1. Introduction
Seeking the most accessible molecular diagnostic tests and developing reliable and cheap procedures is of utmost importance [
1]. In most cases, nucleic acid analysis’s first step is their extraction and purification from biological material. To assure the reliability of detection assays, such as qRT-PCR or genosensors, it is crucial to obtain high-quality purified DNA or RNA [
2]. Classical nucleic acid isolation procedures include phenol-chloroform extraction, salting out and proteinase K treatment, and adsorption on silica–gel membrane [
3]. These efficient but time-consuming methods require hazardous solutions, dedicated equipment, and expensive consumables [
4]. The newly developed procedures should be fast, have throughput, and involve the minimum possible number of reagents or benchtop equipment. Considering the abovementioned issues, nanomaterials gained particular attention as an indispensable part. Their properties, like high surface-to-volume ratio and the easiness of surface properties adjustment, became the reason for their applications, e.g., in genetic material extraction and purification [
5,
6,
7,
8]. Carefully chosen magnetic nanoparticle (MNPs) surfaces can show a high affinity for nucleic acids. In contrast, the presence of a magnetic core allows them to be easily manipulated using an external magnetic field. The DNA should bind preferentially to nanoparticles which facilitate its removal even from complex samples (i.e., proteins or other compounds). Then the application of external magnetic force separates the MNPs. Further changing the solution where MNPs are dispersed induces medium-triggered DNA adsorption reversibility, ensuring its facile recovery back to the solution.
Current research trends targeted at new nano sorbents for the extraction of nucleic acids focus on the development of both magnetic core materials, their shape and morphology [
9], and their surface functionalization [
10,
11]. Several magnetic materials based on iron, cobalt, and nickel have been developed, e.g., magnetic cobalt–zinc ferrite core/SiO
2 shell nano sorbents [
12], cobalt-oxide-based nanoparticles [
13], spinel iron-cobalt oxide compounds [
14], hydrophobic magnetic deep eutectic solvents containing Fe/MnCo/Gd ions [
15], and magnetic ionic liquids including cobalt(II) and nickel(II) complexes [
16]. Cobalt ferrite and other types of novel magnetic nanoparticles are promising due to their magnetocrystalline anisotropy, good parameters of coercivity, and saturation magnetization. Such materials also offer high chemical stability, wear resistance, and generally high physical and chemical stability [
17]. The latest concepts in designing magnetic nano sorbents also include the use of magnetic nanoparticle (MNP) assemblies [
9], the use of porous structures [
18], or the use of specific double helix formation interactions [
19]. However, due to the low cost and simplicity of synthesis, magnetite remains the dominant magnetic material in magnetic nanoparticles dedicated to nucleic acid extraction and purification.
The current scientific literature does not indicate a universal type of surface modification and the conditions that ensure efficient nucleic acid extraction. Therefore, there are several concepts involving different kinds of interactions, including electrostatic attraction between the nanoparticle and polyanionic phosphate backbones of nucleic acids [
20,
21], hydrophobic interactions (
π-π stacking) [
22], hydrogen bonds formation [
23], coordination and salt bridging [
24] or specific, biological affinity [
25,
26]. Nevertheless, as could be found in the literature also, bare Fe
3O
4 nanoparticles, without clearly described surface properties, were successfully employed for DNA isolation [
27]. Recently, Qi et al. proposed a method of capturing DNA adducts from human blood samples through Fe
3O
4@GO nano sorbent, where GO was responsible for improving selectivity by enhancing the interaction with the analyte [
28]. On the other hand, in the recent publication by Zhang, the high and pH-sensitive DNA loading capacity was due to cationic polyethyleneimine [
29]. Paltrinieri et al. coated Fe
3O
4 MNPs with polyallylamine hydrochloride (PAH), and PAH functionalized with guanidinium groups (PAH–Gu) for enhanced phosphate binding [
30]. An interesting and recently reported example of a polycationic ligand is PEDOT. Nanoparticles modified with this polymer showed a unique, high binding capacity in acidic media [
21]. Moreover, silica coatings are commonly used for the solid-phase purification of DNA [
31]. Silanization with modified precursors (APTES/MPTMS) allows easily manipulating the character of functional groups, which also affects affinity towards nucleic acids [
32]. Min et al. described an approach to isolate and purify DNA based on hydrogen bonding via carboxyl groups. These superparamagnetic Fe
3O
4 nanoparticles were modified with
meso-2,3-dimercaptosuccinic acid (DMSA) [
33].
Despite the growing number of reported examples of magnetic nanomaterials as nucleic acid nano sorbents, relatively little attention is paid to the critical evaluation of adsorption mechanisms and their impact on the nucleic acids’ extraction capacity. The comparative studies so far focus on quantitative analysis of solid- and liquid-phase extraction [
34,
35] and the confrontation of manual and automatic approaches [
36]. The variety of conditions and magnetic properties of the Fe
3O
4 cores make a comprehensive comparison of the effects of surface coating difficult. Therefore, the available literature examples concern mainly the comparison of bare and core-shell nanoparticles [
37] or methods of selective and non-selective adsorption of nucleic acids [
38].
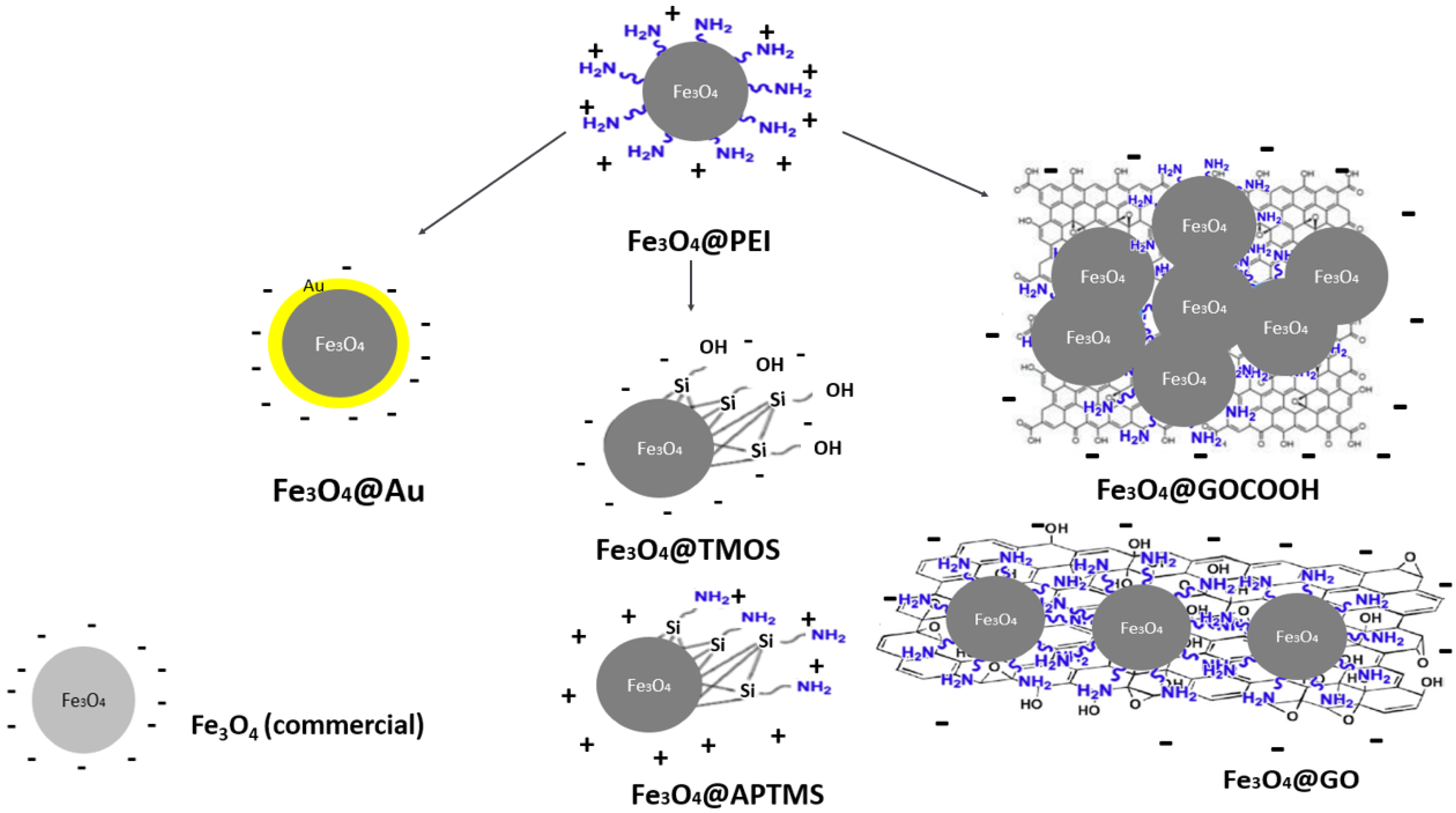
Presented studies focus on the comparative analysis of the influence of the nanoparticle surface type on the efficiency of its interaction with calf thymus DNA. The proposed nanoparticle preparation method allowed several post-synthetic modifications of the starting Fe3O4@PEI nanoparticles. Fe3O4 surface modifications cover the functionalization of a magnetic core with polyethyleneimine (PEI) and further with graphene oxide (GO), carboxylated graphene oxide (GOCOOH), gold (Au), silica/amine-silica (TMOS/APTMS). For comparison, commercially available Fe3O4 nanoparticles without polymer coating were analyzed in the presented studies. Obtained Fe3O4 types (coatings in the form of solid shells as well as surface ligands) were extensively characterized in terms of their morphology, qualitative and quantitative elemental composition, and surface properties by STEM imaging, ICP-MS, and elementary analysis, thermogravimetry, ζ-potential measurements, UV-Vis- and Fourier-transform infrared spectra registering. Additionally, we comparatively characterized nanomaterials’ magnetic properties (specific magnetization and rate of magnetic separation) and determined the iron content in their prepared suspensions using ICP-MS/MS. The latter results allowed for the normalization of MNPs concentration (the same batch of magnetite core). They provided a reliable comparison of the influence of the nanoparticle surface type on the efficiency in its interaction with calf thymus DNA. The adsorption and desorption efficiency from the surface of modified nanoparticles was investigated by UV-Vis spectrophotometry. The effect of various Na+ or Mg2+ concentrations in the adsorption medium and the influence of temperature and concentration of the buffer components used in the DNA desorption protocols were also examined.
2. Results and Discussion
Magnetic nanoparticles’ properties, like good dispersion, high surface area, and ease of surface modification, make them ideal as sorbents, carriers for various biological species, also nucleic acids. Thus, they have broad applicability in developing the DNA/RNA analysis methods or as components in genetic material preparation assays (extraction and purification steps) [
39]. The magnetic core has two essential functions. First, to provide the ability of rapid separation in an external magnetic field from the sample. Second, Fe
3O
4 acts as a platform for further functionalization with compounds expressing the affinity to nucleic acids.
However, due to nanoparticles’ magnetic properties and the tendency to clusterization, maintaining their biological species adsorption efficiency typically requires their stabilization [
40]. A stabilizer is also necessary to secure the Fe
3O
4 nanoparticles as a convenient platform for further modification with various coatings. The presented studies achieved the first use of polyethyleneimine (PEI) as a particle stabilizer introduced in situ during the MNPs co-precipitation synthesis method previously described by Zhou [
41]. Typically, stabilization of PEI is accomplished by post-synthetic ligand exchange from the starting citrate-capped Fe
3O
4 nanoparticles. The already described post-synthetic modification takes more time (an additional 4 h), elevated temperature (80 °C), and strict control of pH during the process [
42,
43]. In situ capping of Fe
3O
4 nanoparticles with PEI significantly simplifies the procedure (one-pot approach) and helps maintain good colloidal stability of nanoparticles by eliminating the risk of aggregation during the ligand exchange of citrate-capped nanoparticles.
The presented research aimed to prepare magnetic nanoparticles of different surface modifications and their comparative analysis in nucleic acids (calf thymus dsDNA) adsorption/desorption process efficiency. For this purpose, seven types of surface coatings of magnetic MNPs that may interact with DNA in various ways were prepared and characterized. As was stated above, PEI provided good stabilization and gave the cationic character of the surface over a wide pH range. Based on magnetic nanoparticles coated with PEI (Fe
3O
4@PEI), a family of functionalized nanoparticles characterized by uniform cores was synthesized. This included: two types of silica shells, anionic silica (Fe
3O
4@TMOS) and cationic amine-silica (Fe
3O
4@APTMS); two types of graphene derivatives, graphene oxide (Fe
3O
4@GO) and carboxylated graphene oxide (Fe
3O
4@GOCOOH). To enable further covalent functionalization of magnetic nanoparticles with thiolated receptors, nanoparticles coated with gold shells (Fe
3O
4@Au) were synthesized. Additionally, for comparison with as-obtained nano sorbents, the commercially available MNPs without polymer shells were considered (the manufacturer did not provide information on the surface coating composition). We expected that in the case of commercial Fe
3O
4 nanoparticles, their core ligands (e.g., adsorbed anions) are directly responsible for the interaction with the nucleic acid. Schemes of nanoparticles used in the framework of this study are depicted in
Figure 1.
It should be emphasized that before MNPs usage for nucleic acid interaction analysis, the concentration of nano sorbents on various surfaces was unified. It was achieved by using the same batch of MNPs for all modifications and the expression of nanoparticle concentrations by iron content (magnetite core). This step was crucial for the reliability of the efficiency of the comparative evaluation of DNA/MNPs interactions.
2.1. Evaluation of Magnetic Properties of Fe3O4-Based Nanosorbents
To assess the influence of surface modification on the rate of magnetic separation, we measured the time from external magnetic field application to obtain the complete MNPs collection. A series of photos before, during, and after applying an external magnetic field were taken, and exemplary images are shown in
Figure 2a. In addition, magnetization curves were recorded for each nanoparticle type (selected examples shown in
Figure 2b), and the saturation magnetization values were determined.
As can be seen, functionalized nanoparticles retain their ability to magnetic separation, but the rate of magnetic collection strongly depends on the type of surface coating. The rates of MNPs separation were compared to MNPs Fe
3O
4@PEI, which were used as an internal benchmark (up to 50 s). The fastest separation (up to 30 s) was observed for nanoparticles coated with silica, amine-silica, and commercial MNPs. This can be attributed to their sizes and morphology. In the case of Fe
3O
4@TMOS and Fe
3O
4@APTMS, partial agglomeration at the silanization stage typically occurs, resulting in large, multi-core structures. On the other hand, commercially available nanoparticles have a larger core diameter than nanoparticles obtained by co-precipitation, which is also reflected in their behavior in a magnetic field. Furthermore, nanoparticles decorated with GO and GOCOOH were characterized by good colloidal stability and rapid separation (up to 40 s) in the magnetic field compared to PEI-coated magnetic cores. Notably, when attached to GO and carboxylated graphene oxide, magnetic nanoparticles gain properties different from those obtained from other modifications. It was noticed that such nanoparticles tend to form macroscopic graphene-like flakes (row 3, column 2 in
Figure 2a). Such behavior of the obtained magnetic nanostructures confirms the effectiveness of the Fe
3O
4 decoration with graphene oxide sheets. However, the tendency of such nanoparticles to agglomerate via π-π stacking does not deteriorate their applicability as nano sorbents, as they can be easily redispersed through sonication.
Macroscopic observations were confirmed by detailed magnetometric analysis. As shown in
Figure 2b, coercivity values were low, indicating that synthesized MNPs show typical superparamagnetic properties. This is also evidenced by the relatively high saturation magnetization values (
Figure 2c), which are slightly lower but comparable to the magnetization of pure magnetite (92 emu/g). As can be seen in the detailed diagrams presented in
Figure 2b, the M(H) magnetization curves in a wide range of magnetic fields correlate with the saturation magnetization values. The similar nanoparticle types compared with each other show a similar course of the magnetization process (see Fe
3O
4@GO vs. Fe
3O
4@GOCOOH and Fe
3O
4@TMOS vs. Fe
3O
4@APTMS). The poor magnetizing ability of Fe
3O
4@Au compared to Fe
3O
4@PEI and Fe
3O
4 (commercial) is also confirmed. Obtained values in most cases were still high enough to employ MNPs as powerful nano sorbents. On the other hand, Fe
3O
4@Au core-shell nanoparticles show a poor capability for magnetic separation (up to 120 s) and relatively low magnetization saturation (44.6 emu/g). This phenomenon can be explained by the presence of a conductive Au shell, which significantly reduces the nanoparticle magnetism [
41] due to the mass effect of the non-magnetic coating. This type of MNPs separation was ineffective; Au-coated MNPs were excluded from detailed studies on DNA adsorption/desorption.
2.2. ζ-Potential Measurements
ζ-potential parameter of synthesized MNPs has been used to portray the changes in their surface modification (
Figure 3). From the family of studied nanoparticles, only Fe
3O
4@PEI (25.3 mV) and Fe
3O
4@APTMS (30.2 mV) are characterized by a positive charge due to the presence of protonated amino groups of the ligands. Commercial MNPs possess an intrinsic negative charge (−22.6 mV). The anionic character of these nanoparticles without polymer shells in near-neutral pH (different from the expected, intrinsically slightly cationic, bare iron(II,III) oxide) comes most likely from the complexation of the surface atoms by anions—either naturally occurring in the sample, or compounds added as a surface stabilizer (e.g., commonly used citrate) [
44]. The negative charge of GO-decorated Fe
3O
4 (−16.3 mV) and GOCOOH-decorated Fe
3O
4 (−23.3 mV) confirms that originally cationic, PEI-coated iron(II,III) oxide nanoparticles are efficiently incorporated into the structure of graphene oxide. The intrinsic negative charge of GO and GOCOOH derives from carboxyl and other oxygen-containing groups on its surface. Fe
3O
4@TMOS (−32.0 mV) are negatively charged due to silanol groups of the silica shell. On the other hand, the negative charge of Fe
3O
4@Au (−16.5 mV) comes from a citrate ion, which acts as a stabilizer of gold nanoparticles. It can be concluded that all nanoparticles gained the expected charge due to their coatings, proving their ligand attachment effectiveness.
2.3. MNPs Morphology (Bright-Field Scanning Transmission Electron Microscopy)
Another critical parameter influencing the MNP’s DNA sorption efficiency (e.g., described above silica or amine-silica coated) is their morphology. For this reason, the family of nanoparticles has been characterized by STEM. Average nanoparticle sizes were investigated based on the analysis of the obtained micrographs (
Figure 4). Despite the same sizes of magnetic cores (except MNPs shown in
Figure 4g), nanoparticles differ significantly in terms of their morphology, which is caused by surface modification. Fe
3O
4@PEI are characterized by the smallest dimensions (average diameter 8.1 nm—
Figure 4a) compared to other structures. Decoration of magnetic MNPs with graphene oxide (b,c), solid silica, or amine-silica shells (d,e) also can be observed in micrographs. Fe
3O
4@PEI nanoparticles have been successfully adsorbed on the surface of GO and GOCOOH by electrostatic self-assembly. Moreover, compared to Fe
3O
4@GO (magnetic core size 8.8 nm), Fe
3O
4@PEI are wrapped more closely at the GOCOOH flake surface (core size 9.0 nm). Based on the core sizes, the decoration process did not affect the average diameter concerning Fe
3O
4@PEI. The formation of silica coatings significantly influences nanoparticle morphology, Fe
3O
4@TMOS, and Fe
3O
4@APTMS. In both cases, larger structures are formed (34.4 and 165.1 nm, respectively), which may be explained by the occlusion of several Fe
3O
4 cores inside a single silica shell. On the other hand, coating MNPs with solid gold results in a slight increase in diameter to 15.7 nm, and no significant agglomeration of nanoparticle cores were visible (
Figure 4f). The only nanoparticles with originally different core diameters are commercial Fe
3O
4 nanoparticles (16.4 nm) shown in
Figure 4g.
2.4. Compositional Analysis by Thermogravimetric Analysis (TGA)
Further characterization of the obtained core-shell nanoparticles in terms of stability and composition involved thermogravimetric analysis. The obtained thermograms show that the MNPs samples differ in thermal stability (
Figure 5). TGA analysis shows that the most stable samples are those covered with a solid layer of gold. The course of thermogram of Fe
3O
4@Au MNPs is similar to parallelly co-precipitated NPs using the same protocol but without the addition of an external stabilizer (such NPs were not examined as nano sorbents in this study and used only for comparative characterization). The nanoparticles covered with a solid gold layer are characterized by the slightest loss of mass, indicating a small contribution of organic matter in their structure. In the case of silane-coated NPs, the oxidation temperature was lower than for the other samples. It can be concluded from the course of the TGA curve that both types of silane coatings have entrapped a higher amount of water than the other shell materials. APTMS-based coating exhibit the most significant weight loss, demonstrating its lower stability compared to TMOS-based silica. Slow oxidation of organic occlusions entrapped in a lattice of aminated silica is visible as the mass loss curve, which does not flatten despite the increase in temperature up to 750 °C. The significant mass loss is visible for MNPs modified with both graphene derivatives, which proves a substantial contribution of organic matter in the final product. Among the MNPs decorated with GO and GOCOOH, a minor loss is observed for the GOCOOH sample. This may be due to the lower water content and a slight reduction of GOCOOH compared to GO. A slight but noticeable weight loss (compared to unmodified MNPs) was observed for PEI-coated MNPs, which proves the effective stabilization of Fe
3O
4@PEI.
2.5. FT-IR Spectroscopy
The successful coating of Fe
3O
4 MNPs with various shells was also confirmed by FT-IR spectroscopy. In
Figure 6, the FT-IR spectra of modified nanoparticles are presented and compared with the spectra of corresponding modifiers in a pure form. As can be easily noticed, the spectra of surface-modified magnetic nanoparticles manifest the characteristic bands derived from the related modifiers.
In all nanoparticle cases, the strong band derived from Fe-O is noticeable, around 500 cm
−1, characteristic of metal-oxygen bonds. Although Fe
3O
4@PEI nanoparticles have been coated with a polymer, as evidenced by previous TGA and ζ-potential analysis, no characteristic bands assigned to the PEI polymer bonds can be observed in
Figure 6a. This can be explained by a relatively thin molecular layer of ligands and thus their low amount in the analyzed sample. Hence, Au and PEI-coated MNPs do not differ significantly in the course of the spectrum from uncoated MNPs. On the other hand, clear bands corresponded to GO or TMOS modifiers shown in
Figure 6b,c may indicate a higher amount deposited at the MNPs surface. Spectra of surface-modified magnetic nanoparticles show specific bands in the exact wavenumber ranges as pure materials. For Fe
3O
4@silica, characteristic bands of Si-O and Si-O-Si bonds are noticeable, which corresponds well to TMOS precursor (except C-H bonds from methyl residuals) C-H. For Fe
3O
4@GO and GO, similar bands assigned to C=O, C-O, and primary and secondary O-H bands, have also been identified. The characteristic bands evidenced the presence of the carboxylate group: at ~1600 cm
−1, the band attributed to the vibration COO
− (v
as), and in the range 1300 cm
−1–1400 cm
−1, the bands attributed to the vibrations COO
− (v
s)– and CO (v
s) + OCO (δ). The bands (COO
− (v
s) are more leading for pure GO in removing Fe
3O
4@GO.
2.6. Analysis of the Nanoparticles Composition
The elemental and ICP-MS/MS analyses were employed to determine the content of the nanoparticle’s main elementary components. As seen in
Table 1, and as expected, the dominant component element of MNPs is iron oxide, represented by the determined iron and estimated oxygen contents (based on the Fe/O molar fraction for nanoparticles without stabilizer). At the same time, the presence of different coatings was reflected by the contributions of different hetero-elements. In the case of nanoparticles decorated with organic coatings (PEI, GO, and GOCOOH), carbon was the dominant hetero-element. Due to the presence of polyethyleneimine in all types of particles (except bare MNPs), a certain proportion of nitrogen was observed, coming from the amine groups of the polymer. At the same time, the GO and GOCOOH decorated nanoparticles have a significantly higher ratio of carbon to nitrogen (4.11 for GO and 3.94 for GOCOOH, respectively) compared to PEI coated NPs (1.35). The shells of Fe
3O
4@TMOS and Fe
3O
4@APTMS MNPs turned out to be much thicker than the remaining ones—the contribution of silicon atoms was in both cases above 40% (
w/
w) and was higher than the iron content. The unmodified silica obtained with TMOS coating was characterized by a low carbon and nitrogen content in contrast to the APTMS coating, in which numerous ω-aminopropyl residues have been trapped. The significant gold coverage of Fe
3O
4@Au magnetic nanoparticles was also confirmed. The proportion of this element at 35.83% by mass is slightly lower than that of Fe as the main core constituent (43.21%). The obtained results unequivocally confirm the modification procedures’ effectiveness for all subjected to test nanoparticles.
2.7. Standardization of Magnetic Nano Sorbents Concentration
The total iron content in MNPs samples was determined using the ICP-MS/MS technique to standardize the dose of DNA nano sorbents taken into further tests. The obtained iron concentrations were used to calculate the amount of MNPs unequivocally. The idea was based on the assumption that all nanoparticle modifications were performed by directly comparing the nucleic acid binding efficiency between variously modified MNPs. As a result, the concentration of nanoparticles in all samples was unified to a total Fe concentration of 72.96 ± 0.08 µg/mL (corresponding to the lowest concentration of iron in the undiluted MNPs sample). Based on the above data, appropriate sample solutions were prepared for further DNA adsorption and recovery studies to ensure the same concentration of magnetic cores within all experiments.
2.8. UV-Vis Absorption Spectra Analysis
The formation of surface coating usually entails a change in the optical properties of MNPs, which results in increased ligand absorption and scattering of the core-shell type structure. Absorption spectra shown in
Figure 7 confirm the attachment of corresponding ligands or the formation of solid shells on the surface of magnetic cores. A slight band around 230–240 nm observed for Fe
3O
4@GO and Fe
3O
4@GOCOOH MNPs can be attributed to π-π* transitions in graphene structure [
45]. On the other hand, Fe
3O
4@TMOS and Fe
3O
4@APTMS MNPs show a high scattering, typical for larger structures [
46]. In turn, the significant absorption at approximately 520–550 nm by Fe
3O
4@Au MNPs can be explained by the occurrence of surface plasmon resonance [
47] (solid dark blue line). The results of the UV-Vis spectra analysis are generally consistent with the conclusions of the STEM and ζ-potential studies.
2.9. Studies of DNA Interactions with Modified MNPs
For the quantitative evaluation of calf thymus DNA adsorption and desorption processes, a spectrophotometric method based on the specific absorption wavelength of nucleobases at 260 nm was used [
29]. The efficiency of the above steps was calculated as Δ
A. It refers to the ratio [%] of DNA amounts in media before adsorption and after nano sorbent separation (
Figure 8).
2.9.1. DNA Adsorption Studies
The efficiency of DNA adsorption can be influenced by several factors such as the nanoparticle surface and ligand type (solid shell, branched polymer), its charge (cationic or anionic), and the concentration of nanoparticles or medium ionic strength. As we standardized the concentration of nanoparticles in the sample, the primary influence on the obtained results should originate only from the type of MNPs modification. This allowed us to observe and compare the effect of surface coating on calf thymus DNA interactions efficiency with modified MNPs. As the nanoparticle residuals in the analyzed sample could influence the registered absorbance value (strong absorption in the UV range,
Figure 7), it was indispensable to provide their quantitative separation. Therefore, only MNPs capable of rapid and complete separation were used for this study. Fe
3O
4@Au, which expressed slow separation kinetics and low efficiency, were excluded. Nonetheless, after the magnetic separation of all tested nanoparticles from the DNA solution, a significant loss of absorbance at 260 nm was observed. This change manifests a relatively high DNA binding capacity of prepared magnetic nano sorbents. In the case of cationic nano sorbent, Fe
3O
4@PEI, a high adsorption ratio (represented by Δ
A values near 99%) was observed even in the medium of low ionic strength (
Figure 9a). Together with increasing the Na
+ or Mg
2+ concentration, this efficiency diminishes to even ~37% when calf thymus DNA was dissolved in an aqueous solution containing 1M Mg
2+. This can be explained that the increase of the medium ionic strength can result in stronger DNA charge shielding, which adversely affects the adsorption process due to the electrostatic attraction. The observed results are in accordance with the postulated interaction mechanism, in which the positively charged surface of nanoparticles attracts negatively charged DNA structure.
As shown in
Figure 9c,d, the opposite trend to Fe
3O
4@PEI in solution ionic strength influence on the dsDNA adsorption can be observed for anionic nanoparticles decorated with graphene oxide and its carboxy-derivative. In this case, the main driving force for DNA adsorption is most likely π-π bonds formation with aromatic rings of carbon nanomaterial surface. However, the negative charge present at its surface can efficiently decrease nucleic acid attraction and adsorption. Nonetheless, a high concentration of cations in a binding medium can reduce the Debye Length of carbon nanomaterials. Its effectively attenuates electrostatic repulsion between DNA and nanoparticles. As observed, the adsorption efficiency increases when the medium ionic strength increase, from 32 to 96% for Fe
3O
4GO and 22 to 99% for Fe
3O
4@GOCOOH (
Figure 9c,d). At a salt (Na
+ and Mg
2+) concentration of 0.5 M, both nanoparticle types showed almost quantitative adsorption of DNA, while the adsorption efficiency in water was significantly lower, approximating 30% for Fe
3O
4@GO and 20% for Fe
3O
4@GOCOOH. It may be explained by the slight difference in surface charge density (see ζ-potential studies) of both GO derivatives. As was mentioned above, the dominant mechanism for DNA adsorption on graphene oxide (and its derivative) is hydrophobic π-π stacking between aromatic rings. However, the hydrogen bond formation and donor-acceptor interactions (between oxygen-bearing moieties of GO and DNA nucleobases) should also be considered. In this point of view, GOCOOH provides oxygen functional groups that most likely can additionally interact with DNA (slightly lower adsorption efficiency in water).
In the case of silica-coated nanoparticles, both with positive (Fe
3O
4@APTMS) and negative (Fe
3O
4@TMOS) surface charge, a moderate ability to DNA binding (not exceeding 58% for Fe
3O
4@APTMS and 47% for Fe
3O
4@TMOS) was observed (
Figure 9e,f). Moreover, for the above efficiency, the ionic strength of the solution was of rather limited influence. We expect that the higher the magnetic core agglomeration during their modification, the smaller the specific surface area available further for DNA adsorption of such nanoparticles, especially versus those where the cores agglomeration was not observed. Therefore we can note weaker adsorption efficiency for large, multi-core MNPs nanostructures coated with silica and amino-silica. The above observations can be explained because the formed, modified magnetic nanoparticles are multi-core constructs (
Figure 4e,f). For this reason, the overall specific area available for DNA adsorption for Fe
3O
4@APTMS and Fe
3O
4@TMOS nanoparticles is highly reduced compared to other investigated single-core MNPs. The last investigated nano sorbent, commercial non-encapsulated nanoparticles, distinguishes the most significant sensitivity to the composition of the adsorption medium—a strong positive effect of divalent cations can be noticed (adsorption ratio increase from 54% for 0 M to 98% for 1 M MgCl
2). However, what is of particular interest in the case of monovalent cations, adsorption decreases with increasing ionic strength (from 0 M to 1 M NaCl) (
Figure 9b). As there is no precise information from the manufacturer regarding nanoparticle surface composition, the obtained results can be explained by the hydration or anion association on the Fe
3O
4 surface, which gained an anionic and polar character.
In general, the DNA extraction efficiency within this study may be influenced by factors such as (i) nanoparticle active surface area—long DNA chains may have a problem attaching to a nanoparticle with very small size; (ii) surface charge—DNA binding by cationic nanoparticle can be driven by electrostatic interactions; (iii) dispersion—the real surface area of MNPs per concentration unit may vary after modification with different coatings, e.g., multi-core particles or their aggregates; (iv) surface coatings types, like in the case of graphene oxide and possible π-π interactions with DNA.
Because of magnetic core concentration standardization in our study, the dominant factor influencing the extraction efficiency is the type of Fe3O4 surface coating, its charge, expressing possible interactions, and dispersion degree. The effect of surface charge is visible in the example of cationic nanoparticles coated with polyethyleneimine (characterized by very good adsorption due to electrostatic attraction of anionic DNA backbones). On the other hand, multi-core and aminated silica-coated MNPs are characterized by a poor DNA extraction efficiency due to the limited surface area available for adsorption.
Na+ and Mg2+ ions influence both binding and desorption processes. Their presence is associated with ion bridging and charge screening during the interaction of DNA and nanoparticle surface. On the other hand, during the desorption process of nucleic acids from modified MNPs, their complexation by EDTA may play a significant role.
2.9.2. Effect of EDTA Concentration on the Obtained Reliability of UV-Vis Results
Magnetic nanoparticles are investigated from the point of view of their application for nucleic acid adsorption, extraction, and its further desorption back to the solution. Both these steps, adsorption and desorption, take place in different environments. Typically, buffered and slightly alkaline media (pH~8.0) containing salt and chelating agents should be used for DNA desorption from nanoparticles [
26,
37]. The most common chelating agent is EDTA, while there are some discrepancies in the literature regarding its appropriate concentration, where typically 0.1–10 mM is used [
26,
37,
48,
49]. Moreover, the presence of EDTA in the desorption medium can have pros and cons. Apart from inhibiting nucleic acids restriction enzymes (DNases, RNases) and facilitating nucleic acid desorption (destabilization of coordination bonds and salt bridging), it can also negatively influence the amplification efficiency of nucleic acids. This was a motivation to initially use EDTA in a desorption medium at 1 mM. However, it had to be diminished even to 0.1 mM.
Figure 10 shows the influence of EDTA concentration in desorption media on obtained results (for Fe
3O
4@PEI nanoparticles as an example). Moreover, the UV-Vis spectra of desorption buffer with two different EDTA concentrations and formed complex of EDTA with Fe
3+ are presented.
First experiments using 10 mM TRIS-HCl of pH 8.0 and 1 mM EDTA as desorption media resulted in unexpectedly high DNA recovery (%), indicating the increase in nucleic acid concentration in the solution after its adsorption and desorption steps (
Figure 10a). These repeated results raised doubts about the validity of the spectrophotometric method of DNA concentration determination in the desorption procedures [
29,
50]. Similar experiments for all nanoparticles without initially adsorbed calf thymus DNA showed a significant increase in UV-Vis absorbance for 260 nm. This confirmed that EDTA in the concentration of 1 mM has a crucial impact on the accuracy of desorption process evaluation using the established UV-Vis method. The reason was probably related to EDTA-induced stripping of Fe
3+ from the magnetite core. The effect was additionally confirmed on UV-Vis spectra registered for 10 mM TRIS-HCl of pH 8.0 with EDTA (1 mM or 0.1 mM) solution with or without the addition of 100 µM FeCl
3 (
Figure 10b). As clearly seen, EDTA forms a complex with Fe
3+ ion with a strong absorption band at around 260 nm (analytical wavelength in desorption analysis). Notably, a 10-fold decrease of the chelating agent concentration (to 0.1 mM) thoroughly eliminated its interference considering all types of MNPs (
Figure 8a, Fe
3O
4@PEI as example). The background absorbance of supernatants was negligible, only slightly more significant than in the case of the complete absence of EDTA in the desorption medium. Therefore, for further studies on the DNA extraction process, TRIS buffer with 0.1 mM EDTA was used [
50]. Such concentration seems to be a trade-off between ensuring the stability of the nucleic acid during the extraction process (by inhibiting nucleases) and suppressing interferences during spectrophotometric DNA determination.
2.9.3. DNA Desorption and Recovery Studies
The final step of the presented investigations covers evaluating the calf thymus DNA recovery from the surface of the analyzed nanoparticles. UV-Vis spectra registered for solution (optimized as described above) after desorption and magnetic separation of nanoparticles enabled the assessment of DNA recovery concerning the amount of DNA adsorbed (100%) on nanoparticles (
Figure 11). The desorption process was examined in various conditions, starting from mild (room temperature, short, 5-min incubation), gradually moving to more drastic (30-min incubation at 70 °C).
The 3D graphs presented in
Figure 11 show the DNA recovery ratios at its individual desorption stages (
Z-axis) versus different ionic strengths of the adsorption media (
X-axis). The desorption studies were carried out for nanoparticles used in adsorption experiments in media of various ionic strengths. The cumulative desorption efficiencies for individual media with different salt contents are presented as corresponding insets in
Figure 11.
The mono- and divalent cations (Na
+ and Mg
2+) influence several factors during DNA interaction with surfaces, e.g., charge screening, interactions with DNA backbone, dsDNA structure stabilization, or with chelating agents. These result from valence, polarizability, and EDTA chelating capacity differences. The DNA sugar-phosphate backbone shows a strongly anionic character in media with a pH close to neutral. Moreover, investigated surfaces express different charges, positive or negative. Multivalent electrolytes, in particular magnesium, are the most effective in charge screening and formation of salt bridges between adjacent anions [
51,
52]. Monovalent cations can bind to the DNA backbone as counterions neutralize the negatively charged DNA surface without bridging [
53]. Cations’ co-adsorption facilitates DNA binding on the modified surfaces, which also possess a negative charge [
54]. It was observed that in the case of the electrostatic mechanism of DNA interaction with cationic nanoparticles (Fe
3O
4@PEI and Fe
3O
4@APTMS), the beneficial effect of magnesium ions is more visible than for nanoparticles characterized by negative surface charge (lack of desorption in 1 M NaCl and 68% and 34% in 1 M MgCl
2, respectively). In previously published works, it has been confirmed that the divalent ions support the formation of dense and more rigid DNA adsorbates due to the increased charge shielding and salt-bridging effect [
51]. What is more, the facility of Mg
2+ ion chelation by EDTA may favor the degradation of nanoparticle-DNA assemblies at the desorption step. Similarly, silica-coated nanoparticles exhibit slightly better DNA recoveries (from 0 to 42% for Fe
3O
4@APTMS and from 0 to 67% for Fe
3O
4@TMOS, respectively) when co-adsorbed with magnesium ions, which is consistent with a few reports in which both flat surfaces and SiO
2-coated nano sorbents were examined [
52,
55].
Nanoparticles decorated with graphene derivatives (GO and GOCOOH), which interact with DNA primarily by attracting aromatic rings or donor-acceptor bonds, exhibit substantially different behavior in the DNA desorption process. In their case, magnesium ion did not promote DNA release. Additionally, significantly better DNA recovery ratios (up to 52% for Fe
3O
4@GOCOOH and from 0 to 78% for Fe
3O
4@GO) can be observed in more aggressive desorption conditions (long desorption times, elevated temperature), which indicates possibly slower desorption kinetics. A slight, beneficial effect of GO carboxylation on DNA desorption was also observed, presumably related to the increased content of polar, oxygen-containing groups available for hydrogen bonding. Commercial nanoparticles with larger diameters and without an additional shell should be considered separately. For such MNPs, the anionic and polar character of the surface is determined by the presence of hydroxyl groups and anions adsorbed on the bare Fe
3O
4 surface. The results obtained for these nanoparticles, e.g., relatively low DNA recoveries and good efficiency of its uptake, indicate a high nanomaterial affinity to DNA, resulting in its hindered desorption. Detailed results of cumulative desorption efficiencies (Insets in
Figure 11) confirm the need for individual selection of optimal desorption parameters for each type of nanoparticle used as a DNA nano sorbent.
4. Conclusions
The presented studies carried out a comprehensive, comparative analysis of the impact of Fe3O4 nanoparticles’ surface modification on their physicochemical properties and DNA binding ability and recovery. A new approach to the co-precipitation method using PEI as a capping agent allowed us to obtain good-quality cationic nanoparticles covered with PEI in a one-step synthesis. The core-shell type nanoparticles synthesized in this way show good stability and shell-dependent magnetism (within limits 44.6–76.8 emu/g Fe, the best for Fe3O4@APTMS), which allowed them to be used as DNA nano sorbents. The efficiency of surface coating for all MNP types was qualitatively and quantitatively characterized by a combination of TGA, FTIR, ICP-MS/MS, and elemental analysis. Thanks to different interaction mechanisms with DNA, their capacity for reversible DNA binding was characterized. Comprehensive characterization of their morphology, composition, and surface properties has proven effective modification with PEI ligands, Au solid coating, silica, amine-silica, and GO/carboxylated GO decorations.
Among the examined nanoparticles, the most efficient in adsorption are Fe3O4@PEI (near 99%), characterized by the electrostatic mechanism of DNA attraction. The most efficient in desorption are Fe3O4@GOCOOH (near 78%), characterized by the hydrophobic and hydrogen bond-derived mechanism of DNA attraction. The significant influence of the ionic strength and valence of the extraction medium cation (Na+ and Mg2+) on DNA binding and recovery for the selected nanoparticle types was demonstrated. Based on studies of the desorption process under various conditions, it was concluded that individual selection of desorption conditions for each nanoparticle type is required to obtain high DNA recovery. During the research, it was also found that high EDTA concentration in the medium adversely affects the desorption process and acts as a potential source of interferences during spectrophotometric DNA determination due to iron(III) stripping from MNPs (inducing nanoparticles degradation). Therefore, a medium containing 0.1 mM EDTA was chosen for desorption process investigation in the presented research. These studies drive a new preparation protocol, which should be implemented during the effective design of new magnetic nano sorbents to rapidly isolate nucleic acids from complex samples.