Nanotechnol. 2019, 15, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different Endocytotic Uptake Mechanisms for Nanoparticles in Epithelial Cells and Macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [Green Version]
- Renard, H.F.; Boucrot, E. Unconventional Endocytic Mechanisms. Curr. Opin. Cell Biol. 2021, 71, 120–129. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Endocytosis and Exocytosis of Nanoparticles in Mammalian Cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar]
- Ivask, A.; Pilkington, E.H.; Blin, T.; Käkinen, A.; Vija, H.; Visnapuu, M.; Quinn, J.F.; Whittaker, M.R.; Qiao, R.; Davis, T.P.; et al. Uptake and Transcytosis of Functionalized Superparamagnetic Iron Oxide Nanoparticles in an in Vitro Blood Brain Barrier. Model. Biomater. Sci. 2018, 6, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Soto-Alvaredo, J.; Montes-Bayón, M.; Bettmer, J. Speciation of Silver Nanoparticles and Silver(I) by Reversed-Phase Liquid Chromatography Coupled to ICPMS. Anal. Chem. 2013, 85, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández García, R.; Fernández-Iglesias, N.; López-Chaves, C.; Sánchez-González, C.; Llopis, J.; Montes-Bayón, M.; Bettmer, J. Complementary Techniques (SpICP-MS, TEM, and HPLC-ICP-MS) Reveal the Degradation of 40 Nm Citrate-Stabilized Au Nanoparticles in Rat Liver after Intraperitoneal Injection. J. Trace Elem. Med. Biol. 2019, 55, 1–5. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic Assay of Cells in Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, J.; Turiel, D.; Bettmer, J.; Jakubowski, N.; Panne, U.; Rivas García, L.; Llopis, J.; Sánchez González, C.; Montes-Bayón, M. In Vitro and in Situ Experiments to Evaluate the Biodistribution and Cellular Toxicity of Ultrasmall Iron Oxide Nanoparticles Potentially Used as Oral Iron Supplements. Nanotoxicology 2020, 14, 388–403. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Appl. Biochem. Biotechnol. Part. B Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Cordelli, E.; Bignami, M.; Pacchierotti, F. Comet Assay: A Versatile but Complex Tool in Genotoxicity Testing. Toxicol. Res. 2021, 10, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Espina, M.; Corte-Rodríguez, M.; Aguado, L.; Montes-Bayón, M.; Sierra, M.I.; Martínez-Camblor, P.; Blanco-González, E.; Sierra, L.M. Cisplatin Resistance in Cell Models Evaluation of Metallomic and Biological Predictive Biomarkers to Address Early Therapy Failure. Metallomics 2017, 9, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.A.V. Genotoxicity: Damage to DNA and Its Consequences. King’s Res. Portal 2009, 99, 87–110. [Google Scholar] [CrossRef] [Green Version]
- Vogel, E.W.; Nivard, M.J.M. A Novel Method for the Parallel Monitoring of Mitotic Recombination and Clastogenicity in Somatic Cells in Vivo. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 431, 141–153. [Google Scholar] [CrossRef]
- García Sar, D.; Aguado, L.; Montes Bayón, M.; Comendador, M.A.; Blanco González, E.; Sanz-Medel, A.; Sierra, L.M. Relationships between Cisplatin-Induced Adducts and DNA Strand-Breaks, Mutation and Recombination in Vivo in Somatic Cells of Drosophila Melanogaster, under Different Conditions of Nucleotide Excision Repair. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 741, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H.J. Understanding Nucleotide Excision Repair and Its Roles in Cancer and Ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Kaygisiz, Ş.Y.; Ciǧerci, I.H. Genotoxic Evaluation of Different Sizes of Iron Oxide Nanoparticles and Ionic Form by SMART, Allium and Comet Assay. Toxicol. Ind. Health 2017, 33, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Nas, B.; Çolak, D.A. Genotoxic Analysis of Nickel–Iron Oxide in Drosophila. Toxicol. Ind. Health 2020, 36, 835–843. [Google Scholar] [CrossRef]
- Sekelsky, J.J.; Hollis, K.J.; Eimerl, A.I.; Burtis, K.C.; Hawley, R.S. Nucleotide Excision Repair Endonuclease Genes in Drosophila Melanogaster. Mutat. Res. DNA Repair 2000, 459, 219–228. [Google Scholar] [CrossRef]
- Marcos, R.; Sierra, L.M.; Gaivão, I. The SMART Assays of Drosophila: Wings and Eyes as Target Tissues. In Genotoxicity and DNA Repair: A Practical Approach; Sierra, L.M., Gaivão, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 283–295. ISBN 978-1-4939-1068-7. [Google Scholar]

| Parameter | Value |
|---|---|
| RF Power [W] | 1550 |
| Coolant gas flow [L min−1] | 14.0 |
| Auxiliary gas flow [L min−1] | 0.8 |
| Carrier gas flow [L min−1] | 0.8 |
| Measurement mode | Single Quadrupole |
| Cell gas flow [mL min−1] | 0.31(H2) |
| Q1 bias [V] | 0 |
| Qcell bias [V] | −5.94 |
| Q3 bias [V] | −12.0 |
| Q1 masses [u] | Open |
| Q3 masses [u] | 56 (56Fe+) |
| Number of spots | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repair | Conc. | Scored | Small | Medium | Large | Total | Spot | Clones/ | |||||
| Status | Sex a | (mM) b | Eyes | N | % | N | % | N | % | N | % | Size c | 104 Cells |
| NER+ | F | 0 | 734 | 61 | 8.31 | 6 | 0.82 | 0 | 0.00 | 67 | 9.13 | 2.14 | 4.88 |
| 0.5 | 392 | 34 | 8.67 | 3 | 0.77 i | 2 | 0.51 i | 39 | 9.95 | 2.49 | 6.19 | ||
| 1 | 416 | 35 | 8.41 | 8 | 1.92 i | 0 | 0.00 i | 43 | 10.34 | 2.19 | 5.66 | ||
| 1.5 | 412 | 33 | 8.01 | 14 | 3.40+ | 1 | 0.24 i | 48 | 11.65 | 2.5 | 7.28 | ||
| 2 | 580 | 70 | 12.07+ | 12 | 2.07 i | 2 | 0.34 i | 84 | 14.48 + | 2.34 | 8.47 | ||
| 5 | 510 | 53 | 10.39 | 15 | 2.94 + | 3 | 0.59 i | 71 | 13.92 + | 2.65 | 9.22 | ||
| MMS | 402 | 81 | 20.15+ | 46 | 11.44 + | 16 | 3.98 + | 143 | 35.57 + | 4.04 | 34.67 | ||
| M | 0 | 752 | 28 | 3.72 | 5 | 0.66 | 0 | 0.00 | 33 | 4.39 | 2.16 | 2.37 | |
| 0.5 | 400 | 14 | 3.50 | 1 | 0.25 i | 0 | 0.00 i | 15 | 3.75 | 2.06 | 1.93 | ||
| 1 | 412 | 19 | 4.61 i | 1 | 0.24 i | 0 | 0.00 i | 20 | 4.85 | 2.05 | 2.49 | ||
| 1.5 | 430 | 17 | 3.95 i | 6 | 1.40 i | 0 | 0.00 i | 23 | 5.35 i | 2.26 | 3.02 | ||
| 2 | 542 | 41 | 7.56 + | 7 | 1.29 i | 0 | 0.00 i | 48 | 8.86 + | 2.06 | 4.56 | ||
| 5 | 508 | 35 | 6.89 + | 10 | 1.97 i | 0 | 0.00 i | 45 | 8.86 + | 2.36 | 5.23 | ||
| MMS | 392 | 46 | 11.73 + | 19 | 4.85 + | 4 | 1.02 + | 69 | 17.60 + | 3.25 | 14.30 | ||
| NER− | F | 0 | 610 | 56 | 9.18 | 12 | 1.97 | 1 | 0.16 | 69 | 11.31 | 2.30 | 3.26 |
| 0.1 | 316 | 35 | 11.08 | 5 | 1.58 i | 3 | 0.95 i | 43 | 13.61 | 3.30 | 5.62 | ||
| 0.25 | 308 | 31 | 10.06 | 6 | 1.95 i | 2 | 0.65 i | 39 | 12.66 | 3.21 | 5.07 | ||
| 0.5 | 492 | 61 | 12.40 | 10 | 2.03 i | 1 | 0.20 i | 72 | 14.63 | 2.27 | 4.09 | ||
| 1 | 310 | 33 | 10.65 | 10 | 3.23 i | 0 | 0.00 i | 43 | 13.87 | 2.28 | 3.95 | ||
| 2.5 | 298 | 30 | 10.07 | 8 | 2.68 i | 3 | 1.01 i | 41 | 13.76 | 4.05 | 6.96 | ||
| 5 | 304 | 27 | 8.88 | 9 | 2.96 i | 0 | 0.00 i | 36 | 11.84 | 2.33 | 3.45 | ||
| MMS | 410 | 102 | 24.88 | 62 | 15.12 + | 45 | 10.98 + | 209 | 50.98 + | 5.25 | 33.48 | ||
| M | 0 | 510 | 32 | 6.27 | 5 | 0.98 | 1 | 0.20 | 38 | 7.45 | 2.38 | 2.28 | |
| 0.1 | 302 | 15 | 4.97 | 1 | 0.33 i | 1 | 0.33 i | 17 | 5.63 | 2.41 | 1.70 | ||
| 0.25 | 302 | 19 | 6.29 | 2 | 0.66 i | 0 | 0.00 i | 21 | 6.95 | 2.19 | 1.90 | ||
| 0.5 | 494 | 33 | 6.68 | 6 | 1.21 i | 0 | 0.00 i | 39 | 7.89 | 2.13 | 2.10 | ||
| 1 | 280 | 16 | 5.71 | 5 | 1.79 i | 1 | 0.36 i | 22 | 7.86 | 3.05 | 2.99 | ||
| 2.5 | 282 | 17 | 6.03 | 0 | 0.00 i | 1 | 0.35 i | 18 | 6.38 | 2.89 | 2.31 | ||
| 5 | 306 | 18 | 5.88 | 2 | 0.65 i | 0 | 0.00 i | 20 | 6.54 | 2.10 | 1.72 | ||
| MMS | 406 | 56 | 13.79 + | 29 | 7.14 + | 7 | 1.72 + | 92 | 22.66 + | 3.19 | 9.05 | ||
| Number of spots | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repair | Conc. | Scored | Small | Medium | Large | Total | Spot | Clones/ | |||||
| Status | Sex a | (mM) b | Eyes | N | % | N | % | N | % | N | % | Size c | 104 Cells |
| NER+ | F | 0 | 682 | 67 | 8.57 | 9 | 1.15 | 0 | 0.00 | 76 | 9.72 | 2.15 | 5.28 |
| 0.1 | 512 | 39 | 7.62 | 9 | 1.76 i | 1 | 0.20 i | 49 | 9.57 | 2.45 | 5.86 | ||
| 0.25 | 350 | 27 | 7.71 | 6 | 1.71 i | 1 | 0.29 i | 34 | 9.71 | 2.97 | 7.85 | ||
| 0.5 | 428 | 33 | 7.71 | 11 | 2.57 i | 1 | 0.23 i | 45 | 10.51 | 2.76 | 7.25 | ||
| 1 | 542 | 52 | 9.59 | 8 | 1.48 i | 2 | 0.37 i | 62 | 11.44 | 2.38 | 6.81 | ||
| 2 | 208 | 33 | 10.71 | 4 | 1.30 i | 0 | 0.00 i | 37 | 12.01 | 2.32 | 6.97 | ||
| MMS | 534 | 103 | 16.25 + | 42 | 6.62 + | 17 | 2.68 + | 162 | 25.55 + | 3.97 | 24.53 | ||
| M | 0 | 592 | 31 | 4.48 | 2 | 0.29 | 0 | 0.00 | 33 | 4.77 | 2.11 | 2.49 | |
| 0.1 | 442 | 18 | 4.07 | 2 | 0.45 i | 0 | 0.00 i | 20 | 4.52 | 2.19 | 2.35 | ||
| 0.25 | 310 | 16 | 5.16 i | 1 | 0.32 i | 0 | 0.00 i | 17 | 5.48 i | 2.06 | 2.99 | ||
| 0.5 | 352 | 15 | 4.26 | 5 | 1.42 i | 0 | 0.00 i | 20 | 5.68 i | 2.45 | 3.48 | ||
| 1 | 508 | 27 | 5.31 i | 1 | 0.20 i | 0 | 0.00 i | 28 | 5.51 | 2.04 | 2.81 | ||
| 2 | 208 | 14 | 4.52 | 2 | 0.65 i | 0 | 0.00 i | 16 | 5.16 | 2.09 | 2.76 | ||
| MMS | 502 | 49 | 8.14 + | 18 | 2.99 + | 2 | 0.33 i | 69 | 11.46 + | 2.53 | 6.80 | ||
| NER− | F | 0 | 302 | 30 | 9.93 | 6 | 1.99 | 0 | 0.00 | 36 | 11.92 | 2.19 | 3.27 |
| 0.1 | 308 | 30 | 9.74 | 5 | 1.62 i | 3 | 0.97 i | 38 | 12.34 | 3.21 | 4.95 | ||
| 0.25 | 302 | 30 | 9.93 | 4 | 1.32 i | 1 | 0.33 i | 35 | 11.59 | 2.40 | 3.48 | ||
| 0.5 | 304 | 31 | 10.20 | 6 | 1.97 i | 2 | 0.66 i | 39 | 12.83 | 3.97 | 6.37 | ||
| 1 | 304 | 32 | 10.53 | 3 | 0.99 i | 0 | 0.00 i | 35 | 11.51 | 2.11 | 3.04 | ||
| MMS | 308 | 68 | 22.08 + | 37 | 12.01 + | 12 | 3.90 + | 117 | 37.99 + | 4.55 | 21.61 | ||
| 0 | 300 | 18 | 6.00 | 2 | 0.67 | 0 | 0.00 | 20 | 6.67 | 2.10 | 1.75 | ||
| 0.1 | 306 | 18 | 5.88 | 1 | 0.33 i | 0 | 0.00 i | 19 | 6.21 | 2.06 | 1.59 | ||
| M | 0.25 | 304 | 22 | 7.24 i | 1 | 0.33 i | 0 | 0.00 i | 23 | 7.57 i | 2.04 | 1.93 | |
| 0.5 | 304 | 19 | 6.25 i | 4 | 1.32 i | 0 | 0.00 i | 23 | 7.57 i | 2.19 | 2.07 | ||
| 1 | 302 | 23 | 7.62 i | 2 | 0.66 i | 0 | 0.00 i | 25 | 8.28 i | 2.12 | 2.19 | ||
| MMS | 300 | 43 | 14.33 + | 15 | 5.00 + | 3 | 1.00 i | 61 | 20.33 + | 3.08 | 7.84 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Pescador, A.; Gutiérrez Romero, L.; Blanco-González, E.; Montes-Bayón, M.; Sierra, L.M. Intracellular Biotransformation of Ultrasmall Iron Oxide Nanoparticles and Their Effect in Cultured Human Cells and in Drosophila Larvae In Vivo. Int. J. Mol. Sci. 2022, 23, 8788. https://doi.org/10.3390/ijms23158788
Rodríguez Pescador A, Gutiérrez Romero L, Blanco-González E, Montes-Bayón M, Sierra LM. Intracellular Biotransformation of Ultrasmall Iron Oxide Nanoparticles and Their Effect in Cultured Human Cells and in Drosophila Larvae In Vivo. International Journal of Molecular Sciences. 2022; 23(15):8788. https://doi.org/10.3390/ijms23158788
Chicago/Turabian StyleRodríguez Pescador, Alonso, Lucía Gutiérrez Romero, Elisa Blanco-González, María Montes-Bayón, and L. María Sierra. 2022. "Intracellular Biotransformation of Ultrasmall Iron Oxide Nanoparticles and Their Effect in Cultured Human Cells and in Drosophila Larvae In Vivo" International Journal of Molecular Sciences 23, no. 15: 8788. https://doi.org/10.3390/ijms23158788







