Hyaluronic Acid Alleviates Oxidative Stress and Apoptosis in Human Tenocytes via Caspase 3 and 7
Abstract
:1. Introduction
2. Results
2.1. Effect of HAs on Cell Proliferation Rate and Viability
2.2. Modulation of Superoxide Anions in the Presence of HAs
2.3. Oxidative Stress-Induced Apoptosis Counteraction by HAs
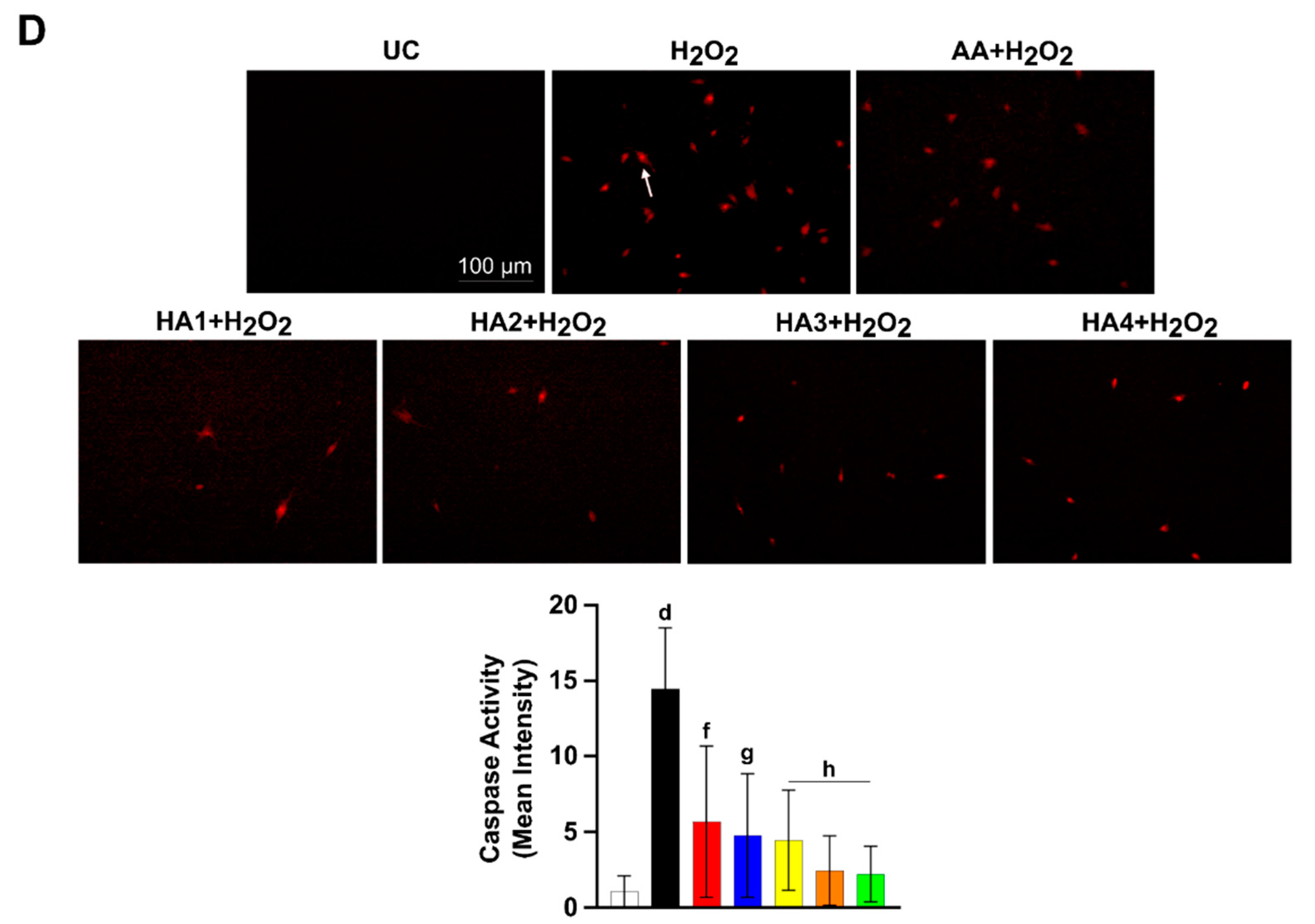
2.4. HAs Treatment Reduces the Activation of Caspase 3 and 7
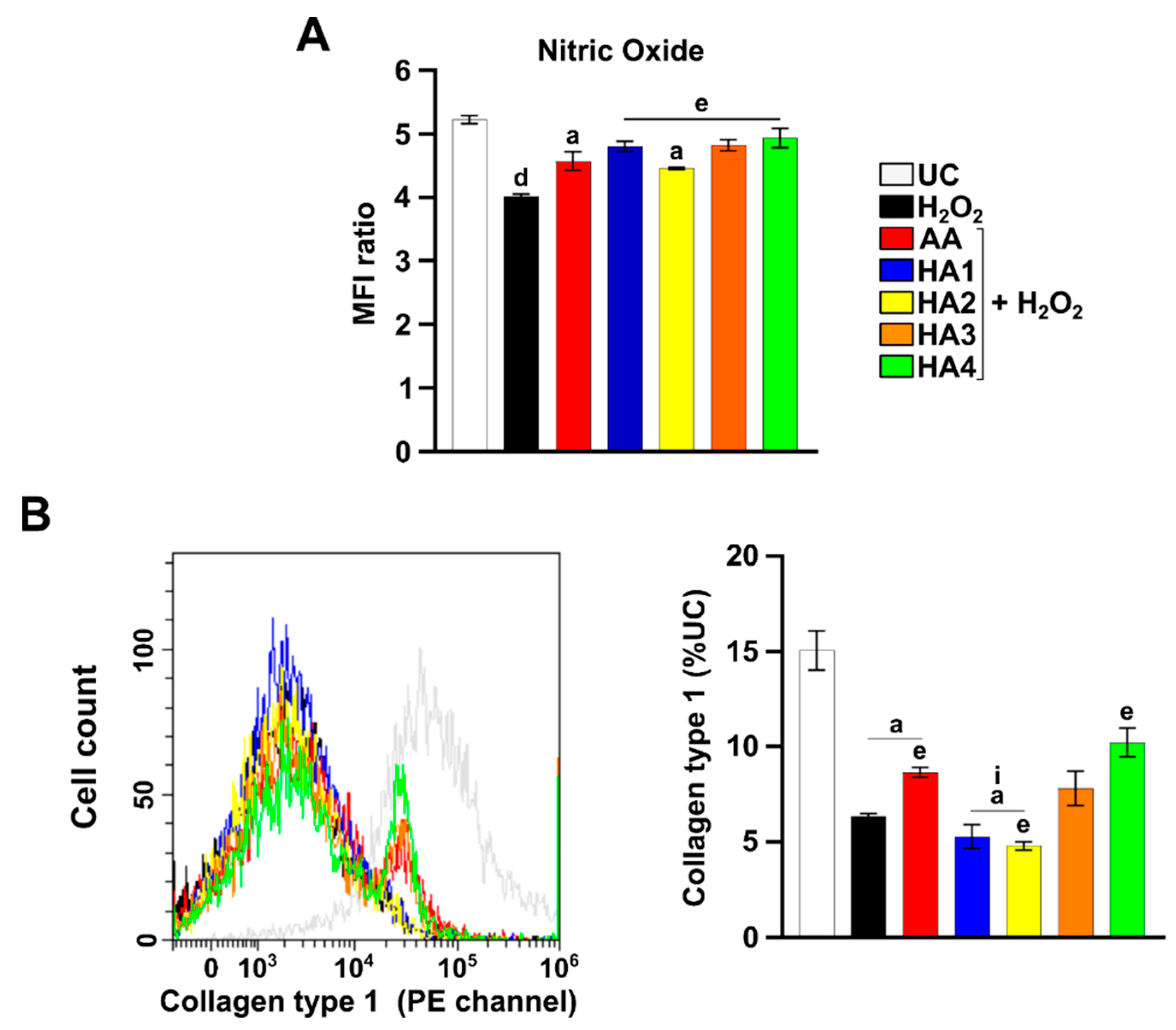
2.5. Modulation of Nitric Oxide (NO) in the Presence of HAs
2.6. Modulation of Collagen 1 Levels by HA Preparations
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Treatment
4.3. Proliferation Assay (alamarBlue)
4.4. Detection of Mitochondrial Superoxide Anions
4.5. Determination of Apoptosis
4.6. Detection of Caspase 3 and 7
4.7. Detection of Intracellular Nitric Oxide
4.8. Expression of Collagen 1 by Flow Cytometry
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yamamoto, A.; Takagishi, K.; Osawa, T.; Yanagawa, T.; Nakajima, D.; Shitara, H.; Kobayashi, T. Prevalence and risk factors of a rotator cuff tear in the general population. J. Shoulder Elb. Surg. 2010, 19, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Osti, L.; Padulo, J.; Maffulli, N. Epidemiology of the rotator cuff tears: A new incidence related to thyroid disease. Muscles Ligaments Tendons J. 2014, 4, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Roesch, Z.K.; Dilisio, M.F.; Radwan, M.M.; Kovilam, A.; Gross, R.M.; Agrawal, D.K. Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci. Rep. 2018, 8, 8918. [Google Scholar] [CrossRef]
- Poulsen, R.C.; Knowles, H.J.; Carr, A.J.; Hulley, P.A. Cell differentiation versus cell death: Extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death Dis. 2014, 5, e1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallorini, M.; Petzel, C.; Bolay, C.; Hiller, K.A.; Cataldi, A.; Buchalla, W.; Krifka, S.; Schweikl, H. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials 2015, 56, 114–128. [Google Scholar] [CrossRef]
- Meng, J.; Yu, P.; Tong, J.; Sun, W.; Jiang, H.; Wang, Y.; Xue, K.; Xie, F.; Qian, H.; Liu, N.; et al. Hydrogen treatment reduces tendon adhesion and inflammatory response. J. Cell. Biochem. 2018, 120, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Osti, L.; Buda, M.; Buono, A.D.; Osti, R.; Massari, L. Clinical evidence in the treatment of rotator cuff tears with hyaluronic acid. Muscles Ligaments Tendons J. 2016, 5, 270–275. [Google Scholar] [CrossRef]
- Oliva, F.; Marsilio, E.; Asparago, G.; Frizziero, A.; Berardi, A.C.; Maffulli, N. The Impact of Hyaluronic Acid on Tendon Physiology and Its Clinical Application in Tendinopathies. Cells 2021, 10, 3081. [Google Scholar] [CrossRef]
- Osti, L.; Berardocco, M.; di Giacomo, V.; Di Bernardo, G.; Oliva, F.; Berardi, A.C. Hyaluronic acid increases tendon derived cell viability and collagen type I expression in vitro: Comparative study of four different Hyaluronic acid preparations by molecular weight. BMC Musculoskelet. Disord. 2015, 16, 284. [Google Scholar] [CrossRef] [Green Version]
- Gallorini, M.; Berardi, A.C.; Gissi, C.; Cataldi, A.; Osti, L. Nrf2-mediated cytoprotective effect of four different hyaluronic acids by molecular weight in human tenocytes. J. Drug Target. 2020, 28, 212–224. [Google Scholar] [CrossRef]
- Muneta, T.; Koga, H.; Ju, Y.J.; Mochizuki, T.; Sekiya, I. Hyaluronan injection therapy for athletic patients with patellar tendinopathy. J. Orthop. 2012, 17, 425–431. [Google Scholar] [CrossRef]
- Flores, C.; Balius, R.; Alvarez, G.; Buil, M.A.; Varela, L.; Cano, C.; Casariego, J. Efficacy and Tolerability of Peritendinous Hyaluronic Acid in Patients with Supraspinatus Tendinopathy: A Multicenter, Randomized, Controlled Trial. Sports Med. Open 2017, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [Green Version]
- Gallorini, M.; Carradori, S. Understanding collagen interactions and their targeted regulation by novel drugs. Expert Opin. Drug Discov. 2021, 16, 1239–1260. [Google Scholar] [CrossRef]
- Oliva, F.; Maffulli, N.; Gissi, C.; Veronesi, F.; Calciano, L.; Fini, M.; Brogini, S.; Gallorini, M.; Antonetti Lamorgese Passeri, C.; Bernardini, R.; et al. Combined ascorbic acid and T3 produce better healing compared to bone marrow mesenchymal stem cells in an Achilles tendon injury rat model: A proof of concept study. J. Orthop. Surg. Res. 2019, 14, 54. [Google Scholar] [CrossRef]
- Li, P.; Zhou, H.; Tu, T.; Lu, H. Dynamic exacerbation in inflammation and oxidative stress during the formation of peritendinous adhesion resulted from acute tendon injury. J. Orthop. Surg. Res. 2021, 16, 293. [Google Scholar] [CrossRef]
- Schweikl, H.; Gallorini, M.; Pöschl, G.; Urmann, V.; Petzel, C.; Bolay, C.; Hiller, K.A.; Cataldi, A.; Buchalla, W. Functions of transcription factors NF-κB and Nrf2 in the inhibition of LPS-stimulated cytokine release by the resin monomer HEMA. Dent. Mater. 2018, 34, 1661–1678. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Shim, M.K.; Yoon, H.Y.; Lee, S.; Jo, M.K.; Park, J.; Kim, J.H.; Jeong, S.Y.; Kwon, I.C.; Kim, K. Caspase-3/-7-Specific Metabolic Precursor for Bioorthogonal Tracking of Tumor Apoptosis. Sci. Rep. 2017, 7, 16635. [Google Scholar] [CrossRef] [Green Version]
- Bokhari, A.R.; Murrell, G.A. The role of nitric oxide in tendon healing. J. Shoulder Elb. Surg. 2012, 21, 238–244. [Google Scholar] [CrossRef]
- Moraes, S.A.; Oliveira, K.R.; Crespo-López, M.E.; Picanço-Diniz, D.L.; Herculano, A.M. Local NO synthase inhibition produces histological and functional recovery in Achilles tendon of rats after tenotomy: Tendon repair and local NOS inhibition. Cell Tissue Res. 2013, 353, 457–463. [Google Scholar] [CrossRef]
- Goshi, E.; Zhou, G.; He, Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 2019, 9, 192–207. [Google Scholar] [CrossRef]
- di Giacomo, V.; Berardocco, M.; Gallorini, M.; Oliva, F.; Colosimo, A.; Cataldi, A.; Maffulli, N.; Berardi, A.C. Combined supplementation of ascorbic acid and thyroid hormone T3 affects tenocyte proliferation. The effect of ascorbic acid in the production of nitric oxide. Muscles Ligaments Tendons J. 2017, 7, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Szomor, Z.; Wang, Y.; Murrell, G.A. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J. Orthop. Res. 2006, 24, 159–172. [Google Scholar] [CrossRef]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. 2008, 33, 102–112. [Google Scholar] [CrossRef]
- Oliva, F.; Berardi, A.C.; Misiti, S.; Verga Falzacappa, C.; Iacone, A.; Maffulli, N. Thyroid hormones enhance growth and counteract apoptosis in human tenocytes isolated from rotator cuff tendons. Cell Death Dis. 2013, 4, e705. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Bestwick, C.S.; Bestwick, L.A.; Maffulli, N.; Aspden, R.M. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006, 12, 1843–1849. [Google Scholar] [CrossRef]
- Berardi, A.C.; Oliva, F.; Berardocco, M.; la Rovere, M.; Accorsi, P.; Maffulli, N. Thyroid hormones increase collagen I and cartilage oligomeric matrix protein (COMP) expression in vitro human tenocytes. Muscles Ligaments Tendons J. 2014, 4, 285–291. [Google Scholar] [CrossRef]
- Gallorini, M.; Berardi, A.C.; Ricci, A.; Antonetti Lamorgese Passeri, C.; Zara, S.; Oliva, F.; Cataldi, A.; Carta, F.; Carradori, S. Dual Acting Carbon Monoxide Releasing Molecules and Carbonic Anhydrase Inhibitors Differentially Modulate Inflammation in Human Tenocytes. Biomedicines 2021, 9, 141. [Google Scholar] [CrossRef]
- Smolewski, P.; Bedner, E.; Du, L.; Hsieh, T.C.; Wu, J.M.; Phelps, D.J.; Darzynkiewicz, Z. Detection of caspases activation by fluorochrome-labeled inhibitors: Multiparameter analysis by laser scanning cytometry. Cytom. J. Int. Soc. Anal. Cytol. 2001, 44, 73–82. [Google Scholar] [CrossRef]
- Alagöz, M.A.; Özdemir, Z.; Uysal, M.; Carradori, S.; Gallorini, M.; Ricci, A.; Zara, S.; Mathew, B. Synthesis, Cytotoxicity and Anti-Proliferative Activity Against AGS Cells of New 3(2H)-Pyridazinone Derivatives Endowed with a Piperazinyl Linker. Pharmaceuticals 2021, 14, 183. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallorini, M.; Antonetti Lamorgese Passeri, C.; Cataldi, A.; Berardi, A.C.; Osti, L. Hyaluronic Acid Alleviates Oxidative Stress and Apoptosis in Human Tenocytes via Caspase 3 and 7. Int. J. Mol. Sci. 2022, 23, 8817. https://doi.org/10.3390/ijms23158817
Gallorini M, Antonetti Lamorgese Passeri C, Cataldi A, Berardi AC, Osti L. Hyaluronic Acid Alleviates Oxidative Stress and Apoptosis in Human Tenocytes via Caspase 3 and 7. International Journal of Molecular Sciences. 2022; 23(15):8817. https://doi.org/10.3390/ijms23158817
Chicago/Turabian StyleGallorini, Marialucia, Cristina Antonetti Lamorgese Passeri, Amelia Cataldi, Anna Concetta Berardi, and Leonardo Osti. 2022. "Hyaluronic Acid Alleviates Oxidative Stress and Apoptosis in Human Tenocytes via Caspase 3 and 7" International Journal of Molecular Sciences 23, no. 15: 8817. https://doi.org/10.3390/ijms23158817
APA StyleGallorini, M., Antonetti Lamorgese Passeri, C., Cataldi, A., Berardi, A. C., & Osti, L. (2022). Hyaluronic Acid Alleviates Oxidative Stress and Apoptosis in Human Tenocytes via Caspase 3 and 7. International Journal of Molecular Sciences, 23(15), 8817. https://doi.org/10.3390/ijms23158817







