Neuroprotective Effect of Dexmedetomidine against Postoperative Cognitive Decline via NLRP3 Inflammasome Signaling Pathway
Abstract
:1. Background
2. Results
2.1. Identification of SMA Surgery-Induced POCD Mice Model
2.2. Effect of Dex on Neurobehavior in POCD Mice Model
2.3. Effect of Dex on Inflammatory Response in the Hippocampus
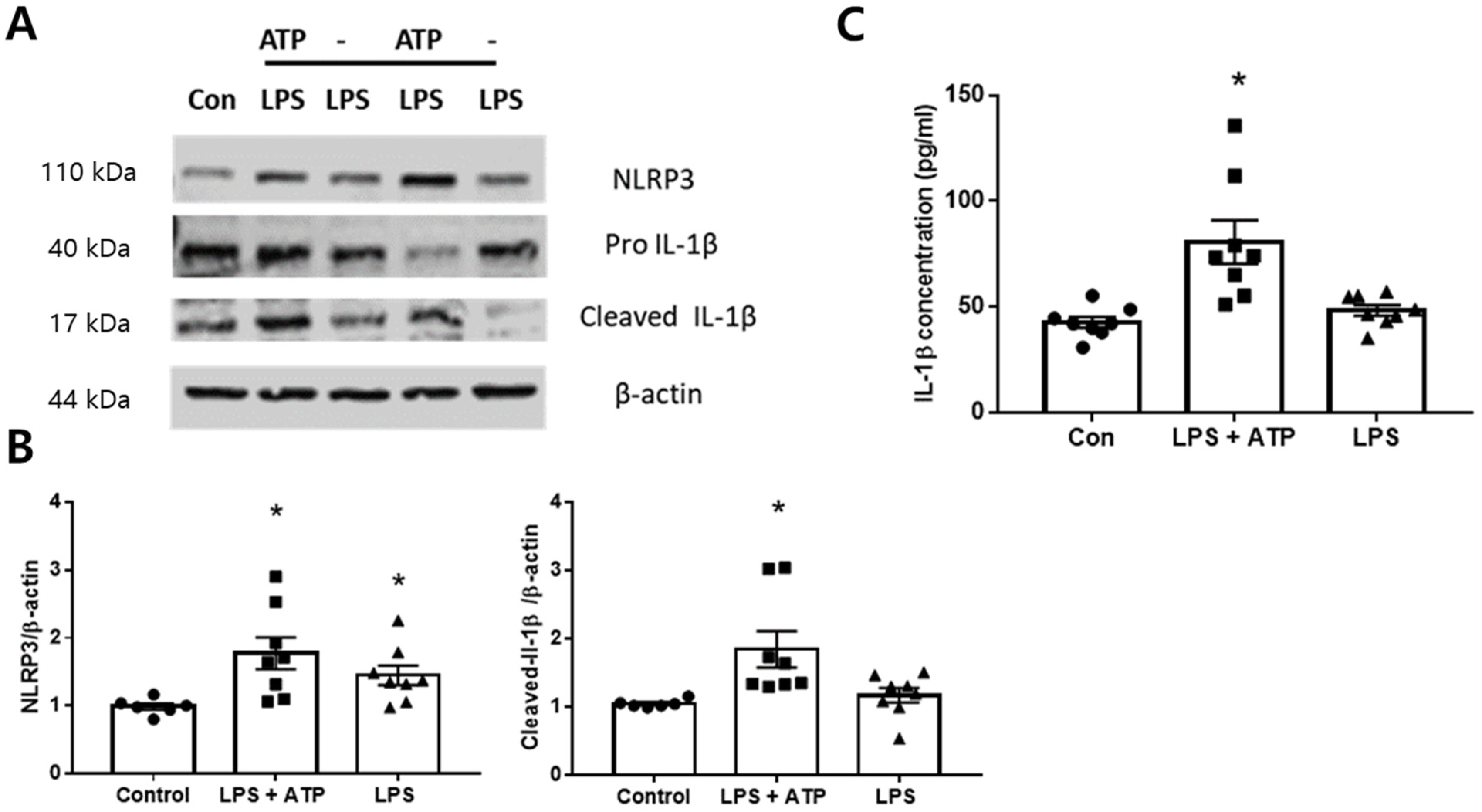
2.4. Expression of NLRP3 Inflammasome in the BV2 Cells
2.5. Dex Inhibited NLRP3 Inflammasome Activation In Vitro
2.6. Dex Ameliorated the Oxidative Stress-Induced Neuronal Cell Death In Vitro
2.7. Dex Inhibits NLRP3 Inflammasome Activation In Vitro
3. Discussion
4. Methods and Materials
4.1. Animals
4.2. Study Groups and Surgical Experimental Protocol
4.3. Neurobehavioral Test
4.4. Cell Culture and Drug Treatment
4.5. Western Blotting and Immunoprecipitation (IP)
4.6. TUNEL Staining and ROS Detection
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
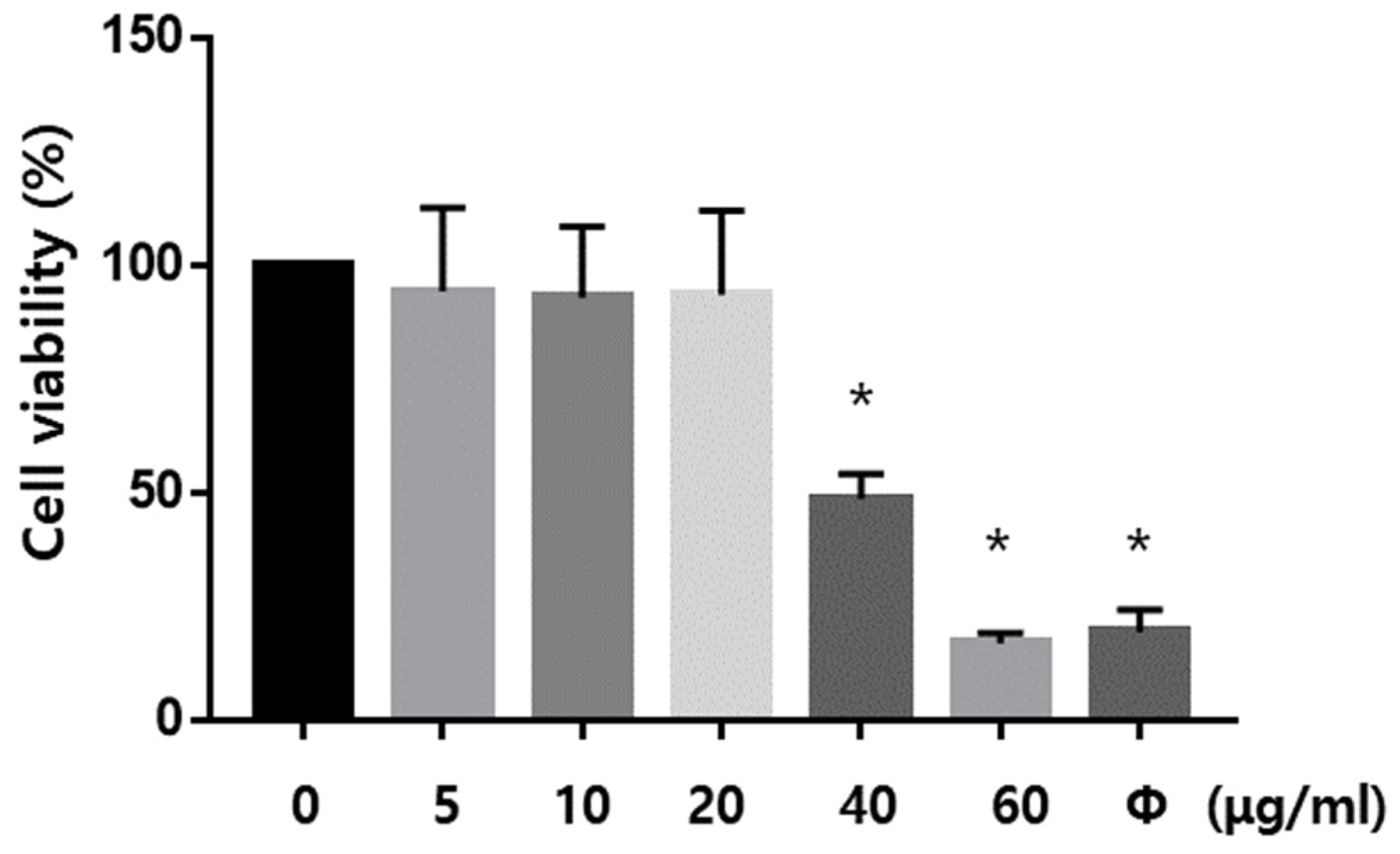
4.8. Cell Viability Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASC | Apoptosis-associated speck-like protein |
| ATP | Adenosine triphosphate |
| CM | Conditioned medium |
| Dex | Dexmedetomidine |
| ELISA | Enzyme-linked immunosorbent assay |
| EPM | Elevated plus maze |
| Het | Dihydroethidium |
| IL-1β | Interleukin (IL)-1-beta |
| IL-6 | Interleukin (IL)-6 |
| IP | Immunoprecipitation |
| LPS | Lipopolysaccharide |
| NLR | NOD-like receptor |
| NLRP3 | The NOD-like receptor pyrin domain-containing 3 |
| NOD | Nucleotide-binding oligomerization domain |
| NORT | Norvel objective recognition test |
| OFT | Open field test |
| POCD | Postoperative cognitive dysfunction |
| ROS | Reactive oxygen species |
| SMA | Superior mesenteric artery |
| TNF-α | Tumor necrosis factor-alpha |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling |
| YOH | Yohimbine hydrochloride |
References
- Gertler, R.; Brown, H.C.; Mitchell, D.H.; Silvius, E.N. Dexmedetomidine: A novel sedative-analgesic agent. Bayl. Univ. Med. Cent. Proc. 2001, 14, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, L.M.; Shane, H.L.; Friend, S.A.; Lukomska, E.; Baur, R.; Anderson, S.E. Topical Application of the Antimicrobial Agent Triclosan Induces NLRP3 Inflammasome Activation and Mitochondrial Dysfunction. Toxicol. Sci. 2020, 176, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.L.; Ibacache, M.; Cortinez, I.; Carrasco-Pozo, C.; Farias, J.G.; Carrasco, R.A.; Méndez, A. Dexmedetomidine Improves Cardiovascular and Ventilatory Outcomes in Critically Ill Patients: Basic and Clinical Approaches. Front. Pharmacol. 2019, 10, 1641. [Google Scholar] [CrossRef]
- Riquelme, J.A.; Westermeier, F.; Hall, A.R.; Vicencio, J.M.; Pedrozo, Z.; Ibacache, M.; Fuenzalida, B.; Sobrevia, L.; Davidson, S.M.; Yellon, D.M.; et al. Dexmedetomidine protects the heart against ischemia-reperfusion injury by an endothelial eNOS/NO dependent mechanism. Pharmacol. Res. 2016, 103, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Y.; Yu, R.; Liu, Y.; Wang, S.; Yuan, D.; Chen, J. Dexmedetomidine Attenuates Monocyte-Endothelial Adherence via Inhibiting Connexin43 on Vascular Endothelial Cells. Mediat. Inflamm. 2020, 2020, 7039854. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, T.; Yu, L.; Zhong, Y.; Dai, H.; Yan, M. Dexmedetomidine protects against oxygen-glucose deprivation-induced injury through the I2 imidazoline receptor-PI3K/AKT pathway in rat C6 glioma cells. J. Pharm. Pharmacol. 2012, 64, 120–127. [Google Scholar] [CrossRef]
- Lei, D.; Sha, Y.; Wen, S.; Xie, S.; Liu, L.; Han, C. Dexmedetomidine May Reduce IL-6 Level and the Risk of Postoperative Cognitive Dysfunction in Patients After Surgery: A Meta-Analysis. Dose-Response 2020, 18, 1559325820902345. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, H.; Zeng, X.; Zhang, C.; Chen, D. Dexmedetomidine reduced cytokine release during postpartum bleeding-induced multiple organ dysfunction syndrome in rats. Mediat. Inflamm. 2013, 2013, 627831. [Google Scholar]
- Chen, R.; Yin, C.; Fang, J.; Liu, B. The NLRP3 inflammasome: An emerging therapeutic target for chronic pain. J. Neuroinflamm. 2021, 18, 84. [Google Scholar] [CrossRef]
- Mashour, G.A.; Woodrum, D.T.; Avidan, M.S. Neurological complications of surgery and anaesthesia. Br. J. Anaesth. 2015, 114, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 688. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Zhao, B.; Ye, Y.; Li, Y.; Zhang, Y.; Xiong, X.; Gu, L. Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke. J. NeuroInflamm. 2021, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Xu, C.B.; Chen, C.J.; Shi, G.N.; Guo, Q.L.; Zhou, Y.; Zhang, T.T. Divanillyl sulfone suppresses NLRP3 inflammasome activation via inducing mitophagy to ameliorate chronic neuropathic pain in mice. J. NeuroInflamm. 2021, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Li, L.L.; Xian, W.B.; Li, M.Y.; Zhang, L.Y.; Xu, J.H.; Hu, X.Q. Chronic colitis exacerbates NLRP3-dependent neuroinflammation and cognitive impairment in middle-aged brain. J. NeuroInflamm. 2021, 18, 153. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, S.; Cao, L.; Chen, Y.; Zuo, Z.; Peng, S. Critical role of NLRP3-caspase-1 pathway in age-dependent isoflurane-induced microglial inflammatory response and cognitive impairment. J. NeuroInflamm. 2018, 15, 109. [Google Scholar] [CrossRef] [Green Version]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; O’neill, L.A. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Yan, F.F.; Liu, Y.P.; Cong, Y.; Sun, K.F.; He, X.M. Dexmedetomidine inhibits the NF-kappaB pathway and NLRP3 inflammasome to attenuate papain-induced osteoarthritis in rats. Pharm. Biol. 2019, 57, 649–659. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, D.; Yang, Y.; Liu, T.; Liu, H. Dexmedetomidine Alleviates Hyperoxia-Induced Acute Lung Injury via Inhibiting NLRP3 Inflammasome Activation. Cell. Physiol. Biochem. 2017, 42, 1907–1919. [Google Scholar] [CrossRef] [Green Version]
- Terrando, N.; Eriksson, L.I.; Ryu, J.K.; Yang, T.; Monaco, C.; Feldmann, M.; Maze, M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011, 70, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, N.; Chen, J.; Xu, Z.; Wang, F.; Ding, C. Effect of Intravenous Dexmedetomidine During General Anesthesia on Acute Postoperative Pain in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. J. Pain 2018, 34, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Talke, P.; Lobo, E.; Brown, R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. J. Am. Soc. Anesthesiol. 2003, 99, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, B.; Zhang, F.; Xue, P.; Cui, R.; Lei, W. The effects of dexmedetomidine on post-operative cognitive dysfunction and inflammatory factors in senile patients. Int. J. Clin. Exp. Med. 2015, 8, 4601–4605. [Google Scholar]
- Li, Y.; He, R.; Chen, S.; Qu, Y. Effect of dexmedetomidine on early postoperative cognitive dysfunction and peri-operative inflammation in elderly patients undergoing laparoscopic cholecystectomy. Exp. Ther. Med. 2015, 10, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- Flukiger, J.; Hollinger, A.; Speich, B.; Meier, V.; Tontsch, J.; Zehnder, T.; Siegemund, M. Dexmedetomidine in prevention and treatment of postoperative and intensive care unit delirium: A systematic review and meta-analysis. Ann. Intensive Care 2018, 8, 92. [Google Scholar] [CrossRef]
- Venn, R.M.; Karol, M.D.; Grounds, R.M. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive caret. Br. J. Anaesth. 2002, 88, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Bekker, A.; Sturaitis, M.; Bloom, M.; Moric, M.; Golfinos, J.; Parker, E.; Pitti, A. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth. Analg. 2008, 107, 1340–1347. [Google Scholar] [CrossRef]
- Ji, F.; Li, Z.; Nguyen, H.; Young, N.; Shi, P.; Fleming, N.; Liu, H. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 2013, 127, 1576–1584. [Google Scholar] [CrossRef] [Green Version]
- Ji, F.; Li, Z.; Young, N.; Moore, P.; Liu, H. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Vacas, S.; Feng, X.; Lutrin, D.; Uchida, Y.; Lai, I.K.; Maze, M. Dexmedetomidine Prevents Cognitive Decline by Enhancing Resolution of High Mobility Group Box 1 Protein-induced Inflammation through a Vagomimetic Action in Mice. Anesthesiology 2018, 128, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Kang, K.; Gao, Y.; Liu, H.; Meng, X.; Yang, S.; Zhao, M. Dexmedetomidine alleviates LPS-induced septic cardiomyopathy via the cholinergic anti-inflammatory pathway in mice. Am. J. Transl. Res. 2017, 9, 5040–5047. [Google Scholar]
- Zhai, Y.; Meng, X.; Ye, T.; Xie, W.; Sun, G.; Sun, X. Inhibiting the NLRP3 Inflammasome Activation with MCC950 Ameliorates Diabetic Encephalopathy in db/db Mice. Molecules 2018, 23, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Du, L.; Fu, Q.; Zhou, Z.; Zhang, J.; Li, G.; Wu, J. Inhibiting the NLRP3 Inflammasome with MCC950 Ameliorates Isoflurane-Induced Pyroptosis and Cognitive Impairment in Aged Mice. Front. Cell. Neurosci. 2018, 12, 426. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lai, I.K.; Pan, J.Z.; Zhang, P.; Maze, M. Dexmedetomidine Exerts an Anti-inflammatory Effect via alpha2 Adrenoceptors to Prevent Lipopolysaccharide-induced Cognitive Decline in Mice. Anesthesiology 2020, 133, 393–407. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, X.; Juan, Z.; Zhang, R.; Wang, R.; Meng, S.; Xie, K. Dexmedetomidine attenuates myocardial ischemia-reperfusion injury in vitro by inhibiting NLRP3 Inflammasome activation. BMC Anesthesiol. 2021, 21, 104. [Google Scholar]
- Ming, T.; Yuan, M.; Kong, Q.; Huang, Q.; Xia, Z.; Wu, X. Dexmedetomidine alleviates blunt chest trauma and hemorrhagic shockresuscitationinduced acute lung injury through inhibiting the NLRP3 inflammasome. Mol. Med. Rep. 2020, 22, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Chen, J.T.; Tsai, H.C.; Chen, T.L.; Liu, S.H.; Chen, R.M. Protection of Dexmedetomidine Against Ischemia/Reperfusion-Induced Apoptotic Insults to Neuronal Cells Occurs Via an Intrinsic Mitochondria-Dependent Pathway. J. Cell. Biochem. 2017, 118, 2635–2644. [Google Scholar] [CrossRef]
- Yuan, F.; Fu, H.; Sun, K.; Wu, S.; Dong, T. Effect of dexmedetomidine on cerebral ischemia-reperfusion rats by activating mitochondrial ATP-sensitive potassium channel. Metab. Brain Dis. 2017, 32, 539–546. [Google Scholar] [CrossRef]
- Wang, X.W.; Cao, J.B.; Lv, B.S.; Mi, W.D.; Wang, Z.Q.; Zhang, C.; Xu, Z. Effect of perioperative dexmedetomidine on the endocrine modulators of stress response: A meta-analysis. Clin. Exp. Pharmacol. Physiol. 2015, 42, 828–836. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, H.; Wang, C.; Sun, J.J.; Lu, K.; Ma, D. Dexmedetomidine attenuates oxidative stress induced lung alveolar epithelial cell apoptosis in vitro. Oxid. Med. Cell. Longev. 2015, 2015, 358396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.D.; Zhang, H.; Wang, H.; Zhang, N.; Du, C.Y.; Yu, J.; Feng, Z.G. Protective effect of dexmedetomidine against glutamate-induced cytotoxicity in PC12 cells and its mechanism. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2016, 37, 150–156. [Google Scholar]
- Zhao, S.; Chen, F.; Yin, Q.; Wang, D.; Han, W.; Zhang, Y. Reactive Oxygen Species Interact with NLRP3 Inflammasomes and Are Involved in the Inflammation of Sepsis: From Mechanism to Treatment of Progression. Front. Physiol. 2020, 11, 571810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, S.; Wang, R. Research Progress of Mitochondrial Mechanism in NLRP3 Inflammasome Activation and Exercise Regulation of NLRP3 Inflammasome. Int. J. Mol. Sci. 2021, 22, 10866. [Google Scholar] [CrossRef]
- Chen, T.C.; Yen, C.K.; Lu, Y.C.; Shi, C.S.; Hsieh, R.Z.; Chang, S.F.; Chen, C.N. The antagonism of 6-shogaol in high-glucose-activated NLRP3 inflammasome and consequent calcification of human artery smooth muscle cells. Cell Biosci. 2020, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Karlidag, R.; Unal, S.; Sezer, O.H.; Bay Karabulut, A.; Battaloglu, B.; But, A.; Ozcan, C. The role of oxidative stress in postoperative delirium. Gen. Hosp. Psychiatry 2006, 28, 418–423. [Google Scholar] [CrossRef]
- Qiu, L.L.; Ji, M.H.; Zhang, H.; Yang, J.J.; Sun, X.R.; Tang, H.; Yang, J.J. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav. Immun. 2016, 51, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kam, E.H.; Kim, S.Y.; Cheon, S.Y.; Kim, E.J.; Chung, S.; Koo, B.N. Erythropoietin Attenuates Postoperative Cognitive Dysfunction by Shifting Macrophage Activation toward the M2 Phenotype. Front. Pharmacol. 2017, 8, 839. [Google Scholar] [CrossRef] [Green Version]
- Hovens, I.B.; Schoemaker, R.G.; van der Zee, E.A.; Absalom, A.R.; Heineman, E.; van Leeuwen, B.L. Postoperative cognitive dysfunction: Involvement of neuroinflammation and neuronal functioning. Brain Behav. Immun. 2014, 38, 202–210. [Google Scholar] [CrossRef]
- Sharma, A.C.; Kulkarni, S.K. Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1992, 16, 117–125. [Google Scholar] [CrossRef]
- Ettcheto, M.; Sanchez-Lopez, E.; Cano, A.; Carrasco, M.; Herrera, K.; Manzine, P.R.; Camins, A. Dexibuprofen ameliorates peripheral and central risk factors associated with Alzheimer’s disease in metabolically stressed APPswe/PS1dE9 mice. Cell Biosci. 2021, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Pan, J.; Shen, Q.; Li, M.; Peng, Y. Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J. Neuroinflamm. 2018, 15, 242. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, I.; Koo, B.-N.; Kim, S.Y.; Park, S.; Kim, E.J.; Kam, E.H.; Kim, J. Neuroprotective Effect of Dexmedetomidine against Postoperative Cognitive Decline via NLRP3 Inflammasome Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 8806. https://doi.org/10.3390/ijms23158806
Cho I, Koo B-N, Kim SY, Park S, Kim EJ, Kam EH, Kim J. Neuroprotective Effect of Dexmedetomidine against Postoperative Cognitive Decline via NLRP3 Inflammasome Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(15):8806. https://doi.org/10.3390/ijms23158806
Chicago/Turabian StyleCho, Inja, Bon-Nyeo Koo, So Yeon Kim, Sujung Park, Eun Jung Kim, Eun Hee Kam, and Jeongmin Kim. 2022. "Neuroprotective Effect of Dexmedetomidine against Postoperative Cognitive Decline via NLRP3 Inflammasome Signaling Pathway" International Journal of Molecular Sciences 23, no. 15: 8806. https://doi.org/10.3390/ijms23158806





