Effects of the Rhizosphere Fungus Cunninghamella bertholletiae on the Solanum lycopersicum Response to Diverse Abiotic Stresses
Abstract
:1. Introduction
2. Results
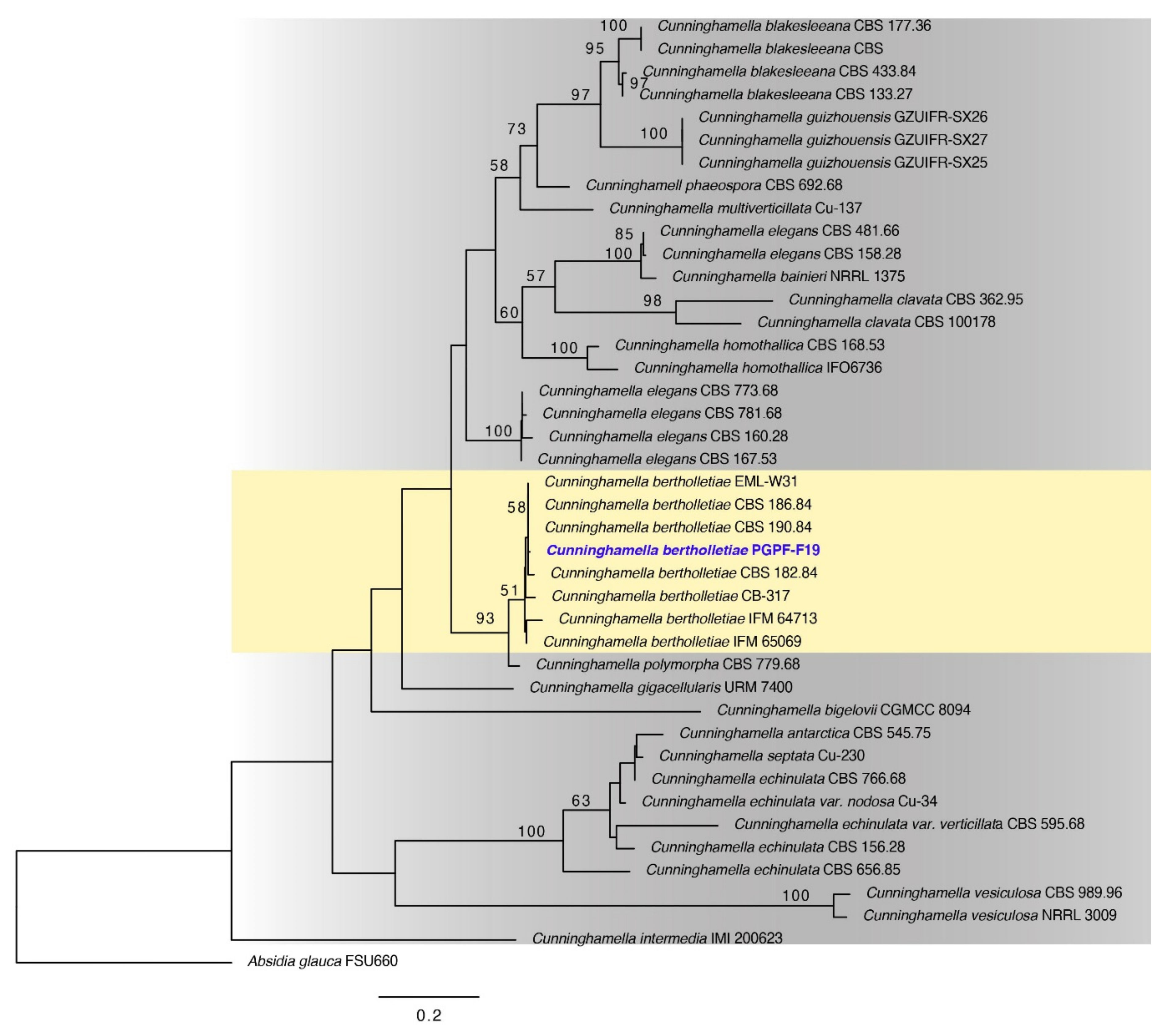
2.1. Identification of Chosen Fungal Strain
2.2. Indole-3-Acetic Acid, Siderophore and Ammonia Production by C. bertholletiae
2.3. Evaluation of C. bertholletiae Stress Resistance on Solid Medium
2.4. Effect of Varied Sodium Chloride Concentrations on Tomato Plant Growth
2.5. Effect of Varied Polyethylene Glycol Concentrations on Tomato Seedling Growth
2.6. Tomato Plants’ Response to PGPF (C. bertholletiae) Inoculant under Varied Abiotic Stresses
2.6.1. Impact of PGPF and Abiotic Stresses on Plant Growth Parameters
2.6.2. Changes in Chlorophyll and Carotenoid Content
2.6.3. Abscisic Acid Response to Abiotic Stresses
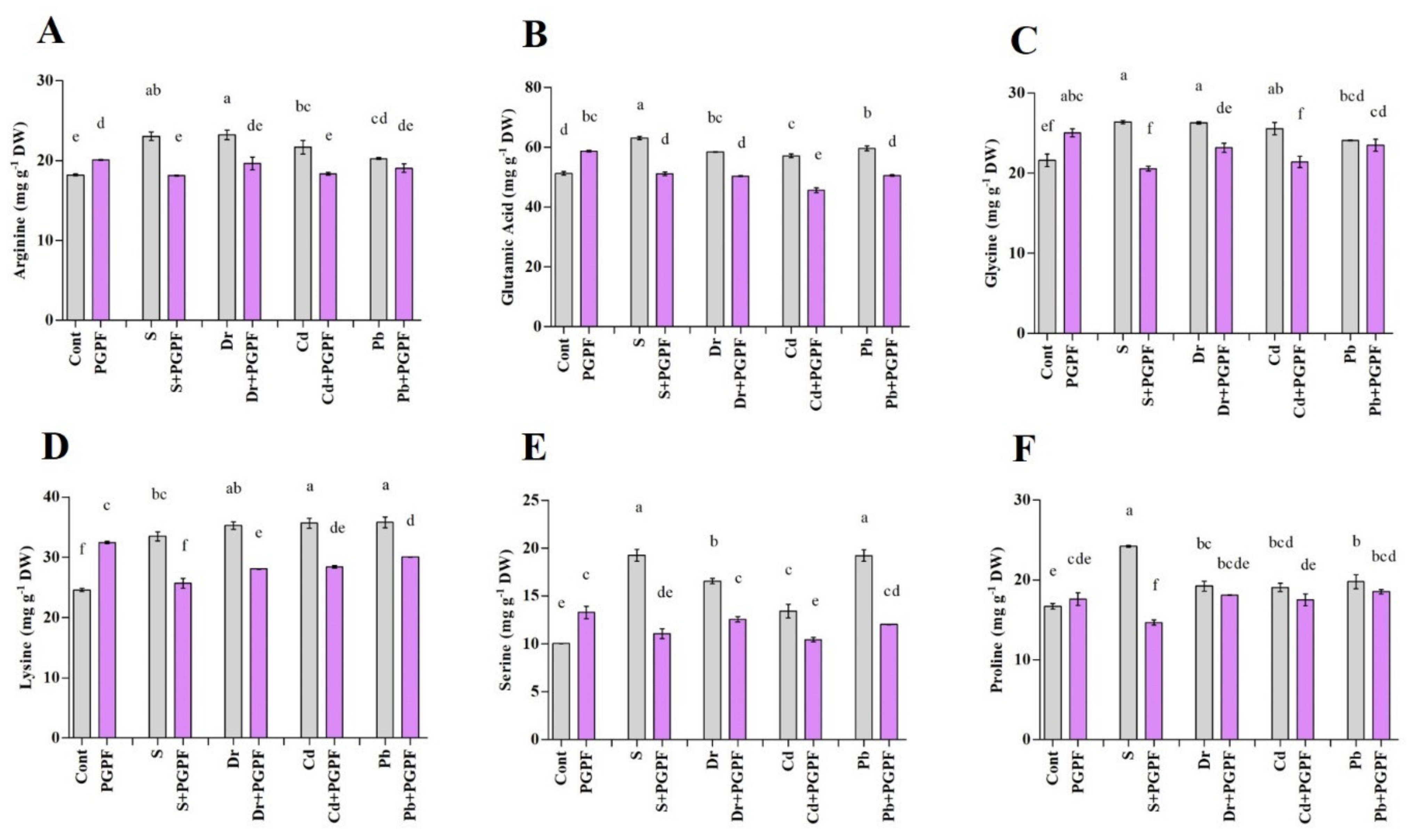
2.6.4. Alteration in Amino Acid Content
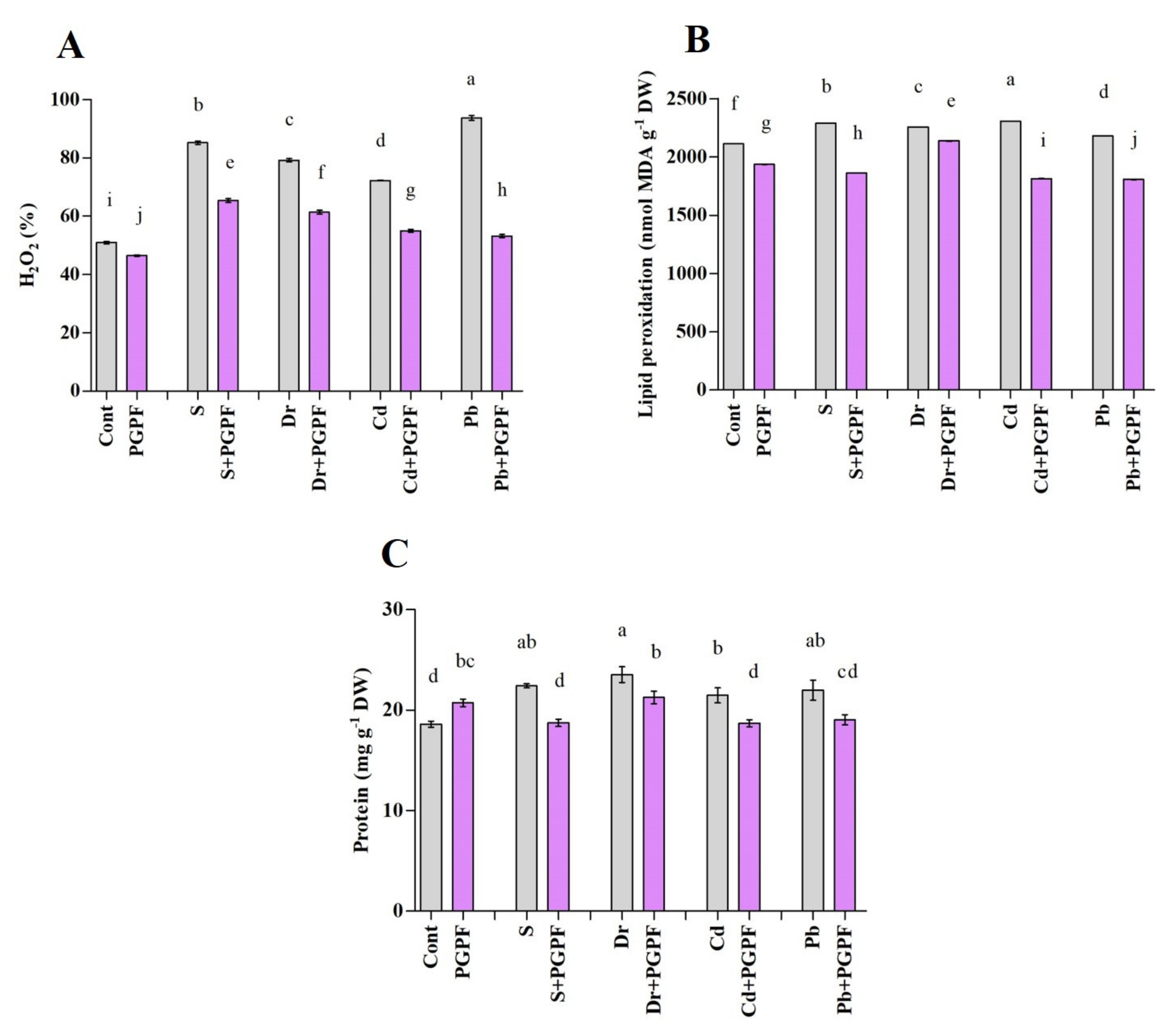
2.6.5. Hydrogen Peroxide and Malondialdehyde Concentrations after Varied Treatments
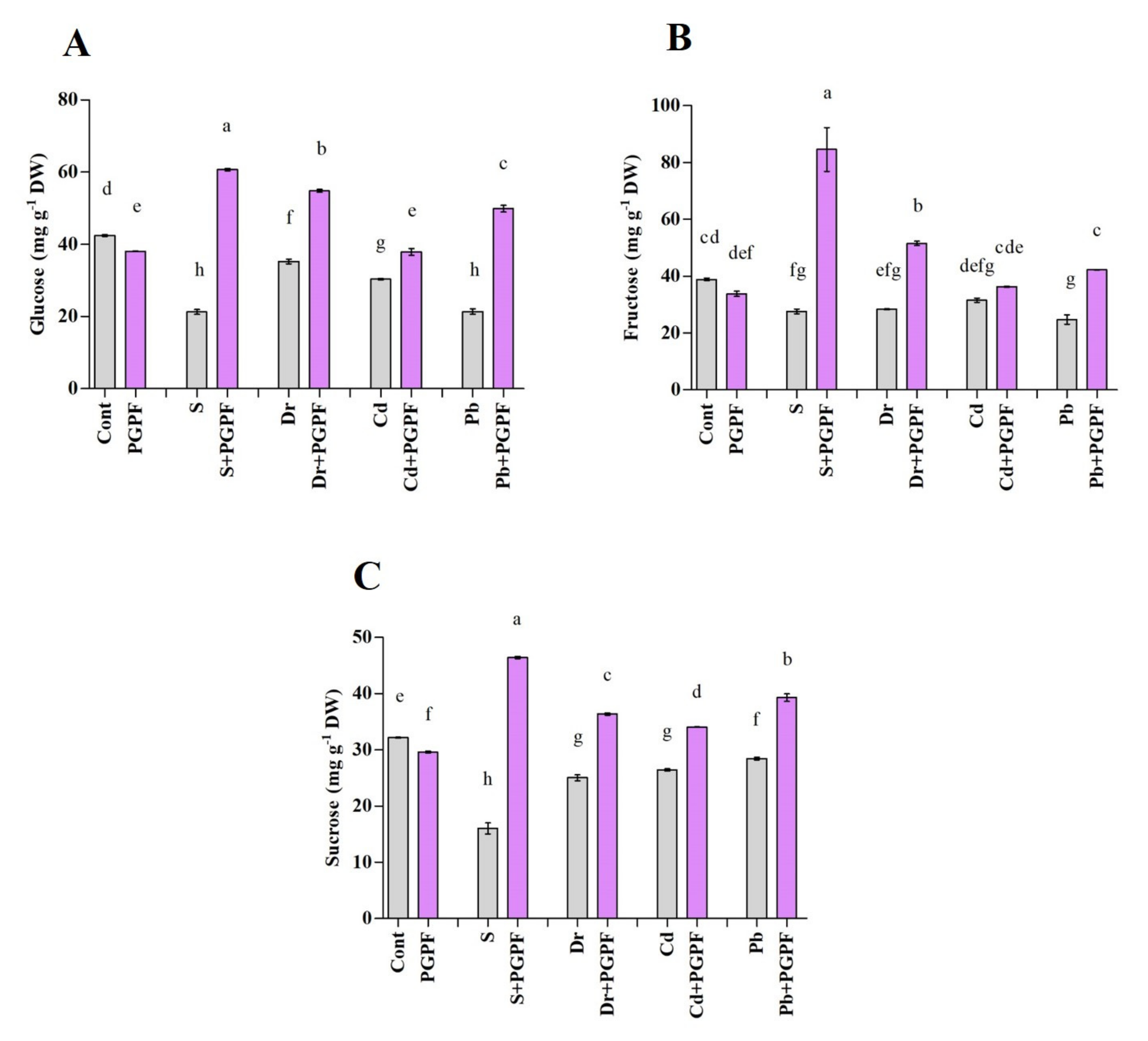
2.6.6. Changes in Protein and Sugar Content
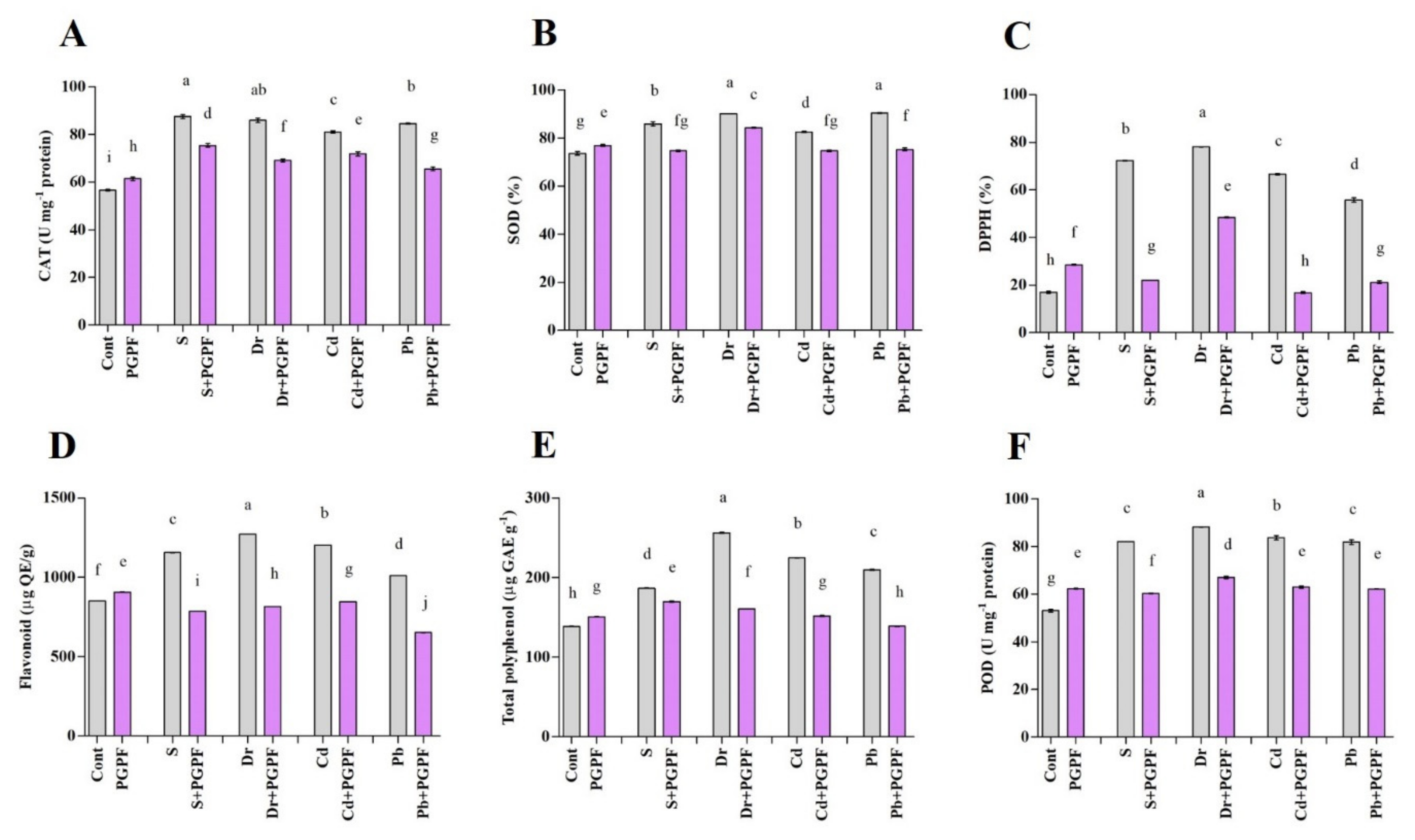
2.6.7. The Activity of Enzymatic and Non-Enzymatic Antioxidants
2.6.8. Nutrient, Sodium and Heavy Metal Contents in Plants
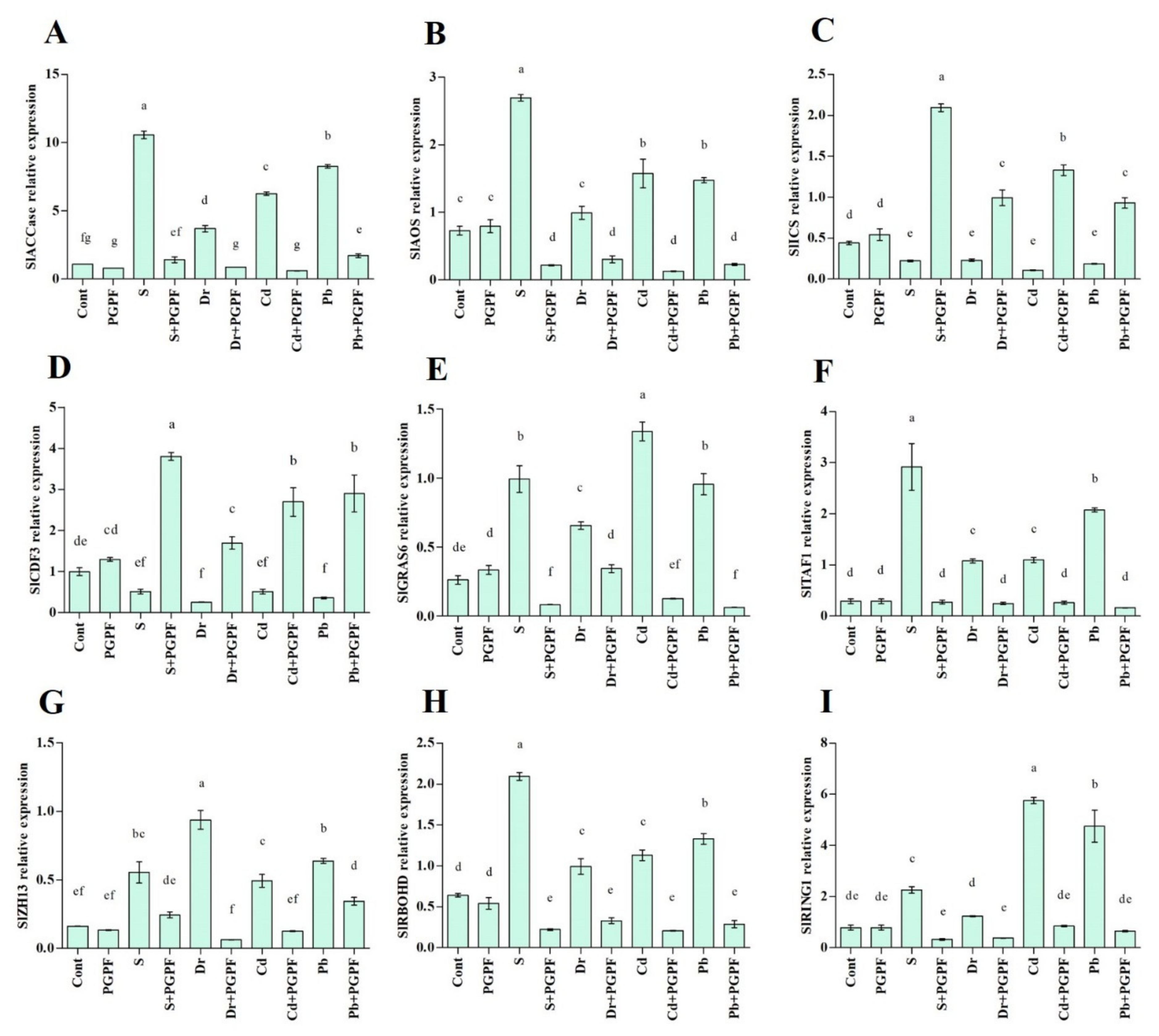
2.7. Expression of Salinity-, Drought- and Heavy Metal-Responsive Genes during the C. bertholletiae Application
2.7.1. Ethylene (SlACCase), Jasmonic Acid (SlAOS) and Salicylic Acid (SlICS) Biosynthesis Genes
2.7.2. Transcription Factors (SlCDF3, SlGRAS6, SlTAF1, SlZH13)
2.7.3. Reactive Oxygen Species Production (SlRBOHD) and E3 Ubiquitin Ligases Activity (SlRING1)
3. Discussion
4. Materials and Methods
4.1. Collection of Rhizospheric Soil and Isolation of Fungus
4.2. Molecular Characterizations of the Fungal Strain
4.3. In Vitro Evaluation of Fungal Strain for Plant Growth-Promoting Traits
4.4. In Vitro Assay of the Fungal Strain for Stress Tolerance
4.5. Greenhouse Experiments
4.6. Influence of C. bertholletiae on Stressed-Tomato Plants
4.7. Measurements of Plant Physiological Traits and Chlorophyll Content
4.8. Determination of Photosynthetic Pigments and Abscisic Acid
4.9. Essential Amino Acid Analysis
4.10. Soluble Protein and Sugar Extraction
4.11. Antioxidant Activity in Inoculated and Non-Inoculated Tomato Plants
4.12. Hydrogen Peroxide and Lipid Peroxidation Quantification of Tomato Plants
4.13. Assessment of Nutrient, Sodium, Cadmium and Lead Contents in Tomato Plants
4.14. RNA Isolation and Gene Expression Analysis
4.15. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Böttner, L.; Grabe, V.; Gablenz, S.; Böhme, N.; Appenroth, K.J.; Gershenzon, J.; Huber, M. Differential localization of flavonoid glucosides in an aquatic plant implicates different functions under abiotic stress. Plant Cell Environ. 2021, 44, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Keep, T.; Sampoux, J.-P.; Barre, P.; Blanco-Pastor, J.-L.; Dehmer, K.J.; Durand, J.-L.; Hegarty, M.; Ledauphin, T.; Muylle, H.; Roldán-Ruiz, I.; et al. To grow or survive: Which are the strategies of a perennial grass to face severe seasonal stress? Funct. Ecol. 2021, 35, 1145–1158. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, M.; Ran, Y.; Yi, X.; Wu, S.; Huang, P. Disentangling the effects of edaphic and vegetational properties on soil aggregate stability in riparian zones along a gradient of flooding stress. Geoderma 2021, 385, 114883. [Google Scholar] [CrossRef]
- Simioniuc, D.P.; Simioniuc, V.; Topa, D.; van den Berg, M.; Prins, U.; Bebeli, P.J.; Gabur, I. Assessment of andean lupin (Lupinus mutabilis) genotypes for improved frost tolerance. Agriculture 2021, 11, 155. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.; Raza, A.; Fatima, E.M.; Baloch, H.; Woodrow, P.; Ciarmiello, L.F. Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Javaid, T.; Farooq, M.A.; Akhtar, J.; Saqib, Z.A.; Anwar-ul-Haq, M. Silicon nutrition improves growth of salt-stressed wheat by modulating flows and partitioning of Na+, Cl− and mineral ions. Plant Physiol. Biochem. 2019, 141, 291–299. [Google Scholar] [CrossRef]
- Zahra, N.; Mahmood, S.; Raza, Z.A. Salinity stress on various physiological and biochemical attributes of two distinct maize (Zea mays L.) genotypes. J. Plant Nutr. 2018, 41, 1368–1380. [Google Scholar] [CrossRef]
- Yadav, T.; Kumar, A.; Yadav, R.; Yadav, G.; Kumar, R.; Kushwaha, M. Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi J. Biol. Sci. 2020, 27, 2010–2017. [Google Scholar] [CrossRef]
- Allen, M.; Babiker, M.; Chen, Y.; de Coninck, H.; Connors, S.; van Diemen, R.; Dube, O.; Ebi, K.; Engelbrecht, F.; Ferrat, M. Global Warming of 1.5 °C. In Summary for Policymakers; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Dey, R.; Lewis, S.C.; Arblaster, J.M.; Abram, N.J. A review of past and projected changes in Australia’s rainfall. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10, e577. [Google Scholar] [CrossRef]
- O’Connell, E. Towards adaptation of water resource systems to climatic and socio-economic change. Water Resour. Manag. 2017, 31, 2965–2984. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef] [Green Version]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Lata, C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avis, T.J.; Gravel, V.; Antoun, H.; Tweddell, R.J. Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol. Biochem. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; Klironomos, J.N.; HilleRisLambers, J.; Kinkel, L.L.; Reich, P.B.; Xiao, K.; Rillig, M.C.; Sikes, B.A.; Callaway, R.M.; Mangan, S.A.; et al. Soil microbes drive the classic plant diversity–productivity pattern. Ecology 2011, 92, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [Green Version]
- Nagaraju, A.; Sudisha, J.; Murthy, S.M.; Ito, S.-I. Seed priming with Trichoderma harzianum isolates enhances plant growth and induces resistance against Plasmopara halstedii, an incitant of sunflower downy mildew disease. Australas. Plant Pathol. 2012, 41, 609–620. [Google Scholar] [CrossRef]
- Saldajeno, M.G.B.; Ito, M.; Hyakumachi, M. Interaction between the plant growth-promoting fungus Phoma sp. GS8-2 and the arbuscular mycorrhizal fungus Glomus mosseae: Impact on biocontrol of soil-borne diseases, microbial population, and plant growth. Australas. Plant Pathol. 2012, 41, 271–281. [Google Scholar] [CrossRef]
- Sultana, F.; Hossain, M.M.; Kubota, M.; Hyakumachi, M. Induction of systemic resistance in Arabidopsis thaliana in response to a culture filtrate from a plant growth-promoting fungus, Phoma sp. GS8-3. Plant Biol. 2009, 11, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.-S.P.; Shin-ichi, I. Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J. Exp. Bot. 2013, 64, 3829–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Arif, M.; Husssin, A.; Khan, S.A. Endophytic Aspergillus niger reprograms the physicochemical traits of tomato under cadmium and chromium stress. Environ. Exp. Bot. 2021, 186, 104456. [Google Scholar] [CrossRef]
- Ishaq, M.; Sultana, N.; Ikram, M.; Iqbal, A.; Shah, F.; Hamayun, M.; Hussain, A. Occurrence of heavy metals and pesticide residues in tomato crop: A threat to public health. Arab. J. Geosci. 2020, 13, 627. [Google Scholar] [CrossRef]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the Health-Promoting Effects of Tomato Fruit for Biofortified Food. Mediat. Inflamm. 2014, 2014, 139873. [Google Scholar] [CrossRef] [PubMed]
- Gerszberg, A.; Hnatuszko-Konka, K. Tomato tolerance to abiotic stress: A review of most often engineered target sequences. Plant Growth Regul. 2017, 83, 175–198. [Google Scholar] [CrossRef] [Green Version]
- da Silva, G.J.; Costa de Oliveira, A. Genes Acting on Transcriptional Control during Abiotic Stress Responses. Adv. Agric. 2014, 2014, 587070. [Google Scholar] [CrossRef] [Green Version]
- Délye, C.; Zhang, X.-Q.; Michel, S.; Matéjicek, A.; Powles, S.B. Molecular bases for sensitivity to acetyl-coenzyme A carboxylase inhibitors in black-grass. Plant Physiol 2005, 137, 794–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, D.; Chen, N.; Chen, J.; Mu, C.; Yin, K.; He, Y.; Liu, H. Plant grafting relieves asymmetry of jasmonic acid response induced by wounding between scion and rootstock in tomato hypocotyl. PLoS ONE 2020, 15, e0241317. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.-R.; Nebauer, S.G.; Carrillo, L.; Fernández-Nohales, P.; Marqués, J.; Renau-Morata, B.; Granell, A.; Pollmann, S.; Vicente-Carbajosa, J.; Molina, R.-V. Characterization of tomato Cycling Dof Factors reveals conserved and new functions in the control of flowering time and abiotic stress responses. J. Exp. Bot. 2014, 65, 995–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimplet, J.; Agudelo-Romero, P.; Teixeira, R.T.; Martinez-Zapater, J.M.; Fortes, A.M. Structural and functional analysis of the GRAS gene family in grapevine indicates a role of GRAS proteins in the control of development and stress responses. Front. Plant Sci. 2016, 7, 353. [Google Scholar] [CrossRef] [Green Version]
- Garapati, P.; Xue, G.-P.; Munné-Bosch, S.; Balazadeh, S. Transcription Factor ATAF1 in Arabidopsis Promotes Senescence by Direct Regulation of Key Chloroplast Maintenance and Senescence Transcriptional Cascades. Plant Physiol 2015, 168, 1122–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.-T.; Wang, Z.-Y.; Bao, Y.-F.; Zhang, X.-C.; Yang, H.-H.; Zhang, D.-Y.; Jiang, J.-B.; Zhang, H.; Li, J.-F.; Chen, Q.-S.; et al. Downregulation of SL-ZH13 transcription factor gene expression decreases drought tolerance of tomato. J. Integr. Agric. 2019, 18, 1579–1586. [Google Scholar] [CrossRef]
- Kissoudis, C.; Sunarti, S.; van de Wiel, C.; Visser, R.G.F.; van der Linden, C.G.; Bai, Y. Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J. Exp. Bot. 2016, 67, 5119–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.-Q.; Xue, H.-W. The ubiquitin-proteasome system in plant responses to environments. Plant Cell Environ. 2019, 42, 2931–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Dey, A.; Kumar, V.; Batiha, G.E.-S.; El-Esawi, M.A.; Tomczyk, M.; Ray, P. Fungal endophyte: An interactive endosymbiont with the capability of modulating host physiology in myriad ways. Front. Plant Sci. 2021, 12, 701800. [Google Scholar] [CrossRef]
- Yanagisawa, S. The Dof family of plant transcription factors. Trends Plant Sci. 2002, 7, 555–560. [Google Scholar] [CrossRef]
- Dong, G.; Ni, Z.; Yao, Y.; Nie, X.; Sun, Q. Wheat Dof transcription factor WPBF interacts with TaQM and activates transcription of an alpha-gliadin gene during wheat seed development. Plant Mol. Biol. 2007, 63, 73–84. [Google Scholar] [CrossRef]
- Santopolo, S.; Boccaccini, A.; Lorrai, R.; Ruta, V.; Capauto, D.; Minutello, E.; Serino, G.; Costantino, P.; Vittorioso, P. DOF AFFECTING GERMINATION 2 is a positive regulator of light-mediated seed germination and is repressed by DOF AFFECTING GERMINATION 1. BMC Plant Biol. 2015, 15, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Cheng, Z.; Xie, L.; Li, X.; Gao, J. Multifaceted role of PheDof12-1 in the regulation of flowering time and abiotic stress responses in moso bamboo (Phyllostachys edulis). Int. J. Mol. Sci. 2019, 20, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaria, N.; Siddappa, S.; Thakur, K.; Tomar, M.; Goutam, U.; Sharma, N.; Sood, S.; Bhardwaj, V.; Singh, B. Solanum tuberosum (CYCLING DOF FACTOR) CDF1. 2 allele: A candidate gene for developing earliness in potato. S. Afr. J. Bot. 2020, 132, 242–248. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; González-Calle, V.; Carbonero, P.; Barrero-Sicilia, C. The family of DOF transcription factors in Brachypodium distachyon: Phylogenetic comparison with rice and barley DOFs and expression profiling. BMC Plant Biol. 2012, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Fornara, F.; Panigrahi, K.C.; Gissot, L.; Sauerbrunn, N.; Rühl, M.; Jarillo, J.A.; Coupland, G. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev. Cell 2009, 17, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2016, 15, 101–123. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal. Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef] [Green Version]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 13, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef]
- Yasuda, M.; Ishikawa, A.; Jikumaru, Y.; Seki, M.; Umezawa, T.; Asami, T.; Maruyama-Nakashita, A.; Kudo, T.; Shinozaki, K.; Yoshida, S. Antagonistic interaction between systemic acquired resistance and the abscisic acid–mediated abiotic stress response in Arabidopsis. Plant Cell 2008, 20, 1678–1692. [Google Scholar] [CrossRef] [Green Version]
- Madhaiyan, M.; Poonguzhali, S.; Ryu, J.; Sa, T. Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta 2006, 224, 268–278. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.-G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Q.; Ervin, E.H.; Yang, Z.; Zhang, X. Physiological Mechanism of Enhancing Salt Stress Tolerance of Perennial Ryegrass by 24-Epibrassinolide. Front. Plant Sci. 2017, 8, 1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchisaka, A.; Yu, G.; Jin, H.; Alonso, J.M.; Ecker, J.R.; Zhang, X.; Gao, S.; Theologis, A. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 2009, 183, 979–1003. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Baek, K.-H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Abeles, F.; Morgan, P.; Saltveit, M.E., Jr. Plant Biology, 2nd ed.; Academic Press: New York, NY, USA, 1992. [Google Scholar]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa-Balderas, R.E.; García-Ponce, B.; Rocha-Sosa, M. Hormonal and stress induction of the gene encoding common bean acetyl-coenzyme A carboxylase. Plant Physiol. 2006, 142, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Habben, J.E.; Bao, X.; Bate, N.J.; DeBruin, J.L.; Dolan, D.; Hasegawa, D.; Helentjaris, T.G.; Lafitte, R.H.; Lovan, N.; Mo, H.; et al. Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol. J. 2014, 12, 685–693. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, S.; Bonsegna, S.; Horres, R.; Pastor, V.; Taurino, M.; Poltronieri, P.; Imtiaz, M.; Kahl, G.; Flors, V.; Winter, P.; et al. Transcriptomic analysis of oxylipin biosynthesis genes and chemical profiling reveal an early induction of jasmonates in chickpea roots under drought stress. Plant Physiol. Biochem. 2012, 61, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.S.P.; Nakashima, K.; Sakuma, Y.; Osakabe, Y.; Qin, F.; Simpson, S.D.; Maruyama, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Co-expression of the stress-inducible zinc finger homeodomain ZFHD1 and NAC transcription factors enhances expression of the ERD1 gene in Arabidopsis. Plant J. 2007, 49, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, P.; Li, Y.; Hou, X. Genome-wide analysis and expression patterns of ZF-HD transcription factors under different developmental tissues and abiotic stresses in Chinese cabbage. Mol. Genet. Genom. 2016, 291, 1451–1464. [Google Scholar] [CrossRef]
- Peng, J.; Carol, P.; Richards, D.E.; King, K.E.; Cowling, R.J.; Murphy, G.P.; Harberd, N.P. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997, 11, 3194–3205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolle, C.; Koncz, C.; Chua, N.-H. PAT1, a new member of the GRAS family, is involved in phytochrome A signal transduction. Genes Dev. 2000, 14, 1269–1278. [Google Scholar] [CrossRef]
- Helariutta, Y.; Fukaki, H.; Wysocka-Diller, J.; Nakajima, K.; Jung, J.; Sena, G.; Hauser, M.-T.; Benfey, P.N. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 2000, 101, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Stuurman, J.; Jäggi, F.; Kuhlemeier, C. Shoot meristem maintenance is controlled by a GRAS-gene mediated signal from differentiating cells. Genes Dev. 2002, 16, 2213–2218. [Google Scholar] [CrossRef] [Green Version]
- Mayrose, M.; Ekengren, S.K.; Melech-Bonfil, S.; Martin, G.B.; Sessa, G. A novel link between tomato GRAS genes, plant disease resistance and mechanical stress response. Mol. Plant Pathol. 2006, 7, 593–604. [Google Scholar] [CrossRef]
- Ma, H.-S.; Liang, D.; Shuai, P.; Xia, X.-L.; Yin, W.-L. The salt-and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. J. Exp. Bot. 2010, 61, 4011–4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H. Killing two birds with one stone: Transcriptional regulators coordinate development and stress responses in plants. Plant Signal. Behav. 2012, 7, 701–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenabeele, S.; Van Der Kelen, K.; Dat, J.; Gadjev, I.; Boonefaes, T.; Morsa, S.; Rottiers, P.; Slooten, L.; Van Montagu, M.; Zabeau, M. A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc. Natl. Acad. Sci. USA 2003, 100, 16113–16118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.; Shulaev, V.; Dangl, J.; Mittler, R. The Plant NADPH Oxidase RBOHD Mediates Rapid Systemic Signaling in Response to Diverse Stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [Green Version]
- Egbichi, I.; Keyster, M.; Jacobs, A.; Klein, A.; Ludidi, N. Modulation of antioxidant enzyme activities and metabolites ratios by nitric oxide in short-term salt stressed soybean root nodules. S. Afr. J. Bot. 2013, 88, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Lee, K. Physiological responses of soybean-inoculation of Bradyrhizobium japonicum with PGPR in saline soil conditions. Res. J. Agric. Biol. Sci. 2005, 1, 216–221. [Google Scholar]
- Singh, R.P.; Jha, P.; Jha, P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015, 184, 57–67. [Google Scholar] [CrossRef]
- Banik, P.; Zeng, W.; Tai, H.; Bizimungu, B.; Tanino, K. Effects of drought acclimation on drought stress resistance in potato (Solanum tuberosum L.) genotypes. Environ. Exp. Bot. 2016, 126, 76–89. [Google Scholar] [CrossRef]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Abid, M.; Rizwan, M.; Shahid, M.R.; Alotaibi, M.; et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Lechno, S.; Zamski, E.; Tel-Or, E. Salt stress-induced responses in cucumber plants. J. Plant Physiol. 1997, 150, 206–211. [Google Scholar] [CrossRef]
- Meneguzzo, S.; Navam-Izzo, F.; Izzo, R. Antioxidative responses of shoots and roots of wheat to increasing NaCI concentrations. J. Plant Physiol. 1999, 155, 274–280. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Wang, B.; Xie, H.; Wang, J.; Nan, Z. Transcriptome analysis of two soybean cultivars identifies an aluminum respon-sive antioxidant enzyme GmCAT1. Biosci. Biotechnol. Biochem. 2020, 84, 1394–1400. [Google Scholar] [CrossRef]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.H.; Li, X.Z.; Guo, D.L.; Zhang, H.L.; Li, G.R.; Li, X.Q.; Zhang, G.H. Genome-wide identification and analysis of the U-box family of E3 ligases in grapevine. Russ. J. Plant Physiol. 2016, 63, 835–848. [Google Scholar] [CrossRef]
- Stone, S.L. Chapter Three-Role of the Ubiquitin Proteasome System in Plant Response to Abiotic Stress. In International Review of Cell and Molecular Biology; Galluzzi, L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 343, pp. 65–110. [Google Scholar]
- Zhang, Z.; Li, J.; Liu, H.; Chong, K.; Xu, Y. Roles of ubiquitination-mediated protein degradation in plant responses to abiotic stresses. Environ. Exp. Bot. 2015, 114, 92–103. [Google Scholar] [CrossRef]
- Qin, F.; Sakuma, Y.; Tran, L.-S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.-I.; et al. Arabidopsis DREB2A-Interacting Proteins Function as RING E3 Ligases and Negatively Regulate Plant Drought Stress–Responsive Gene Expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef] [Green Version]
- Chapagain, S.; Park, Y.C.; Jang, C.S. Functional diversity of RING E3 ligases of major cereal crops in response to abiotic stresses. J. Crop Sci. Biotechnol. 2017, 20, 351–357. [Google Scholar] [CrossRef]
- Qi, Z.-Y.; Ahammed, G.J.; Jiang, C.-Y.; Li, C.-X.; Zhou, J. The E3 ubiquitin ligase gene SlRING1 is essential for plant tolerance to cadmium stress in Solanum lycopersicum. J. Biotechnol. 2020, 324, 239–247. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Fenando, E.; Boero, C.; Gallardo, M.; Gonzalez, J. Effect of NaCl on germination, growth, and soluble suger content in Chenopodium quinonaseeds. Bot. Bull. Acad. Sin. 2000, 41, 27–34. [Google Scholar]
- Sánchez, F.J.; Manzanares, M.; de Andres, E.F.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Murakeözy, É.P.; Nagy, Z.; Duhazé, C.; Bouchereau, A.; Tuba, Z. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J. Plant Physiol. 2003, 160, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ruan, Y.-L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef] [Green Version]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- D’Mello, J.F. Amino Acids in Higher Plants; CABI: Wallingford, UK, 2015. [Google Scholar]
- Nasir Khan, M.; Siddiqui, M.H.; Mohammad, F.; Naeem, M.; Khan, M.M.A. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant. 2009, 32, 121. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biológica Colomb. 2015, 20, 223–235. [Google Scholar] [CrossRef]
- Kim, J.; Liu, Y.; Zhang, X.; Zhao, B.; Childs, K.L. Analysis of salt-induced physiological and proline changes in 46 switchgrass (Panicum virgatum) lines indicates multiple response modes. Plant Physiol. Biochem. 2016, 105, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Trotel, P.; Bouchereau, A.; Niogret, M.F.; Larher, F. The fate of osmo-accumulated proline in leaf discs of Rape (Brassica napus L.) incubated in a medium of low osmolarity. Plant Sci. 1996, 118, 31–45. [Google Scholar] [CrossRef]
- Nanjo, T.; Kobayashi, M.; Yoshiba, Y.; Sanada, Y.; Wada, K.; Tsukaya, H.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. Cell Mol. Biol. 1999, 18, 185–193. [Google Scholar] [CrossRef]
- Wang, X.e.; Basnayake, B.M.V.S.; Zhang, H.; Li, G.; Li, W.; Virk, N.; Mengiste, T.; Song, F. The Arabidopsis ATAF1, a NAC Transcription Factor, Is a Negative Regulator of Defense Responses Against Necrotrophic Fungal and Bacterial Pathogens. Mol. Plant-Microbe Interact. 2009, 22, 1227–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.-L.; Chen, N.-Z.; An, R.; Su, Z.; Qi, B.-S.; Ren, F.; Chen, J.; Wang, X.-C. A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Mol. Biol. 2007, 63, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Hagedorn, P.H.; De Torres-Zabala, M.; Grant, M.R.; Rung, J.H.; Collinge, D.B.; Lyngkjaer, M.F. Transcriptional regulation by an NAC (NAM–ATAF1,2–CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp. hordei in Arabidopsis. Plant J. 2008, 56, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Devkar, V.; Thirumalaikumar, V.P.; Xue, G.-P.; Vallarino, J.G.; Turečková, V.; Strnad, M.; Fernie, A.R.; Hoefgen, R.; Mueller-Roeber, B.; Balazadeh, S. Multifaceted regulatory function of tomato SlTAF1 in the response to salinity stress. New Phytol. 2020, 225, 1681–1698. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sarkar, A.; Dutta, S. Characterisation of plant growth promoting traits and stress tolerant properties of Aspergillus terreus (MCC 1819) isolated from the rhizosphere of Aloe barbadensis Mill. and its application. J. Pharmacogn. Phytochem. 2019, 8, 2303–2310. [Google Scholar]
- Al-Sadi, A.M.; Al-Masoodi, R.S.; Al-Ismaili, M.; Al-Mahmooli, I.H. Population structure and development of resistance to hymexazol among Fusarium solani populations from date palm, citrus and cucumber. J. Phytopathol. 2015, 163, 947–955. [Google Scholar] [CrossRef]
- Al-Balushi, Z.M.; Agrama, H.; Al-Mahmooli, I.H.; Maharachchikumbura, S.S.; Al-Sadi, A.M. Development of resistance to hymexazol among Pythium species in cucumber greenhouses in Oman. Plant Dis. 2018, 102, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horisawa, S.; Sakuma, Y.; Doi, S. Identification and species-typing of wood rotting fungi using melting curve analysis. J. Wood Sci. 2013, 59, 432–441. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.; Gelfand, D.; Sninsky, J. PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Acuña, J.J.; Jorquera, M.A.; Martínez, O.A.; Menezes-Blackburn, D.; Fernández, M.T.; Marschner, P.; Greiner, R.; Mora, M. Indole acetic acid and phytase activity produced by rhizosphere bacilli as affected by pH and metals. J. Soil Sci. Plant Nutr. 2011, 11, 1–12. [Google Scholar]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Szilagyi-Zecchin, V.J.; Ikeda, A.C.; Hungria, M.; Adamoski, D.; Kava-Cordeiro, V.; Glienke, C.; Galli-Terasawa, L.V. Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. AMB Express 2014, 4, 26. [Google Scholar] [CrossRef]
- Daniel, A.L.; Praveen Kumar, G.; Desai, S.; Mir Hassan, A. In vitro characterization of Trichoderma viride for abiotic stress tolerance and field evaluation against root rot disease in Vigna mungo L. J. Biofertil. Biopestic. 2011, 2, 1–5. [Google Scholar]
- Ripa, F.A.; Cao, W.-D.; Tong, S.; Sun, J.-G. Assessment of Plant Growth Promoting and Abiotic Stress Tolerance Properties of Wheat Endophytic Fungi. Biomed. Res. Int. 2019, 2019, 6105865. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Biocontrol potential of a native species of Trichoderma longibrachiatum against Meloidogyne incognita. Appl. Soil Ecol. 2015, 94, 21–29. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Simões, I.M.; Baptista, J.O.; Bighi, K.N.; Fontes, M.M.P.; Schmildt, E.R.; Lopes, J.C.; Caldeira, M.V.W.; Alexandre, R.S. In vitro germination of Melanoxylon brauna Schott. and evaluation of the toxicity of disinfecting agents in the Lactuca sativa L. model plant. Cerne 2020, 25, 375–385. [Google Scholar] [CrossRef]
- Ke, Q.; Ye, J.; Wang, B.; Ren, J.; Yin, L.; Deng, X.; Wang, S. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 2018, 9, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentovic, P.; Luxova, M.; Kolarovic, L.; Gasparikova, O. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ. 2006, 52, 184. [Google Scholar]
- Hiscox, J.; Israelstam, G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Al-Sadi, A.M.; Kim, I.-D.; Imran, M.; Lee, I.-J. Ampelopsin Confers Endurance and Rehabilitation Mechanisms in Glycine max cv. Sowonkong Under Mult. Abiotic Stresses 2021, 22, 10943. [Google Scholar]
- Qi, Q.; Rose, P.A.; Abrams, G.D.; Taylor, D.C.; Abrams, S.R.; Cutler, A.J. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme a synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis inBrassica napus embryos. Plant Physiol. 1998, 117, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Iram, A. Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora-Morphol. Distrib. Funct. Ecol. Plants 2005, 200, 535–546. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhu, J.; Qi, J.; Fang, Y.; Xiao, X.; Li, J.; Lan, J.; Tang, C. Characterization of Sugar Contents and Sucrose Metabolizing Enzymes in Developing Leaves of Hevea brasiliensis. Front. Plant Sci. 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putter, J. Peroxidase. In Methods of Enzymatic Analysis; Hu, B., Ed.; Verlag Chemie: Weinan, China, 1974; pp. 685–690. [Google Scholar]
- Soffan, A.; Alghamdi, S.; Aldawood, A. Peroxidase and polyphenol oxidase activity in moderate resistant and susceptible Vicia faba induced by Aphis craccivora (Hemiptera: Aphididae) infestation. J. Insect Sci. 2014, 14, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; Rolim Medeiros, J.-V.; Gomes-Filho, E. Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J. Plant Physiol. 2005, 162, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Hu, L.; Liao, W.; Dawuda, M.M.; Li, C. Carbon Monoxide Is Involved in Hydrogen Gas-Induced Adventitious Root Development in Cucumber under Simulated Drought Stress. Front. Plant Sci. 2017, 8, 128. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, W.; Wang, Q.; Eneji, A.; Li, Z.; Duan, L. Coronatine enhances chilling tolerance in cucumber (Cucumis sativus L.) seedlings by improving the antioxidative defence system. J. Agron. Crop Sci. 2009, 195, 377–383. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- López-Serrano, L.; Canet-Sanchis, G.; Vuletin Selak, G.; Penella, C.; San Bautista, A.; López-Galarza, S.; Calatayud, Á. Pepper Rootstock and Scion Physiological Responses under Drought Stress. Front. Plant Sci. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.-G.; Toyota, M.; Kim, S.-H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oñate-Sánchez, L.; Vicente-Carbajosa, J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes 2008, 1, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]

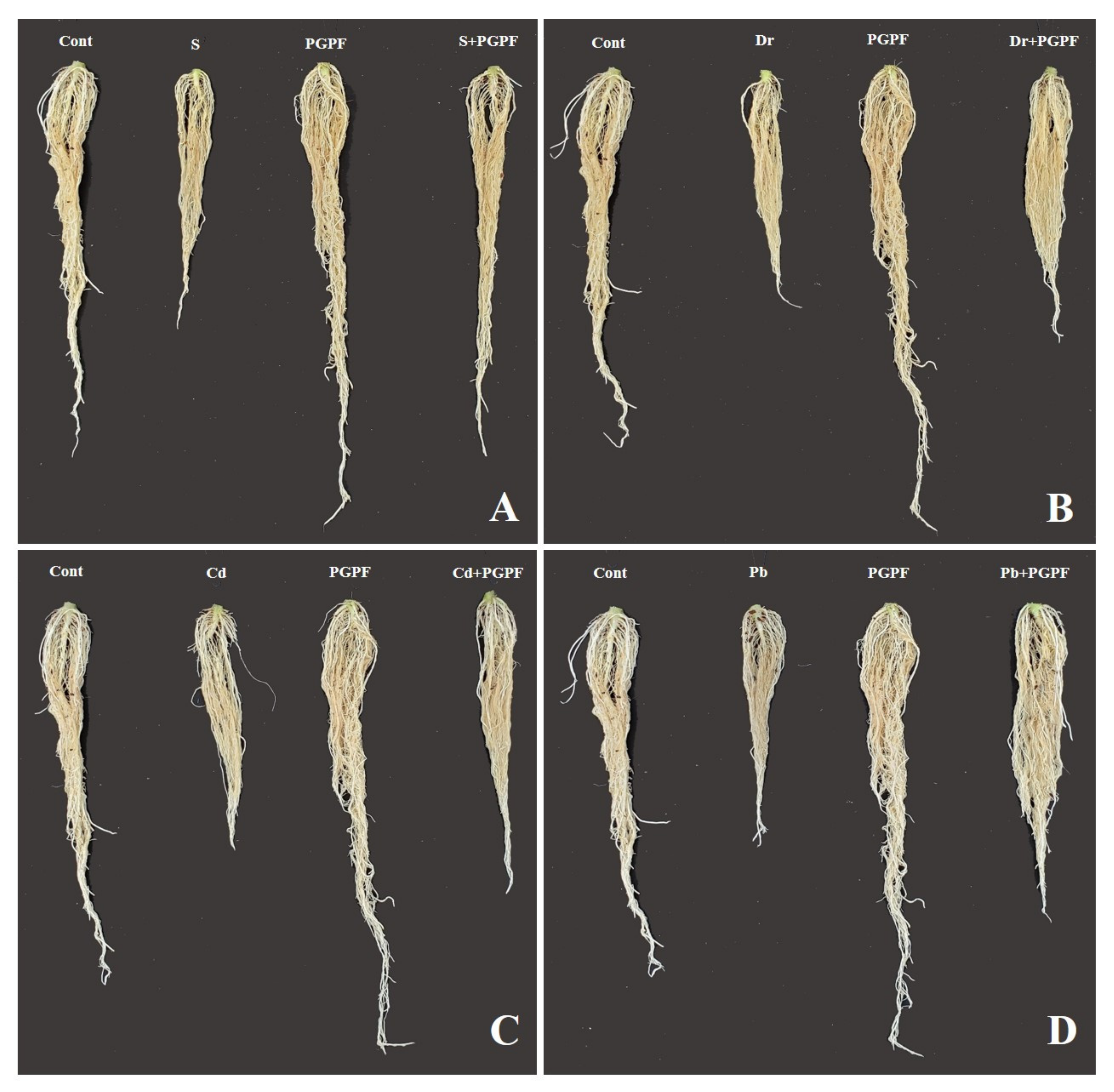


| Treatment | Plant Height | Root Length | Stem Diameter | Leaf Length | Leaf Width | Plant Fresh Weight | Plant Dry Weight | Root Fresh Weight | Root Dry Weight | No. Leaf |
|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (cm) | (cm) | (cm) | (cm) | (g) | (g) | (g) | (g) | ||
| Cont | 27.5 ± 0.75 b | 22.75 ± 0.37 b | 0.75 ± 0.07 b | 24.5 ± 0.25 b | 18.5 ± 0.0 ab | 24.91 ± 0.45 b | 1.22 ± 0.01 b | 4.73 ± 0.36 b | 0.17 ± 0.01 b | 11.0 ± 0.5 ab |
| PGPF | 36.5 ± 0.25 a | 27.75 ± 0.87 a | 0.87 ± 0.03 a | 28.5 ± 0.0 a | 19.75 ± 0.87 a | 31.57 ± 0.78 a | 1.98 ± 0.01 a | 5.72 ± 0.86 a | 0.28 ± 0.0 a | 12.0 ± 0.5 a |
| S | 12.5 ± 0.25 f | 14.5 ± 0.25 e | 0.34 ± 0.0 f | 11.5 ± 0.75 fg | 7.25 ± 0.62 f | 5.75 ± 0.87 h | 0.45 ± 0.02 f | 1.62 ± 0.0 c | 0.07 ± 0.0 c | 6.5 ± 0.25 de |
| S + PGPF | 25.5 ± 0.75 c | 22.5 ± 0.22 b | 0.48 ± 0.0 e | 17.5 ± 1.0 d | 14.5 ± 0.25 d | 12.6 ± 0.0 ef | 1.07 ± 0.03 c | 4.25 ± 0.12 b | 0.16 ± 0.01 b | 10.5 ± 0.0 b |
| Dr | 19.5 ± 0.75 d | 13.5 ± 0.65 e | 0.32 ± 0.04 f | 13.5 ± 1.0 ef | 10.5 ± 0.0 e | 9.58 ± 0.79 g | 0.51 ± 0.04 ef | 1.81 ± 0.09 c | 0.04 ± 0.0 c | 7.5 ± 0.75 cd |
| Dr + PGPF | 26.5 ± 0.25 bc | 16.5 ± 0.0 d | 0.60 ± 0.0 d | 18.75 ± 0.37 d | 14.75 ± 0.37 d | 15.59 ± 0.79 d | 0.91 ± 0.04 d | 3.98 ± 0.01 b | 0.15 ± 0.0 b | 10.5 ± 0.0 b |
| Cd | 15.5 ± 0.75 e | 13.5 ± 0.65 e | 0.49 ± 0.01 e | 11.0 ± 0.5 g | 7.5 ± 0.75 f | 14.44 ± 0.22 de | 0.60 ± 0.0 e | 2.42 ± 0.21 c | 0.07 ± 0.0 c | 6.0 ± 0.5 e |
| Cd + PGPF | 27.0 ± 0.5 bc | 17.75 ± 0.87 cd | 0.65 ± 0.0 cd | 22.5 ± 0.0 bc | 17.25 ± 0.62 bc | 20.54 ± 0.3 c | 1.10 ± 0.05 c | 3.98 ± 0.01 b | 0.15 ± 0.0 b | 10.0 ± 0.5 b |
| Pb | 19.5 ± 0.75 d | 13.5 ± 0.65 e | 0.32 ± 0.02 f | 14.0 ± 0.5 e | 10.5 ± 0.25 e | 11.91 ± 0.95 f | 0.56 ± 0.0 e | 2.05 ± 0.02 c | 0.08 ± 0.01 c | 8.5 ± 0.0 c |
| Pb + PGPF | 26.5 ± 0.5 bc | 18.75 ± 0.37 c | 0.73 ± 0.0 bc | 21.75 ± 0.87 c | 16.5 ± 0.0 c | 24.54 ± 0.04 b | 1.15 ± 0.02 bc | 4.3 ± 0.15 b | 0.16 ± 0.04 b | 11.0 ± 0.5 ab |
| Sample Name | Ca (µg/kg) | K (µg/kg) | P (µg/kg) | Na (µg/kg) | Cd (µg/kg) | Pb (µg/kg) |
|---|---|---|---|---|---|---|
| Cont | 8.25 ± 0.12 de | 51.18 ± 0.59 h | 6.31 ± 0.15 c | 4.02 ± 0.01 c | N.D | N.D |
| PGPF | 10.28 ± 0.14 c | 59.94 ± 0.97 g | 7.05 ± 0.52 bc | 4.60 ± 0.3 c | N.D | N.D |
| S | 14.38 ± 0.19 a | 64.81 ± 0.4 f | 7.51 ± 0.75 abc | 15.44 ± 0.72 a | N.D | N.D |
| S + PGPF | 7.22 ± 0.61 e | 73.05 ± 0.52 c | 8.81 ± 0.4 a | 7.64 ± 0.82 b | N.D | N.D |
| Dr | 8.44 ± 0.22 de | 60.18 ± 0.09 g | 7.79 ± 0.89 abc | 4.20 ± 0.1 c | N.D | N.D |
| Dr + PGPF | 5.58 ± 0.79 f | 65.84 ± 0.92 ef | 8.20 ± 0.1 ab | 4.51 ± 0.25 c | N.D | N.D |
| Cd | 13.02 ± 0.51 ab | 67.41 ± 0.7 e | 6.80 ± 0.4 bc | 4.45 ± 0.22 c | 2.78 ± 0.05 a | N.D |
| Cd + PGPF | 9.21 ± 0.6 cd | 77.93 ± 0.96 a | 7.93 ± 0.96 abc | 5.0 ± 0.5 c | 0.89 ± 0.01 b | N.D |
| Pb | 12.41 ± 0.2 b | 70.13 ± 0.06 d | 6.83 ± 0.41 bc | 4.36 ± 0.18 c | N.D | 2.24 ± 0.03 a |
| Pb + PGPF | 8.69 ± 0.34 d | 75.33 ± 0.66 b | 8.03 ± 0.01 abc | 5.27 ± 0.63 c | N.D | 1.03 ± 0.01 b |
| Symbol | Treatment |
|---|---|
| Cont | irrigated with sterile distilled water |
| PGPF | irrigated with Cunninghamella bertholletiae |
| S | irrigated with sodium chloride (1.5% NaCl) |
| S + PGPF | irrigated with sodium chloride (1.5% NaCl) + Cunninghamella bertholletiae |
| Dr | irrigated with polyethylene glycol (25%PEG; −0.73 Mpa) |
| Dr + PGPF | irrigated with polyethylene glycol (25%PEG; −0.73 Mpa) + Cunninghamella bertholletiae |
| Cd | irrigated with cadmium (3 mM Cd) |
| Cd + PGPF | irrigated with cadmium (3 mM Cd) + Cunninghamella bertholletiae |
| Pb | irrigated with lead (3 mM Pb) |
| Pb + PGPF | irrigated with lead (3 mM Pb) + Cunninghamella bertholletiae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M.; Rashid, U.; Kim, I.-D.; Kang, S.-M.; Lee, I.-J. Effects of the Rhizosphere Fungus Cunninghamella bertholletiae on the Solanum lycopersicum Response to Diverse Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 8909. https://doi.org/10.3390/ijms23168909
Kazerooni EA, Maharachchikumbura SSN, Al-Sadi AM, Rashid U, Kim I-D, Kang S-M, Lee I-J. Effects of the Rhizosphere Fungus Cunninghamella bertholletiae on the Solanum lycopersicum Response to Diverse Abiotic Stresses. International Journal of Molecular Sciences. 2022; 23(16):8909. https://doi.org/10.3390/ijms23168909
Chicago/Turabian StyleKazerooni, Elham Ahmed, Sajeewa S. N. Maharachchikumbura, Abdullah Mohammed Al-Sadi, Umer Rashid, Il-Doo Kim, Sang-Mo Kang, and In-Jung Lee. 2022. "Effects of the Rhizosphere Fungus Cunninghamella bertholletiae on the Solanum lycopersicum Response to Diverse Abiotic Stresses" International Journal of Molecular Sciences 23, no. 16: 8909. https://doi.org/10.3390/ijms23168909
APA StyleKazerooni, E. A., Maharachchikumbura, S. S. N., Al-Sadi, A. M., Rashid, U., Kim, I.-D., Kang, S.-M., & Lee, I.-J. (2022). Effects of the Rhizosphere Fungus Cunninghamella bertholletiae on the Solanum lycopersicum Response to Diverse Abiotic Stresses. International Journal of Molecular Sciences, 23(16), 8909. https://doi.org/10.3390/ijms23168909










