Osteoporosis Preclinical Research: A Systematic Review on Comparative Studies Using Ovariectomized Sheep
Abstract
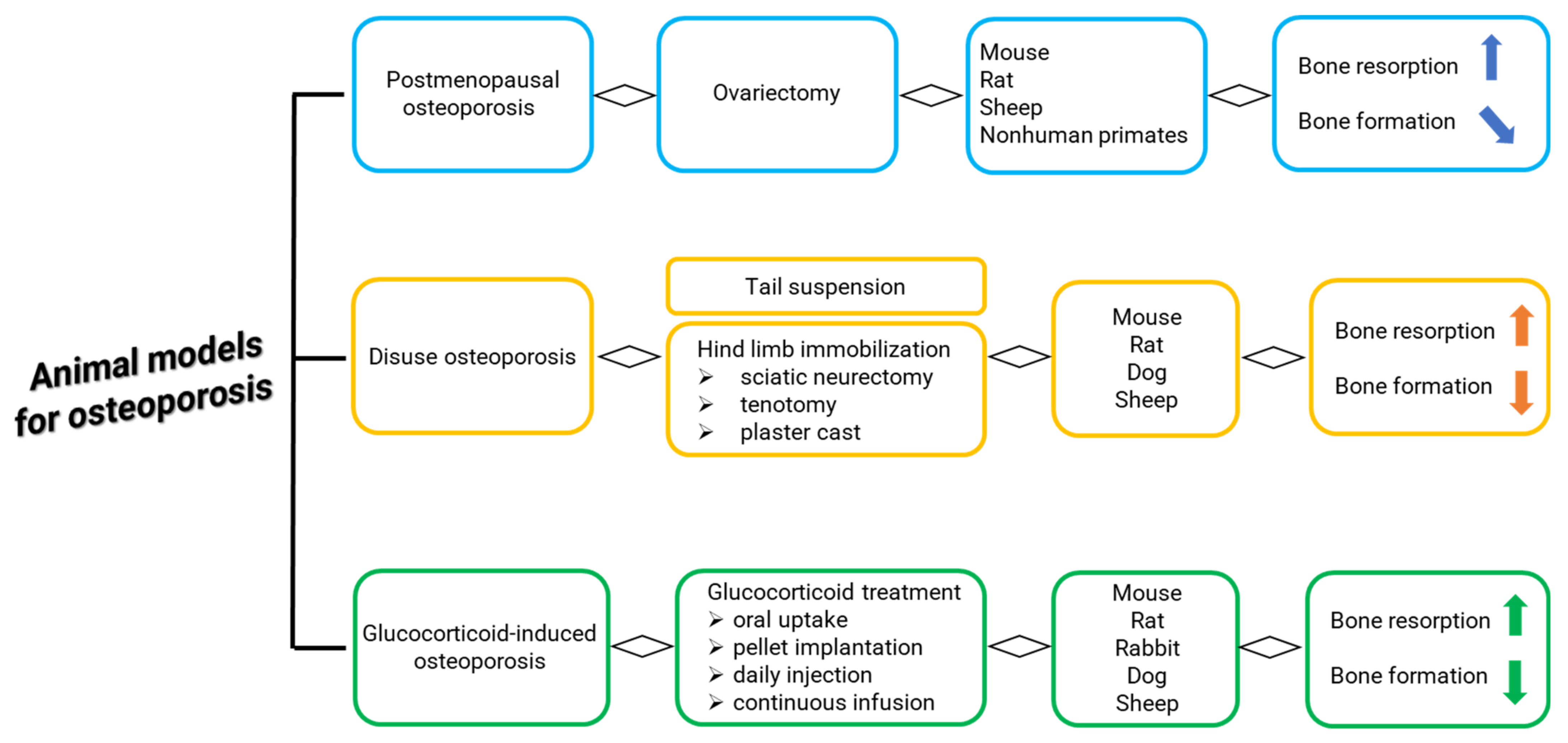
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategies
2.3. Selection Process
2.4. Data Collection Process and Synthesis Methods
2.5. Assessment of Methodological Quality
| Ref. | Objectives | Study Design (Groups) | Animal Number and Age | OVX (Time from OVX) | Experimental Time | Analyses and Measuraments | Main Results |
|---|---|---|---|---|---|---|---|
| Brennan et al., 2012 [23] | Evaluation of the timing of changes in bone | - Control (intact); - OVX | n = 19 mixed-breed 4 years | NR | 12 and 31 mo after OVX | - Micro-CT: proximal femur BV/TV, Tb.N., Tb.Th, Tb.Sp; - Biomechanics: trabecular bone ultimate compressive strength; - RT-PCR: right metacarpal RANKL, OPG, COL1A1, COL1A2, OCN, OPN; - FTIR: mineral-to-matrix ratio, Crystallinity, Collagen crosslinking | OVX induced: ↑ RANKL, OPG, COL1A1, COL1A2, OCN, OPN by 12 and 32 mo; ↓ mineral-to-matrix ratio, ratio of mature to immature collagen cross-linking than Control at 31 mo; =Tb.N., Tb.Th, Tb.Sp by 12 mo; ↓ Tb.N, Tb.Th and ↑ Tb.Sp at 31 mo; ↓ compressive strength at 31 mo |
| Oheim et al., 2013 [24] | Relevance of low turnover OP induced by OVX and HPD | - Control (intact) 24 mo; - OVX 24 mo; - HPD + OVX 12 mo; - HPD + OVX 24 mo | n = 20 Corriedale 6–7 years | OVX performed 1 week before HPD | 12 and 24 mo after HPD | - Biochemistry urine (deoxypyridinoline) and serum parameters (sodium, potassium, chloride, Ca, P, Crea, BAP) - X-rays, histology: iliac crest, spines (L3–L5) - histomorphometry (BV/TV, Tb.N, Tb.Sp, Tb.Th, Ob/B.Pm, N.Oc/B.Pm, Ob.S/BS and Oc.S/BS, ES/BS, OS/BS, MS/BS, BFR/BS - HR-pQCT: BV/TV, Tb.N, Tb.Sp, Tb.Th, Ct.Th - qBEI: BMDD - biomechanical test: femora three-point-bending test | OVX + HPD lead to a persisting low turnover status with negative turnover balance in sheep followed by cortical and trabecular bone loss with biomechanical impairment |
| Zhang et al., 2014 [25] | Variation of cancellous bones at four skeletal sites: lumbar vertebra, femoral neck, mandibular angle, and rib | - Control (intact) - OVX | n = 16 4 ± 0.5 years | 12 mo after OVX | 12 mo after OVX | - X-ray absorptiometry: lumbar vertebra BMD - micro-CT: BV/TV, Tb.N, Tb.Sp, Tb.N - histology - histomorphometry: Tb.Ar/T.Ar - biomechanical test: compression test | The sensibility of cancellous bones in OVX sheep was site-specific on a pattern as follows: lumbar vertebra, femoral neck, mandibular angle (↓ BV/TV by 45.6%, 36.1% 21.3% and 18.7% in lumbar vertebrae, femoral necks, mandibular angles, and ribs; Tb.N have the same downtrend; ↓ Tb.Ar/T.Ar by 32.1%, 23.2% and 20.7% in lumbar vertebrae, femoral necks, and mandibular angles) |
| Kreipke et al., 2014 [26] | Changes in microarchitectural and mechanical parameters in femoral condyles and vertebral bodies | - Control (intact) - OVX-1 (euthanized at 1 year) - OVX-2 group (euthanized at 2 years) | n = 19 | 1 or 2 years following the OVX | 2 years | micro-CT (BV/TV, SMI, Tb.Th., Tb.Sp., BMD, TMD, DA) | ↓ BV/TV, Tb.Th., BMD, ↑ SMI, Tb.Sp. in OVX-1 and OVX-2 vs control. ↑ mechanical anisotropy in OVX groups. OVX had minimal effects on trabecular architecture of the distal femur even after 2 years. Medial condyle: ↑ BV/TV, BMD, TMD in OVX-1 group vs. control. Lateral condyle: ↓ DA in OVX-1 group vs control. ↑ TMD in OVX groups vs control |
| Andreasen et al., 2015 [27] | How GC affects the cancellous bone and the cellular events of the bone remodeling process | - Control (intact) OVX + deficient diet + GC | n = 20 9 ± 1 years | 2 weeks after OVX, deficient diet and s.i. of methylprednisolone | 7 mo after GC treatment | - Biochemistry: Serum CTX and OC; - Micro-CT: BV/TV, Tb.Th; - Histology; - IHC: TRAcP, SMA, Sp7, Runx2, ALP; - Histomorphometry: ES/BS, OS/BS, Oc.S/BS, Rv.S/BS | GC ↑ bone loss with ↓ BV/TV, Tb.Th, OC, OS/BS, cell density, Sp7, Runx2 and SMA; ↑ Rv.S/BS |
| Kiełbowicz et al., 2015a [28] | Changes in bone parameters in OVX and OVX + methylprednisol sheep in comparison to healthy sheep | - Control (intact) - OVX - OVX + methylprednisolone | n = 49 Merino 5–6 years | Methylprednisolone 30 days after OVX | 21 days after the last application of steroidal medication (injections repeated 4 times at 20-day intervals) | Blood tests (estradiol, cortisol, progesterone, parathormone), diagnostic arthroscopy, micro-CT, and X-ray (BMD, BV/TV, BS/BV, porosity, Tb.N, Tb.Th, Tb. Sp, Conn.Dn) | ↓ estrogens and progesterone levels, and ↑ parathormone and cortisol levels, OVX + methylprednisolone. ↓ bone turnover markers (b-ALP) in all groups. ↑ bone resorption markers (CTX), and ↓ radiological density in OVX and OVX + methylprednisolone groups |
| Kiełbowicz et al., 2015b [29] | Impact of steroidal medications on the structure and mechanical properties of OP animal model | - Control (intact) - OVX - OVX + glucocorticosteroid | n = 21 Merino 5–6 years | Treatment with GC (for 80 days) 1 mo after OVX | 2 weeks after glucocorticosteroid administration | Quantitative CT | ↓ radiological bone density in OVX + glucocorticosteroid group vs. control group |
| Kiełbowicz et al., 2016 [30] | Changes in bone parameters in OVX and OVX + methylprednisolone as opposed to parameters in healthy sheep | - Control (intact) - OVX - OVX + methylprednisolone | n = 49 Merino 5–6 years | Methylprednisolone 30 days after OVX | 21 days after the last application of steroidal medication (injections repeated 4 times at 20-day intervals) | Mechanical tests (force/strength), SEM X-ray microanalysis (Ca/P), morphometric analysis (bone formation, porosity, thickness) | ↑ bone formation, porosity and thickness, and ↓ Ca and P levels, strength, Young’s modulus, compressive strength of bone tissue, and deformation (strain) energy in OVX + methylprednisolone group |
| Kreipke et al., 2016 [31] | Effects of microarchitecture and estrogen depletion on microdamage susceptibility in trabecular bone | - Control (intact) - OVX | n = 19 | 2 years following OVX | 2 years | Sequence of compressive and torsional overloads (propensity for microdamage formation in trabecular bone of the distal femur), mechanical testing, micro-CT (BMD, B.Ar., Dx.Ar., Cr.Dn., Cr.Ln., Cr.S.Dn.) | ↓ BV/TV and ↑ SMI in the lateral condyle following OVX. ↓ Young’s modulus in OVX. ↓ Pre-existing Cr.Dn. with ↑ BV/TV in both Control and OVX, with a more negative slope in OVX. ↑ Cr.Dn. with ↑ SMI in OVX. Dx.Ar. correlated with ↑ SMI for OVX. In OVX Cr.Dn. from the compressive load correlated with preexisting Cr.Dn. as Dx.Ar., and both ↑ with ↓ BV/TV |
| Oheim et al., 2017 [32] | Effects of peripheral hormone therapy on centrally induced systemic bone loss | - Control (intact) - OVX - OVX + HPD; - OVX + HPD + estrogen - OVX + HPD + thyroxin - OVX + HPD + thyroxin + estrogen | n = 30 Corriedale 4–5 years | - OVX performed 1 week before HPD - Hormone therapy started 2 weeks after HPD | At the end of the 9-mo of hormone therapy | - Biochemistry urine (deoxypyridinoline) and serum parameters (sodium, potassium, chloride, Ca, P, Crea, BAP) - histology: iliac crest, spines (L3–L5) - X-rays, micro-CT, HR-pQCT: BV/TV, Tb.N, Tb.Sp, Tb.Th, Ct.Th - histomorphometry: BV/TV, Tb.N, Tb.Sp, Tb.Th - biomechanical test: femora three-point-bending test | Bone loss in OVX+HPD. Treatment with thyroxin alone ↑ bone resorption, ↓ bone volume. Treatment with estrogen and the combined treatment with estrogen and thyroxin prevent OVX-HPD-induced bone loss |
| Schulze et al., 2017 [33] | Selection of reference genes for quantitative real-time PCR | - Control (intact) - OVX - OVX + deficient diet (OVXD) - OVX + deficient diet + i.m. steroid-suspension (OVXDS) | n = 31 Merino 5.5 years | 2 weeks after OVX diet and/or i.m steroid were given | 8 mo after OVX | PCR L5 vertebra: GAPDH, ALAS1, HPRT, EF-2, G6PDH, ACTB, RPL19, B2M, YWHAZ, SDHA, PGK1, and TFRC | B2M, GAPDH, RPL19, and YWHAZ are the genes recommended for relative quantification of gene expression studies in ovine bone for evaluating bone-substituents in OP |
| Heiss et al., 2017 [34] | Clinically similar T-score standard to diagnose OP | - Control (sham) - OVX - OVX+deficient diet - OVX + deficient diet + GC | n = 32 Merino 3–9 years | 2 weeks after OVX, deficient diet and i.m. injection of methylprednisolone | 8 mo after GC | - DXA: BMD, BMC, and T-score and Z-score | OVX+GC ↓ LV, femur BMD, BMC. OVX+deficient diet ↓ LV, BMC, T-score during time |
| El Khassawna et al., 2017 [35] | Evaluation of RANKL/OPG ratio correlation to the method of OP induction | - Control (intact) (group 1) - OVX (group 2) - OVX + deficient diet (group 3) - OVX + deficient die t+ GC (group 4) | n = 32 Merino 5.5 years | 2 weeks after OVX, deficient diet and i.m. injection of methylprednisolone | 8 mo after GC | - DXA: BMD, Fat % for IC and LV; - Biomechanics: The maximum strength of the vertebrae, maximum load peak in the stiffness; - Histology; - IHC: ALP, TRAP, RANKL, OPG; - Histomorphometry: nonmineralized and mineralized matrix portion in IC and LV, Quantification of toluidine blue staining on LV, Spindle, spherical, and empty lacunae, RANKL or OPG activity; serum OCN, BAP, NTX; RT-PCR: ALP, CA2, OPG, RANKL, COL1A2, FN1 of L1 | (Group 4): ↓ Z-score, maximum load at failure, stiffness, mineralized bone area, RANKL/OPG; ↑ OCN, empty lacunae, COL1A2, Fat% than the other groups. (Groups 3,4): ↓ NTX than (Groups 1,2). (Group 2): ↓ CA2 than the other groups. (Groups 2,4): ↓ OPG than (Groups 1,3) |
| Cabrera et al., 2018 [36] | Validation of the combination of OVX and GC | - Control (intact) - OVX - OVX + GC (2 and 5 mo) | n = 28 Merino 7–9 years | 2 weeks after OVX, methylprednisolone administered for 2 or 5 mo | 2 and 5 mo after OVX | - Biochemistry: serum OC, CTX-1; - DXA: Lumbar spine and femur BMC and aBMD. - pQCT: left tibia vBMD, bone area, BMC, cortical/subcortical and trabecular vBMD, cortical/subcortical and trabecular bone area, cortical/subcortical, and trabecular BMC | OVX + GC induces bone loss in a short period of time. OVX ↑ CTx-1 and OC. OVX + GC ↑ CTx-1 and ↓ OC at 5 mo. OVX and OVX + GC ↓ BMD, total and trabecular vBMD in the proximal tibia |
| Muller et al., 2019 [37] | Characterization of bone quality | - Control (sham) - OVX - OVX + deficient diet of Ca and vitamin D - OVX + deficient diet + GC injections | n = 28 5.5 years | NR | 8 mo | Biomechanical testing and mathematical modelling, compression tests and finite-element analysis of stress states (stiffness, strength, averaged microscopic Young’s modulus at tissue level), micro-CT, and time-of-flight secondary ion mass spectrometry (trabecular structure, mineral and collagen distribution) | ↓ BV/TV, Tb.Th, stiffness, strength and ↑ BS/BV in OVX + deficient diet + GC. ↑ Tb.Sp in OVX and OVX + deficient diet of Ca and vitamin D groups. ↓ Tb.N and ↑ SMI in OVX group |
| Cabrera et al., 2020 [38] | Effects of short- or long-term GC on plasma metabolites and lipids | - Control (intact) - OVX - OVX + GC (2 and 5 mo) | n = 28 Merino 7–9 years | 2 weeks after OVX, methylprednisolone administered for 2 or 5 mo | 2 and 5 mo after OVX | - Liquid chromatography–mass spectrometry untargeted metabolomic analysis | OVX + GC altered the metabolite and lipid profiles |
| Coelho et al., 2020 [39] | Characterization of GC-treated OVX sheep | - Control (sham) - OVX | n = 12 Portuguese Serra-da-Estrela 3–4 years | OVX + 1/week injections of dexamethasone (1 mg/kg) for 5 mo | 6 mo after OVX | ELISA: serum TRAP, estradiol, BUN, Crea, TC, Ca, P, Mg, Glu, ALP activity, AST, ALT, GGT, TP; Micro-CT: BMD, BV/TV, BS/BV, Tb.Th, Tb.N, Tb.Sp, Po(cl), Po(op), Po(tot) of L4 vertebra body; Histology; Histomorphometry: Ct.Po % and Ct.Th were assessed in the cortical bone and the BV/TV, Tb.Th, Tb.Sp and Tb.N in the trabecular bone | OVX ↑ osteopenia, mean corpuscular volume, mean cell hemoglobin and monocytes, ALP, GGT, Mg, α1-globulin; ↓ red blood count and eosinophils, Crea, albumin, sodium, and estradiol |
| Rupp et al., 2021 [40] | Clinical relevance of a fracture defect model in the iliac crest | - Control (intact) - OVX - OVX + deficient diet (OVXD) - OVX + deficient diet + i.m. steroid-suspension (OVXDS) | n = 31 Merino 5.5 years | 2 weeks after OVX diet and/or i.m steroid were given, and iliac crest bone defects (7.5 mm diameter and 25 mm long) were created | 5 and 8 mo after OVX | - DXA: BMD, BMC - Micro-CT - Histology and histomorphometry: Tb.Th, Ct.Th, size callus formation, cartilage tissue, mineral deposition rate | Significant ↓ BMD and BMC in OVXDS. OVX and OVXD showed complete healing after 8 mo. Bone quality affected mostly in the OVXDS group with irregular trabecular network, ↓ Ct.Th, ↑ cartilaginous tissue at 8 mo. The mineral deposition rate showed a declining pattern in the control, OVX, and OVXD from 5 mo to 8 mo. OVXDS group showed an incremental pattern from 5 mo to 8 mo |
3. Results
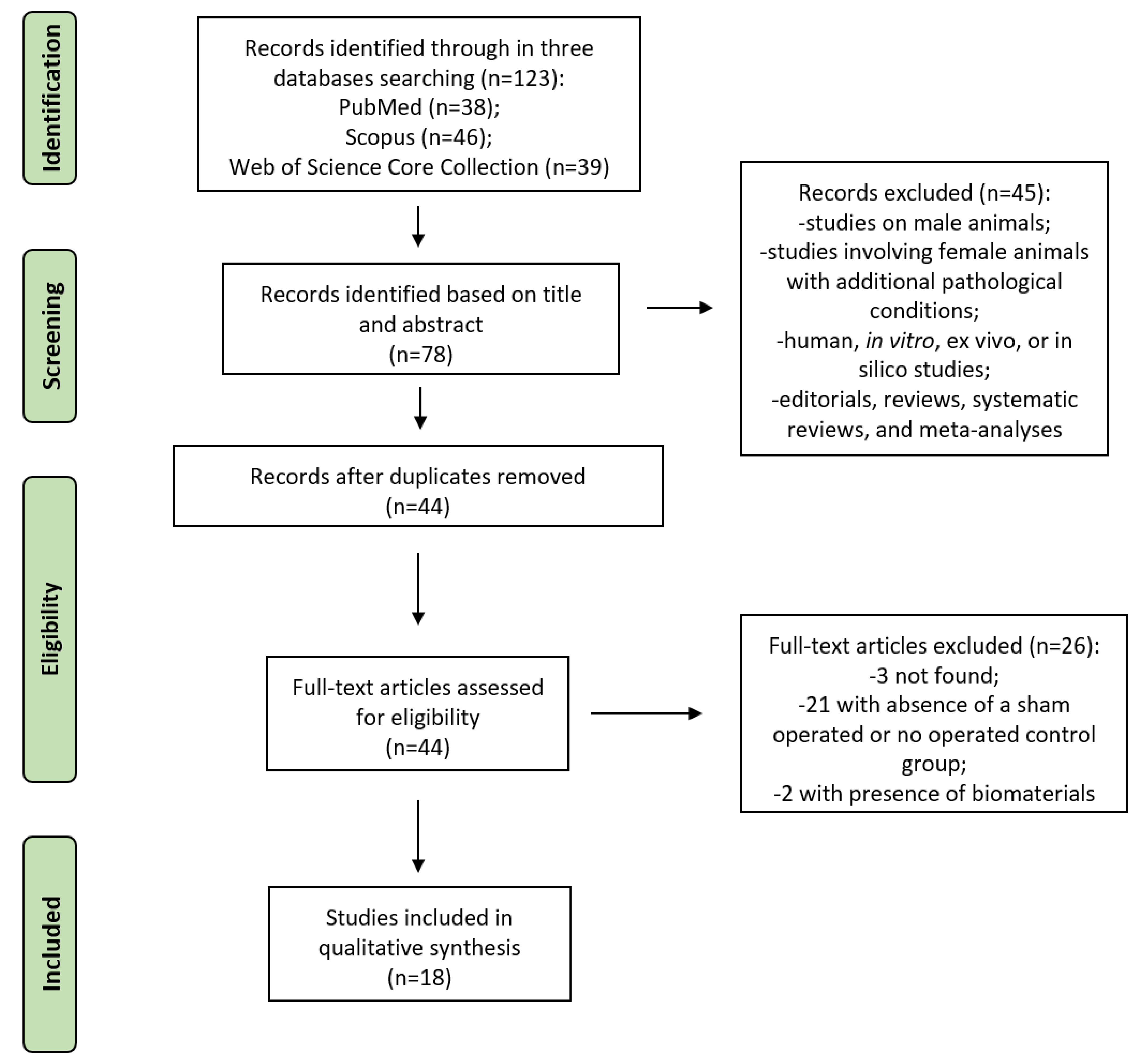
3.1. Study Selection and Characteristics
3.2. Assessment of Methodological Quality
3.3. Studies Results
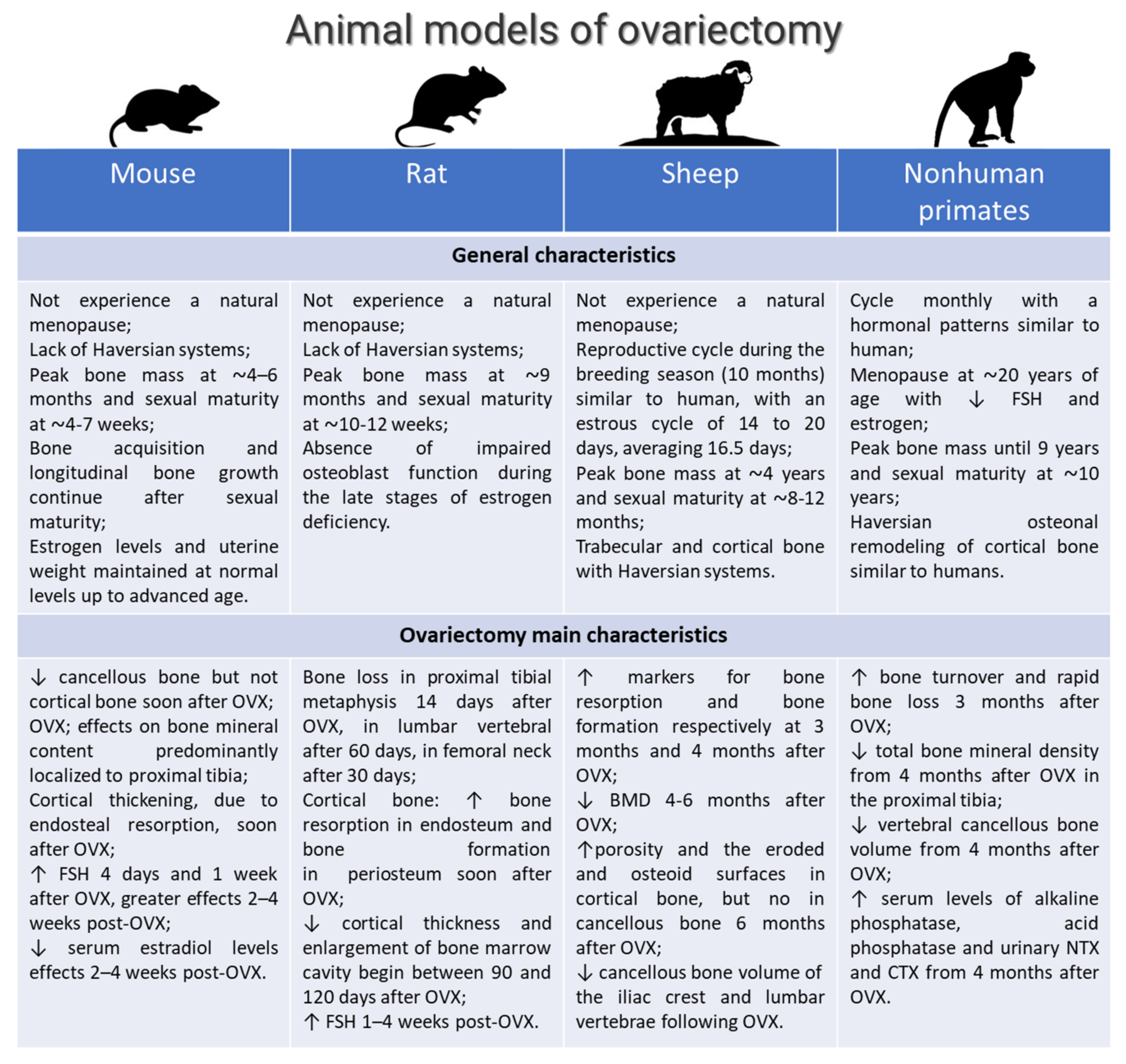
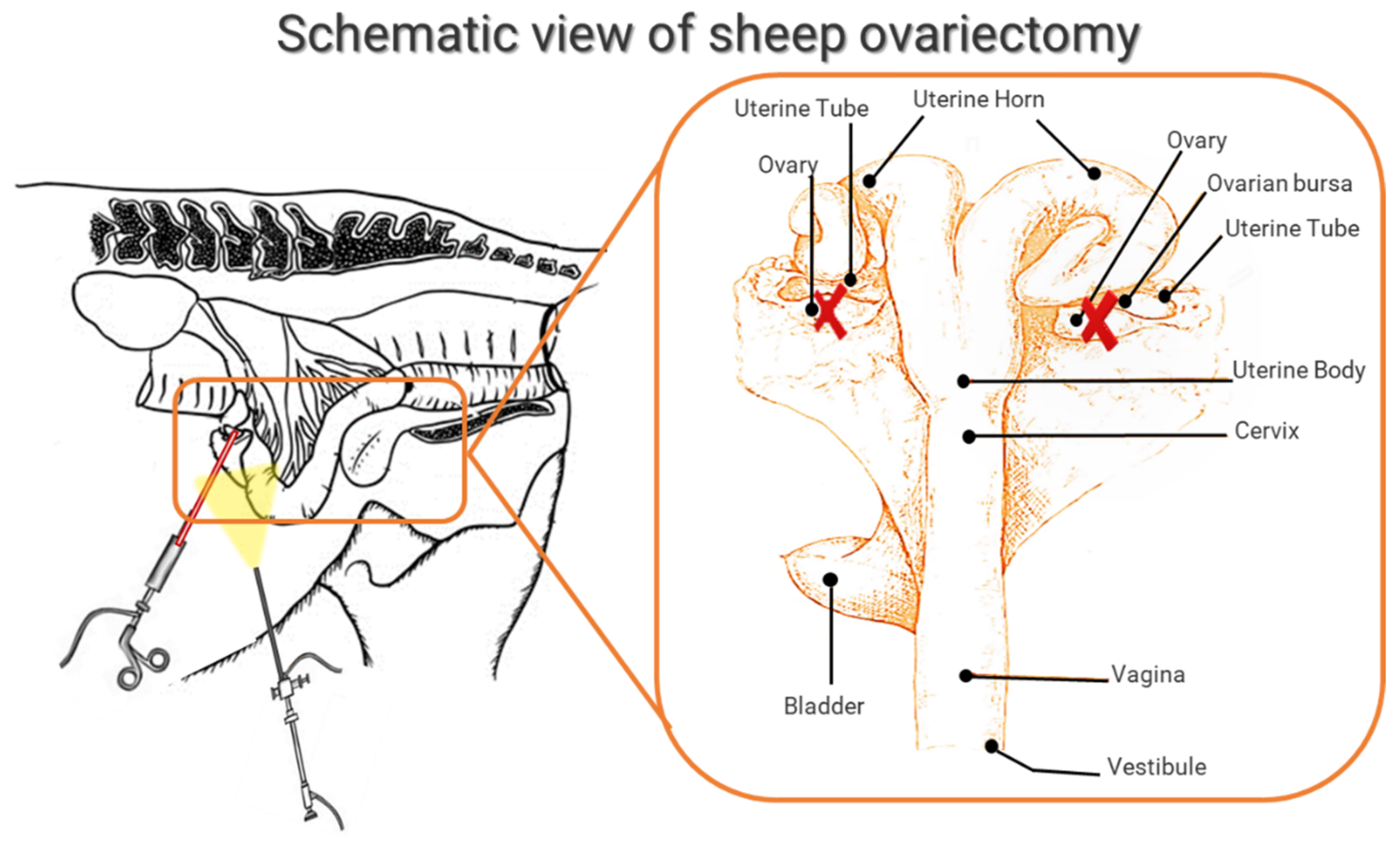
3.3.1. General Characteristics: Breed, Age, Surgical Methods for OVX
3.3.2. Control Group
3.3.3. Verification of OVX
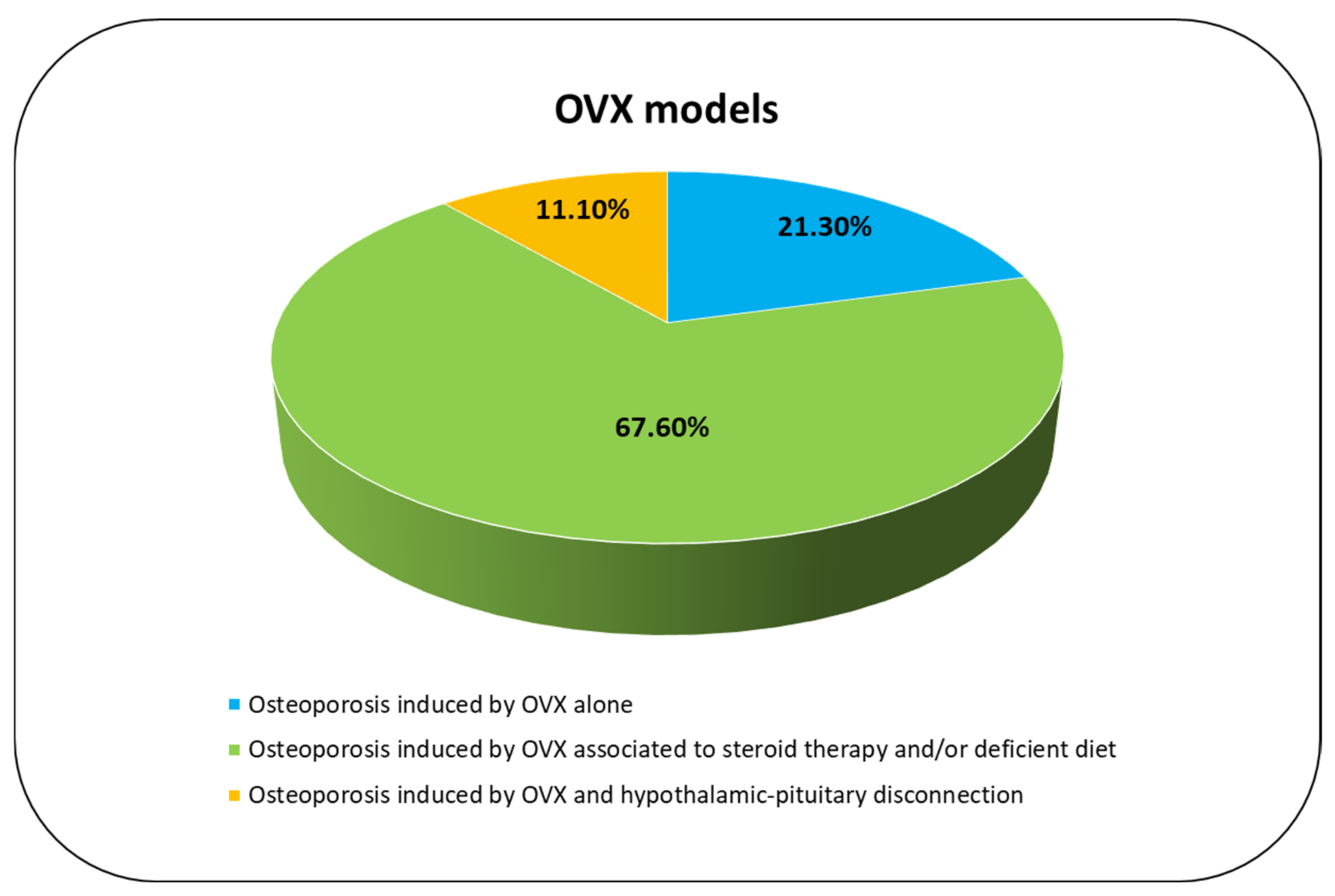
3.3.4. Osteoporosis Induced by OVX Alone
3.3.5. Osteoporosis Induced by OVX Associated to Steroid Therapy and/or Deficient Diet
3.3.6. Osteoporosis Induced by OVX and Hypothalamic–Pituitary Disconnection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crandall, C.J. Risk Assessment Tools for Osteoporosis Screening in Postmenopausal Women: A Systematic Review. Curr. Osteoporos Rep. 2015, 13, 287–301. [Google Scholar] [CrossRef]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Curtis, E.M.; van der Velde, R.; Moon, R.J.; van den Bergh, J.P.W.; Geusens, P.; de Vries, F.; van Staa, T.P.; Cooper, C.; Harvey, N.C. Epidemiology of fractures in the United Kingdom 1988-2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone 2016, 87, 19–26. [Google Scholar] [CrossRef]
- Song, S.; Guo, Y.; Yang, Y.; Fu, D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 2022, 237, 108168. [Google Scholar] [CrossRef]
- Brent, M.B.; Brüel, A.; Thomsen, J.S. A Systematic Review of Animal Models of Disuse-Induced Bone Loss. Calcif. Tissue Int. 2021, 108, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Oheim, R.; Schinke, T.; Amling, M.; Pogoda, P. Can we induce osteoporosis in animals comparable to the human situation? Injury 2016, 47 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- Dias, I.R.; Camassa, J.A.; Bordelo, J.A.; Babo, P.S.; Viegas, C.A.; Dourado, N.; Reis, R.L.; Gomes, M.E. Preclinical and Translational Studies in Small Ruminants (Sheep and Goat) as Models for Osteoporosis Research. Curr. Osteoporos Rep. 2018, 16, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, P.; Egermann, M.; Schnell, J.C.; Priemel, M.; Schilling, A.F.; Alini, M.; Schinke, T.; Rueger, J.M.; Schneider, E.; Clarke, I.; et al. Leptin inhibits bone formation not only in rodents, but also in sheep. J. Bone Miner. Res. 2006, 21, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, I.M.; Gerhardt, C.; Alini, M.; Schneider, E.; Egermann, M. The long-term effects of ovariectomy on bone metabolism in sheep. J. Bone Miner. Metab 2007, 25, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Augat, P.; Schorlemmer, S.; Gohl, C.; Iwabu, S.; Ignatius, A.; Claes, L. Glucocorticoid-treated sheep as a model for osteopenic trabecular bone in biomaterials research. J. Biomed. Mater. Res. A 2003, 66, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Macleay, J.M.; Olson, J.D.; Turner, A.S. Effect of dietary-induced metabolic acidosis and ovariectomy on bone mineral density and markers of bone turnover. J. Bone Miner. Metab. 2004, 22, 561–568. [Google Scholar] [CrossRef]
- Johnson, R.B.; Gilbert, J.A.; Cooper, R.C.; Parsell, D.E.; Stewart, B.A.; Dai, X.; Nick, T.G.; Streckfus, C.F.; Butler, R.A.; Boring, J.G. Effect of estrogen deficiency on skeletal and alveolar bone density in sheep. J. Periodontol. 2002, 73, 383–391. [Google Scholar] [CrossRef]
- Newton, B.I.; Cooper, R.C.; Gilbert, J.A.; Johnson, R.B.; Zardiackas, L.D. The ovariectomized sheep as a model for human bone loss. J. Comp. Pathol. 2004, 130, 323–326. [Google Scholar] [CrossRef]
- Holland, J.C.; Brennan, O.; Kennedy, O.D.; Rackard, S.M.; O’Brien, F.J.; Lee, T.C. Subchondral trabecular structural changes in the proximal tibia in an ovine model of increased bone turnover. J. Anat. 2011, 218, 619–624. [Google Scholar] [CrossRef]
- Fini, M.; Pierini, G.; Giavaresi, G.; Biagini, G.; Mattioli Belmonte, M.M.; Nicoli Aldini, N.; Rocca, M.; Martini, L.; Giardino, R. The ovariectomised sheep as a model for testing biomaterials and prosthetic devices in osteopenic bone: A preliminary study on iliac crest biopsies. Int. J. Artif. Organs 2000, 23, 275–281. [Google Scholar] [CrossRef]
- Giavaresi, G.; Fini, M.; Torricelli, P.; Martini, L.; Giardino, R. The ovariectomized ewe model in the evaluation of biomaterials for prosthetic devices in spinal fixation. Int. J. Artif. Organs 2001, 24, 814–820. [Google Scholar] [CrossRef]
- Chavassieux, P.; Garnero, P.; Duboeuf, F.; Vergnaud, P.; Brunner-Ferber, F.; Delmas, P.D.; Meunier, P.J. Effects of a new selective estrogen receptor modulator (MDL 103,323) on cancellous and cortical bone in ovariectomized ewes: A biochemical, histomorphometric, and densitometric study. J. Bone Miner. Res. 2001, 16, 89–96. [Google Scholar] [CrossRef]
- Turner, A.S.; Mallinckrodt, C.H.; Alvis, M.R.; Bryant, H.U. Dose-response effects of estradiol implants on bone mineral density in ovariectomized ewes. Bone 1995, 17 (Suppl. 4), 421S–427S. [Google Scholar] [CrossRef]
- Tugwell, P.; Tovey, D. Prisma 2020. J. Clin. Epidemiol. 2021, 134, A5–A6. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Moher, D.; Schulz, K.F.; Altman, D.G. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001, 357, 1191–1194. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Brennan, O.; Kuliwaba, J.S.; Lee, T.C.; Parkinson, I.H.; Fazzalari, N.L.; McNamara, L.M.; O’Brien, F.J. Temporal changes in bone composition, architecture, and strength following estrogen deficiency in osteoporosis. Calcif Tissue Int. 2012, 91, 440–449. [Google Scholar] [CrossRef]
- Oheim, R.; Beil, F.T.; Köhne, T.; Wehner, T.; Barvencik, F.; Ignatius, A.; Amling, M.; Clarke, I.J.; Pogoda, P. Sheep model for osteoporosis: Sustainability and biomechanical relevance of low turnover osteoporosis induced by hypothalamic-pituitary disconnection. J. Orthop. Res. 2013, 31, 1067–1074. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Gao, Q.; Shao, B.; Xiao, J.; Zhou, H.; Niu, Q.; Shen, M.; Liu, B.; Hu, K.; et al. The variation of cancellous bones at lumbar vertebra, femoral neck, mandibular angle and rib in ovariectomized sheep. Arch. Oral Biol. 2014, 59, 663–669. [Google Scholar] [CrossRef]
- Kreipke, T.C.; Rivera, N.C.; Garrison, J.G.; Easley, J.T.; Turner, A.S.; Niebur, G.L. Alterations in trabecular bone microarchitecture in the ovine spine and distal femur following ovariectomy. J. Biomech. 2014, 47, 1918–1921. [Google Scholar] [CrossRef]
- Andreasen, C.M.; Ding, M.; Overgaard, S.; Bollen, P.; Andersen, T.L. A reversal phase arrest uncoupling the bone formation and resorption contributes to the bone loss in glucocorticoid treated ovariectomised aged sheep. Bone 2015, 75, 32–39. [Google Scholar] [CrossRef]
- Kiełbowicz, Z.; Piątek, A.; Bieżyński, J.; Skrzypczak, P.; Chmielewska, E.; Kafarski, P.; Kuryszko, J. Improvement of large animal model for studying osteoporosis. Bull. Vet. Inst. Pulawy 2015, 59, 123–128. [Google Scholar] [CrossRef]
- Kiełbowicz, Z.; Piątek, A.; Bieżyński, J.; Skrzypczak, P.; Kuropka, P.; Kuryszko, J.; Nikodem, A.; Kafarski, P.; Pezowicz, C. The experimental osteoporosis in sheep—Clinical approach. Pol. J. Vet. Sci. 2015, 18, 645–654. [Google Scholar] [CrossRef][Green Version]
- Kiełbowicz, Z.; Piątek, A.; Kuropka, P.; Mytnik, E.; Nikodem, A.; Bieżyński, J.; Skrzypczak, P.; Pezowicz, C.; Kuryszko, J.; Reichert, P. Experimental osteoporosis in sheep—Mechanical and histological approach. Pol. J. Vet. Sci. 2016, 19, 109–118. [Google Scholar] [CrossRef][Green Version]
- Kreipke, T.C.; Garrison, J.G.; Easley, J.; Turner, A.S.; Niebur, G.L. The roles of architecture and estrogen depletion in microdamage risk in trabecular bone. J. Biomech. 2016, 49, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Oheim, R.; Simon, M.J.K.; Steiner, M.; Vettorazzi, E.; Barvencik, F.; Ignatius, A.; Amling, M.; Clarke, I.J.; Pogoda, P.; Beil, F.T. Sheep model for osteoporosis: The effects of peripheral hormone therapy on centrally induced systemic bone loss in an osteoporotic sheep model. Injury 2017, 48, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Malhan, D.; El Khassawna, T.; Heiss, C.; Seckinger, A.; Hose, D.; Rösen-Wolff, A. A tissue-based approach to selection of reference genes for quantitative real-time PCR in a sheep osteoporosis model. BMC Genomics 2017, 18, 975. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Kern, S.; Malhan, D.; Böcker, W.; Engelhardt, M.; Daghma, D.E.S.; Stoetzel, S.; Schmitt, J.; Ivo, M.; Kauschke, V.; et al. A New Clinically Relevant T-Score Standard to Interpret Bone Status in a Sheep Model. Med. Sci Monit. Basic Res. 2017, 23, 326–335. [Google Scholar] [CrossRef]
- El Khassawna, T.; Merboth, F.; Malhan, D.; Böcker, W.; Daghma, D.E.S.; Stoetzel, S.; Kern, S.; Hassan, F.; Rosenbaum, D.; Langenstein, J.; et al. Osteocyte Regulation of Receptor Activator of NF-κB Ligand/Osteoprotegerin in a Sheep Model of Osteoporosis. Am. J. Pathol. 2017, 187, 1686–1699. [Google Scholar] [CrossRef]
- Cabrera, D.; Wolber, F.M.; Dittmer, K.; Rogers, C.; Ridler, A.; Aberdein, D.; Parkinson, T.; Chambers, P.; Fraser, K.; Roy, N.C.; et al. Glucocorticoids affect bone mineral density and bone remodelling in OVX sheep: A pilot study. Bone Rep. 2018, 9, 173–180. [Google Scholar] [CrossRef]
- Müller, R.; Henss, A.; Kampschulte, M.; Rohnke, M.; Langheinrich, A.C.; Heiss, C.; Janek, J.; Voigt, A.; Wilke, H.J.; Ignatius, A.; et al. Analysis of microscopic bone properties in an osteoporotic sheep model: A combined biomechanics, FE and ToF-SIMS study. J. R Soc. Interface 2019, 16, 20180793. [Google Scholar] [CrossRef]
- Cabrera, D.; Kruger, M.; Wolber, F.M.; Roy, N.C.; Fraser, K. Effects of short- and long-term glucocorticoid-induced osteoporosis on plasma metabolome and lipidome of ovariectomized sheep. BMC Musculoskelet Disord. 2020, 21, 349. [Google Scholar] [CrossRef]
- Coelho, C.A.; Bordelo, J.P.; Camassa, J.A.; Barros, V.A.; Babo, P.S.; Gomes, M.E.; Reis, R.L.; Azevedo, J.T.D.E.; Requicha, J.F.; FaÍsca, P.; et al. Evaluation of hematology, general serum biochemistry, bone turnover markers and bone marrow cytology in a glucocorticoid treated ovariectomized sheep model for osteoporosis research. An. Acad. Bras. Cienc. 2020, 92, e20200435. [Google Scholar] [CrossRef]
- Rupp, M.; Biehl, C.; Malhan, D.; Hassan, F.; Attia, S.; Rosch, S.; Schäfer, A.B.; McMahon, E.; Kampschulte, M.; Heiss, C.; et al. Large Animal Model of Osteoporotic Defect Healing: An Alternative to Metaphyseal Defect Model. Life 2021, 11, 254. [Google Scholar] [CrossRef]
- Barone, R. Anatomia Comparata Dei Mammiferi Domestici. In Splancnologia; Edagricole-New Business Media: Milano, Italy, 2003; Volume 3. [Google Scholar]
- Turner, A.S. The sheep as a model for osteoporosis in humans. Vet. J. 2002, 163, 232–239. [Google Scholar] [CrossRef]
- Wilke, H.J.; Kettler, A.; Claes, L.E. Are sheep spines a valid biomechanical model for human spines? Spine 1997, 22, 2365–2374. [Google Scholar] [CrossRef]
- Zarrinkalam, M.R.; Beard, H.; Schultz, C.G.; Moore, R.J. Validation of the sheep as a large animal model for the study of vertebral osteoporosis. Eur. Spine J. 2009, 18, 244–253. [Google Scholar] [CrossRef]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Arens, D.; Sigrist, I.; Alini, M.; Schawalder, P.; Schneider, E.; Egermann, M. Seasonal changes in bone metabolism in sheep. Vet. J. 2007, 174, 585–591. [Google Scholar] [CrossRef]
- Beil, F.T.; Oheim, R.; Barvencik, F.; Hissnauer, T.N.; Pestka, J.M.; Ignatius, A.; Rueger, J.M.; Schinke, T.; Clarke, I.J.; Amling, M.; et al. Low turnover osteoporosis in sheep induced by hypothalamic-pituitary disconnection. J. Orthop. Res. 2012, 30, 1254–1262. [Google Scholar] [CrossRef]
| Database | Free-Vocabulary and/or Medical Subject Headings (MeSH) Terms |
|---|---|
| PubMed | (“ovariectomied” [All Fields] OR “ovariectomy” [MeSH Terms] OR “ovariectomy” [All Fields] OR “ovariectomies” [All Fields]) AND (“sheep” [MeSH Terms] OR “sheep” [All Fields] OR “sheeps” [All Fields] OR “sheep s” [All Fields] OR “sheep, domestic” [MeSH Terms] OR (“sheep” [All Fields] AND “domestic” [All Fields]) OR “domestic sheep” [All Fields]) AND (“bone and bones” [MeSH Terms] OR (“bone” [All Fields] AND “bones” [All Fields]) OR “bone and bones” [All Fields] OR “bone” [All Fields]) AND ((y_10[Filter]) AND (english[Filter])) |
| Web of Science Core Collection | (TS = ovariectomied OR TS = ovariectomy) AND (TS = sheep OR TS = sheep, domestic) AND (TS = bone and OR TS = bones)—With Publication Year from 2012 to 2022 |
| Scopus | (TITLE-ABS-KEY (ovariectomy) OR TITLE-ABS-KEY (ovariectomied) AND TITLE-ABS-KEY (sheep) OR TITLE-ABS-KEY (sheep, domestic) OR TITLE-ABS-KEY (bone AND bones)) AND PUBYEAR > 2012 |
| Ref. | Title | Abstract | Introduction | Methods | Results | Discussion | Total |
|---|---|---|---|---|---|---|---|
| Brennan et al., 2012 [23] | 0 | 1 | 3 | 14 | 4 | 5 | 27 |
| Oheim et al., 2013 [24] | 1 | 1 | 3 | 8 | 3 | 4 | 20 |
| Zhang et al., 2014 [25] | 1 | 2 | 3 | 6 | 5 | 3 | 20 |
| Kreipke et al., 2014 [26] | 1 | 1 | 3 | 11 | 3 | 2 | 21 |
| Andreasen et al., 2015 [27] | 1 | 2 | 3 | 16 | 7 | 4 | 33 |
| Kiełbowicz et al., 2015a [28] | 1 | 1 | 2 | 12 | 4 | 3 | 23 |
| Kiełbowicz et al., 2015b [29] | 1 | 1 | 3 | 10 | 3 | 2 | 20 |
| Kiełbowicz et al., 2016 [30] | 1 | 1 | 2 | 12 | 4 | 3 | 23 |
| Kreipke et al., 2016 [31] | 0 | 1 | 3 | 7 | 2 | 4 | 17 |
| Oheim et al., 2017 [32] | 1 | 1 | 3 | 8 | 6 | 5 | 24 |
| Schulze et al., 2017 [33] | 1 | 2 | 3 | 8 | 3 | 3 | 20 |
| Heiss et al., 2017 [34] | 1 | 2 | 3 | 13 | 3 | 3 | 25 |
| El Khassawna et al., 2017 [35] | 1 | 2 | 3 | 14 | 3 | 1 | 24 |
| Cabrera et al., 2018 [36] | 1 | 2 | 3 | 15 | 3 | 6 | 30 |
| Muller et al., 2019 [37] | 1 | 2 | 3 | 13 | 3 | 3 | 25 |
| Cabrera et al., 2020 [38] | 1 | 2 | 3 | 15 | 4 | 5 | 30 |
| Coelho et al., 2020 [39] | 1 | 2 | 3 | 14 | 3 | 4 | 27 |
| Rupp et al., 2021 [40] | 1 | 2 | 3 | 8 | 6 | 6 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanna, F.; Contartese, D.; Veronesi, F.; Martini, L.; Fini, M. Osteoporosis Preclinical Research: A Systematic Review on Comparative Studies Using Ovariectomized Sheep. Int. J. Mol. Sci. 2022, 23, 8904. https://doi.org/10.3390/ijms23168904
Salamanna F, Contartese D, Veronesi F, Martini L, Fini M. Osteoporosis Preclinical Research: A Systematic Review on Comparative Studies Using Ovariectomized Sheep. International Journal of Molecular Sciences. 2022; 23(16):8904. https://doi.org/10.3390/ijms23168904
Chicago/Turabian StyleSalamanna, Francesca, Deyanira Contartese, Francesca Veronesi, Lucia Martini, and Milena Fini. 2022. "Osteoporosis Preclinical Research: A Systematic Review on Comparative Studies Using Ovariectomized Sheep" International Journal of Molecular Sciences 23, no. 16: 8904. https://doi.org/10.3390/ijms23168904
APA StyleSalamanna, F., Contartese, D., Veronesi, F., Martini, L., & Fini, M. (2022). Osteoporosis Preclinical Research: A Systematic Review on Comparative Studies Using Ovariectomized Sheep. International Journal of Molecular Sciences, 23(16), 8904. https://doi.org/10.3390/ijms23168904







