Ethyl Vinyl Ketone Activates K+ Efflux to Regulate Stomatal Closure by MRP4-Dependent eATP Accumulation Working Upstream of H2O2 Burst in Arabidopsis
Abstract
:1. Introduction
2. Results
2.1. MRP4/5-Dependent eATP Buildup is Required for Evk-Induced Stomatal Closure
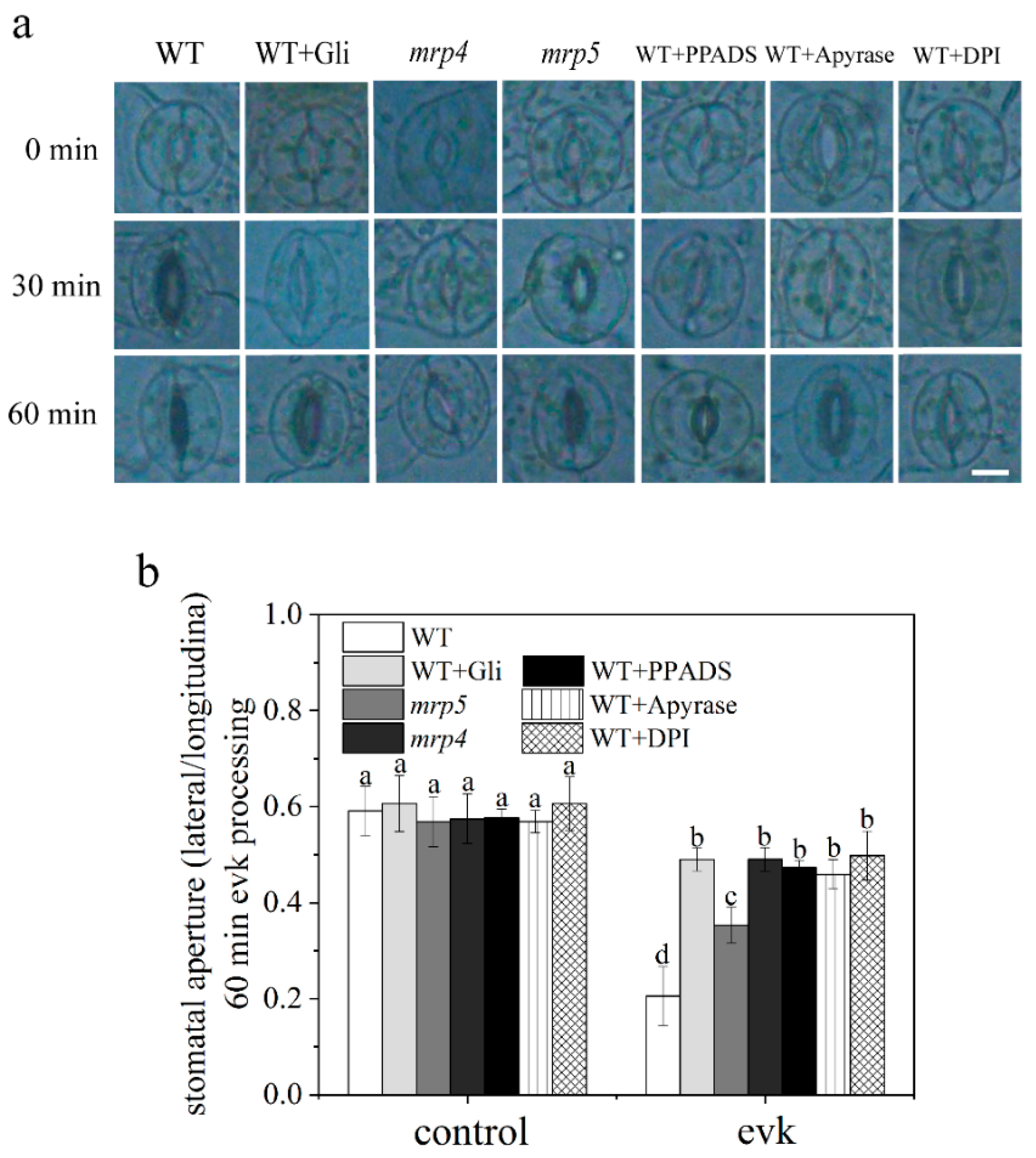
2.1.1. Evk-Induced Stomatal Closure Was Suppressed by Gli, PPADS, and Apyrase in Arabidopsis
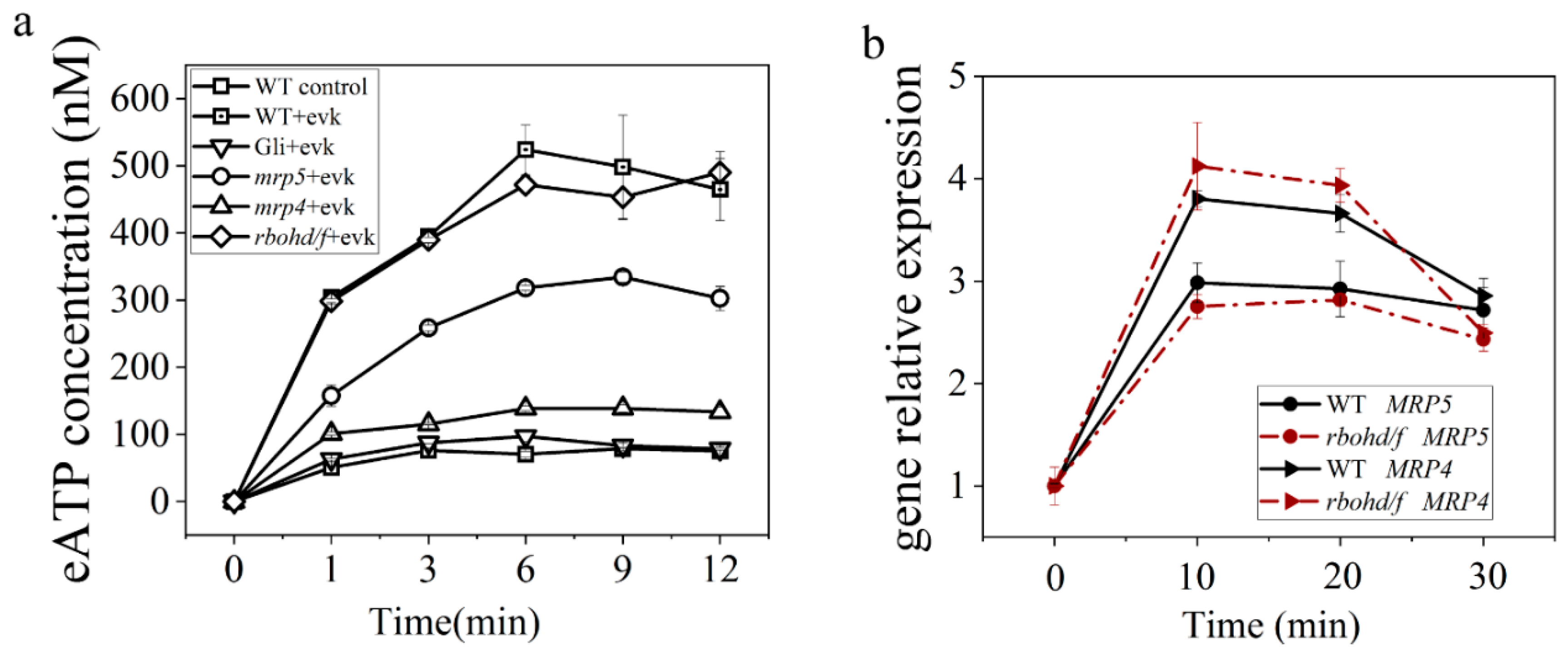
2.1.2. eATP Accumulation Was Induced by Evk and Reversed by Gli
2.1.3. Evk-Induced eATP Accumulation and Stomatal Closure Were Impaired in Mrp4, Mrp5 Mutants
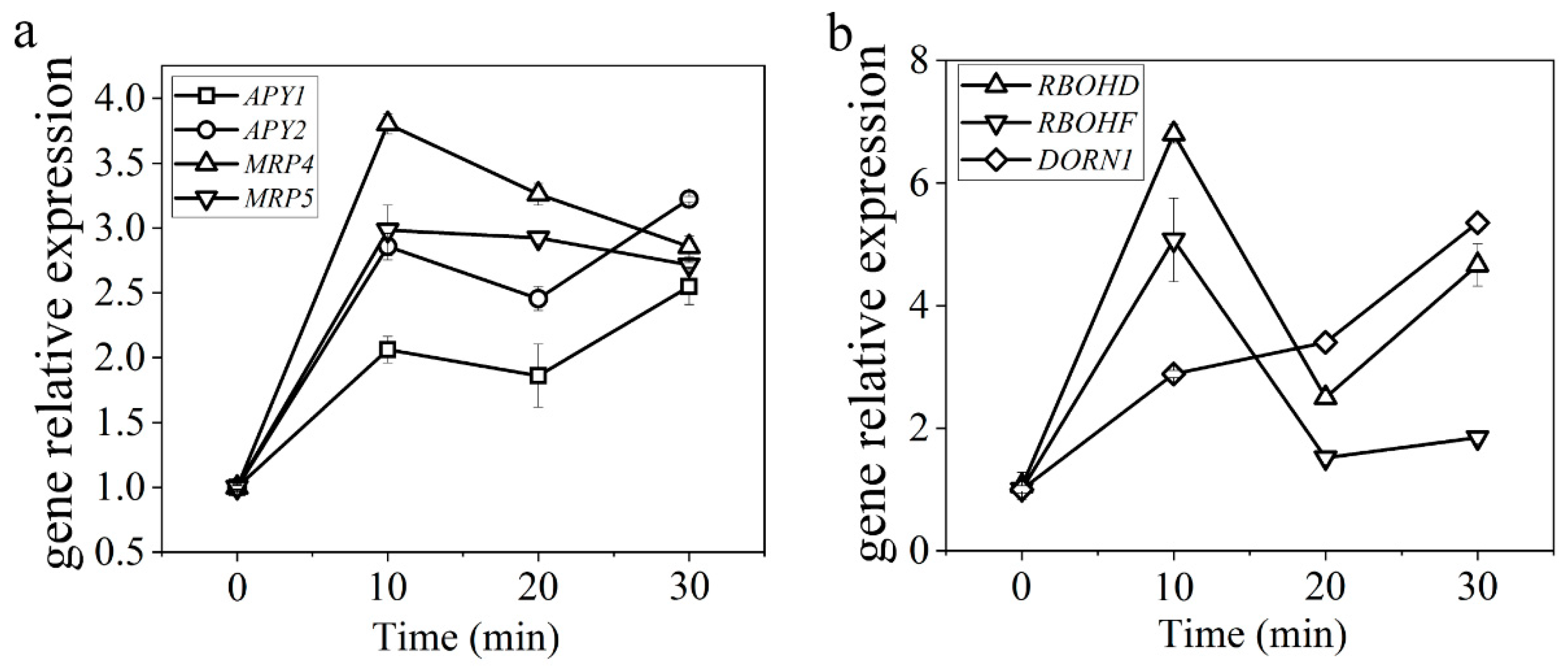
2.1.4. Evk Treatment Enhanced the Expression of ATP-Related Genes in Arabidopsis Leaves
2.2. eATP is Up Stream of H2O2 in the Process of Evk-Induced Stomatal Closure in Arabidopsis
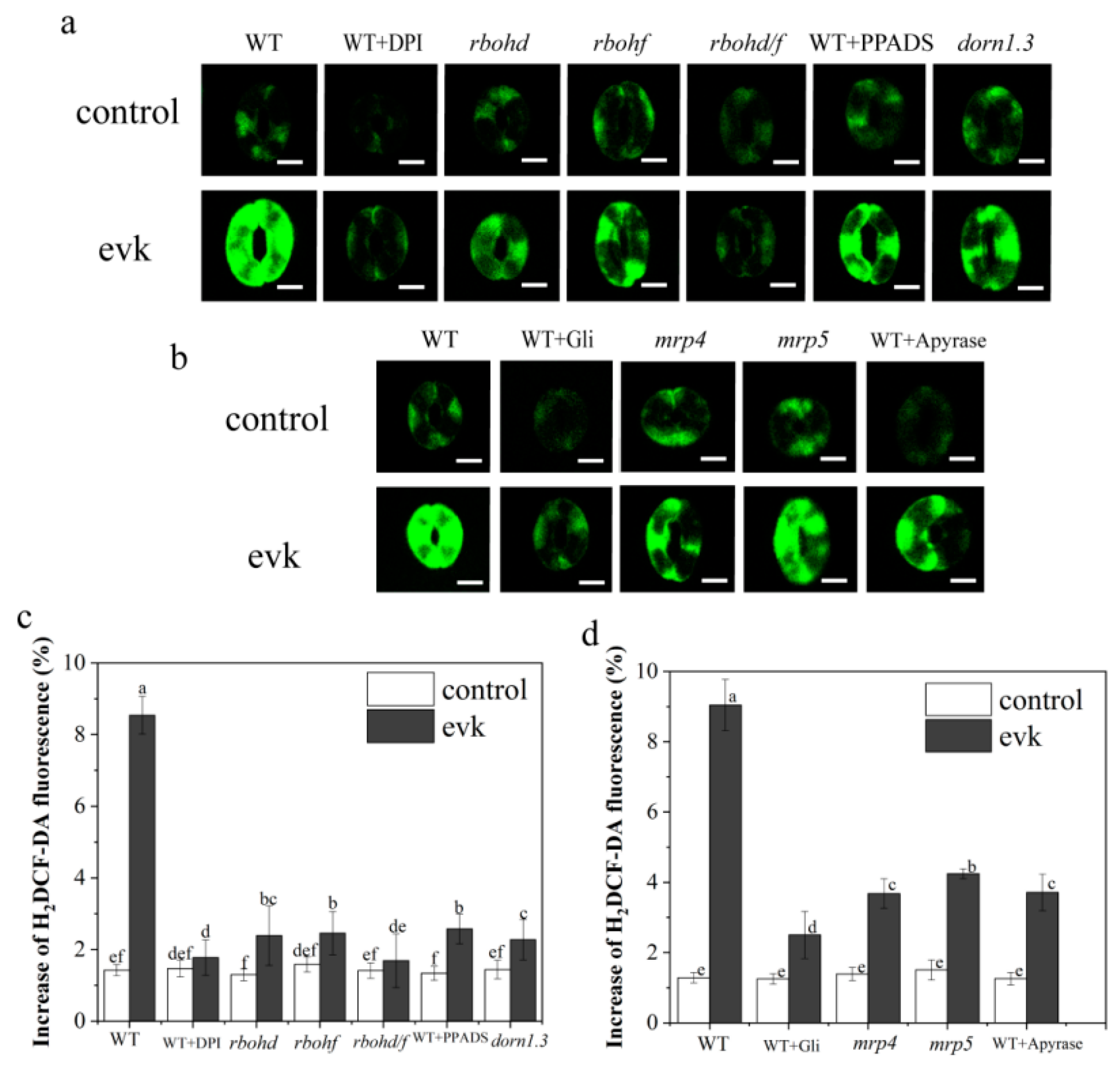
2.2.1. Evk-Induced H2O2 Burst is NADPH-Dependent
2.2.2. The Effects of Gli, PPADS, and Apyrase on Evk-Induced H2O2 Synthesis in Arabidopsis
2.2.3. In Mrp4, Mrp5 Mutants, Evk-Induced H2O2 Production Was Diminished
2.2.4. Mutations in RBOHD/F did not Affect Evk-Mediated eATP Accumulation and Evk-Mediated MRP Upregulation
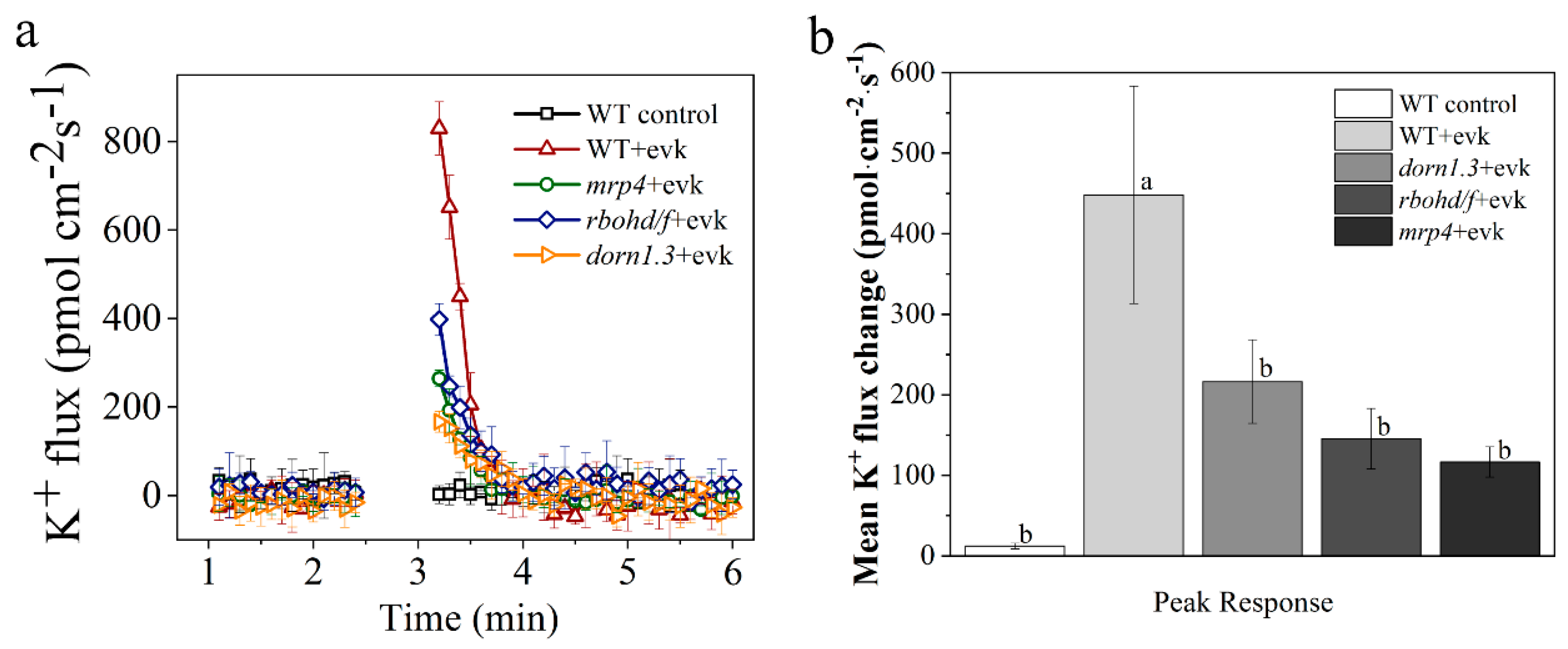
2.3. In Wild Arabidopsis Guard Cells, Evk Stimulated Outward K+ Currents, but This Activation Was Impaired in Mrp4 and Rbohd/f Mutants
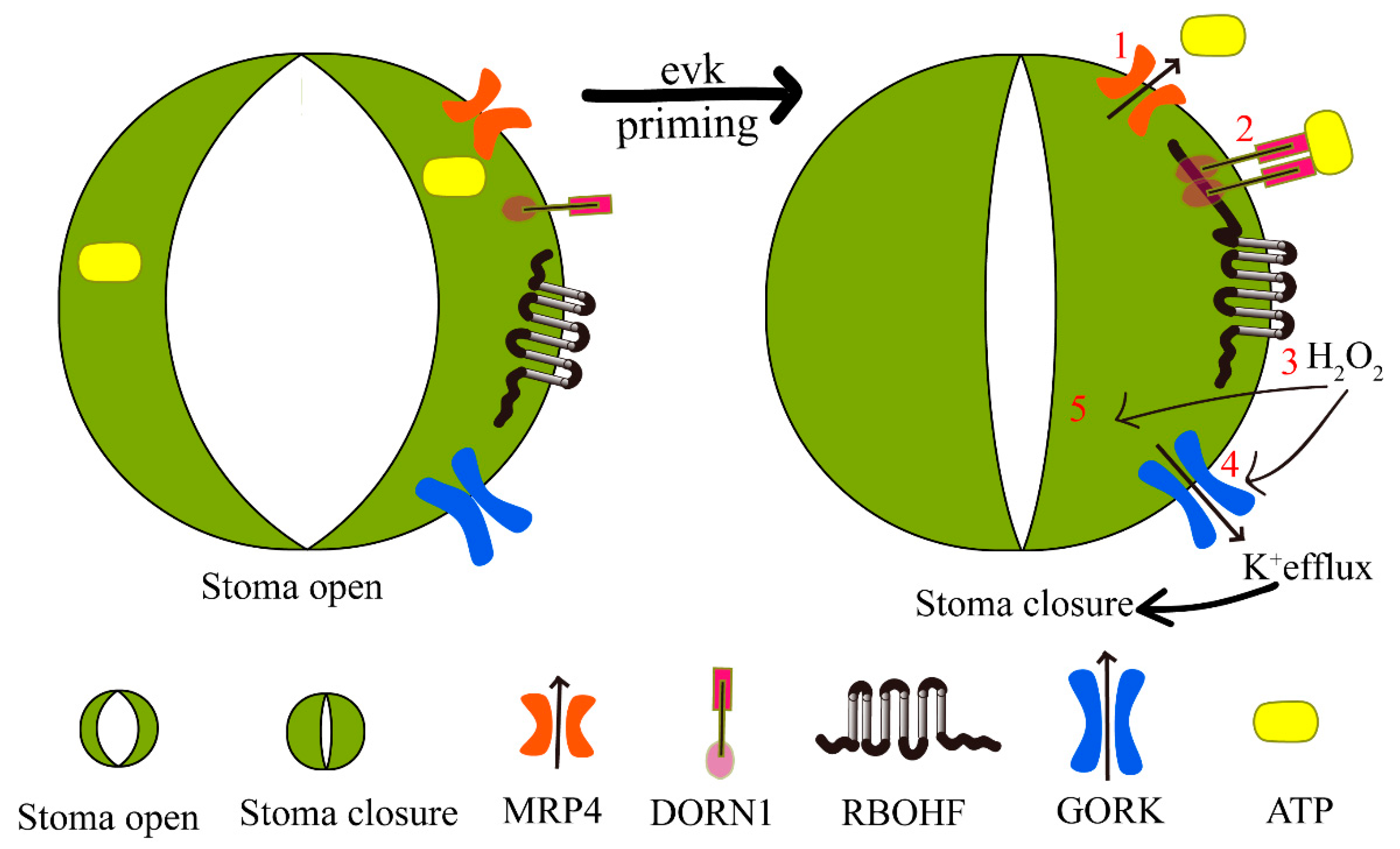
2.4. RBOHF Interacts with DORN1
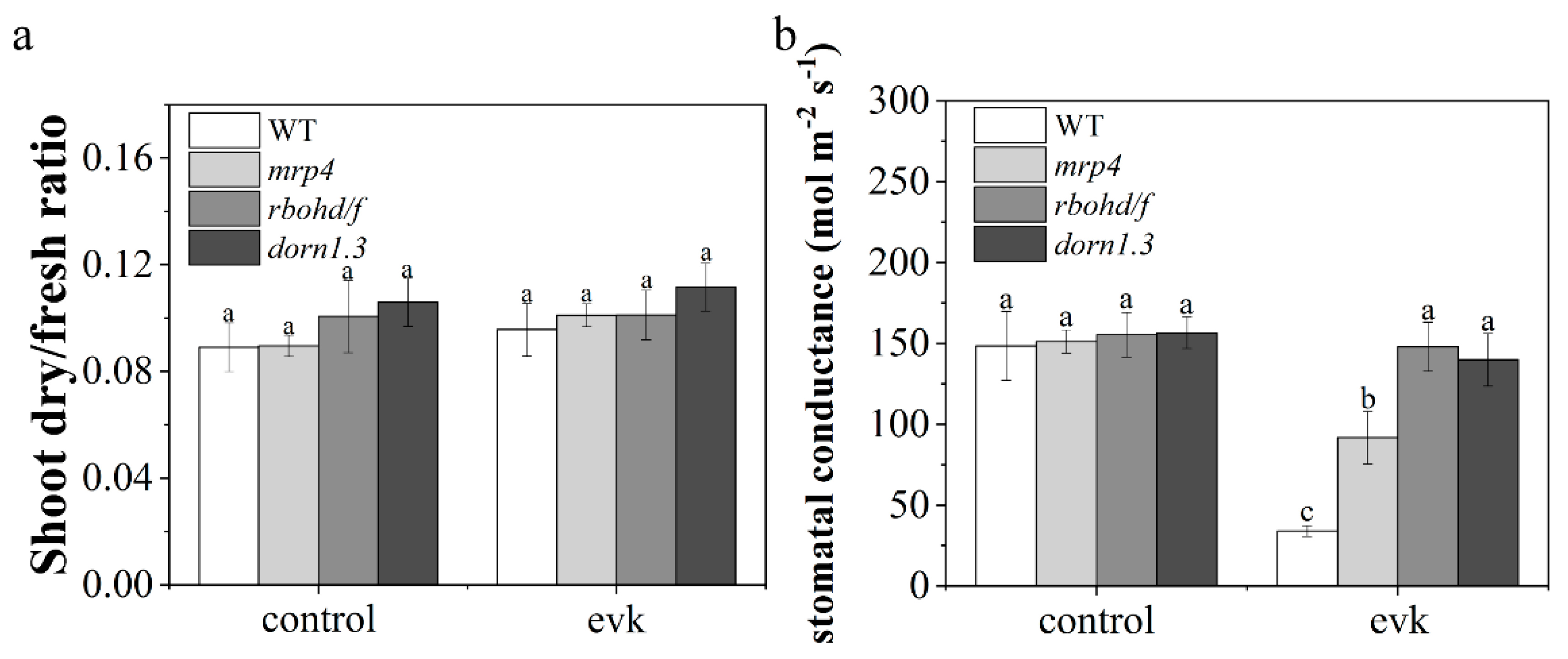
2.5. Evk Reduced Stomatal Conductance
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Measurement of Stomatal Aperture
4.3. Detection of eATP Content in Arabidopsis
4.4. qRT-PCR
4.5. H2O2 Detection in Guard Cells
4.6. Evk Fumigation
4.7. K+ Flux Measurements in Guard Cells
- J is the flux of K+ (pmol cm–2 s–1);
- D is the diffusion coefficient (1.96 × 10–5 cm2 s–1);
- ΔC is the difference between the concentrations near and far from the cells;
- ΔX is 10 µm. Each group contained 3–4 replicates.
4.8. Yeast Two-Hybrid (Y2H) Assay
4.9. Firefly Luciferase Complementation Imaging (LCI) Assay
4.10. In Vitro Pulldown Assay
4.11. Bimolecular Fluorescence Complementation Assay
4.12. Fresh/Dry Weight Measurements
4.13. Measurement of Stomatal Conductance
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| evk | Ethyl vinyl ketone |
| RES | Reactive electrophilic substances |
| WT | Wild type |
| NMT | Non-invasive micro-test technology |
| Gli | Glibenclamide |
| ABC transporters | ATP-binding cassette transporters |
| VOCs | Volatiles |
| HP | (Z)-3-hexylpropionate |
| HB | (Z)-3-hexylbutyrate |
| MDA | Malondialdehyde |
| JA | Jasmonic acid |
| SA | Salicylic acid |
| eATP | Extracellular ATP |
| DAMP | Damage-associated molecular pattern |
| MRPs | Multidrug resistance-associated proteins |
| PAMPs | Pathogen-associated molecular patterns |
| ROS | Reactive oxygen species |
| AS | Acetosyringone |
References
- Gudesblat, G.E.; Torres, P.S.; Vojnov, A.A. Xanthomonas campestris Overcomes Arabidopsis Stomatal Innate Immunity through a DSF Cell-to-Cell Signal-Regulated Virulence Factor. Plant Physiol. 2009, 149, 1017–1027. [Google Scholar] [CrossRef]
- Lin, P.A.; Chen, Y.; Ponce, G.; Acevedo, F.E.; Lynch, J.P.; Anderson, C.T.; Ali, J.G.; Felton, G.W. Stomata-mediated interactions between plants, herbivores, and the environment. Trends Plant Sci. 2021, 27, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E. Surface-to-air signals. Nature 2001, 411, 854–856. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Payá, C.; Ozáez, M.; Rodrigo, I.; Conejero, V.; Klee, H.; Bellés, J.M.; Lisón, P. A new role for green leaf volatile esters in tomato stomatal defense against Pseudomonas syringe pv. tomato. Front. Plant Sci. 2018, 9, 1855. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Davoine, C. Reactive electrophile species. Curr. Opin. Plant Biol. 2007, 10, 380–386. [Google Scholar] [CrossRef]
- Matsui, K.; Koeduka, T. Green Leaf Volatiles in Plant Signaling and Response. Sub-Cell. Biochem. 2016, 86, 427–443. [Google Scholar]
- Paré, P.W.; Tumlinson, J.H. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997, 114, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Heiden, A.C.; Kobel, K.; Langebartels, C.; Schuh-Thomas, G.; Wildt, J. Emissions of oxygenated volatile organic compounds from plants part I: Emissions from lipoxygenase activity. J. Atmos. Chem. 2003, 45, 143–172. [Google Scholar] [CrossRef]
- Shiojiri, K.; Ozawa, R.; Matsui, K.; Kishimoto, K.; Kugimiya, S.; Takabayashi, J. Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J. Chem. Ecol. 2006, 32, 969–979. [Google Scholar] [CrossRef]
- Schmid-Siegert, E.; Loscos, J.; Farmer, E.E. Inducible malondialdehyde pools in zones of cell proliferation and developing tissues in Arabidopsis. J. Biol. Chem. 2012, 287, 8954–8962. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Hilbert, B.; Dueckershoff, K.; Roitsch, T.; Krischke, M.; Mueller, M.J.; Berger, S. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 2008, 20, 768–785. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Weber, H.; Reymond, P.; Browse, J.; Farmer, E.E. Plant defense in the absence of jasmonic acid: The role of cyclopentenones. Proc. Natl. Acad. Sci. USA 2001, 98, 12837–12842. [Google Scholar] [CrossRef] [PubMed]
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005, 139, 1268–1283. [Google Scholar] [CrossRef] [PubMed]
- Mattick, L.R.; Hand, D.B. Identification of a volatile component in soybeans that contributes to the raw bean flavor. J. Agric. Food Chem. 1969, 17, 15–17. [Google Scholar] [CrossRef]
- Salch, Y.P.; Grove, M.J.; Takamura, H.; Gardner, H.W. Characterization of a C-5,13-Cleaving Enzyme of 13(S)-Hydroperoxide of Linolenic Acid by Soybean Seed. Plant Physiol. 1995, 108, 1211–1218. [Google Scholar] [CrossRef]
- Alméras, E.; Stolz, S.; Vollenweider, S.; Reymond, P.; Mène-Saffrané, L.; Farmer, E.E. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. Cell Mol. Biol. 2003, 34, 205–216. [Google Scholar] [CrossRef]
- Van Poecke, R.M.P.; Posthumus, M.A.; Dicke, M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 2001, 27, 1911–1928. [Google Scholar] [CrossRef]
- Fishera, A.J.; Grimesb, H.D.; Fall, R. The biochemical origin of pentenol emissions from wounded leaves. Phytochemistry 2003, 62, 159–163. [Google Scholar] [CrossRef]
- Meucci, A.; Shiriaev, A.; Rosellini, I.; Malorgio, F.; Pezzarossa, B. Se-Enrichment Pattern, Composition, and Aroma Profile of Ripe Tomatoes after Sodium Selenate Foliar Spraying Performed at Different Plant Developmental Stages. Plants 2021, 10, 1050. [Google Scholar] [CrossRef]
- Chen, D.; Cao, Y.; Li, H.; Kim, D.; Ahsan, N.; Thelen, J.; Stacey, G. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat. Commun. 2017, 8, 2265. [Google Scholar] [CrossRef]
- Chivasa, S.; Ndimba, B.K.; Simon, W.J.; Lindsey, K.; Slabas, A.R. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell 2005, 17, 3019–3034. [Google Scholar] [CrossRef]
- Thomas, C.; Rajagopal, A.; Windsor, B.; Dudler, R.; Lloyd, A.; Roux, S.J. A role for ectophosphatase in xenobiotic resistance. Plant Cell 2000, 12, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Sun, J.; Zhao, R.; Ding, M.; Zhang, Y.; Sun, Y.; Wang, W.; Tan, Y.; Liu, D.; Ma, X.; et al. Populus euphratica APYRASE2 Enhances Cold Tolerance by Modulating Vesicular Trafficking and Extracellular ATP in Arabidopsis Plants. Plant Physiol. 2015, 169, 530–548. [Google Scholar] [CrossRef] [PubMed]
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Jeter, C.R.; Tang, W.; Henaff, E.; Butterfield, T.; Roux, S.J. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 2004, 16, 2652–2664. [Google Scholar] [CrossRef]
- Clark, G.; Wu, M.; Wat, N.; Onyirimba, J.; Pham, T.; Herz, N.; Ogoti, J.; Gomez, D.; Canales, A.A.; Aranda, G.; et al. Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygen species. Plant Mol. Biol. 2010, 74, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Sivaguru, M.; Stacey, G. Extracellular ATP in plants. Visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol. 2006, 142, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Perfus-Barbeoch, L.; Frelet, A.; Gaedeke, N.; Reinhardt, D.; Mueller-Roeber, B.; Martinoia, E.; Forestier, C. The plant multidrug resistance ABC transporter AtMRP5 is involved in guard cell hormonal signalling and water use. Plant J. Cell Mol. Biol. 2003, 33, 119–129. [Google Scholar] [CrossRef]
- Gaedeke, N.; Klein, M.; Kolukisaoglu, U.; Forestier, C.; Müller, A.; Ansorge, M.; Becker, D.; Mamnun, Y.; Kuchler, K.; Schulz, B.; et al. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 2001, 20, 1875–1887. [Google Scholar] [CrossRef]
- Bodin, P.; Burnstock, G. Purinergic signalling: ATP release. Neurochem. Res. 2001, 26, 959–969. [Google Scholar] [CrossRef]
- Lin, P.A.; Chen, Y.; Chaverra-Rodriguez, D.; Heu, C.C.; Zainuddin, N.B.; Sidhu, J.S.; Peiffer, M.; Tan, C.W.; Helms, A.; Kim, D.; et al. Silencing the alarm: An insect salivary enzyme closes plant stomata and inhibits volatile release. New Phytol. 2021, 230, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Musser, R.O.; Kwon, H.S.; Williams, S.A.; White, C.J.; Romano, M.A.; Holt, S.M.; Bradbury, S.; Brown, J.K.; Felton, G.W. Evidence that caterpillar labial saliva suppresses infectivity of potential bacterial pathogens. Arch. Insect Biochem. Physiol. 2005, 58, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.; Last, K.; Harrett-Williams, R.; Tagliavia, C.; Harter, K.; Hooley, R.; Hancock, J.T.; Neill, S.J. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. Cell Mol. Biol. 2006, 47, 907–916. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radic. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Cao, Y.; Tanaka, K.; Nguyen, C.T.; Stacey, G. Extracellular ATP is a central signaling molecule in plant stress responses. Curr. Opin. Plant Biol. 2014, 20, 82–87. [Google Scholar] [CrossRef]
- Ferrari, D.; McNamee, E.N.; Idzko, M.; Gambari, R.; Eltzschig, H.K. Purinergic signaling during immune cell trafficking. Trends Immunol. 2016, 37, 399–411. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Hosy, E.; Vavasseur, A.; Mouline, K.; Dreyer, I.; Gaymard, F.; Porée, F.; Boucherez, J.; Lebaudy, A.; Bouchez, D.; Véry, A.A.; et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 2003, 100, 5549–5554. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Breitling, R.; Amtmann, A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Newman, I.; Zhou, M.; Mendham, N.; Zhang, G.; Shabala, S. Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant Cell Environ. 2005, 28, 1230–1246. [Google Scholar] [CrossRef]
- Meharg, A. Marschner’s Mineral Nutrition of Higher Plants. Exp. Agric. 2012, 48, 305. [Google Scholar] [CrossRef]
- Blatt, M.R. Cellular signalling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 221–241. [Google Scholar] [CrossRef]
- Hills, A.; Chen, Z.H.; Amtmann, A.; Blatt, M.R.; Lew, V.L. OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol. 2012, 159, 1026–1042. [Google Scholar] [CrossRef]
- Humble, G.D.; Raschke, K. Stomatal opening quantitatively related to potassium transport: Evidence from electron probe analysis. Plant Physiol. 1971, 48, 447–453. [Google Scholar] [CrossRef]
- Assmann, S.M. Signal Transduction in Guard Cells. Annu. Rev. Cell Biol. 1993, 9, 345–375. [Google Scholar] [CrossRef]
- Zhao, X.; Qiao, X.R.; Yuan, J.; Ma, X.F.; Zhang, X. Nitric oxide inhibits blue light-induced stomatal opening by regulating the K+ influx in guard cells. Plant Sci. 2012, 184, 29–35. [Google Scholar] [CrossRef]
- Kim, S.H.; Yang, S.H.; Kim, T.J.; Han, J.S.; Sum, J.W. Hypertonic Stress Increased Extracellular ATP Levels and the Expression of Stress-Responsive Genes in Arabidopsis thaliana Seedlings. J. Agric. Chem. Soc. Jpn. 2009, 73, 1252–1256. [Google Scholar]
- Wortelboer, H.M.; Usta, M.; van der Velde, A.E.; Boersma, M.G.; Spenkelink, B.; van Zanden, J.J.; Rietjens, I.M.C.M.; van Bladeren, P.J.; Cnubben, N.H.P. Interplay between MRP inhibition and metabolism of MRP inhibitors: The case of curcumin. Chem. Res. Toxicol. 2003, 16, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, X.; Che, Y.; Hou, L.; Liu, X.; Zhang, W. Extracellular ATP mediates H2S-regulated stomatal movements and guard cell K+ current in a H2O2-dependent manner in Arabidopsis. Sci. Bull. 2015, 60, 419–427. [Google Scholar] [CrossRef]
- Obsilova, V.; Kopecka, M.; Kosek, D.; Kacirova, M.; Kylarova, S.; Rezabkova, L.; Obsil, T. Mechanisms of the 14-3-3 protein function: Regulation of protein function through conformational modulation. Physiol. Res. 2014, 63 (Suppl. 1), S155–S164. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Jing, W.; Xu, N.; Shen, L.; Zhang, Q.; Zhang, W. Arabidopsis thaliana constitutively active ROP11 interacts with the NADPH oxidase respiratory burst oxidase homologue F to regulate reactive oxygen species production in root hairs. Funct. Plant Biol. FPB 2016, 43, 221–231. [Google Scholar] [CrossRef]
- Yun, B.W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; Bihan, T.L.; Yu, M.; Moore1, J.W.; Kang, J.G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Gong, B.; Yan, Y.; Wen, D.; Shi, Q. Hydrogen peroxide produced by NADPH oxidase: A novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum. Physiol. Plant 2017, 160, 396–409. [Google Scholar] [CrossRef]
- Drerupa, M.M.; Schlückinga, K.; Hashimotoa, K.; Manishankara, P.; Steinhorsta, L.; Kuchitsub, K.; Kudla, J. The Calcineurin B-Like Calcium Sensors CBL1 and CBL9 Together with Their Interacting Protein Kinase CIPK26 Regulate the Arabidopsis NADPH Oxidase RBOHF. Mol. Plant 2013, 6, 559–569. [Google Scholar] [CrossRef]
- Han, J.P.; Köster, P.; Drerup, M.M.; Scholz, M.; Li, S.; Edel, K.H.; Hashimoto, K.; Kuchitsu, K.; Hippler, M.; Kudla, J. Fine-tuning of RBOHF activity is achieved by differential phosphorylation and Ca2+ binding. New Phytol. 2019, 221, 1935–1949. [Google Scholar] [CrossRef]
- Kawarazaki, T.; Kimura, S.; Iizuka, A.; Hanamata, S.; Nibori, H.; Michikawa, M.; Imai, A.; Abe, M.; Kaya, H.; Kuchitsu, K. A low temperature-inducible protein AtSRC2 enhances the ROS-producing activity of NADPH oxidase AtRbohF. BBA Mol. Cell Res. 2013, 1833, 2775–2780. [Google Scholar] [CrossRef]
- Sirichandra, C.; Gu, D.; Hu, H.C.; Davanture, M.; Lee, S.; Djaoui, M.; Valot, B.; Zivy, M.; Leung, J.; Merlot, S.; et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009, 583, 2982–2986. [Google Scholar] [CrossRef]
- Förster, S.; Schmidt, L.K.; Kopic, E.; Anschütz, U.; Huang, S.; Schlücking, K.; Köster, P.; Waadt, R.; Larrieu, A.; Batistič, O.; et al. Wounding-Induced Stomatal Closure Requires Jasmonate-Mediated Activation of GORK K+ Channels by a Ca2+ Sensor-Kinase CBL1-CIPK5 Complex. Dev. Cell 2018, 48, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Adem, G.D.; Chen, G.; Shabala, L.; Chen, Z.H.; Shabala, S. GORK Channel: A Master Switch of Plant Metabolism? Trends Plant Sci. 2020, 25, 434–445. [Google Scholar] [CrossRef]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Wang, Y.; Wang, J.W.; Babla, M.; Zhao, C.; García-Mata, C.; Sani, E.; Differ, C.; Mak, M.; Hills, A.; et al. Nitrate reductase mutation alters potassium nutrition as well as nitric oxide-mediated control of guard cell ion channels in Arabidopsis. New Phytol. 2016, 209, 1456–1469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Barrett, J.P.; Alvarez-Croda, D.M.; Stoica, B.A.; Faden, A.I.; Loane, D.J. NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav. Immun. 2016, 291–309. [Google Scholar] [CrossRef]
- Nogués, S.; Allen, D.; Baker, R. Ultraviolet-B Radiation Effects on Water Relations, Leaf Development, and Photosynthesis in Droughted Pea Plants. Plant Physiol. 1998, 117, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Melillo, M.T.; Leonetti, P.; Bongiovanni, M.; Castagnone-Sereno, P.; Bleve-Zacheo, T. Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato–root-knot nematode interactions. New Phytol. 2006, 170, 501–511. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Wang, J.; Cao, Q.; Wen, Z. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 2014, 251, 899–912. [Google Scholar] [CrossRef]
- Chun, Y.J.; Gong, J.Q.; Guo, Z.J.; Li, S.W.; Zuo, Y.X.; Shen, Y.B. Linalool Activates Oxidative and Calcium Burst and CAM3-ACA8 Participates in Calcium Recovery in Arabidopsis Leaves. Int. J. Mol. Sci. 2022, 23, 5357. [Google Scholar]
- Brown, R.L.; Kazan, K.; McGrath, K.C.; Maclean, D.J.; Manners, J.M. A role for the GCC-Box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol. 2003, 132, 1020–1032. [Google Scholar] [CrossRef]
- Yan, S.; Jiao, C.; McLamore, E.S.; Wang, N.; Yao, H.; Shen, Y. Insect Herbivory of Leaves Affects the Auxin Flux along Root Apices in Arabidopsis thaliana. Plant Growth Regul. 2017, 36, 846–854. [Google Scholar] [CrossRef]
- Yan, S.; Luo, S.; Dong, S.; Zhang, T.; Sun, J.; Wang, N.; Yao, H.; Shen, Y. Heterotrimeric G-proteins involved in the MeJA regulated ion flux and stomatal closure in Arabidopsis thaliana. Funct. Plant Biol. FPB 2015, 42, 126–135. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| DORN1 | AACCACTCACCTTACGCTTGG | AGTCGCCGTTTTTCCCTCT |
| APY1 | ACACGATGAAAAACCACGAGG | AAGAGTTTGCTGATTGCCGAG) |
| APY2 | GGATAACCATCAACGCACTAAAAG | GGGACGAACTGTAGCAACAGG |
| MRP4 | CAAATCTCCACTGACGCTCG | CCAACGTACATGACGCCGA |
| MRP5 | GCCGCAGTTACATTCGCTAC | CCAGATCAGGAAAGTTCCGAAG |
| RBOHF | TTCGCATCATTTGTTCGTCA | TGTAGCGTTAGAACATTACCAGGA |
| RBOHD | ATCAAGGTGGCTGTTTACCC | GGGAGCTGATGTGATTGAGA |
| ACTIN2 | AGTGGTCGTACAACCGGTATTGT | GATGGCATGAGGAAGAGAGAAAC |
| EF1α | TCCAGCTAAGGGTGCC | GGTGGGTACTCGGAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Yao, L.; Jiao, C.; Guo, Z.; Li, S.; Zuo, Y.; Shen, Y. Ethyl Vinyl Ketone Activates K+ Efflux to Regulate Stomatal Closure by MRP4-Dependent eATP Accumulation Working Upstream of H2O2 Burst in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 9002. https://doi.org/10.3390/ijms23169002
Gong J, Yao L, Jiao C, Guo Z, Li S, Zuo Y, Shen Y. Ethyl Vinyl Ketone Activates K+ Efflux to Regulate Stomatal Closure by MRP4-Dependent eATP Accumulation Working Upstream of H2O2 Burst in Arabidopsis. International Journal of Molecular Sciences. 2022; 23(16):9002. https://doi.org/10.3390/ijms23169002
Chicago/Turabian StyleGong, Junqing, Lijuan Yao, Chunyang Jiao, Zhujuan Guo, Shuwen Li, Yixin Zuo, and Yingbai Shen. 2022. "Ethyl Vinyl Ketone Activates K+ Efflux to Regulate Stomatal Closure by MRP4-Dependent eATP Accumulation Working Upstream of H2O2 Burst in Arabidopsis" International Journal of Molecular Sciences 23, no. 16: 9002. https://doi.org/10.3390/ijms23169002
APA StyleGong, J., Yao, L., Jiao, C., Guo, Z., Li, S., Zuo, Y., & Shen, Y. (2022). Ethyl Vinyl Ketone Activates K+ Efflux to Regulate Stomatal Closure by MRP4-Dependent eATP Accumulation Working Upstream of H2O2 Burst in Arabidopsis. International Journal of Molecular Sciences, 23(16), 9002. https://doi.org/10.3390/ijms23169002






