Effects of Human Activity on Markers of Oxidative Stress in the Intestine of Holothuria tubulosa, with Special Reference to the Presence of Microplastics
Abstract
:1. Introduction
2. Results
2.1. Biometric Measurements
2.2. Microplastic Abundance in H. tubulosa
2.3. Microplastic Abundance in the Sediments of the Collection Site
2.4. MPs Characterization
2.5. Mesoplastic Characterization
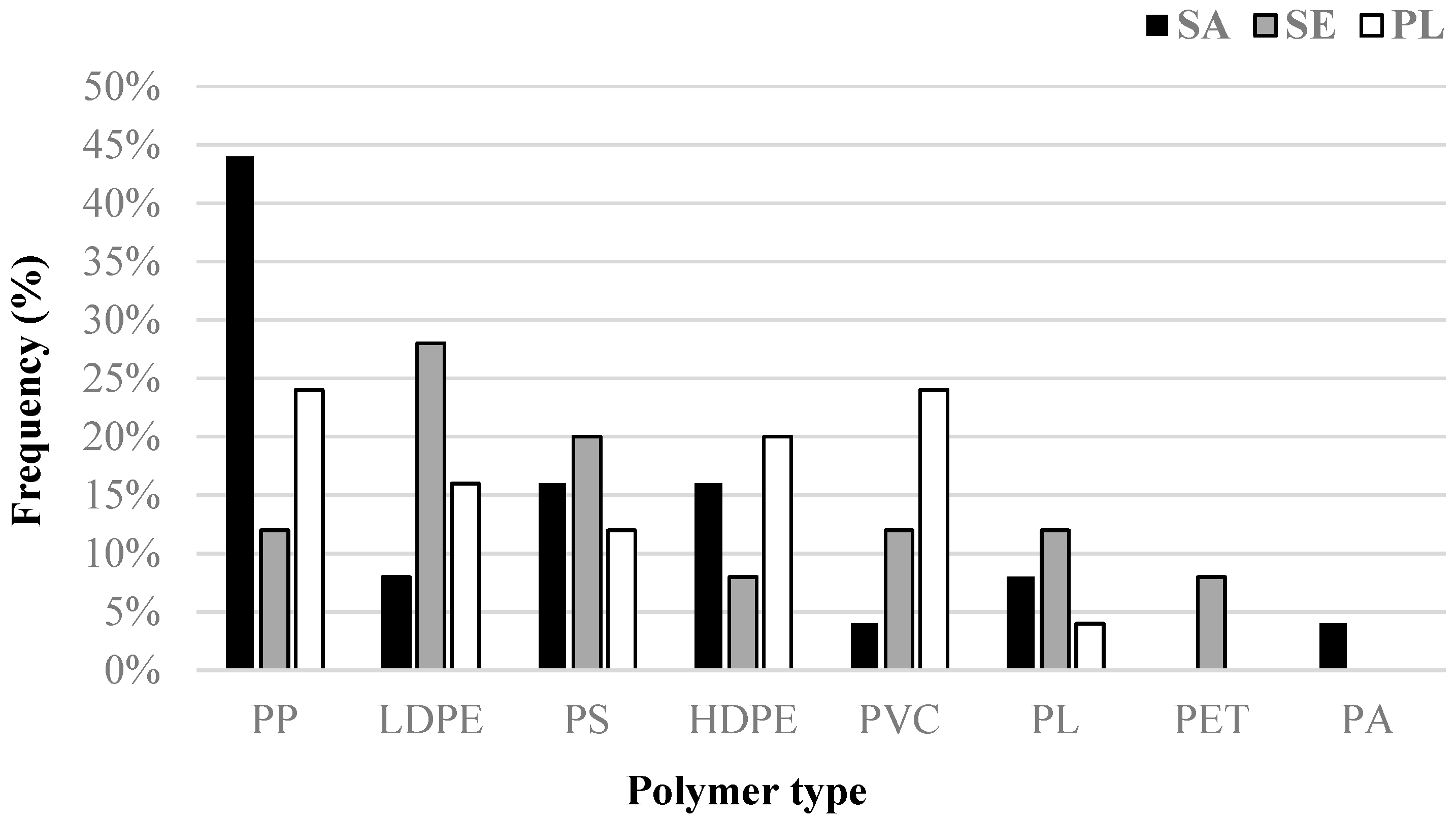
2.6. Polymer Characterization
2.7. Oxidative Stress Biomarkers
3. Discussion
4. Materials and Methods
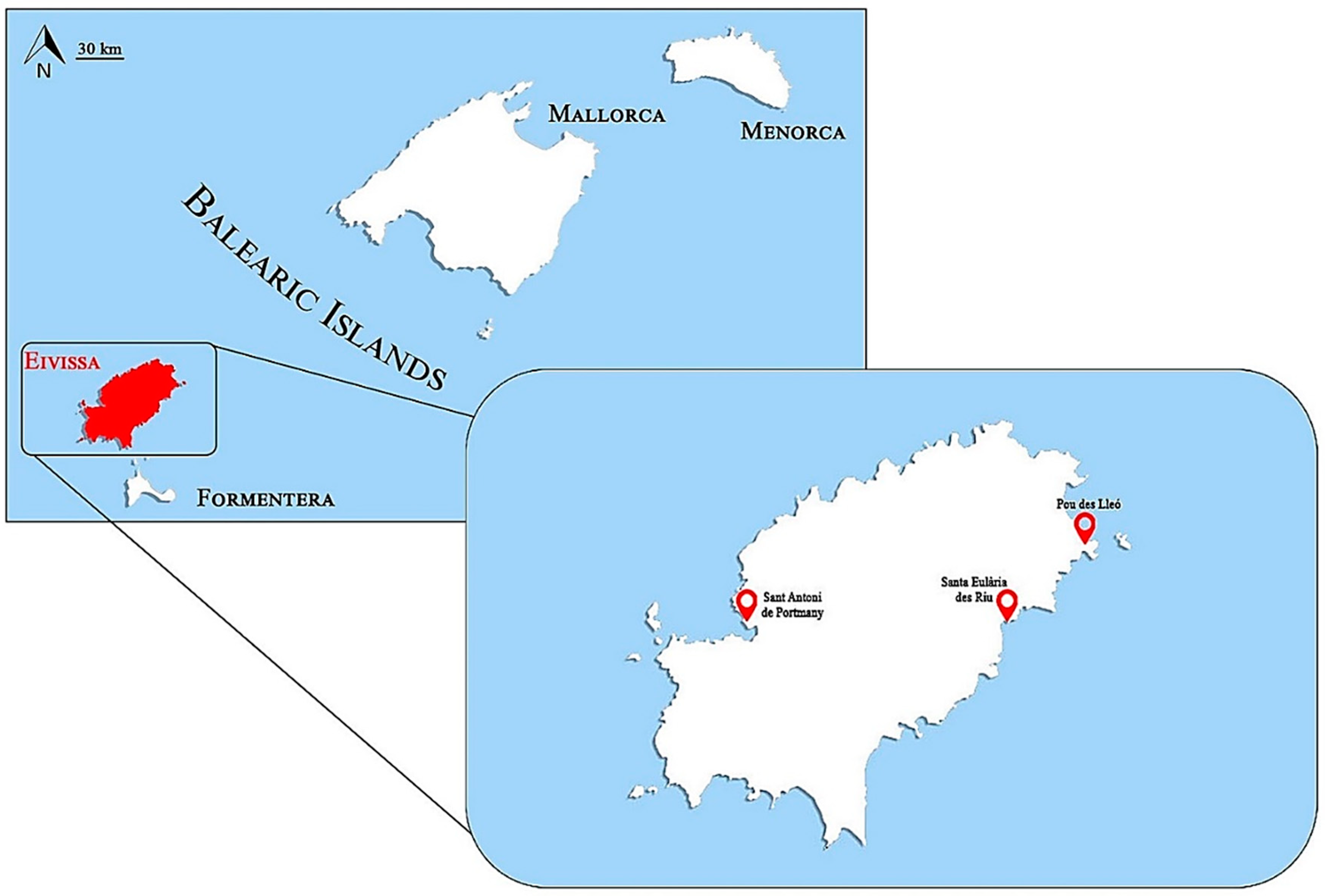
4.1. Holothuria Sampling and Study Area
4.2. Mesoplastic Characterization
4.3. Microplastic Ingestion
4.4. Biomarkers
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2020. Brussels, Plastics Europe: Association of Plastics Manufacturers. 2020. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 19 July 2022).
- World Economic Forum. The new plastics economy: Rethinking the Future of Plastics. Ellen MacArthur Foundation. 2016. (Issue January). Available online: https://www.weforum.org/reports/the-new-plastics-economy-rethinking-the-future-of-plastics/ (accessed on 19 July 2022).
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar]
- Parker, L. Plastic pollution facts and information. The world’s plastic pollution crisis explained. Natl. Geogr. 2019, 7, 6. [Google Scholar]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Lusher, A.; Thompson, R.C.; Morley, A. The Deposition and Accumulation of Microplastics in Marine Sediments and Bottom Water from the Irish Continental Shelf. Sci. Rep. 2017, 7, 10772. [Google Scholar] [CrossRef] [PubMed]
- Tosin, M.; Weber, M.; Siotto, M.; Lott, C.; Innocenti, F.D. Laboratory test methods to determine the degradation of plastics in marine environmental conditions. Front. Microbiol. 2012, 3, 225. [Google Scholar] [CrossRef]
- Suaria, G.; Perold, V.; Lee, J.R.; Lebouard, F.; Aliani, S.; Ryan, P.G. Floating macro-and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020, 136, 105494. [Google Scholar] [CrossRef]
- Balearic Sea Report. Informe Mar Balear (IBM). 2021. Available online: https://marilles.org (accessed on 18 July 2022).
- Alomar, C. Plastic Litter in Seafloor Habitats of the Balearic Islands and Its Implications for Marine Species. Ph.D. Thesis, Universitat de les Illes Balears, Palma, Spain, 2020. [Google Scholar]
- PlasticsEurope Market Research Group. Plastics—The Facts 2018. Plastics Europe. Association of Plastics Manufacturers. 2018. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2018/ (accessed on 15 July 2022).
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Duncan, E.M.; Broderick, A.C.; Fuller, W.J.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Limpus, C.J.; Lindeque, P.K.; Mayes, A.G.; Omeyer, L.C.M.; et al. Microplastic ingestion ubiquitous in marine turtles. Glob. Chang. Biol. 2019, 25, 744–752. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Compa, M.; March, D.; Deudero, S. Spatio-temporal monitoring of coastal floating marine debris in the Balearic Islands from sea-cleaning boats. Mar. Pollut. Bull. 2019, 141, 205–214. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Wegner, A.; Foekema, E.M. Plastic as a carrier of POPs to aquatic organisms: A model analysis. Environ. Sci. Technol. 2013, 47, 7812–7820. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Estarellas, F.; Deudero, S. Microplastics in the Mediterranean Sea: Deposition in coastal shallow sediments, spatial variation and preferential grain size. Mar. Environ. Res. 2016, 115, 1–10. [Google Scholar] [CrossRef]
- Ling, L.B.; Chang, Y.; Liu, C.W.; Lai, P.L.; Hsu, T. Oxidative stress intensity-related effects of cadmium (Cd) and paraquat (PQ) on UV-damaged-DNA binding and excision repair activities in zebrafish (Danio rerio) embryos. Chemosphere 2017, 167, 10–18. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olson, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings- entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Capo, X.; Rubio, M.; Solomando, A.; Alomar, C.; Compa, M.; Sureda, A.; Deudero, S. Microplastic intake and enzymatic responses in Mytilus galloprovincialis reared at the vicinities of an aquaculture station. Chemosphere 2021, 280, 130575. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the effect of long-term exposure to microplastics and depuration period in Sparus aurata Linnaeus, 1758: Liver and blood biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef]
- Sureda, A.; Box, A.; Enseñat, M.; Alou, E.; Tauler, P.; Deudero, S.; Pons, A. Enzymatic antioxidant response of a labrid fish (Coris julis) liver to environmental caulerpenyne. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 144, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Cossu, C.; Doyotte, A.; Jacquin, M.C.; Babut, M.; Exinger, A.; Vasseur, P. Glutathione reductase, selenium-dependent glutathione peroxidase, glutathione levels, and lipid peroxidation in freshwater bivalves, Unio tumidus, as biomarkers of aquatic contamination in field studies. Ecotoxicol. Environ. Saf. 1997, 38, 122–131. [Google Scholar] [CrossRef]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.R.; Thompson, J.T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol. 2009, 368, 22–29. [Google Scholar] [CrossRef]
- Bulleri, F.; Ravaglioli, C.; Anselmi, S.; Renzi, M. The sea cucumber Holothuria tubulosa does not reduce the size of microplastics but enhances their resuspension in the water column. Sci. Total Environ. 2021, 781, 146650. [Google Scholar] [CrossRef]
- Coc, C.; Rogers, A.; Barrientos, E.; Sanchez, H. Micro and Macroplastics Analysis in the Digestive Tract of a Sea Cucumber (Holothuriidae, Holothuria floridana) of the Placencia Lagoon, Belize. Caribb. J. Sci. 2021, 51, 166–174. [Google Scholar] [CrossRef]
- Tejedor-Junco, M.T.; Díaz, V.C.; González-Martín, M.; Tuya, F. Presence of microplastics and antimicrobial-resistant bacteria in sea cucumbers under different anthropogenic influences in Gran Canaria (Canary Islands, Spain). Mar. Biol. Res. 2021, 17, 537–544. [Google Scholar] [CrossRef]
- Ocaña, A.; Tocino, L.S. Spawning of Holothuria tubulosa (Holothurioidea, Echinodermata) in the Alboran Sea (Mediterranean Sea). Zool. Baetica 2005, 16, 147–150. [Google Scholar]
- Bay-Nouailhat, A. Description of Holothuria (Holothuria) tubulosa. Available online: https://www.european-marine-life.org (accessed on 19 July 2022).
- Navarro, P.G.; García-Sanz, S.; Barrio, J.M.; Tuya, F. Feeding and movement patterns of the sea cucumber Holothuria sanctori. Mar. Biol. 2013, 160, 2957–2966. [Google Scholar] [CrossRef]
- Peng, G.; Bellerby, R.; Zhang, F.; Sun, X.; Li, D. The ocean’s ultimate trashcan: Hadal trenches as major depositories for plastic pollution. Water Res. 2020, 168, 115121. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Álvarez-Muñoz, D.; Ábalos, M.; Rodríguez-Mozaz, S.; Santos, L.H.; León, V.M.; Campillo, J.A.; Martínez-Gómez, C.; Abad, E.; Farré, M. Microplastics in Mediterranean coastal area: Toxicity and impact for the environment and human health. Trends Environ. Anal. Chem. 2020, 27, e00090. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Gago, J.; Campillo, J.A.; León, V.M. Microplastic distribution in surface sediments along the Spanish Mediterranean continental shelf. Environ. Sci. Pollut. Res. 2019, 26, 21264–21273. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Gago, J.; Otero, V.; Sobral, P. Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar. Environ. Res. 2016, 114, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.; Wang, Q.; Zhang, L.; Sun, L.; Lin, C.; Yang, H. Microplastic ingestion by the farmed sea cucumber Apostichopus japonicus in China. Environ. Pollut. 2019, 245, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Renzi, M.; Blašković, A.; Bernardi, G.; Russo, G.F. Plastic litter transfer from sediments towards marine trophic webs: A case study on holothurians. Mar. Pollut. Bull. 2018, 135, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.L.; Gwinnett, C.; Robinson, L.F.; Woodall, L.C. Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 2016, 6, 33997. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- de Haan, W.P.; Sanchez-Vidal, A.; Canals, M. Floating microplastics and aggregate formation in the Western Mediterranean Sea. Mar. Pollut. Bull. 2019, 140, 523–535. [Google Scholar] [CrossRef]
- Expósito, N.; Rovira, J.; Sierra, J.; Folch, J.; Schuhmacher, M. Microplastics levels, size, morphology and composition in marine water, sediments and sand beaches. Case study of Tarragona coast (western Mediterranean). Sci. Total Environ. 2021, 786, 147453. [Google Scholar] [CrossRef]
- Renzi, M.; Blašković, A.; Broccoli, A.; Bernardi, G.; Grazioli, E.; Russo, G. Chemical composition of microplastic in sediments and protected detritivores from different marine habitats (Salina Island). Mar. Pollut. Bull. 2020, 152, 110918. [Google Scholar] [CrossRef]
- Abidli, S.; Antunes, J.C.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; Trigui El Menif, N. Microplastics in sediments from the littoral zone of the north Tunisian coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 205, 1–9. [Google Scholar] [CrossRef]
- Cannas, S.; Fastelli, P.; Guerranti, C.; Renzi, M. Plastic litter in sediments from the coasts of south Tuscany (Tyrrhenian Sea). Mar. Pollut. Bull. 2017, 119, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Cannas, S.; Scopetani, C.; Fastelli, P.; Cincinelli, A.; Renzi, M. Plastic litter in aquatic environments of Maremma Regional Park (Tyrrhenian Sea, Italy): Contribution by the Ombrone river and levels in marine sediments. Mar. Pollut. Bull. 2017, 117, 366–370. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.; Fogelberg, V.; Nilsson, P.A.; Hollander, J. Microplastics in a freshwater mussel (Anodonta anatina) in Northern Europe. Sci. Total Environ. 2019, 697, 134192. [Google Scholar] [CrossRef] [PubMed]
- Hara, J.; Frias, J.; Nash, R. Quantification of microplastic ingestion by the decapod crustacean Nephrops norvegicus from Irish waters. Mar. Pollut. Bull. 2020, 152, 110905. [Google Scholar] [CrossRef]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse effects of plastic ingestion on the Mediterranean small-spotted catshark (Scyliorhinus canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef]
- Pinya, S.; Renga, E.; Fernández, G.; Mateu-Vicens, G.; Tejada, S.; Capó, X.; Sureda, A. Physiological biomarkers in loggerhead turtles (Caretta caretta) as a tool for monitoring sanitary evolution in marine recovery centres. Sci. Total Environ. 2021, 757, 143930. [Google Scholar] [CrossRef]
- Jeong, C.B.; Kang, H.M.; Lee, M.C.; Kim, D.H.; Han, J.; Hwang, D.S.; Souissi, S.; Lee, S.J.; Shin, K.H.; Park, H.G.; et al. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 2017, 7, 41323. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; da Costa, J.P.; Rocha-Santos, T.; Duarte, A.C.; Pereira, R. Oxidative stress, energy metabolism and molecular responses of earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics. Environ. Sci. Pollut. Res. 2018, 25, 33599–33610. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquatic. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, D.; Chen, Y.; Zhao, C.; Liu, L.; Gu, Y.; Ren, Y.; Xia, B. Adverse effects of dietary virgin (nano)microplastics on growth performance, immune response, and resistance to ammonia stress and pathogen challenge in juvenile sea cucumber Apostichopus japonicus (Selenka). J. Hazard. Mater. 2022, 423, 127038. [Google Scholar] [CrossRef]
- Rabeh, I.; Telahigue, K.; Bejaoui, S.; Hajji, T.; Chouba, L.; el Cafsi, M.; Soudani, N. Effects of mercury graded doses on redox status, metallothionein levels and genotoxicity in the intestine of sea cucumber Holothuria forskali. Chem. Ecol. 2019, 35, 204–218. [Google Scholar] [CrossRef]
- Stephensen, E.; Sturve, J.; Förlin, L. Effects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2022, 133, 435–442. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Li, N.; Gao, Y.; Ju, Z.; Liao, G.; Xiong, D. Combined effects of elevated temperature and crude oil pollution on oxidative stress and apoptosis in sea cucumber (Apostichopus japonicus, selenka). Int. J. Environ. Res. Public Health 2021, 18, 801. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Wang, Q.; Li, B.; Zhang, H.; Pi, Y. The effects of benzo[a]pyrene on the composition of gut microbiota and the gut health of the juvenile sea cucumber Apostichopus japonicus Selenka. Fish Shellfish Immunol. 2019, 93, 369–379. [Google Scholar] [CrossRef] [PubMed]
- IBESTAT. 2021 (Institut d’Estadística de les Illes Balears). Available online: https://ibestat.caib.es/ibestat/inici (accessed on 15 July 2022).
- Iwalaye, O.A.; Moodley, G.K.; Robertson-Andersson, D.V. Microplastic occurrence in marine invertebrates sampled from Kwazulu-Natal, South Africa in different seasons. Nat. Environ. Pollut. Technol. 2020, 19, 1811–1819. [Google Scholar] [CrossRef]
- Tuit, C.B.; Wait, A.D. A review of marine sediment sampling methods. Environ. Forensics 2020, 21, 291–309. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef] [PubMed]
- Cordova, M.R.; Riani, E.; Shiomoto, A. Microplastics ingestion by blue panchax fish (Aplocheilus sp.) from Ciliwung Estuary, Jakarta, Indonesia. Mar. Pollut. Bull. 2020, 161, 111763. [Google Scholar] [CrossRef] [PubMed]
- Cordova, M.R.; Riani, E. Micro- and mesoplastics release from the Indonesian municipal solid waste landfill leachate to the aquatic environment: Case study in Galuga Landfill Area, Indonesia. Mar. Pollut. Bull. 2021, 163, 111986. [Google Scholar]
- Klangnurak, W.; Chunniyom, S. Screening for microplastics in marine fish of Thailand: The accumulation of microplastics in the gastrointestinal tract of different foraging preferences. Environ. Sci. Pollut. Res. 2020, 27, 27161–27168. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Flohé, L.; Ötting, F. Superoxide Dismutase Assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzymol 1984, 105, 121–126. [Google Scholar]
- Goldberg, D.M.; Spooner, R. Glutathione reductase. Methods Enzym. Anal. 1984, 3, 258–265. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pinya, S.; Tejada, S.; Capó, X.; Sureda, A. Invasive predator snake induces oxidative stress responses in insular amphibian species. Sci. Total Environ. 2016, 566, 57–62. [Google Scholar] [CrossRef] [PubMed]






| H. tubulosa (N = 104) | Sediments (N = 137) | ||
|---|---|---|---|
| Shape | FO (%) | Shape | FO (%) |
| Fibers | 83.7% | Fibers | 96.4% |
| Fragments | 16.30% | Fragments | 3.6% |
| Color | FO (%) | Color | FO (%) |
| Blue | 43.3% | Black | 39.8% |
| Transparent | 13.5% | Blue | 35.8% |
| Black | 13.5% | Red | 11.4% |
| Sant Antoni de Portmany (n = 25) | Santa Eulària des Riu (n = 25) | Pou des Lleó (n = 25) | ||
|---|---|---|---|---|
| Shape | FO (%) | |||
| Fragments | 17 | 20 | 20 | 76% |
| Sheet | 5 | 3 | 3 | 15% |
| Thread | 3 | 2 | 2 | 9% |
| Color | FO (%) | |||
| Blue | 9 | 8 | 12 | 39% |
| Green | 4 | 3 | 4 | 15% |
| Red | 2 | 2 | 3 | 9% |
| Transparent | 2 | 2 | 1 | 7% |
| Yellow | 1 | 1 | 3 | 7% |
| Grey | 1 | 2 | 2 | 7% |
| Black | 2 | 2 | 0 | 5% |
| White | 1 | 3 | 0 | 5% |
| Orange | 1 | 2 | 0 | 4% |
| Violet | 1 | 0 | 0 | 1% |
| Gold | 1 | 0 | 0 | 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, J.; Solomando, A.; Cohen-Sánchez, A.; Pinya, S.; Tejada, S.; Ferriol, P.; Mateu-Vicens, G.; Box, A.; Faggio, C.; Sureda, A. Effects of Human Activity on Markers of Oxidative Stress in the Intestine of Holothuria tubulosa, with Special Reference to the Presence of Microplastics. Int. J. Mol. Sci. 2022, 23, 9018. https://doi.org/10.3390/ijms23169018
Lombardo J, Solomando A, Cohen-Sánchez A, Pinya S, Tejada S, Ferriol P, Mateu-Vicens G, Box A, Faggio C, Sureda A. Effects of Human Activity on Markers of Oxidative Stress in the Intestine of Holothuria tubulosa, with Special Reference to the Presence of Microplastics. International Journal of Molecular Sciences. 2022; 23(16):9018. https://doi.org/10.3390/ijms23169018
Chicago/Turabian StyleLombardo, Jessica, Antònia Solomando, Amanda Cohen-Sánchez, Samuel Pinya, Silvia Tejada, Pere Ferriol, Guillem Mateu-Vicens, Antonio Box, Caterina Faggio, and Antoni Sureda. 2022. "Effects of Human Activity on Markers of Oxidative Stress in the Intestine of Holothuria tubulosa, with Special Reference to the Presence of Microplastics" International Journal of Molecular Sciences 23, no. 16: 9018. https://doi.org/10.3390/ijms23169018







