Behavioral, Neural, and Molecular Mechanisms of Conditioned Mate Preference: The Role of Opioids and First Experiences of Sexual Reward
Abstract
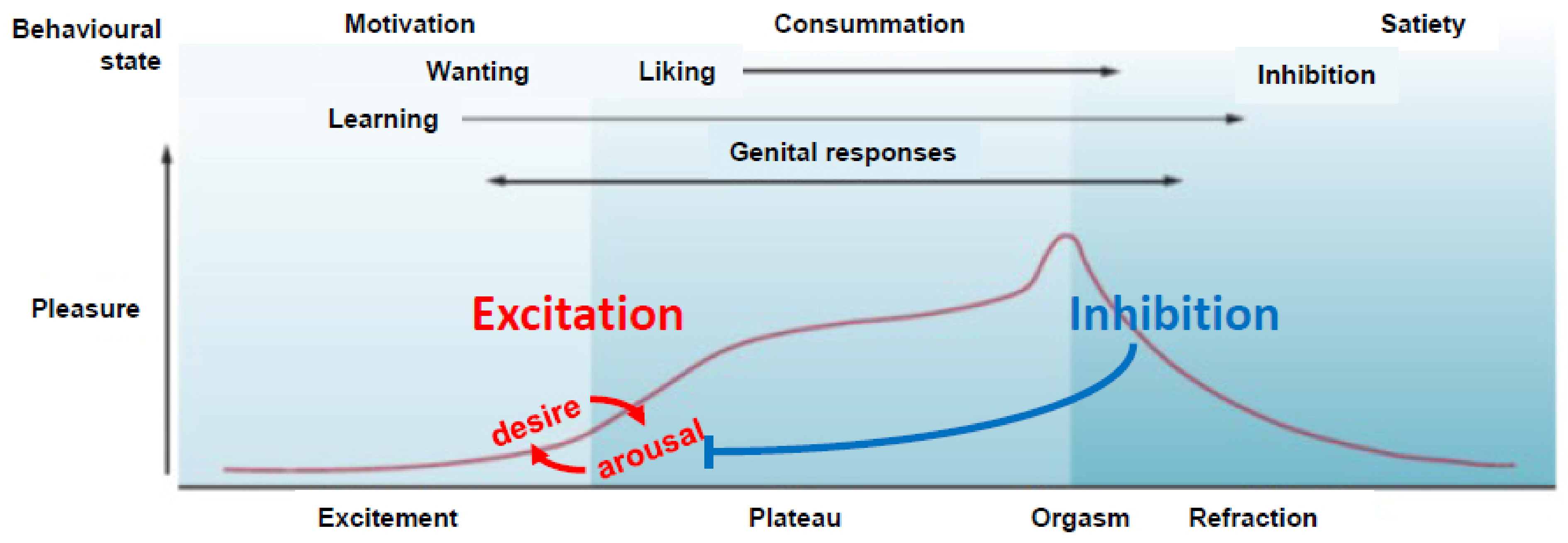
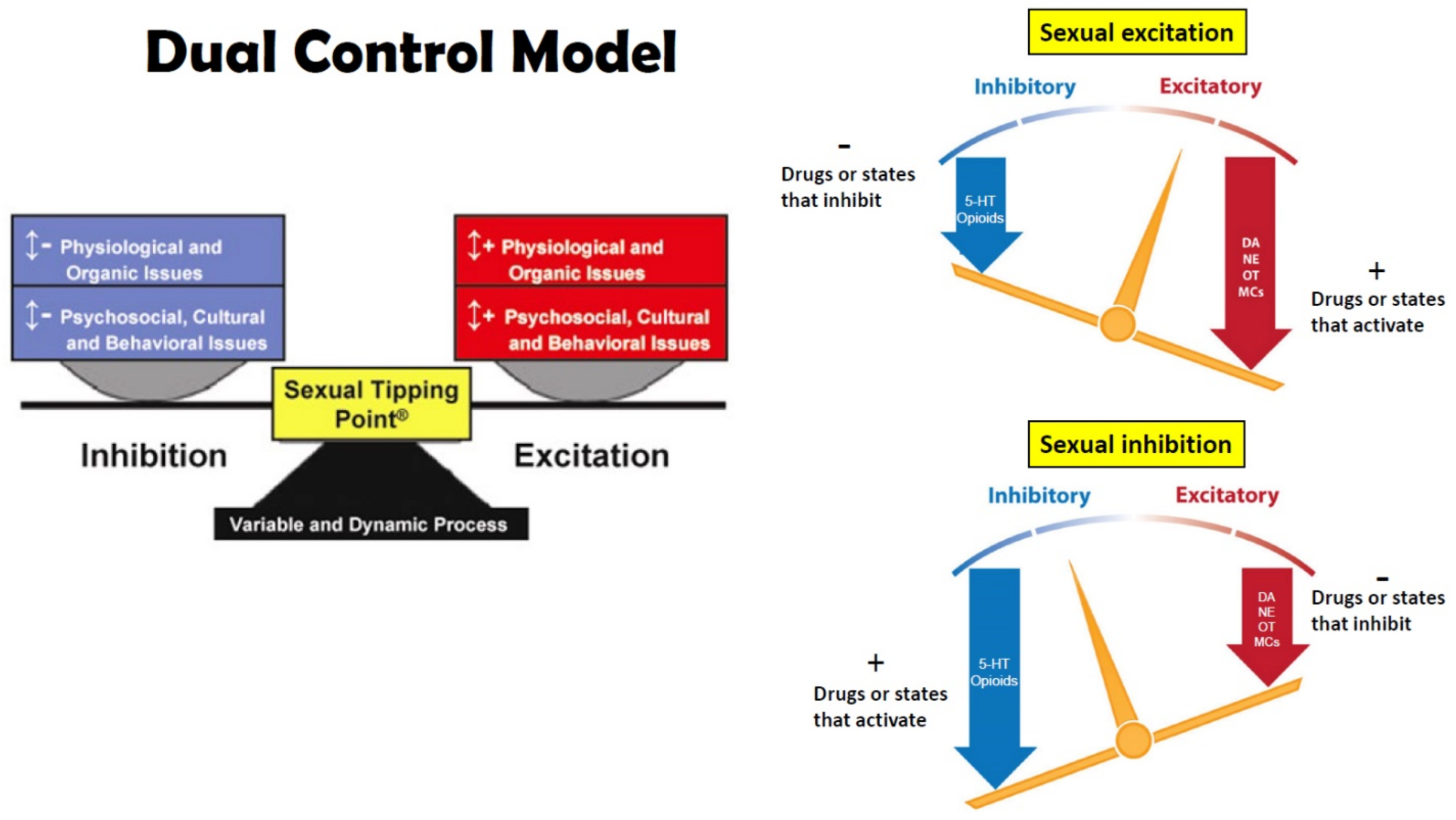
1. Sexual Excitation and Inhibition
2. Learning and Sexual Experience
2.1. Classical Conditioning of Sexual Behavior
2.2. Instrumental Learning and Sexual Behavior
2.3. First Experiences of Sexual Reward
2.4. Potentiating or Inhibiting Conditioning during First Experiences with Reward
3. Brain Activation by Sexual Experience and Cues Associated with Sexual Reward
4. Dual Role of Endogenous Opioids in Sexual Behavior
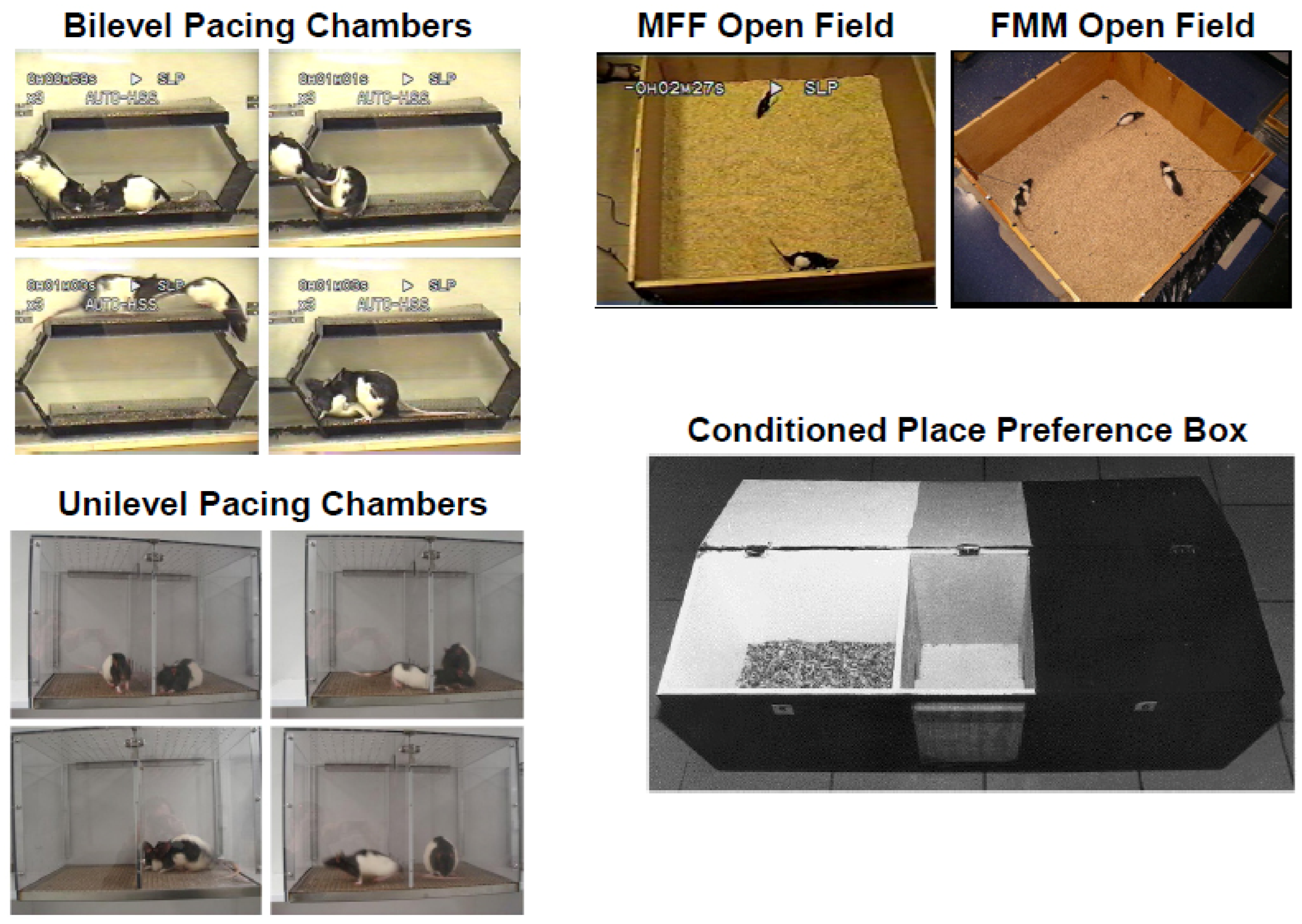
4.1. Inhibition and Refractoriness or Disinhibition
4.2. Reward-Related Sensitization of Incentive Cues
4.3. Antagonism of Opioid Reward
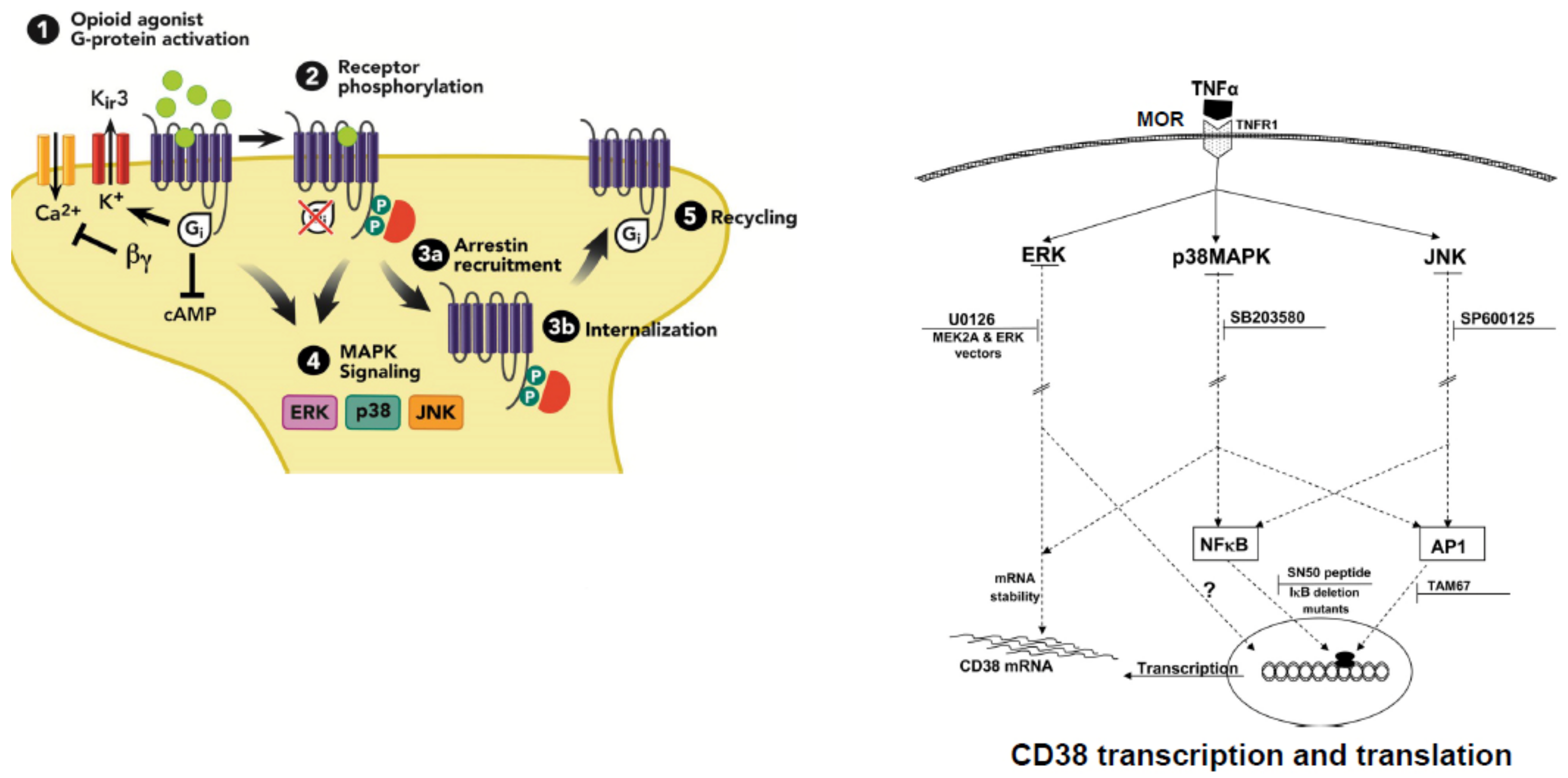
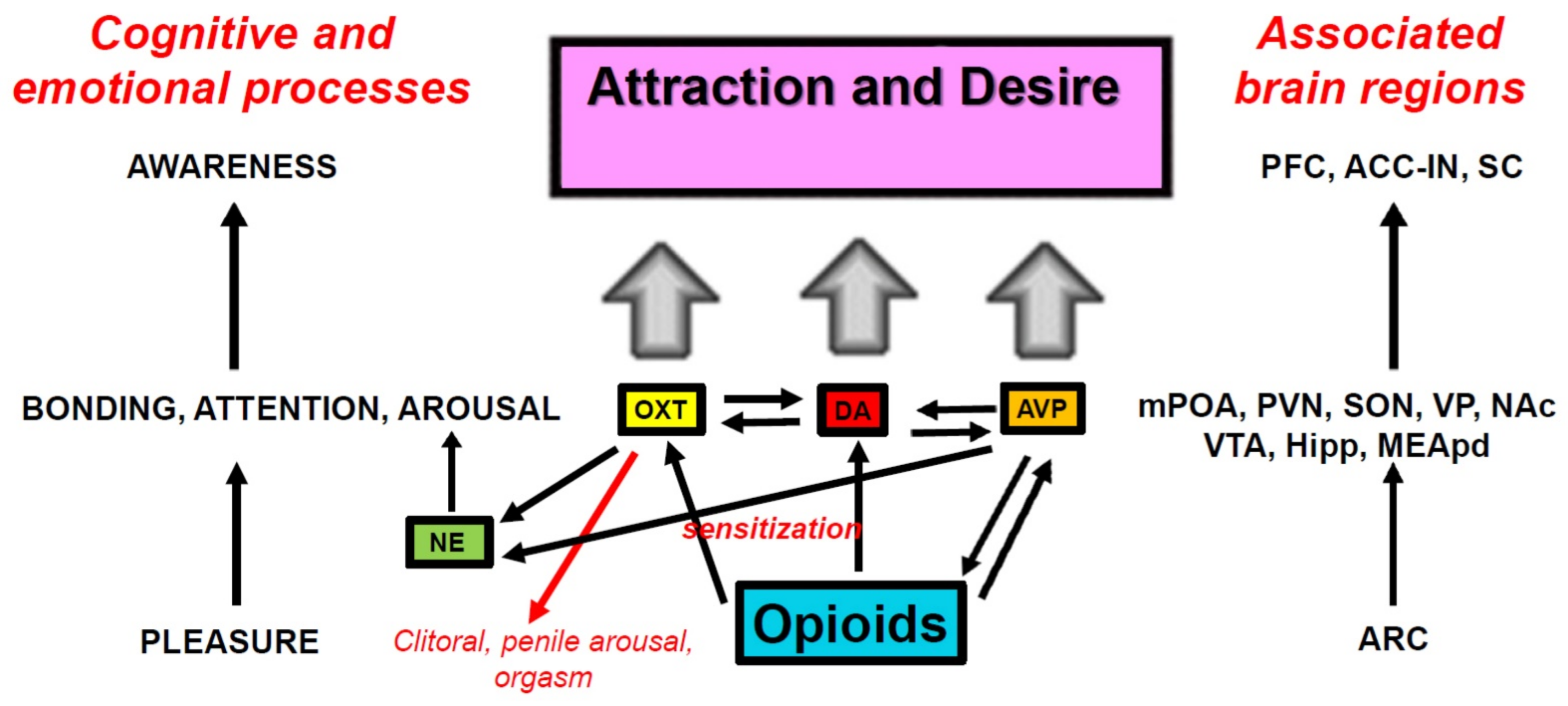
5. Neuropharmacological, Signal Transduction, and Molecular Mechanisms of Opioid Sensitization
5.1. Mesolimbic DA Sensitization
5.2. DA Related Signal Transduction and Genomic Mechanisms
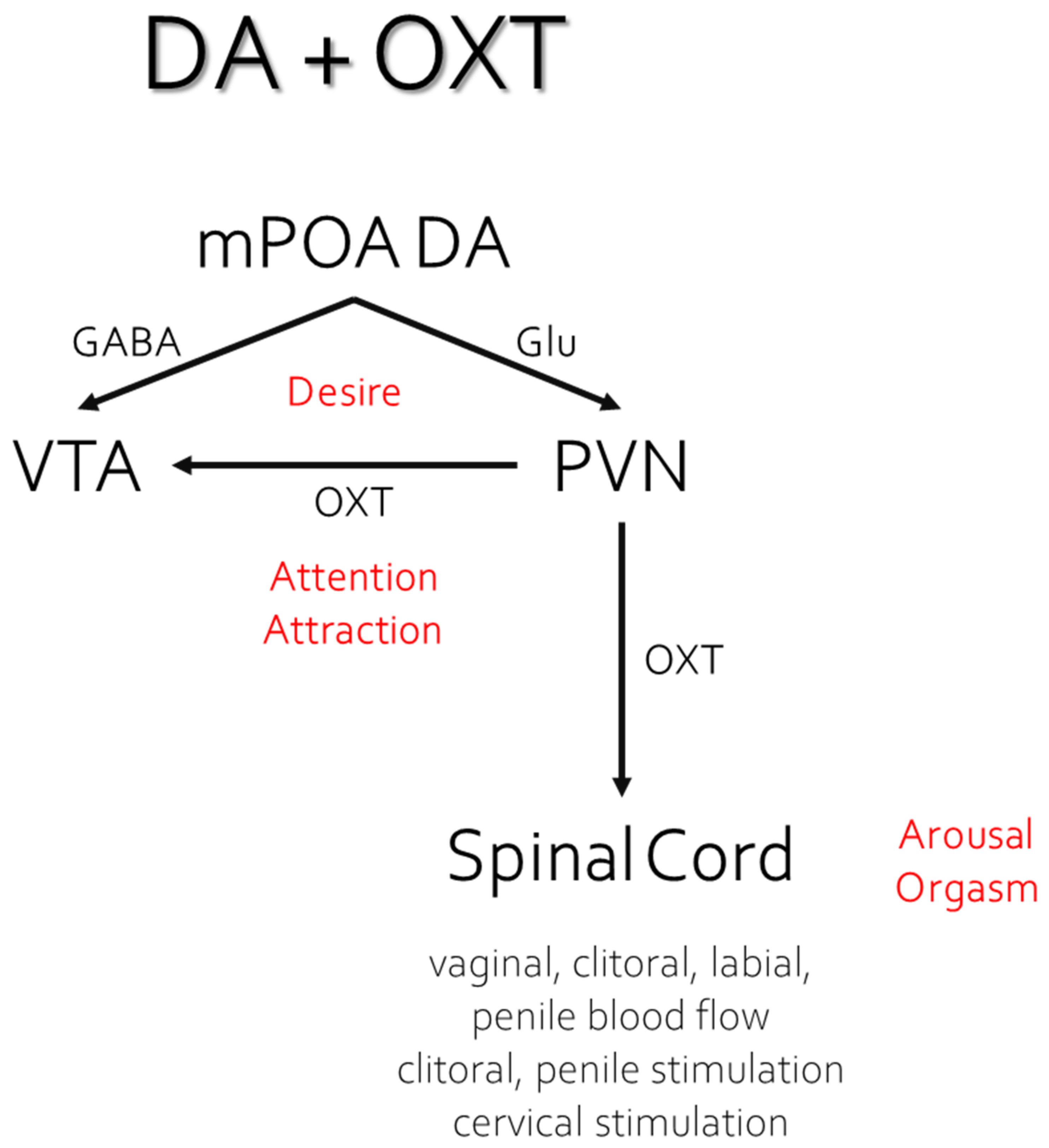
5.3. Downstream Activation of OXT and AVP Neurons
5.4. Epigenetic Mechanisms
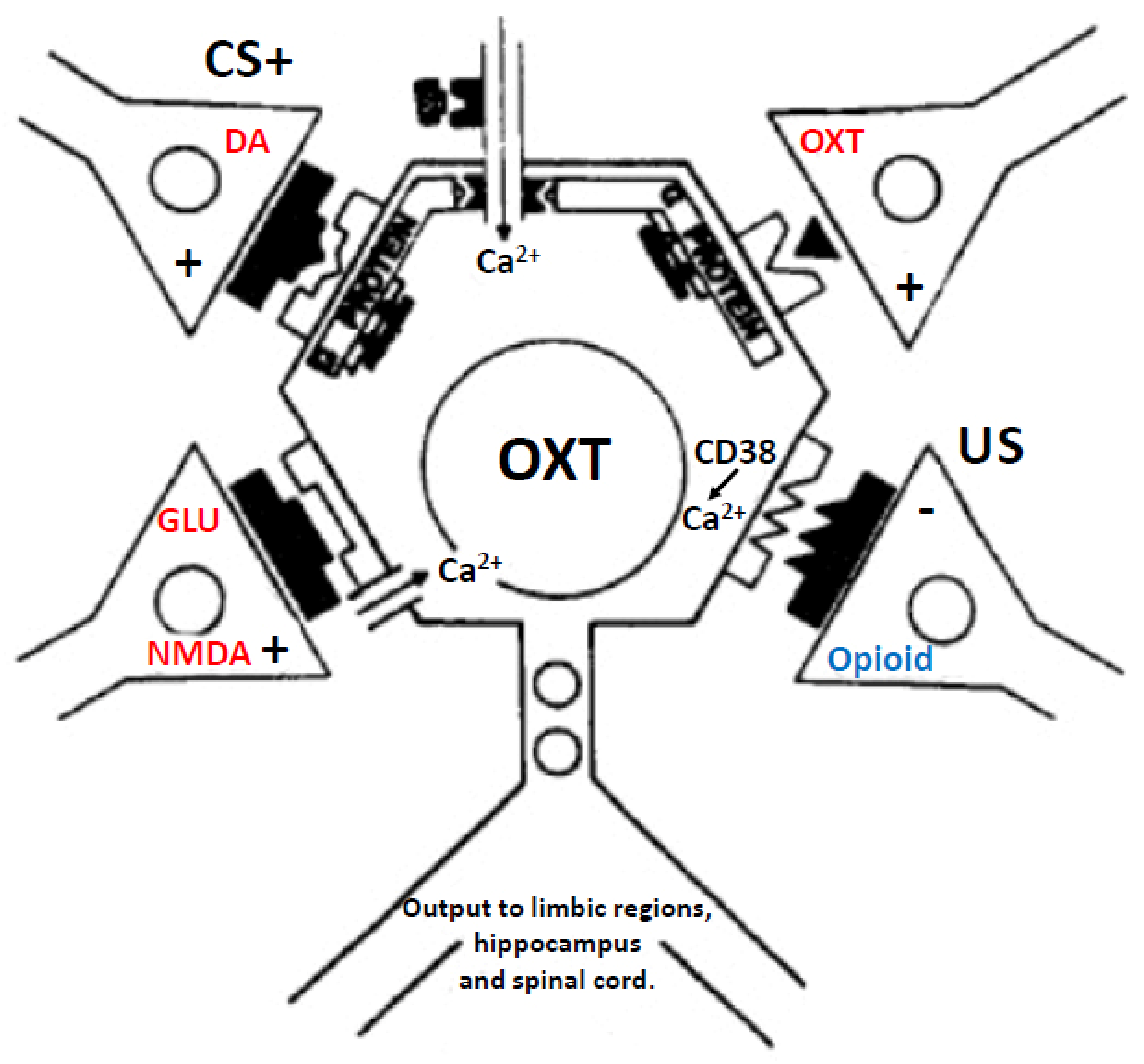
5.5. Molecular Mechanisms of Opioid Sensitization of OXT Neurons and the Role of DA
6. First Experiences of Sexual Reward in Humans; or the Path of Cupid’s Arrow
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Perelman, M.A. A New Combination Treatment for Premature Ejaculation: A Sex Therapist’s Perspective. J. Sex. Med. 2006, 3, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G. Neurobiology of Sexual Behavior. Curr. Opin. Neurobiol. 1999, 9, 751–758. [Google Scholar] [CrossRef]
- Pfaus, J.G. REVIEWS: Pathways of Sexual Desire. J. Sex. Med. 2009, 6, 1506–1533. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.; Janssen, E. The Dual Control Model of Male Sexual Response: A Theoretical Approach to Centrally Mediated Erectile Dysfunction. Neurosci. Biobehav. Rev. 2000, 24, 571–579. [Google Scholar] [CrossRef]
- Moll, A. Das Sexualleben Des Kindes; Vogel: Eindhoven, The Netherlands, 1908. [Google Scholar]
- Masters, W.H.; Johnson, V.E. Human Sexual Response; Bantam Books: New York, NY, USA, 1966; ISBN 978-0-923891-21-3. [Google Scholar]
- Whalen, R.E. Sexual Motivation. Psychol. Rev. 1966, 73, 151. [Google Scholar] [CrossRef]
- Salu, Y. The Roots of Sexual Arousal and Sexual Orientation. Med. Hypotheses 2011, 76, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Bindra, D. Neuropsychological Interpretation of the Effects of Drive and Incentive-Motivation on General Activity and Instrumental Behavior. Psychol. Rev. 1968, 75, 1–22. [Google Scholar] [CrossRef]
- Bindra, D. A Motivational View of Learning, Performance, and Behavior Modification. Psychol. Rev. 1974, 81, 199. [Google Scholar] [CrossRef]
- Bolles, R.C. Reinforcement, Expectancy, and Learning. Psychol. Rev. 1972, 79, 394. [Google Scholar] [CrossRef]
- Toates, F.M. Motivational Systems; Cambridge University Press: Cambridge, UK, 1986; ISBN 0-521-31894-7. [Google Scholar]
- Pfaus, J.G.; Kippin, T.E.; Coria-Avila, G. What Can Animal Models Tell Us about Human Sexual Response? Annu. Rev. Sex Res. 2003, 14, 1–63. [Google Scholar]
- Georgiadis, J.R.; Kringelbach, M.L.; Pfaus, J.G. Sex for Fun: A Synthesis of Human and Animal Neurobiology. Nat. Rev. Urol. 2012, 9, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Stendhal, H.B. De l’amour; Le Devian: Paris, France, 1822. [Google Scholar]
- Von Krafft-Ebing, R.F. Psychopathia Sexualis: Eine Klinisch-Forensische Studie; Adamant Media Corporation: Chestnut Hill, MA, USA, 1886. [Google Scholar]
- Money, J. Lovemaps: Clinical Concepts of Sexual/Erotic Health and Pathology, Paraphilia, and Gender Transposition in Childhood, Adolescence, and Maturity; Prometheus Books: Buffalo, NY, USA, 1986; ISBN 1-61614-045-3. [Google Scholar]
- Díaz-Estrada, V.X.; Barradas-Moctezuma, M.; Herrera-Covarrubias, D.; Manzo, J.; Coria-Avila, G.A. Nature and Nurture of Sexual Partner Preference: Teachings from Prenatal Administration of Acetaminophen in Male Rats. Horm. Behav. 2020, 124, 104775. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Kippin, T.E.; Coria-Avila, G.A.; Gelez, H.; Afonso, V.M.; Ismail, N.; Parada, M. Who, What, Where, When (and Maybe Even Why)? How the Experience of Sexual Reward Connects Sexual Desire, Preference, and Performance. Arch. Sex. Behav. 2012, 41, 31–62. [Google Scholar] [CrossRef]
- Pfaff, D.W. Drive: Neurobiological and Molecular Mechanisms of Sexual Motivation; The MIT Press: Cambridge, MA, USA, 1999; ISBN 978-0-262-28154-6. [Google Scholar]
- Toates, F. How Sexual Desire Works; Cambridge University Press: Cambridge, UK, 2014; ISBN 1-107-05001-4. [Google Scholar]
- Pfaus, J.G.; Gorzalka, B.B. Opioids and Sexual Behavior. Neurosci. Biobehav. Rev. 1987, 11, 1–34. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Quintana, G.R.; Mac Cionnaith, C.E.; Gerson, C.A.; Dubé, S.; Coria-Avila, G.A. Conditioning of Sexual Interests and Paraphilias in Humans Is Difficult to See, Virtually Impossible to Test, and Probably Exactly How It Happens: A Comment on Hsu and Bailey (2020). Arch. Sex. Behav. 2020, 49, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; Hinde, R. The Psychology of Fear and Stress; CUP Archive: Cambridge, UK, 1987; Volume 5, ISBN 0-521-27098-7. [Google Scholar]
- Pavlov, I. Conditioned Reflexes; An Investigation of the Physiological Activity of the Cerebral Cortex; Oxford University Press: London, UK, 1927. [Google Scholar]
- Dominguez, J.M.; Hull, E.M. Dopamine, the Medial Preoptic Area, and Male Sexual Behavior. Physiol. Behav. 2005, 86, 356–368. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A. Dopamine and Sexual Behavior. Neurosci. Biobehav. Rev. 1995, 19, 19–38. [Google Scholar] [CrossRef]
- Tagliamonte, A.; Fratta, W.; Del Fiacco, M.; Gessa, G.L. Possible Stimulatory Role of Brain Dopamine in the Copulatory Behavior of Male Rats. Pharmacol. Biochem. Behav. 1974, 2, 257–260. [Google Scholar] [CrossRef]
- Rodríguez-Manzo, G. Yohimbine Interacts with the Dopaminergic System to Reverse Sexual Satiation: Further Evidence for a Role of Sexual Motivation in Sexual Exhaustion. Eur. J. Pharmacol. 1999, 372, 1–8. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Phillips, A.G. Role of Dopamine in Anticipatory and Consummatory Aspects of Sexual Behavior in the Male Rat. Behav. Neurosci. 1991, 105, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Laroche, C.; Girard-Bériault, F.; Ménard, S.; Greggain-Mohr, J.A.; Pfaus, J.G. Conditioned Ejaculatory Preference in Male Rats Paired with Haloperidol-Treated Females. Physiol. Behav. 2010, 100, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Hull, E.M.; Rodríguez-Manzo, G. Male Sexual Behavior; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Clark, J.T. Sexual Arousal and Performance Are Modulated by Adrenergic-Neuropeptide-Steroid Interactions. In The Pharmacology of Sexual Function and Dysfunction; Elsevier: Amsterdam, The Netherlands, 1995; Volume 1995, pp. 55–68. [Google Scholar]
- McIntosh, T.K.; Barfield, R.J. Brain Monoaminergic Control of Male Reproductive Behavior. III. Norepinephrine and the Post-Ejaculatory Refractory Period. Behav. Brain Res. 1984, 12, 275–281. [Google Scholar] [CrossRef]
- Carter, C.S. Oxytocin and Sexual Behavior. Neurosci. Biobehav. Rev. 1992, 16, 131–144. [Google Scholar] [CrossRef]
- Cantor, J.M.; Binik, Y.M.; Pfaus, J.G. Chronic Fluoxetine Inhibits Sexual Behavior in the Male Rat: Reversal with Oxytocin. Psychopharmacology 1999, 144, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kita, I.; Yoshida, Y.; Nishino, S. An Activation of Parvocellular Oxytocinergic Neurons in the Paraventricular Nucleus in Oxytocin-Induced Yawning and Penile Erection. Neurosci. Res. 2006, 54, 269–275. [Google Scholar] [CrossRef]
- Meyerson, B.J. The effect of neuropharmacological agents on hormone-activated estrus behaviour in ovariectomised rats. Arch. Int. Pharmacodyn. Ther. 1964, 150, 4–33. [Google Scholar] [PubMed]
- Dalló, J. Effect of Two Brain Serotonin Depletors on the Sexual Behavior of Male Rats. Pol. J. Pharmacol. Pharm. 1977, 29, 247–251. [Google Scholar]
- Paredes, R.G. Opioids and Sexual Reward. Pharmacol. Biochem. Behav. 2014, 121, 124–131. [Google Scholar] [CrossRef]
- Garduño-Gutiérrez, R.; León-Olea, M.; Rodríguez-Manzo, G. Different Amounts of Ejaculatory Activity, a Natural Rewarding Behavior, Induce Differential Mu and Delta Opioid Receptor Internalization in the Rat’s Ventral Tegmental Area. Brain Res. 2013, 1541, 22–32. [Google Scholar] [CrossRef]
- Szechtman, H.; Hershkowitz, M.; Simantov, R. Sexual Behavior Decreases Pain Sensitivity and Stimulated Endogenous Opioids in Male Rats. Eur. J. Pharmacol. 1981, 70, 279–285. [Google Scholar] [CrossRef]
- Coolen, L.M.; Fitzgerald, M.E.; Yu, L.; Lehman, M. Activation of μ Opioid Receptors in the Medial Preoptic Area Following Copulation in Male Rats. Neuroscience 2004, 124, 11–21. [Google Scholar] [CrossRef]
- Balfour, M.E.; Yu, L.; Coolen, L.M. Sexual Behavior and Sex-Associated Environmental Cues Activate the Mesolimbic System in Male Rats. Neuropsychopharmacology 2004, 29, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Van Ree, J.M.; Gerrits, M.A.F.M.; Vanderschuren, L.J.M.J. Opioids, Reward and Addiction: An Encounter of Biology, Psychology, and Medicine. Pharmacol. Rev. 1999, 51, 341–396. [Google Scholar] [PubMed]
- Hughes, A.M.; Everitt, B.J.; Herbert, J. Selective Effects of β-Endorphin Infused into the Hypothalamus, Preoptic Area and Bed Nucleus of the Stria Terminalis on the Sexual and Ingestive Behaviour of Male Rats. Neuroscience 1987, 23, 1063–1073. [Google Scholar] [CrossRef]
- Chessick, R.D. The “Pharmacogenic Orgasm” in the Drug Addict. Arch. Gen. Psychiatry 1960, 3, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Boyse, E.A.; Miké, V.; Thaler, H.T.; Mathieson, B.J.; Abbott, J.; Boyse, J.; Zayas, Z.A.; Thomas, L. Control of Mating Preferences in Mice by Genes in the Major Histocompatibility Complex. J. Exp. Med. 1976, 144, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, K.M.; Hinton, M.R.; Atkins, K.; Haupt, M.A.; Skinner, J.D. Mothers Determine Sexual Preferences. Nature 1998, 395, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Rescorla, R.; Wagner, A. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement; Appleton-Century: New York, NY, USA, 1972. [Google Scholar]
- Domjan, M.; Hall, S. Determinants of Social Proximity in Japanese Quail (Coturnix Coturnix Japonica): Male Behavior. J. Comp. Psychol. 1986, 100, 59–67. [Google Scholar] [CrossRef]
- Agmo, A.; Berenfeld, R. Reinforcing Properties of Ejaculation in the Male Rat: Role of Opioids and Dopamine. Behav. Neurosci. 1990, 104, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Jones, S.L.; Flanagan-Cato, L.M.; Blaustein, J.D. Female Sexual Behavior. Knobil Neills Physiol. Reprod. 2015, 2, 2287–2370. [Google Scholar]
- Paredes, R.G.; Vazquez, B. What Do Female Rats like about Sex? Paced Mating. Behav. Brain Res. 1999, 105, 117–127. [Google Scholar] [CrossRef]
- Kippin, T.; Talianakis, S.; Schattmann, L.; Bartholomew, S.; Pfaus, J. Olfactory Conditioning of Sexual Behavior in the Male Rat (Rattus Norvegicus). J. Comp. Psychol. 1998, 112, 389–399. [Google Scholar] [CrossRef]
- Graham, J.M.; Desjardins, C. Classical Conditioning: Induction of Luteinizing Hormone and Testosterone Secretion in Anticipation of Sexual Activity. Science 1980, 210, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Kippin, T.; Pfaus, J. The Development of Olfactory Conditioned Ejaculatory Preferences in the Male Rat: I. Nature of the Unconditioned Stimulus. Physiol. Behav. 2001, 73, 457–469. [Google Scholar] [CrossRef]
- Ménard, S.; Gelez, H.; Jacubovitch, M.; Coria-Avila, G.A.; Pfaus, J.G. Appetitive Olfactory Conditioning in the Neonatal Male Rat Facilitates Subsequent Sexual Partner Preference. Psychoneuroendocrinology 2020, 121, 104858. [Google Scholar] [CrossRef]
- Domjan, M.; Huber-McDonald, M.; Holloway, K.S. Conditioning Copulatory Behavior to an Artificial Object: Efficacy of Stimulus Fading. Anim. Learn. Behav. 1992, 20, 350–362. [Google Scholar] [CrossRef][Green Version]
- Köksal, F.; Domjan, M.; Kurt, A.; Sertel, O.; Orüng, S.; Bowers, R.; Kumru, G. An Animal Model of Fetishism. Behav. Res. Ther. 2005, 42, 1421–1434. [Google Scholar] [CrossRef]
- Ismail, N.; Jones, S.L.; Graham, M.D.; Sylvester, S.; Pfaus, J.G. Partner Preference for Strain of Female in Long–Evans Male Rats. Physiol. Behav. 2011, 102, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Quintana, G.R.; Desbiens, S.; Marceau, S.; Kalantari, N.; Bowden, J.; Pfaus, J.G. Conditioned Partner Preference in Male and Female Rats for a Somatosensory Cue. Behav. Neurosci. 2019, 133, 188–197. [Google Scholar] [CrossRef]
- Quintana, G.R.; González, B.; Borduas, E.; Lemay, V.; Yarur, F.; Pfaus, J.G. Naloxone Disrupts the Development of a Conditioned Ejaculatory Preference Based on a Somatosensory Cue in Male Rats. Behav Neurosci 2019, 133, 198–202. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Erickson, K.A.; Talianakis, S. Somatosensory Conditioning of Sexual Arousal and Copulatory Behavior in the Male Rat: A Model of Fetish Development. Physiol. Behav. 2013, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.G.; Alonso, A. Sexual Behavior Regulated (Paced) by the Female Induces Conditioned Place Preference. Behav. Neurosci. 1997, 111, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Smith, W.J.; Coopersmith, C.B. Appetitive and Consummatory Sexual Behaviors of Female Rats in Bilevel Chambers. I. A Correlational and Factor Analysis and the Effects of Ovarian Hormones. Horm. Behav. 1999, 35, 224–240. [Google Scholar] [CrossRef]
- Erskine, M.S. Effects of Paced Coital Stimulation on Estrus Duration in Intact Cycling Rats and Ovariectomized and Ovariectomized-Adrenalectomized Hormone-Primed Rats. Behav. Neurosci. 1985, 99, 151. [Google Scholar] [CrossRef] [PubMed]
- Coria-Avila, G.A.; Ouimet, A.J.; Pacheco, P.; Manzo, J.; Pfaus, J.G. Olfactory Conditioned Partner Preference in the Female Rat. Behav. Neurosci. 2005, 119, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Coria-Avila, G.A.; Solomon, C.E.; Vargas, E.B.; Lemme, I.; Ryan, R.; Ménard, S.; Gavrila, A.M.; Pfaus, J.G. Neurochemical Basis of Conditioned Partner Preference in the Female Rat: I. Disruption by Naloxone. Behav. Neurosci. 2008, 122, 385–395. [Google Scholar] [CrossRef]
- Coria-Avila, G.A.; Jones, S.L.; Solomon, C.E.; Gavrila, A.M.; Jordan, G.J.; Pfaus, J.G. Conditioned Partner Preference in Female Rats for Strain of Male. Physiol. Behav. 2006, 88, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Meerts, S.H.; Clark, A.S. Artificial Vaginocervical Stimulation Induces a Conditioned Place Preference in Female Rats. Horm. Behav. 2009, 55, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Parada, M.; Chamas, L.; Censi, S.; Coria-Avila, G.; Pfaus, J.G. Clitoral Stimulation Induces Conditioned Place Preference and Fos Activation in the Rat. Horm. Behav. 2010, 57, 112–118. [Google Scholar] [CrossRef]
- Parada, M.; Abdul-Ahad, F.; Censi, S.; Sparks, L.; Pfaus, J.G. Context Alters the Ability of Clitoral Stimulation to Induce a Sexually-Conditioned Partner Preference in the Rat. Horm. Behav. 2011, 59, 520–527. [Google Scholar] [CrossRef]
- Ismail, N.; Gelez, H.; Lachapelle, I.; Pfaus, J.G. Pacing Conditions Contribute to the Conditioned Ejaculatory Preference for a Familiar Female in the Male Rat. Physiol. Behav. 2009, 96, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mac Cionnaith, C.E.; Lemay, A.; Gomez-Perales, E.L.; Robert, G.; Cernik, R.; Brake, W.G.; Pfaus, J.G. Fos Expression Is Increased in Oxytocin Neurons of Female Rats with a Sexually Conditioned Mate Preference for an Individual Male Rat. Horm. Behav. 2020, 117, 104612. [Google Scholar] [CrossRef] [PubMed]
- Thorndike, E.L. Animal Intelligence: Experimental Studies; Macmillan Press: New York, NY, USA, 1911. [Google Scholar]
- Skinner, B.F. The Behavior of Organisms: An Experimental Analysis; Appleton-Century: Oxford, UK, 1938. [Google Scholar]
- Ferster, C.B.; Skinner, B.F. Schedules of Reinforcement; Appleton-Century: New York, NY, USA, 1957. [Google Scholar]
- Beck, J. Instrumental Conditioned Reflexes with Sexual Reinforcement in Rats. Acta Neurobiol. Exp. 1971, 313, 251–252. [Google Scholar]
- Jowaisas, D.; Taylor, J.; Dewsbury, D.A.; Malagodi, E.F. Copulatory Behavior of Male Rats under an Imposed Operant Requirement. Psychon. Sci. 1971, 25, 287–290. [Google Scholar] [CrossRef]
- Sheffield, F.D.; Wulff, J.J.; Backer, R. Reward Value of Copulation without Sex Drive Reduction. J. Comp. Physiol. Psychol. 1951, 44, 3–8. [Google Scholar] [CrossRef]
- Anderson, E.E. Interrelationship of Drives in the Male Albino Rat. I. Intercorrelations of Measures of Drives. J. Comp. Psychol. 1937, 24, 73–118. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Zheng, X.; Luo, H.; Liu, Z.; Bai, Y. A Conflict Model of Reward-Seeking Behavior in Male Rats. JoVE 2019, 144, e59141. [Google Scholar] [CrossRef]
- Warner, L.H. A Study of Sex Behavior in the White Rat by Means of the Obstruction Method. Comp. Psychol. Monogr. 1927, 4, 58. [Google Scholar]
- Everitt, B.J.; Cador, M.; Robbins, T.W. Interactions between the Amygdala and Ventral Striatum in Stimulus-Reward Associations: Studies Using a Second-Order Schedule of Sexual Reinforcement. Neuroscience 1989, 30, 63–75. [Google Scholar] [CrossRef]
- Everitt, B.J.; Stacey, P. Studies of Instrumental Behavior with Sexual Reinforcement in Male Rats (Rattus Norvegicus): II. Effects of Preoptic Area Lesions, Castration, and Testosterone. J. Comp. Psychol. 1987, 101, 407–419. [Google Scholar] [CrossRef]
- Randich, A.; LoLordo, V.M. Associative and Nonassociative Theories of the UCS Preexposure Phenomenon: Implications for Pavlovian Conditioning. Psychol. Bull. 1979, 86, 523–548. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A. Level of Conditioning and Intensity of the Adaptation Stimulus. J. Exp. Psychol. 1956, 51, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Rankin, C.H.; Abrams, T.; Barry, R.J.; Bhatnagar, S.; Clayton, D.F.; Colombo, J.; Coppola, G.; Geyer, M.A.; Glanzman, D.L.; Marsland, S. Habituation Revisited: An Updated and Revised Description of the Behavioral Characteristics of Habituation. Neurobiol. Learn. Mem. 2009, 92, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Quintana, G.R.; Guizar, A.; Rassi, S.; Pfaus, J.G. First Sexual Experiences Determine the Development of Conditioned Ejaculatory Preference in Male Rats. Learn. Mem. 2018, 25, 522–532. [Google Scholar] [CrossRef]
- Parada, M.; Jafari, N.; Pfaus, J.G. Sexual Experience Blocks the Ability of Clitoral Stimulation to Induce a Conditioned Place Preference in the Rat. Physiol. Behav. 2013, 119, 97–102. [Google Scholar] [CrossRef]
- Lubow, R.E.; Moore, A.U. Latent Inhibition: The Effect of Nonreinforced Pre-Exposure to the Conditional Stimulus. J. Comp. Physiol. Psychol. 1959, 52, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Alonso, G. Latent Inhibition as a Function of CS Intensity in Taste Aversion Learning. Behav. Processes 2002, 60, 61–67. [Google Scholar] [CrossRef]
- Schmajuk, N.A. Latent Inhibition and Its Neural Substrates; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; ISBN 0-7923-7610-2. [Google Scholar]
- Zamble, E.; Mitchell, J.B.; Findlay, H. Pavlovian Conditioning of Sexual Arousal: Parametric and Background Manipulations. J. Exp. Psychol. Anim. Behav. Processes 1986, 12, 403–411. [Google Scholar] [CrossRef]
- Quintana, G.R.; Jackson, M.; Nasr, M.; Pfaus, J.G. Effect of CS Preexposure on the Conditioned Ejaculatory Preference of the Male Rat: Behavioral Analyses and Neural Correlates. Learn. Mem. 2018, 25, 513–521. [Google Scholar] [CrossRef]
- Ménard, S.; Gelez, H.; Girard-Bériault, F.; Coria-Avila, G.; Pfaus, J.G. Differential Role of Oxytocin and Vasopressin in the Conditioned Ejaculatory Preference of the Male Rat. Physiol. Behav. 2019, 208, 112577. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.; Shalev, S.; Bellevue, S.; Pfaus, J.G. Conditioned Mate-Guarding Behavior in the Female Rat. Physiol. Behav. 2014, 131, 136–141. [Google Scholar] [CrossRef] [PubMed]
- McClintock, M.K. Group Mating in the Domestic Rat as a Context for Sexual Selection: Consequences for the Analysis of Sexual Behavior and Neuroendocrine Responses. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 1984; Volume 14, pp. 1–50. ISBN 0065-3454. [Google Scholar]
- Holley, A.; Bellevue, S.; Vosberg, D.; Wenzel, K.; Roorda, S.; Pfaus, J.G. The Role of Oxytocin and Vasopressin in Conditioned Mate Guarding Behavior in the Female Rat. Physiol. Behav. 2015, 144, 7–14. [Google Scholar] [CrossRef]
- Holley, A.; Joulakian, L.; Wenzel, K.; Roorda, S., Jr.; Gonzalez, B.; Sparks, L.; Pfaus, J.G. Inhibition of Lysine-Specific Demethylase Enzyme Disrupts Sexually Conditioned Mate Guarding in the Female Rat. Physiol. Behav. 2018, 196, 78–83. [Google Scholar] [CrossRef]
- Herrera-Morales, W.V.; Herrera-Solís, A.; Núñez-Jaramillo, L. Sexual Behavior and Synaptic Plasticity. Arch. Sex. Behav. 2019, 48, 2617–2631. [Google Scholar] [CrossRef]
- Piergies, A.M.H.; Hicks, M.E., Jr.; Schwartz, J.P.; Meerts, S.H. Sexually Experienced, but Not Naïve, Female Rats Show a Conditioned Object Preference (COP) for Mating after a Single Training Trial. Physiol. Behav. 2019, 198, 42–47. [Google Scholar] [CrossRef]
- Sanna, F.; Poddighe, L.; Serra, M.P.; Boi, M.; Bratzu, J.; Sanna, F.; Corda, M.G.; Giorgi, O.; Melis, M.R.; Argiolas, A. C-Fos, ΔFosB, BDNF, TrkB and Arc Expression in the Limbic System of Male Roman High-and Low-Avoidance Rats That Show Differences in Sexual Behavior: Effect of Sexual Activity. Neuroscience 2019, 396, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sigl-Glöckner, J.; Maier, E.; Takahashi, N.; Sachdev, R.; Larkum, M.; Brecht, M. Effects of Sexual Experience and Puberty on Mouse Genital Cortex Revealed by Chronic Imaging. Curr. Biol. 2019, 29, 3588–3599. [Google Scholar] [CrossRef]
- Meerts, S.H.; Park, J.H.; Sekhawat, R. Sexual Experience Modulates Partner Preference and MPOA Nitric Oxide Synthase in Female Rats. Behav. Neurosci. 2016, 130, 490. [Google Scholar] [CrossRef] [PubMed]
- Nutsch, V.L.; Will, R.G.; Hattori, T.; Tobiansky, D.J.; Dominguez, J.M. Sexual Experience Influences Mating-Induced Activity in Nitric Oxide Synthase-Containing Neurons in the Medial Preoptic Area. Neurosci. Lett. 2014, 579, 92–96. [Google Scholar] [CrossRef]
- Ménard, S.; Gelez, H.; Coria-Avila, G.; Pfaus, J.G. Sexual Experience Increases Oxytocin, but Not Vasopressin, Receptor Densities in the Ventromedial Hypothalamus and Central Amygdala of Male Rats. 2022; Submitted. [Google Scholar]
- Pfaus, J.G.; Damsma, G.; Nomikos, G.G.; Wenkstern, D.G.; Blaha, C.D.; Phillips, A.G.; Fibiger, H.C. Sexual Behavior Enhances Central Dopamine Transmission in the Male Rat. Brain Res. 1990, 530, 345–348. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Damsma, G.; Wenkstern, D.; Fibiger, H.C. Sexual Activity Increases Dopamine Transmission in the Nucleus Accumbens and Striatum of Female Rats. Brain Res. 1995, 693, 21–30. [Google Scholar] [CrossRef]
- Sanna, F.; Bratzu, J.; Piludu, M.A.; Corda, M.G.; Melis, M.R.; Giorgi, O.; Argiolas, A. Dopamine, Noradrenaline and Differences in Sexual Behavior between Roman High and Low Avoidance Male Rats: A Microdialysis Study in the Medial Prefrontal Cortex. Front. Behav. Neurosci. 2017, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Wenkstern, D.; Pfaus, J.G.; Fibiger, H.C. Dopamine Transmission Increases in the Nucleus Accumbens of Male Rats during Their First Exposure to Sexually Receptive Female Rats. Brain Res. 1993, 618, 41–46. [Google Scholar] [CrossRef]
- Pitchers, K.; Di Sebastiano, A.; Coolen, L. MGluR5 Activation in the Nucleus Accumbens Is Not Essential for Sexual Behavior or Cross-Sensitization of Amphetamine Responses by Sexual Experience. Neuropharmacology 2016, 107, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Coria-Avila, G.A.; Pfaus, J.G. Neuronal Activation by Stimuli That Predict Sexual Reward in Female Rats. Neuroscience 2007, 148, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kippin, T.E.; Cain, S.W.; Pfaus, J.G. Estrous Odors and Sexually Conditioned Neutral Odors Activate Separate Neural Pathways in the Male Rat. Neuroscience 2003, 117, 971–979. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Heeb, M.M. Implications of Immediate-Early Gene Induction in the Brain Following Sexual Stimulation of Female and Male Rodents. Brain Res. Bull. 1997, 44, 397–407. [Google Scholar] [CrossRef]
- Goldstein, A.; Tachibana, S.; Lowney, L.I.; Hunkapiller, M.; Hood, L. Dynorphin-(1-13), an Extraordinarily Potent Opioid Peptide. Proc. Natl. Acad. Sci. USA 1979, 76, 6666–6670. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.V.; Stern, A.S.; Kimura, S.; Rossier, J.; Stein, S.; Udenfriend, S. An about 50,000-Dalton Protein in Adrenal Medulla: A Common Precursor of [Met]- and [Leu]Enkephalin. Science 1980, 208, 1459–1461. [Google Scholar] [CrossRef]
- Nakanishi, S.; Inoue, A.; Kita, T.; Numa, S.; Chang, A.C.; Cohen, S.N.; Nunberg, J.; Schimke, R.T. Construction of Bacterial Plasmids That Contain the Nucleotide Sequence for Bovine Corticotropin-Beta-Lipotropin Precursor. Proc. Natl. Acad. Sci. USA 1978, 75, 6021–6025. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, G.C.; Brawer, J.R.; Beaudet, A. Distribution of Mu, Delta, and Kappa Opioid Receptors in the Hypothalamus of the Rat. Brain Res. 1990, 536, 114–123. [Google Scholar] [CrossRef]
- Mansour, A.; Fox, C.A.; Burke, S.; Meng, F.; Thompson, R.C.; Akil, H.; Watson, S.J. Mu, Delta, and Kappa Opioid Receptor MRNA Expression in the Rat CNS: An in Situ Hybridization Study. J. Comp. Neurol. 1994, 350, 412–438. [Google Scholar] [CrossRef] [PubMed]
- Gaudriault, G.; Nouel, D.; Dal Farra, C.; Beaudet, A.; Vincent, J.-P. Receptor-Induced Internalization of Selective Peptidic μ and δ Opioid Ligands. J. Biol. Chem. 1997, 272, 2880–2888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pfaus, J.G.; Smith, W.J.; Byrne, N.; Stephens, G. Appetitive and Consummatory Sexual Behaviors of Female Rats in Bilevel Chambers. Horm. Behav. 2000, 37, 96–107. [Google Scholar] [CrossRef][Green Version]
- Van Furth, W.R.; van Emst, M.G.; van Ree, J.M. Opioids and Sexual Behavior of Male Rats: Involvement of the Medial Preoptic Area. Behav. Neurosci. 1995, 109, 123–134. [Google Scholar] [CrossRef]
- Rodrıguez-Manzo, G.; Asai, M.; Fernández-Guasti, A. Evidence for Changes in Brain Enkephalin Contents Associated to Male Rat Sexual Activity. Behav. Brain Res. 2002, 131, 47–55. [Google Scholar] [CrossRef]
- Arletti, R.; Calza, L.; Giardino, L.; Benelli, A.; Cavazzuti, E.; Bertolini, A. Sexual Impotence Is Associated with a Reduced Production of Oxytocin and with an Increased Production of Opioid Peptides in the Paraventricular Nucleus of Male Rats. Neurosci. Lett. 1997, 233, 65–68. [Google Scholar] [CrossRef]
- Matuszewich, L.; Dornan, W.A. Bilateral Injections of a Selectiveμ-Receptor Agonist (Morphiceptin) into the Medial Preoptic Nucleus Produces a Marked Delay in the Initiation of Sexual Behavior in the Male Rat. Psychopharmacology 1992, 106, 391–396. [Google Scholar] [CrossRef]
- Leyton, M.; Stewart, J. The Stimulation of Central κ Opioid Receptors Decreases Male Sexual Behavior and Locomotor Activity. Brain Res. 1992, 594, 56–74. [Google Scholar] [CrossRef]
- Gessa, G.; Paglietti, E.; Quarantotti, B.P. Induction of Copulatory Behavior in Sexually Inactive Rats by Naloxone. Science 1979, 204, 203–205. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Wilkins, M.F. A Novel Environment Disrupts Copulation in Sexually Naive but Not Experienced Male Rats: Reversal with Naloxone. Physiol. Behav. 1995, 57, 1045–1049. [Google Scholar] [CrossRef]
- Rodriguez-Manzo, G.; Fernández-Guasti, A. Opioid Antagonists and the Sexual Satiation Phenomenon. Psychopharmacology 1995, 122, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.M.; Baum, M.J. Facilitation by Opiate Antagonists of Sexual Performance in the Male Rat. Pharmacol. Biochem. Behav. 1979, 10, 615–618. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Pfaff, D.W. Mu-, Delta-, and Kappa-Opioid Receptor Agonists Selectively Modulate Sexual Behaviors in the Female Rat: Differential Dependence on Progesterone. Horm. Behav. 1992, 26, 457–473. [Google Scholar] [CrossRef]
- Acosta-Martinez, M.; Etgen, A.M. Activation of μ-Opioid Receptors Inhibits Lordosis Behavior in Estrogen and Progesterone-Primed Female Rats. Horm. Behav. 2002, 41, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Hong, W.; Micevych, P. Optogenetic Activation of β-Endorphin Terminals in the Medial Preoptic Nucleus Regulates Female Sexual Receptivity. Eneuro 2020, 7, ENEURO.0315-19.2019. [Google Scholar] [CrossRef] [PubMed]
- Bozarth, M.A.; Wise, R.A. Intracranial Self-Administration of Morphine into the Ventral Tegmental Area in Rats. Life Sci. 1981, 28, 551–555. [Google Scholar] [CrossRef]
- Shippenberg, T.S.; Elmer, G.I. The Neurobiology of Opiate Reinforcement. Crit. Rev. Neurobiol. 1998, 12, 267–303. [Google Scholar] [CrossRef] [PubMed]
- Stolerman, I. Motivational Effects of Opioids: Evidence on the Role of Endorphins in Mediating Reward or Aversion. Pharmacol. Biochem. Behav. 1985, 23, 877–881. [Google Scholar] [CrossRef]
- Van Ree, J.M.; Niesink, R.J.; Van Wolfswinkel, L.; Ramsey, N.F.; Van Furth, W.R.; Vanderschuren, L.J.; Gerrits, M.A.; Van den Berg, C.L. Endogenous Opioids and Reward. Eur. J. Pharmacol. 2000, 405, 89–101. [Google Scholar] [CrossRef]
- Wise, R.A. The Brain and Reward; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Ågmo, A.; Gómez, M. Conditioned Place Preference Produced by Infusion of Met-Enkephalin into the Medial Preoptic Area. Brain Res. 1991, 550, 343–346. [Google Scholar] [CrossRef]
- Garduño-Gutiérrez, R.; León-Olea, M.; Rodríguez-Manzo, G. Opioid Receptor and β-Arrestin2 Densities and Distribution Change after Sexual Experience in the Ventral Tegmental Area of Male Rats. Physiol. Behav. 2018, 189, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Herz, A. Opioid Reward Mechanisms: A Key Role in Drug Abuse? Can. J. Physiol. Pharmacol. 1998, 76, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Morse, A.K.; Balleine, B.W. The Role of Opioid Processes in Reward and Decision-making. Br. J. Pharmacol. 2015, 172, 449–459. [Google Scholar] [CrossRef]
- Raehal, K.M.; Schmid, C.L.; Groer, C.E.; Bohn, L.M. Functional Selectivity at the μ-Opioid Receptor: Implications for Understanding Opioid Analgesia and Tolerance. Pharm. Rev 2011, 63, 1001–1019. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Mendelson, S.D.; Phillips, A.G. A Correlational and Factor Analysis of Anticipatory and Consummatory Measures of Sexual Behavior in the Male Rat. Psychoneuroendocrinology 1990, 15, 329–340. [Google Scholar] [CrossRef]
- Van Furth, W.R.; Wolterink-Donselaar, I.G.; van Ree, J.M. Endogenous Opioids Are Differentially Involved in Appetitive and Consummatory Aspects of Sexual Behavior of Male Rats. Am. J. Physiol. 1994, 266, R606–R613. [Google Scholar] [CrossRef]
- Burkett, J.P.; Spiegel, L.L.; Inoue, K.; Murphy, A.Z.; Young, L.J. Activation of μ-Opioid Receptors in the Dorsal Striatum Is Necessary for Adult Social Attachment in Monogamous Prairie Voles. Neuropsychopharmacol 2011, 36, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Quintana, G.R.; Birrel, M.; Marceau, S.; Kalantari, N.; Bowden, J.; Bachoura, Y.; Borduas, E.; Lemay, V.; Payne, J.W.; Cionnaith, C.M.; et al. Differential Disruption of Conditioned Ejaculatory Preference in the Male Rat Based on Different Sensory Modalities by Micro-Infusions of Naloxone to the Medial Preoptic Area or Ventral Tegmental Area. Psychopharmacology 2019, 236, 3613–3623. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P. Effects of Daily Cocaine and Morphine Treatment on Somatodendritic and Terminal Field Dopamine Release. J. Neurochem. 1988, 50, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; Stewart, J. Dopamine Transmission in the Initiation and Expression of Drug-and Stress-Induced Sensitization of Motor Activity. Brain Res. Rev. 1991, 16, 223–244. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Weber, B. Amphetamine Injection into the Ventral Mesencephalon Sensitizes Rats to Peripheral Amphetamine and Cocaine. J. Pharmacol. Exp. Ther. 1988, 245, 1095–1102. [Google Scholar] [PubMed]
- Robinson, T.E.; Berridge, K.C. The Neural Basis of Drug Craving: An Incentive-Sensitization Theory of Addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Pert, A.; Post, R.; Weiss, S.R. Conditioning as a Critical Determinant of Sensitization Induced by Psychomotor Stimulants. NIDA Res. Monogr. 1990, 97, 208–241. [Google Scholar]
- Deniau, J.; Thierry, A.; Feger, J. Electrophysiological Identification of Mesencephalic Ventromedial Tegmental (VMT) Neurons Projecting to the Frontal Cortex, Septum and Nucleus Accumbens. Brain Res. 1980, 189, 315–326. [Google Scholar] [CrossRef]
- Oades, R.D.; Halliday, G.M. Ventral Tegmental (A10) System: Neurobiology. 1. Anatomy and Connectivity. Brain Res. Rev. 1987, 12, 117–165. [Google Scholar] [CrossRef]
- Swanson, L.W. nd The Projections of the Ventral Tegmental Area and Adjacent Regions: A Combined Fluorescent Retrograde Tracer and Immunofluorescence Study in the Rat. Brain Res. Bull. 1982, 9, 321–353. [Google Scholar] [CrossRef]
- Aragona, B.J.; Liu, Y.; Curtis, J.T.; Stephan, F.K.; Wang, Z. A Critical Role for Nucleus Accumbens Dopamine in Partner-Preference Formation in Male Prairie Voles. J. Neurosci. 2003, 23, 3483–3490. [Google Scholar] [CrossRef]
- Curtis, J.T.; Wang, Z. Ventral Tegmental Area Involvement in Pair Bonding in Male Prairie Voles. Physiol. Behav. 2005, 86, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, G. Nucleus Accumbens Shell and Core Dopamine: Differential Role in Behavior and Addiction. Behav. Brain Res. 2002, 137, 75–114. [Google Scholar] [CrossRef]
- Li, Y.-L.; Wei, S.; Liu, Q.; Gong, Q.; Zhang, Q.-J.; Zheng, T.-G.; Yong, Z.; Chen, F.; Lawrence, A.J.; Liang, J.-H. Mu-Opioid Receptors in Septum Mediate the Development of Behavioural Sensitization to a Single Morphine Exposure in Male Rats. Addict. Biol. 2022, 27, e13066. [Google Scholar] [CrossRef]
- Stewart, J. Conditioned and Unconditioned Drug Effects in Relapse to Opiate and Stimulant Drug Self-Adminstration. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1983, 7, 591–597. [Google Scholar] [CrossRef]
- Graham, M.D.; Gardner Gregory, J.; Hussain, D.; Brake, W.G.; Pfaus, J.G. Ovarian Steroids Alter Dopamine Receptor Populations in the Medial Preoptic Area of Female Rats: Implications for Sexual Motivation, Desire, and Behaviour. Eur. J. Neurosci. 2015, 42, 3138–3148. [Google Scholar] [CrossRef] [PubMed]
- Tobiansky, D.J.; Will, R.G.; Lominac, K.D.; Turner, J.M.; Hattori, T.; Krishnan, K.; Martz, J.R.; Nutsch, V.L.; Dominguez, J.M. Estradiol in the Preoptic Area Regulates the Dopaminergic Response to Cocaine in the Nucleus Accumbens. Neuropsychopharmacology 2016, 41, 1897–1906. [Google Scholar] [CrossRef]
- Barrot, M.; Sesack, S.R.; Georges, F.; Pistis, M.; Hong, S.; Jhou, T.C. Braking Dopamine Systems: A New GABA Master Structure for Mesolimbic and Nigrostriatal Functions. J. Neurosci. 2012, 32, 14094–14101. [Google Scholar] [CrossRef] [PubMed]
- Fields, H.L.; Margolis, E.B. Understanding Opioid Reward. Trends Neurosci. 2015, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Phasic versus Tonic Dopamine Release and the Modulation of Dopamine System Responsivity: A Hypothesis for the Etiology of Schizophrenia. Neuroscience 1991, 41, 1–24. [Google Scholar] [CrossRef]
- Blackburn, J.R.; Pfaus, J.G.; Phillips, A.G. Dopamine Functions in Appetitive and Defensive Behaviours. Prog. Neurobiol. 1992, 39, 247–279. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Stewart, J. Facilitation of Sexual Behaviors in the Male Rat Associated with Intra-VTA Injections of Opiates. Pharmacol. Biochem. Behav. 1990, 35, 643–650. [Google Scholar] [CrossRef]
- McConnell, S.K.; Baum, M.J.; Badger, T.M. Lack of Correlation between Naloxone-Induced Changes in Sexual Behavior and Serum LH in Male Rats. Horm. Behav. 1981, 15, 16–35. [Google Scholar] [CrossRef]
- Childers, S.R. Opioid Receptor-Coupled Second Messenger Systems. Life Sci. 1991, 48, 1991–2003. [Google Scholar] [CrossRef]
- Kramer, H.K.; Simon, E.J. μ and δ-Opioid Receptor Agonists Induce Mitogen-Activated Protein Kinase (MAPK) Activation in the Absence of Receptor Internalization. Neuropharmacology 2000, 39, 1707–1719. [Google Scholar] [CrossRef]
- Samways, D.S.; Henderson, G. Opioid Elevation of Intracellular Free Calcium: Possible Mechanisms and Physiological Relevance. Cell. Signal. 2006, 18, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ammon-Treiber, S.; Höllt, V. Morphine-induced Changes of Gene Expression in the Brain. Addict. Biol. 2005, 10, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Björk, K.; Tronci, V.; Thorsell, A.; Tanda, G.; Hirth, N.; Heilig, M.; Hansson, A.C.; Sommer, W.H. β-Arrestin 2 Knockout Mice Exhibit Sensitized Dopamine Release and Increased Reward in Response to a Low Dose of Alcohol. Psychopharmacology 2013, 230, 439–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Argiolas, A.; Melis, M.R. Neuropeptides and Central Control of Sexual Behaviour from the Past to the Present: A Review. Prog. Neurobiol. 2013, 108, 80–107. [Google Scholar] [CrossRef]
- Bratzu, J.; Bharatiya, R.; Manca, E.; Cocco, C.; Argiolas, A.; Melis, M.R.; Sanna, F. Oxytocin Induces Penile Erection and Yawning When Injected into the Bed Nucleus of the Stria Terminalis: A Microdialysis and Immunohistochemical Study. Behav. Brain Res. 2019, 375, 112147. [Google Scholar] [CrossRef]
- Corona, G.; Isidori, A.M.; Aversa, A.; Burnett, A.L.; Maggi, M. Endocrinologic Control of Men’s Sexual Desire and Arousal/Erection. J. Sex. Med. 2016, 13, 317–337. [Google Scholar] [CrossRef]
- Gelez, H.; Poirier, S.; Facchinetti, P.; Allers, K.A.; Wayman, C.; Alexandre, L.; Giuliano, F. Neuroanatomical Evidence for a Role of Central Melanocortin-4 Receptors and Oxytocin in the Efferent Control of the Rodent Clitoris and Vagina. J. Sex. Med. 2010, 7, 2056–2067. [Google Scholar] [CrossRef]
- Carter, C.S.; Williams, J.R.; Witt, D.M.; Insel, T.R. Oxytocin and Social Bonding. Ann. N. Y. Acad. Sci. 1992, 652, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Young, L.; Wang, Z. Central Oxytocin and Reproductive Behaviours. Rev. Reprod. 1997, 2, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Winslow, J.T.; Wang, Z.; Young, L.J. Oxytocin, Vasopressin, and the Neuroendocrine Basis of Pair Bond Formation. Vasopressin Oxytocin 1998, 449, 215–224. [Google Scholar]
- Johnson, Z.V.; Young, L.J. Oxytocin and Vasopressin Neural Networks: Implications for Social Behavioral Diversity and Translational Neuroscience. Neurosci. Biobehav. Rev. 2017, 76, 87–98. [Google Scholar] [CrossRef]
- Winslow, J.T.; Hastings, N.; Carter, C.S.; Harbaugh, C.R.; Insel, T.R. A Role for Central Vasopressin in Pair Bonding in Monogamous Prairie Voles. Nature 1993, 365, 545–548. [Google Scholar] [CrossRef]
- Young, L.J.; Lim, M.M.; Gingrich, B.; Insel, T.R. Cellular Mechanisms of Social Attachment. Horm. Behav. 2001, 40, 133–138. [Google Scholar] [CrossRef]
- Young, L.J.; Wang, Z. The Neurobiology of Pair Bonding. Nat. Neurosci. 2004, 7, 1048–1054. [Google Scholar] [CrossRef]
- Panaro, M.A.; Benameur, T.; Porro, C. Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. J. Clin. Med. 2020, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Althammer, F.; Grinevich, V. Diversity of Oxytocin Neurones: Beyond Magno- and Parvocellular Cell Types? J. Neuroendocrinol. 2018, 30, e12549. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked Axonal Oxytocin Release in the Central Amygdala Attenuates Fear Response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef]
- Landgraf, R.; Neumann, I.D. Vasopressin and Oxytocin Release within the Brain: A Dynamic Concept of Multiple and Variable Modes of Neuropeptide Communication. Front. Neuroendocr. 2004, 25, 150–176. [Google Scholar] [CrossRef] [PubMed]
- Eliava, M.; Melchior, M.; Knobloch-Bollmann, H.S.; Wahis, J.; da Silva Gouveia, M.; Tang, Y.; Ciobanu, A.C.; Triana del Rio, R.; Roth, L.C.; Althammer, F.; et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron 2016, 89, 1291–1304. [Google Scholar] [CrossRef]
- Lim, M.M.; Murphy, A.Z.; Young, L.J. Ventral Striatopallidal Oxytocin and Vasopressin V1a Receptors in the Monogamous Prairie Vole (Microtus Ochrogaster). J. Comp. Neurol. 2004, 468, 555–570. [Google Scholar] [CrossRef]
- Zhang, X.; Filippi, S.; Vignozzi, L.; Morelli, A.; Mancina, R.; Luconi, M.; Donati, S.; Marini, M.; Vannelli, G.; Forti, G. Identification, Localization and Functional in Vitro and in Vivo Activity of Oxytocin Receptor in the Rat Penis. J. Endocrinol. 2005, 184, 567–576. [Google Scholar] [CrossRef]
- Mac Cionnaith, C.E.; Gomez-Perales, E.L.; Ismail, H.; Lacasse, J.; Pfaus, J.G.; Brake, W.G. Peripheral Oxytocin Signalling Promotes Estrous Termination and Conditioned Mate Preferences in Long-Evans Female Rats. 2022; Submitted. [Google Scholar]
- Coria-Avila, G.A.; Pfaus, J.G.; Hernandez, M.E.; Manzo, J.; Pacheco, P. Timing between Ejaculations Changes Paternity Success. Physiol. Behav. 2004, 80, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Argiolas, A. Central Control of Penile Erection: A Re-Visitation of the Role of Oxytocin and Its Interaction with Dopamine and Glutamic Acid in Male Rats. Neurosci. Biobehav. Rev. 2011, 35, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Crews, D. Epigenetics and Its Implications for Behavioral Neuroendocrinology. Front. Neuroendocrinol. 2008, 29, 344–357. [Google Scholar] [CrossRef]
- Elvir, L.; Duclot, F.; Wang, Z.; Kabbaj, M. Epigenetic Regulation of Motivated Behaviors by Histone Deacetylase Inhibitors. Neurosci. Biobehav. Rev. 2019, 105, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L. Epigenetic Mechanisms in the Development of Behavior: Advances, Challenges, and Future Promises of a New Field. Dev. Psychopathol. 2013, 25, 1279–1291. [Google Scholar] [CrossRef]
- Wang, H.; Duclot, F.; Liu, Y.; Wang, Z.; Kabbaj, M. Histone Deacetylase Inhibitors Facilitate Partner Preference Formation in Female Prairie Voles. Nat. Neurosci. 2013, 16, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Metzger, E.; Wissmann, M.; Yin, N.; Müller, J.M.; Schneider, R.; Peters, A.H.; Günther, T.; Buettner, R.; Schüle, R. LSD1 Demethylates Repressive Histone Marks to Promote Androgen-Receptor-Dependent Transcription. Nature 2005, 437, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Yang, Y.; Meng, Y.; Li, Y.; Zhou, L.; Wang, Z.; Zhu, J. Distribution of D1 and D2 Receptor-Immunoreactive Neurons in the Paraventricular Nucleus of the Hypothalamus in the Rat. J. Chem. Neuroanat. 2019, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Lian, H.; Li, Y.; Wang, Z. Colocalization of Dopamine Receptors in BDNF-Expressing Peptidergic Neurons in the Paraventricular Nucleus of Rats. J. Chem. Neuroanat. 2020, 106, 101794. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.E.; Hess, S.; Meyer, K.D.; Verhagen, L.A.; Koch, L.; Brönneke, H.S.; Dietrich, M.O.; Jordan, S.D.; Saletore, Y.; Elemento, O. The Fat Mass and Obesity Associated Gene (Fto) Regulates Activity of the Dopaminergic Midbrain Circuitry. Nat. Neurosci. 2013, 16, 1042–1048. [Google Scholar] [CrossRef]
- Lee, H.C.; Deng, Q.W.; Zhao, Y.J. The Calcium Signaling Enzyme CD38—A Paradigm for Membrane Topology Defining Distinct Protein Functions. Cell Calcium 2022, 101, 102514. [Google Scholar] [CrossRef]
- Chong, A.; Tolomeo, S.; Xiong, Y.; Angeles, D.; Cheung, M.; Becker, B.; Lai, P.S.; Lei, Z.; Malavasi, F.; Tang, Q. Blending Oxytocin and Dopamine with Everyday Creativity. Sci. Rep. 2021, 11, 16185. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H. Somato-Axodendritic Release of Oxytocin into the Brain Due to Calcium Amplification Is Essential for Social Memory. J. Physiol. Sci. 2016, 66, 275–282. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.; Lee, H.-R.; Jang, E.-H.; Ryu, H.-H.; Kang, M.; Rah, S.-Y.; Yoo, J.; Lee, B.; Kim, J.-I. Impaired Learning and Memory in CD38 Null Mutant Mice. Mol. Brain 2016, 9, 16. [Google Scholar] [CrossRef]
- Quintana, D.S.; Rokicki, J.; van der Meer, D.; Alnæs, D.; Kaufmann, T.; Córdova-Palomera, A.; Dieset, I.; Andreassen, O.A.; Westlye, L.T. Oxytocin Pathway Gene Networks in the Human Brain. Nat. Commun. 2019, 10, 668. [Google Scholar] [CrossRef]
- Sadikaj, G.; Moskowitz, D.; Zuroff, D.C.; Bartz, J.A. CD38 Is Associated with Communal Behavior, Partner Perceptions, Affect and Relationship Adjustment in Romantic Relationships. Sci. Rep. 2020, 10, 12926. [Google Scholar] [CrossRef]
- Zhong, J.; Amina, S.; Liang, M.; Akther, S.; Yuhi, T.; Nishimura, T.; Tsuji, C.; Tsuji, T.; Liu, H.-X.; Hashii, M. Cyclic ADP-Ribose and Heat Regulate Oxytocin Release via CD38 and TRPM2 in the Hypothalamus during Social or Psychological Stress in Mice. Front. Neurosci. 2016, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Peñagarikano, O. Oxytocin in Animal Models of Autism Spectrum Disorder. Dev. Neurobiol. 2017, 77, 202–213. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.-F.; Han, J.-S.; Han, S.-P. Genes Related to Oxytocin and Arginine-Vasopressin Pathways: Associations with Autism Spectrum Disorders. Neurosci. Bull. 2017, 33, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Flood, M. Exposure to Pornography Among Youth in Australia. J. Sociol. 2007, 43, 45–60. [Google Scholar] [CrossRef]
- Wright, P.J. U.S. Males and Pornography, 1973–2010: Consumption, Predictors, Correlates. J. Sex Res. 2013, 50, 60–71. [Google Scholar] [CrossRef]
- Bonierbale-branchereau, M.; Hontanx, J.; Boubli, L. The Sexual Behavior of Young French People. Contracept. Fertil. Sex. 1987, 15, 61–67. [Google Scholar]
- Darling, C.A.; Davidson, J.K. Coitally Active University Students: Sexual Behaviors, Concerns, and Challenges. Adolescence 1986, 21, 403. [Google Scholar] [PubMed]
- Meston, C.M.; Levin, R.J.; Sipski, M.L.; Hull, E.M.; Heiman, J.R. Women’s Orgasm. Annu. Rev. Sex Res. 2004, 15, 173–257. [Google Scholar] [PubMed]
- Opperman, E.; Braun, V.; Clarke, V.; Rogers, C. “It Feels so Good It Almost Hurts”: Young Adults’ Experiences of Orgasm and Sexual Pleasure. J. Sex Res. 2014, 51, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Scardochio, T.; Parada, M.; Gerson, C.; Quintana, G.R.; Coria-Avila, G.A. Do Rats Have Orgasms? Socioaffect. Neurosci. Psychol. 2016, 6, 31883. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, S.B.; Francisco, M.; van Anders, S.M. When Orgasms Do Not Equal Pleasure: Accounts of “Bad” Orgasm Experiences during Consensual Sexual Encounters. Arch. Sex. Behav. 2019, 48, 2435–2459. [Google Scholar] [CrossRef] [PubMed]
- Kingsberg, S.A.; Wysocki, S.; Magnus, L.; Krychman, M.L. Vulvar and Vaginal Atrophy in Postmenopausal Women: Findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) Survey. J. Sex. Med. 2013, 10, 1790–1799. [Google Scholar] [CrossRef]
- Rowland, D.; Donarski, A.; Graves, V.; Caldwell, C.; Hevesi, B.; Hevesi, K. The Experience of Orgasmic Pleasure during Partnered and Masturbatory Sex in Women with and without Orgasmic Difficulty. J. Sex Marital Ther. 2019, 45, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Rowland, D.L.; Kolba, T.N. Relationship of Specific Sexual Activities to Orgasmic Latency, Pleasure, and Difficulty during Partnered Sex. J. Sex. Med. 2019, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.A.; Graham, C.A.; Milhausen, R.R. Predicting Sexual Problems in Women: The Relevance of Sexual Excitation and Sexual Inhibition. Arch. Sex. Behav. 2008, 37, 241–251. [Google Scholar] [CrossRef]
- Vandereycken, W. On Desire, Excitement, and Impotence in Modern Sex Therapy. Psychother. Psychosom. 1987, 47, 175–180. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Quintana, G.R.; Mac Cionnaith, C.; Parada, M. The Whole versus the Sum of Some of the Parts: Toward Resolving the Apparent Controversy of Clitoral versus Vaginal Orgasms. Socioaffect. Neurosci. Psychol. 2016, 6, 32578. [Google Scholar] [CrossRef] [PubMed]
- Van Anders, S.M.; Herbenick, D.; Brotto, L.A.; Harris, E.A.; Chadwick, S.B. The Heteronormativity Theory of Low Sexual Desire in Women Partnered with Men. Arch. Sex. Behav. 2022, 51, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.M.; Brody, S. Women’s Relationship Quality Is Associated with Specifically Penile-Vaginal Intercourse Orgasm and Frequency. J. Sex Marital Ther. 2007, 33, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Van Roijen, J.H.; Slob, A.K.; Gianotten, W.L.; Dohle, G.R.; van der Zon, A.T.; Vreeburg, J.T.; Weber, R.F. Sexual Arousal and the Quality of Semen Produced by Masturbation. Hum. Reprod. 1996, 11, 147–151. [Google Scholar] [CrossRef] [PubMed]
- El Amiri, S.; Brassard, A.; Rosen, N.O.; Rossi, M.A.; Beaulieu, N.; Bergeron, S.; Péloquin, K. Sexual Function and Satisfaction in Couples with Infertility: A Closer Look at the Role of Personal and Relational Characteristics. J. Sex. Med. 2021, 18, 1984–1997. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, W.S.; Gherman, B.R.; Abdo, C.H.N.; Coutinho, E.S.F.; Nardi, A.E.; Appolinario, J.C. Prevalence of Sexual Dysfunction in Depressive and Persistent Depressive Disorders: A Systematic Review and Meta-Analysis. Int. J. Impot. Res. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, D.A.; Ciesla, J.A.; Janata, J.W.; Kingsberg, S.A. Specificity of Anhedonic Depression and Anxious Arousal with Sexual Problems among Sexually Healthy Young Adults. J. Sex. Med. 2012, 9, 505–513. [Google Scholar] [CrossRef]
- Trovao, J.N.; Serefoglu, E.C. Neurobiology of Male Sexual Dysfunctions in Psychiatric Disorders: The Cases of Depression, Anxiety, Mania and Schizophrenia. Int. J. Impot. Res. 2018, 30, 279–286. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Manson, C.; Nowak, M. Impact of Antidepressant Drugs on Sexual Function and Satisfaction. CNS Drugs 2015, 29, 905–913. [Google Scholar] [CrossRef]
- Clayton, A.H.; Croft, H.A.; Handiwala, L. Antidepressants and Sexual Dysfunction: Mechanisms and Clinical Implications. Postgrad. Med. 2014, 126, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Grimsley, S.; Jann, M.W. Paroxetine, Sertraline, and Fluvoxamine: New Selective Serotonin Reuptake Inhibitors. Clin. Pharm. 1992, 11, 930–957. [Google Scholar]
- Hirschfeld, R.M. Efficacy of SSRIs and Newer Antidepressants in Severe Depression: Comparison with TCAs. J. Clin. Psychiatry 1999, 60, 6242. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, T.; Rullo, J.; Faubion, S. Antidepressant-Induced Female Sexual Dysfunction; Elsevier: Amsterdam, The Netherlands, 2016; Volume 91, pp. 1280–1286. [Google Scholar]
- Rothmore, J. Antidepressant-induced Sexual Dysfunction. Med. J. Aust. 2020, 212, 329–334. [Google Scholar] [CrossRef]
- Zajecka, J.; Fawcett, J.; Schaff, M.; Jeffriess, H.; Guy, C. The Role of Serotonin in Sexual Dysfunction: Fluoxetine-Associated Orgasm Dysfunction. J. Clin. Psychiatry 1991, 52, 66–68. [Google Scholar]
- Segraves, R.T. Antidepressant-Induced Orgasm Disorder. J. Sex Marital Ther. 1995, 21, 192–201. [Google Scholar] [CrossRef]
- Kinsey, A.C.; Pomeroy, W.B.; Martin, C.E. Sexual Behavior in the Human Male; Saunders: Oxford, UK, 1948. [Google Scholar]
- Kinsey, A.C.; Pomeroy, W.B.; Martin, C.E.; Gebhard, P.H. Sexual Behavior in the Human Female; Saunders: Oxford, UK, 1953. [Google Scholar]
- Faulkenberry, J.R.; Vincent, M.; James, A.; Johnson, W. Coital Behaviors, Attitudes, and Knowledge of Students Who Experience Early Coitus. Adolescence 1987, 22, 321. [Google Scholar] [PubMed]
- Meston, C.M.; Hamilton, L.D.; Harte, C.B. Sexual Motivation in Women as a Function of Age. J. Sex. Med. 2009, 6, 3305–3319. [Google Scholar] [CrossRef]
- Kleinplatz, P.J.; Ménard, A.D.; Paquet, M.-P.; Paradis, N.; Campbell, M.; Zuccarino, D.; Mehak, L. The Components of Optimal Sexuality: A Portrait of “Great Sex”. Can. J. Hum. Sex. 2009, 18, 1–13. [Google Scholar]
- Boul, L.; Hallam-Jones, R.; Wylie, K.R. Sexual Pleasure and Motivation. J. Sex Marital Ther. 2008, 35, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Bischof-Campbell, A.; Hilpert, P.; Burri, A.; Bischof, K. Body Movement Is Associated with Orgasm during Vaginal Intercourse in Women. J. Sex Res. 2019, 56, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Buss, D.M. Sex Differences in Human Mate Selection Criteria: An Evolutionary Perspective. In Sociobiology and Psychology: Ideas, Issues, and Applications; Lawrence Erlbaum: Mahwah, NJ, USA, 1987; pp. 335–352. [Google Scholar]
- Meston, C.M.; Buss, D.M. Why Humans Have Sex. Arch. Sex. Behav. 2007, 36, 477–507. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P. Sexual Imprinting and Optimal Outbreeding. Nature 1978, 273, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P. Mate Choice; Cambridge University Press: Cambridge, UK, 1983; ISBN 978-0-521-27207-0. [Google Scholar]
- Lorenz, K. Der Kumpan in Der Umwelt Des Vogels. J. Ornithol. 1935, 83, 137–213. [Google Scholar] [CrossRef]
- Fillion, T.J.; Blass, E.M. Infantile Experience with Suckling Odors Determines Adult Sexual Behavior in Male Rats. Science 1986, 231, 729–731. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana, G.R.; Mac Cionnaith, C.E.; Pfaus, J.G. Behavioral, Neural, and Molecular Mechanisms of Conditioned Mate Preference: The Role of Opioids and First Experiences of Sexual Reward. Int. J. Mol. Sci. 2022, 23, 8928. https://doi.org/10.3390/ijms23168928
Quintana GR, Mac Cionnaith CE, Pfaus JG. Behavioral, Neural, and Molecular Mechanisms of Conditioned Mate Preference: The Role of Opioids and First Experiences of Sexual Reward. International Journal of Molecular Sciences. 2022; 23(16):8928. https://doi.org/10.3390/ijms23168928
Chicago/Turabian StyleQuintana, Gonzalo R., Conall E. Mac Cionnaith, and James G. Pfaus. 2022. "Behavioral, Neural, and Molecular Mechanisms of Conditioned Mate Preference: The Role of Opioids and First Experiences of Sexual Reward" International Journal of Molecular Sciences 23, no. 16: 8928. https://doi.org/10.3390/ijms23168928
APA StyleQuintana, G. R., Mac Cionnaith, C. E., & Pfaus, J. G. (2022). Behavioral, Neural, and Molecular Mechanisms of Conditioned Mate Preference: The Role of Opioids and First Experiences of Sexual Reward. International Journal of Molecular Sciences, 23(16), 8928. https://doi.org/10.3390/ijms23168928





