Functional Impairment of Endothelial Colony Forming Cells (ECFC) in Patients with Severe Atherosclerotic Cardiovascular Disease (ASCVD)
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Population
2.2. Characterization of EPCs in Patients with ASCVD
2.3. ECFC Functionality According to the ASCVD Presence and Severity
2.4. Associations between PC Subpopulations Counts and Functionality, Arterial Phenotypes, and Circulating Markers
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Cells Characterization
4.3. Enumeration and Characterization of Circulating Progenitor Cells by Flow Cytometry
4.4. Assessment of Colony-Forming Unit-Endothelial Cells
4.5. Assessment of Endothelial Colony Forming Cells
4.6. ECFC Functional Tests (Proliferation, Senescence, and Vasculogenic Properties)
4.7. IL-6 and VEGF Plasmatic Levels Determination
4.8. Characterization of Arterial Phenotype
4.8.1. Carotid Femoral Pulse Wave Velocity (PWV)
4.8.2. Carotid Artery Intima-Media Thickness (IMT) and Presence of Carotid Atherosclerotic Plaque
4.9. Measurement of Blood Pressure
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Feig, J.E. Regression of Atherosclerosis: Insights from Animal and Clinical Studies. Ann. Glob. Health 2014, 80, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-A.; Park, J.B.; O’Rourke, M.F. Vasculopathy of Aging and the Revised Cardiovascular Continuum. Pulse 2015, 3, 141–147. [Google Scholar] [CrossRef]
- Altabas, V.; Altabas, K.; Kirigin, L. Endothelial progenitor cells (EPCs) in ageing and age-related diseases: How currently available treatment modalities affect EPC biology, atherosclerosis, and cardiovascular outcomes. Mech. Ageing Dev. 2016, 159, 49–62. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. Available online: https://www.nejm.org/doi/10.1056/NEJMoa022287 (accessed on 15 December 2021).
- Werner, N.; Kosiol, S.; Schiegl, T.; Ahlers, P.; Walenta, K.; Link, A.; Böhm, M.; Nickenig, G. Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. Available online: https://www.nejm.org/doi/10.1056/NEJMoa043814 (accessed on 15 December 2021).
- Tahhan, A.; Hammadah, M.; Raad, M.; Almuwaqqat, Z.; Alkhoder, A.; Sandesara, P.B.; Mohamed-Kelli, H.; Hayek, S.S.; Kim, J.H.; O’Neal, W.T.; et al. Progenitor Cells and Clinical Outcomes in Patients with Acute Coronary Syndromes. Circ. Res. 2018, 122, 1565–1575. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, L.; Liao, J.; Luo, Y.; He, L. Early risk assessment of circulating endothelial progenitor cells and plasma stromal cell-derived factor-1 for nondisabling ischemic cerebrovascular events. BMC Neurol. 2019, 19, 22. [Google Scholar] [CrossRef]
- Kukumberg, M.; Zaw, A.M.; Wong, D.H.C.; Toh, C.M.; Chan, B.P.L.; Seet, R.C.S.; Wong, P.T.H.; Yim, E.K.F. Characterization and Functional Assessment of Endothelial Progenitor Cells in Ischemic Stroke Patients. Stem Cell Rev. Rep. 2021, 17, 952–967. [Google Scholar] [CrossRef]
- Psaltis, P.J.; Simari, R.D. Vascular Wall Progenitor Cells in Health and Disease. Circ. Res. 2015, 116, 1392–1412. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Tsai, W.-C.; Huang, T.-S.; Su, S.-H.; Chang, C.-Y.; Ma, H.-Y.; Wu, C.-H.; Yang, C.-Y.; Lin, C.-H.; Huang, P.-H.; et al. Dysregulation of endothelial colony-forming cell function by a negative feedback loop of circulating miR-146a and -146b in cardiovascular disease patients. PLoS ONE 2017, 12, e0181562. [Google Scholar] [CrossRef]
- Goligorsky, M.; Hirschi, K. Stress-Induced Premature Senescence of Endothelial and Endothelial Progenitor Cells. Adv. Pharm. 2016, 77, 281–306. [Google Scholar] [CrossRef]
- Toshner, M.; Voswinckel, R.; Southwood, M.; Al-Lamki, R.; Howard, L.S.; Marchesan, D.; Yang, J.; Suntharalingam, J.; Soon, E.; Exley, A.; et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2009, 180, 780–787. [Google Scholar] [CrossRef]
- Paschalaki, K.E.; Starke, R.D.; Hu, Y.; Mercado, N.; Margariti, A.; Gorgoulis, V.G.; Randi, A.M.; Barnes, P.J. Dysfunction of Endothelial Progenitor Cells from Smokers and Chronic Obstructive Pulmonary Disease Patients Due to Increased DNA Damage and Senescence. Stem Cells 2013, 31, 2813–2826. [Google Scholar] [CrossRef]
- Blandinières, A.; Gendron, N.; Bacha, N.; Bièche, I.; Chocron, R.; Nunes, H.; Nevo, N.; Rossi, E.; Crestani, B.; Lecourt, S.; et al. Interleukin-8 release by endothelial colony-forming cells isolated from idiopathic pulmonary fibrosis patients might contribute to their pathogenicity. Angiogenesis 2019, 22, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Gager, G.M.; Biesinger, B.; Hofer, F.; Winter, M.-P.; Hengstenberg, C.; Jilma, B.; Eyileten, C.; Postula, M.; Lang, I.M.; Siller-Matula, J.M. Interleukin-6 level is a powerful predictor of long-term cardiovascular mortality in patients with acute coronary syndrome. Vasc. Pharmacol. 2020, 135, 106806. [Google Scholar] [CrossRef]
- Rizza, S.; Longo, S.; Piciucchi, G.; Romanello, D.; Mavilio, M.; Montagna, M.; Coppeta, L.; Martelli, E.; Magrini, A.; Federici, M. Carotid intimal medial thickness in rotating night shift is related to IL1β/IL6 axis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1826–1832. [Google Scholar] [CrossRef]
- Barajas-Gómez, B.A.; Rosas-Carrasco, O.; Morales-Rosales, S.L.; Pedraza Vázquez, G.; González-Puertos, V.Y.; Juárez-Cedillo, T.; García-Álvarez, J.A.; López-Diazguerrero, N.E.; Damián-Matsumura, P.; Königsberg, M.; et al. Relationship of inflammatory profile of elderly patients serum and senescence-associated secretory phenotype with human breast cancer cells proliferation: Role of IL6/IL8 ratio. Cytokine 2017, 91, 13–29. [Google Scholar] [CrossRef]
- Lacina, L.; Brábek, J.; Král, V.; Kodet, O.; Smetana, K. Interleukin-6: A molecule with complex biological impact in cancer. Histol. Histopathol. 2019, 34, 125–136. [Google Scholar] [CrossRef]
- Yamashita, T.; Abe, K. Mechanisms of Endogenous Endothelial Repair in Stroke. Curr. Pharm. Des. 2012, 18, 3649–3652. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The Role of the VEGF Family in Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef]
- Xue, L.; Chen, H.; Zhang, T.; Chen, J.; Geng, Z.; Zhao, Y. Changes in serum vascular endothelial growth factor and endostatin concentrations associated with circulating endothelial progenitor cells after acute ischemic stroke. Metab. Brain Dis. 2017, 32, 641–648. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, S.-H.; Li, X.-P.; Shen, X.-Q.; Fang, Z.-F. A novel hypothesis of atherosclerosis: EPCs-mediated repair-to-injury. Med. Hypotheses 2008, 70, 838–841. [Google Scholar] [CrossRef]
- Vemparala, K.; Roy, A.; Bahl, V.K.; Prabhakaran, R.; Nath, N.; Sinha, S.; Nandi, P.; Pandey, R.M.; Reddy, K.S.; Manhapra, A.; et al. Early accelerated senescence of circulating endothelial progenitor cells in premature coronary artery disease patients in a developing country—A case control study. BMC Cardiovasc. Disord. 2013, 13, 104. [Google Scholar] [CrossRef]
- Fadini, G.P.; Agostini, C.; Sartore, S.; Avogaro, A. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis 2007, 194, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Leal, V.; Ribeiro, C.F.; Oliveiros, B.; António, N.; Silva, S. Intrinsic Vascular Repair by Endothelial Progenitor Cells in Acute Coronary Syndromes: An Update Overview. Stem Cell Rev. Rep. 2019, 15, 35–47. [Google Scholar] [CrossRef]
- Benyamine, A.; Magalon, J.; Cointe, S.; Lacroix, R.; Arnaud, L.; Bardin, N.; Rossi, P.; Francès, Y.; Bernard-Guervilly, F.; Kaplanski, G.; et al. Increased serum levels of fractalkine and mobilisation of CD34+CD45− endothelial progenitor cells in systemic sclerosis. Arthritis Res. Ther. 2017, 19, 60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vega, F.M.; Gautier, V.; Fernandez-Ponce, C.M.; Extremera, M.; Altelaar, A.; Millan, J.; Tellez, J.C.; Hernandez-Campos, J.A.; Conejero, R.; Bolivar, J.; et al. The atheroma plaque secretome stimulates the mobilization of endothelial progenitor cells ex vivo. J. Mol. Cell. Cardiol. 2017, 105, 12–23. [Google Scholar] [CrossRef]
- Kou, F.; Zhu, C.; Wan, H.; Xue, F.; Wang, J.; Xiang, L.; Li, J. Endothelial progenitor cells as the target for cardiovascular disease prediction, personalized prevention, and treatments: Progressing beyond the state-of-the-art. EPMA J. 2020, 11, 629–643. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, B.; Gu, D.; Zhang, L.; Han, Z. Endothelial progenitor cell therapy in atherosclerosis: A double-edged sword? Ageing Res. Rev. 2009, 8, 83–93. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lee, W.-S.; Kim, J. Therapeutic strategy for atherosclerosis based on bone-vascular axis hypothesis. Pharmacol. Ther. 2020, 206, 107436. [Google Scholar] [CrossRef]
- Foteinos, G.; Hu, Y.; Xiao, Q.; Metzler, B.; Xu, Q. Rapid Endothelial Turnover in Atherosclerosis-Prone Areas Coincides with Stem Cell Repair in Apolipoprotein E–Deficient Mice. Circulation 2008, 117, 1856–1863. [Google Scholar] [CrossRef]
- Smadja, D.M.; Melero-Martin, J.M.; Eikenboom, J.; Bowman, M.; Sabatier, F.; Randi, A.M. Standardization of methods to quantify and culture endothelial colony-forming cells derived from peripheral blood: Position paper from the international society on thrombosis and haemostasis SSC. J. Thromb. Haemost. 2019, 17, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Nickenig, G. Endothelial progenitor cells in health and atherosclerotic disease. Ann. Med. 2007, 39, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Komuro, I. Vascular Cell Senescence: Contribution to Atherosclerosis. Circ. Res. 2007, 100, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef]
- Goligorsky, M.S.; Chen, J.; Patschan, S. Stress-induced premature senescence of endothelial cells: A perilous state between recovery and point of no return. Curr. Opin. Hematol. 2009, 16, 215–219. [Google Scholar] [CrossRef]
- Bürrig, K.F. The endothelium of advanced arteriosclerotic plaques in humans. Arter. Thromb 1991, 11, 1678–1689. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef]
- Honda, S.; Ikeda, K.; Urata, R.; Yamazaki, E.; Emoto, N.; Matoba, S. Cellular senescence promotes endothelial activation through epigenetic alteration, and consequently accelerates atherosclerosis. Sci. Rep. 2021, 11, 14608. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; Van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Childs, B.G.; Zhang, C.; Shuja, F.; Sturmlechner, I.; Trewartha, S.; Velasco, R.F.; Baker, D.J.; Li, H.; van Deursen, J.M. Senescent cells suppress innate smooth muscle cell repair functions in atherosclerosis. Nat. Aging 2021, 1, 698–714. [Google Scholar] [CrossRef]
- Toupance, S.; Simonici, S.; Labat, C.; Dumoulin, C.; Lai, T.; Lakomy, C.; Regnault, V.; Lacolley, P.; George, F.D.; Sabatier, F.; et al. Number and Replating Capacity of Endothelial Colony-Forming Cells are Telomere Length Dependent: Implication for Human Atherogenesis. J. Am. Heart Assoc. 2021, 10, e020606. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A.; Levy, D. Telomeres, Atherosclerosis, and the Hemothelium: The Longer View. Annu. Rev. Med. 2012, 63, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhang, J.; Ju, Z.; Rudolph, K.L. Telomere dysfunctional environment induces loss of quiescence and inherent impairments of hematopoietic stem cell function. Aging Cell 2012, 11, 449–455. [Google Scholar] [CrossRef]
- Fali, T.; Fabre-Mersseman, V.; Yamamoto, T.; Bayard, C.; Papagno, L.; Fastenackels, S.; Zoorab, R.; Koup, R.A.; Boddaert, J.; Sauce, D.; et al. Elderly human hematopoietic progenitor cells express cellular senescence markers and are more susceptible to pyroptosis. JCI Insight 2018, 3, 95319. [Google Scholar] [CrossRef] [PubMed]
- Edwards, N.; Langford-Smith, A.W.W.; Wilkinson, F.L.; Alexander, M.Y. Endothelial Progenitor Cells: New Targets for Therapeutics for Inflammatory Conditions with High Cardiovascular Risk. Front. Med. 2018, 5, 200. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Ehrhart, J.; Sanberg, P.R.; Borlongan, C.V. Potential Role of Humoral IL-6 Cytokine in Mediating Pro-Inflammatory Endothelial Cell Response in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2018, 19, 423. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Ye, J.; Sun, G.; Sun, X. Mechanism overview and target mining of atherosclerosis: Endothelial cell injury in atherosclerosis is regulated by glycolysis (Review). Int. J. Mol. Med. 2021, 47, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mancera, P.A.; Young, A.R.J.; Narita, M. Inside and out: The activities of senescence in cancer. Nat. Rev. Cancer 2014, 14, 547–558. [Google Scholar] [CrossRef]
- Simoncini, S.; Chateau, A.-L.; Robert, S.; Todorova, D.; Yzydorzick, C.; Lacroix, R.; Ligi, I.; Louis, L.; Bachelier, R.; Simeoni, U.; et al. Biogenesis of Pro-senescent Microparticles by Endothelial Colony Forming Cells from Premature Neonates is driven by SIRT1-Dependent Epigenetic Regulation of MKK6. Sci. Rep. 2017, 7, 8277. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Yoder, M.C. Circulating and tissue resident endothelial progenitor cells. J. Cell. Physiol. 2014, 229, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in Cardiomyocytes and Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef]
- Sandhu, K.; Mamas, M.; Butler, R. Endothelial progenitor cells: Exploring the pleiotropic effects of statins. World J. Cardiol. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Meyer, N.; Brodowski, L.; Richter, K.; Von Kaisenberg, C.S.; Schröder-Heurich, B.; Von Versen-Höynck, F. Pravastatin Promotes Endothelial Colony-Forming Cell Function, Angiogenic Signaling and Protein Expression in vitro. J. Clin. Med. 2021, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Toupance, S.; Gautier, S.; Labat, C.; Kimura, M.; Rossi, P.M.; Settembre, N.; Hubert, J.; Frimat, L.; Bertrand, B.; et al. Short Leukocyte Telomere Length Precedes Clinical Expression of Atherosclerosis: The Blood-and-Muscle Model. Circ. Res. 2018, 122, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.R.; Anderson, L.; Keeney, M.; Nayar, R.; Chin-Yee, I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996, 5, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.; Mauge, L.; Gaussem, P.; D’Audigier, C.; Israel-Biet, D.; Celermajer, D.; Bonnet, D.; Lévy, M. Treprostinil increases the number and angiogenic potential of endothelial progenitor cells in children with pulmonary hypertension. Angiogenesis 2011, 14, 17–27. [Google Scholar] [CrossRef]
- Ligi, I.; Simoncini, S.; Tellier, E.; Vassallo, P.F.; Sabatier, F.; Guillet, B.; Lamy, E.; Sarlon, G.; Quemener, C.; Bikfalvi, A.; et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood 2011, 118, 1699–1709. [Google Scholar] [CrossRef]
- Stea, F.; Bozec, E.; Millasseau, S.; Khettab, H.; Boutouyrie, P.; Laurent, S. Comparison of the Complior Analyse device with Sphygmocor and Complior SP for pulse wave velocity and central pressure assessment. J. Hypertens. 2014, 32, 873–880. [Google Scholar] [CrossRef]
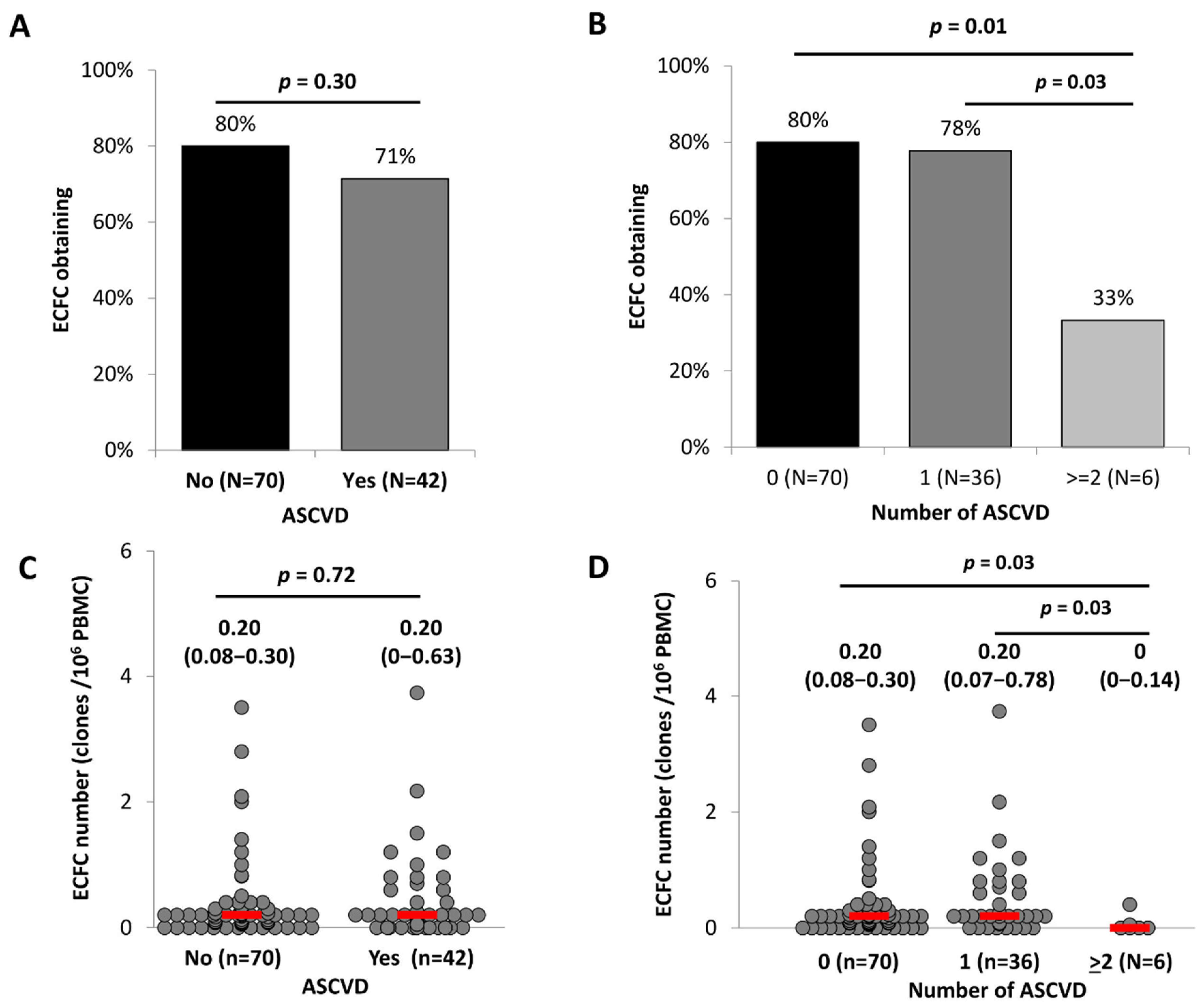
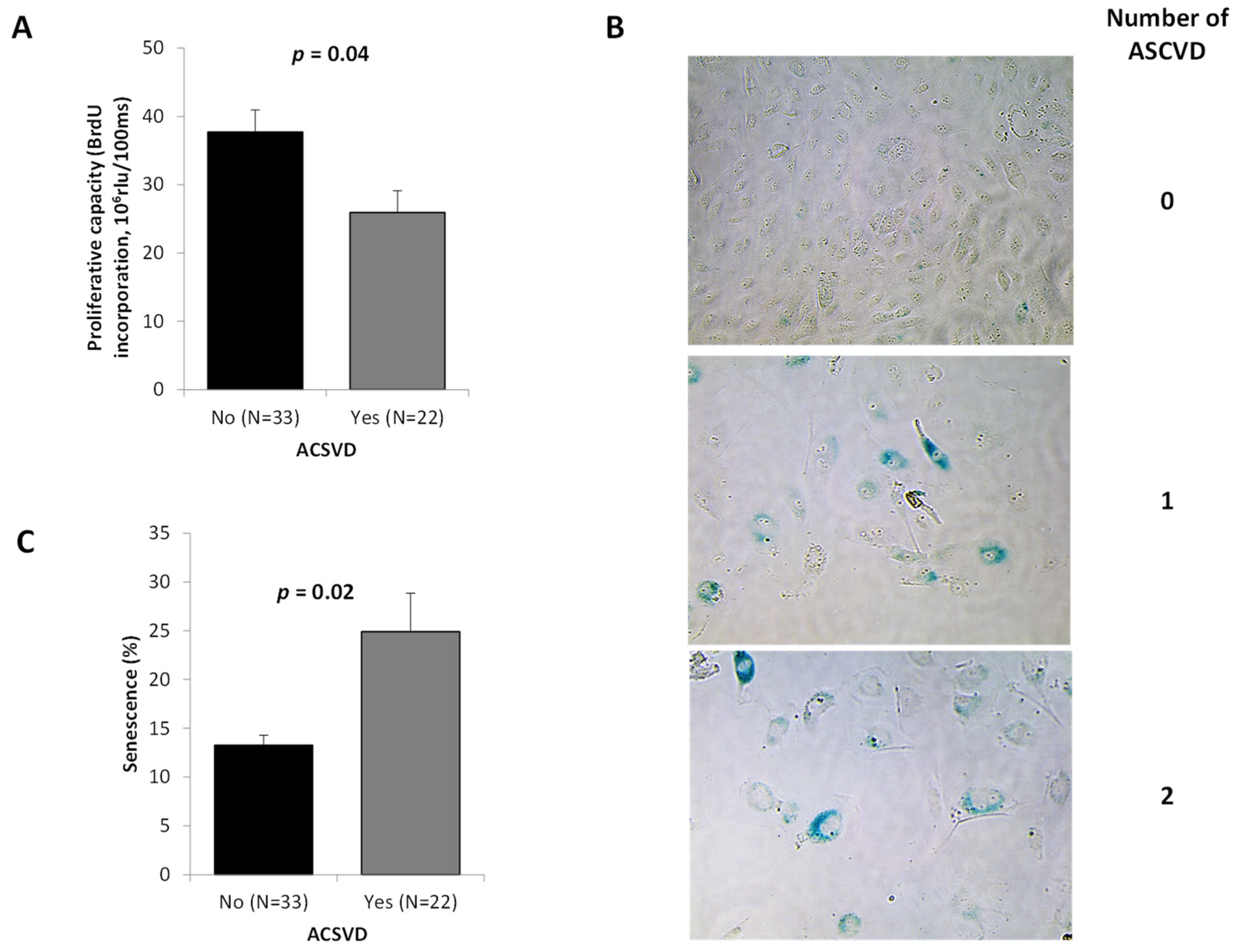



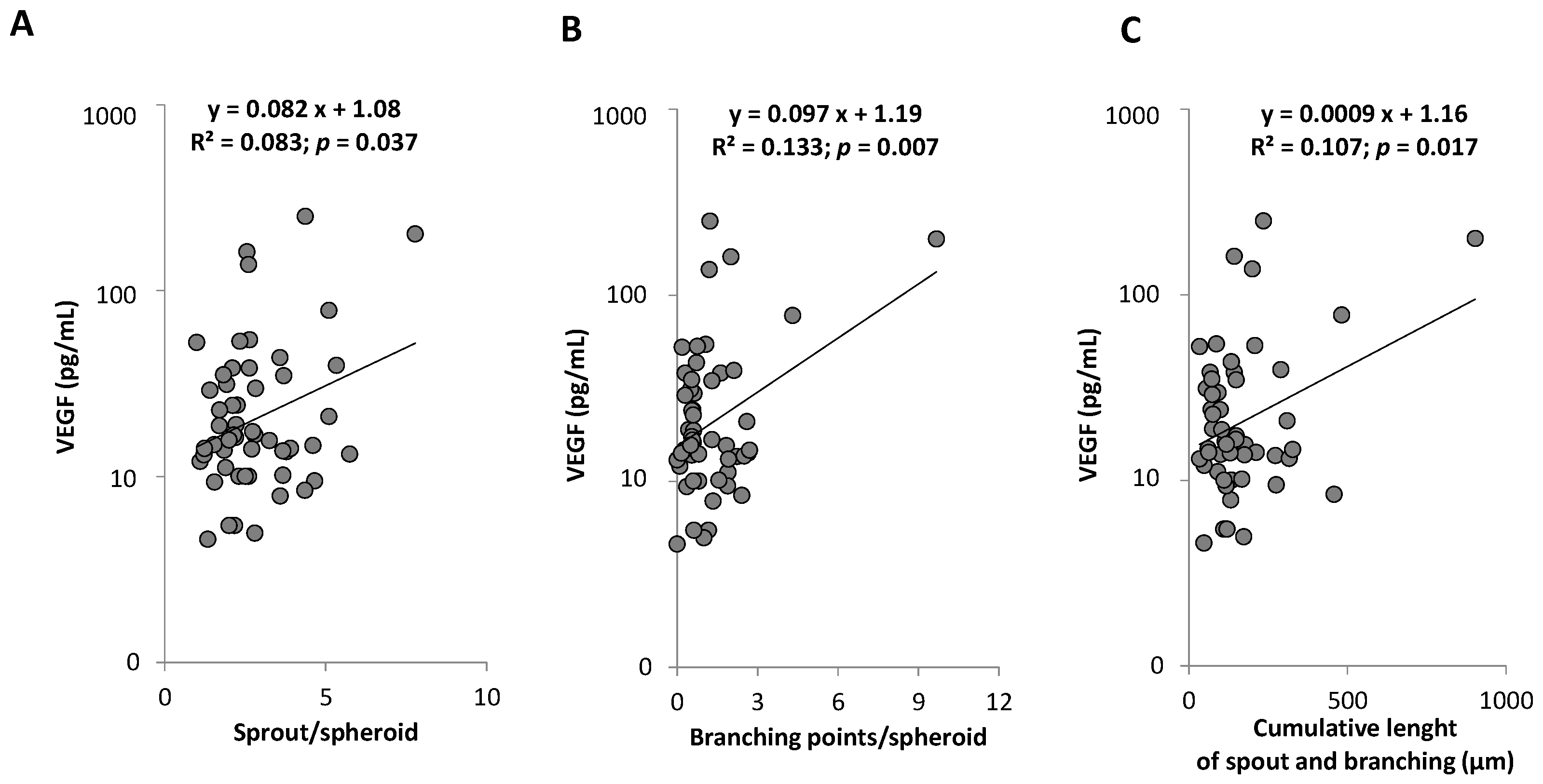
| All Participants | N | ASCVD | ||||
|---|---|---|---|---|---|---|
| No | N | Yes | N | |||
| Gender (women%) | 35% | 243 | 52% | 124 | 17% *** | 119 |
| Age (years) | 62 ± 14 | 243 | 57 ± 16 | 124 | 68 ± 10 *** | 119 |
| BMI (kg/m2) | 26.9 ± 6.1 | 240 | 27.0 ± 5.8 | 124 | 26.8 ± 6.4 | 116 |
| ASCVD sites | ||||||
| Coronary | 24% | 243 | - | 124 | 49% | 119 |
| Carotido-cerebral | 16% | 243 | - | 124 | 32% | 119 |
| Femoro-popliteal | 23% | 243 | - | 124 | 47% | 119 |
| Others | 2% | 243 | - | 124 | 3% | 119 |
| Number of ASCVD sites | 0.64 ± 0.74 | 243 | - | 124 | 1.31 ± 0.50 | 119 |
| ACSVD | 49% | 243 | - | 124 | 100% | 119 |
| ASCVD Risk factors | ||||||
| Hypertension | 45% | 242 | 34% | 123 | 57% *** | 119 |
| Diabetes | 18% | 242 | 10% | 123 | 27% *** | 119 |
| Dyslipidaemia | 23% | 242 | 12% | 123 | 34% *** | 119 |
| Smoking | 50% | 242 | 29% | 123 | 71% *** | 119 |
| Treatments | ||||||
| Anti-hypertensive drugs | 59% | 243 | 40% | 124 | 79% *** | 119 |
| Anti-diabetic drugs | 15% | 243 | 7% | 124 | 24% *** | 119 |
| Anti-coagulant drugs | 12% | 243 | 7% | 124 | 17% * | 119 |
| Statins | 41% | 243 | 15% | 124 | 68% *** | 119 |
| Number of CV and metabolic drugs | 2.9 ± 2.7 | 243 | 1.4 ± 1.9 | 124 | 4.4 ± 2.5 *** | 119 |
| Total number of drugs | 3.1 ± 2.8 | 243 | 1.6 ± 2.0 | 124 | 4.7 ± 2.6 *** | 119 |
| Blood pressure | ||||||
| SBP (mmHg) | 135 ± 18 | 179 | 133 ± 17 | 95 | 136 ± 20 | 84 |
| DBP (mmHg) | 76 ± 10 | 179 | 78 ± 10 | 95 | 74 ± 10 ** | 84 |
| MBP (mmHg) | 95 ± 11 | 179 | 96 ± 11 | 95 | 94 ± 11 | 84 |
| PP (mmHg) | 59 ± 16 | 179 | 55 ± 14 | 95 | 63 ± 17 ** | 84 |
| HR (bpm) | 73 ± 14 | 179 | 75 ± 13 | 95 | 72 ± 15 | 84 |
| Arterial phenotypes | ||||||
| PWV (m/s) | 12.30 ± 2.74 | 169 | 11.77 ± 2.50 | 89 | 12.88 ± 2.89 * | 80 |
| Carotid thickness (mm) | 0.79 ± 0.18 | 129 | 0.74 ± 0.18 | 71 | 0.85 ± 0.15 *** | 58 |
| Carotid plaque presence | 65% | 127 | 51% | 69 | 83% *** | 58 |
| Plasmatic markers | ||||||
| IL-6 (pg/mL) | 1.90 (0.95–4.78) | 237 | 1.16 (0.71–2.76) | 120 | 3.13 (1.36–6.48) *** | 117 |
| VEGF (pg/mL) | 15.7 (10.1–27.0) | 237 | 17.3 (12.26–30.89) | 120 | 14.06 (7.32–23.08) *** | 117 |
| (A) | All Participants | N | ASCVD | p | |||
| No | N | Yes | N | ||||
| CD34+ cells (/mL) | 1324 (802–2023) | 240 | 1338 (880–2032) | 122 | 1297 (722–2021) | 118 | 0.75 |
| CD45+CD34+KDR+ (/mL) | 8.09 (0–24.85) | 84 | 0 (0–20.56) | 33 | 13.68 (0–31.84) | 51 | 0.02 |
| CFU-EC (/million cells) | 0.49 (0–3.45) | 220 | 0.40 (0–3.27) | 112 | 0.51 (0–3.80) | 108 | 0.68 |
| (B) | Number of ASCVD Sites | Trend p | |||||
| 0 | N | 1 | N | >2 | N | ||
| CD34+ cells (/mL) | 1338 (880–2032) | 122 | 1256 (701–2025) | 83 | 1423 (1036–1846) | 35 | 0.93 |
| CD45+CD34+KDR+ (/mL) | 0 (0–20.56) | 33 | 11.56 (0–23.53) | 32 | 22.79 (5.55–37.70) | 19 | 0.004 |
| CFU-EC (/million cells) | 0.40 (0–3.27) | 112 | 0.20 (0–3.06) | 75 | 1.29 (0–9.99) | 33 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simoncini, S.; Toupance, S.; Labat, C.; Gautier, S.; Dumoulin, C.; Arnaud, L.; Stathopoulou, M.G.; Visvikis-Siest, S.; Rossi, P.M.; Benetos, A.; et al. Functional Impairment of Endothelial Colony Forming Cells (ECFC) in Patients with Severe Atherosclerotic Cardiovascular Disease (ASCVD). Int. J. Mol. Sci. 2022, 23, 8969. https://doi.org/10.3390/ijms23168969
Simoncini S, Toupance S, Labat C, Gautier S, Dumoulin C, Arnaud L, Stathopoulou MG, Visvikis-Siest S, Rossi PM, Benetos A, et al. Functional Impairment of Endothelial Colony Forming Cells (ECFC) in Patients with Severe Atherosclerotic Cardiovascular Disease (ASCVD). International Journal of Molecular Sciences. 2022; 23(16):8969. https://doi.org/10.3390/ijms23168969
Chicago/Turabian StyleSimoncini, Stéphanie, Simon Toupance, Carlos Labat, Sylvie Gautier, Chloé Dumoulin, Laurent Arnaud, Maria G. Stathopoulou, Sophie Visvikis-Siest, Pascal M. Rossi, Athanase Benetos, and et al. 2022. "Functional Impairment of Endothelial Colony Forming Cells (ECFC) in Patients with Severe Atherosclerotic Cardiovascular Disease (ASCVD)" International Journal of Molecular Sciences 23, no. 16: 8969. https://doi.org/10.3390/ijms23168969
APA StyleSimoncini, S., Toupance, S., Labat, C., Gautier, S., Dumoulin, C., Arnaud, L., Stathopoulou, M. G., Visvikis-Siest, S., Rossi, P. M., Benetos, A., Dignat-George, F., & Sabatier, F. (2022). Functional Impairment of Endothelial Colony Forming Cells (ECFC) in Patients with Severe Atherosclerotic Cardiovascular Disease (ASCVD). International Journal of Molecular Sciences, 23(16), 8969. https://doi.org/10.3390/ijms23168969







