Nuclease Triggered “Signal-On” and Amplified Fluorescent Sensing of Fumonisin B1 Incorporating Graphene Oxide and Specific Aptamer
Abstract
:1. Introduction
2. Results and Discussion
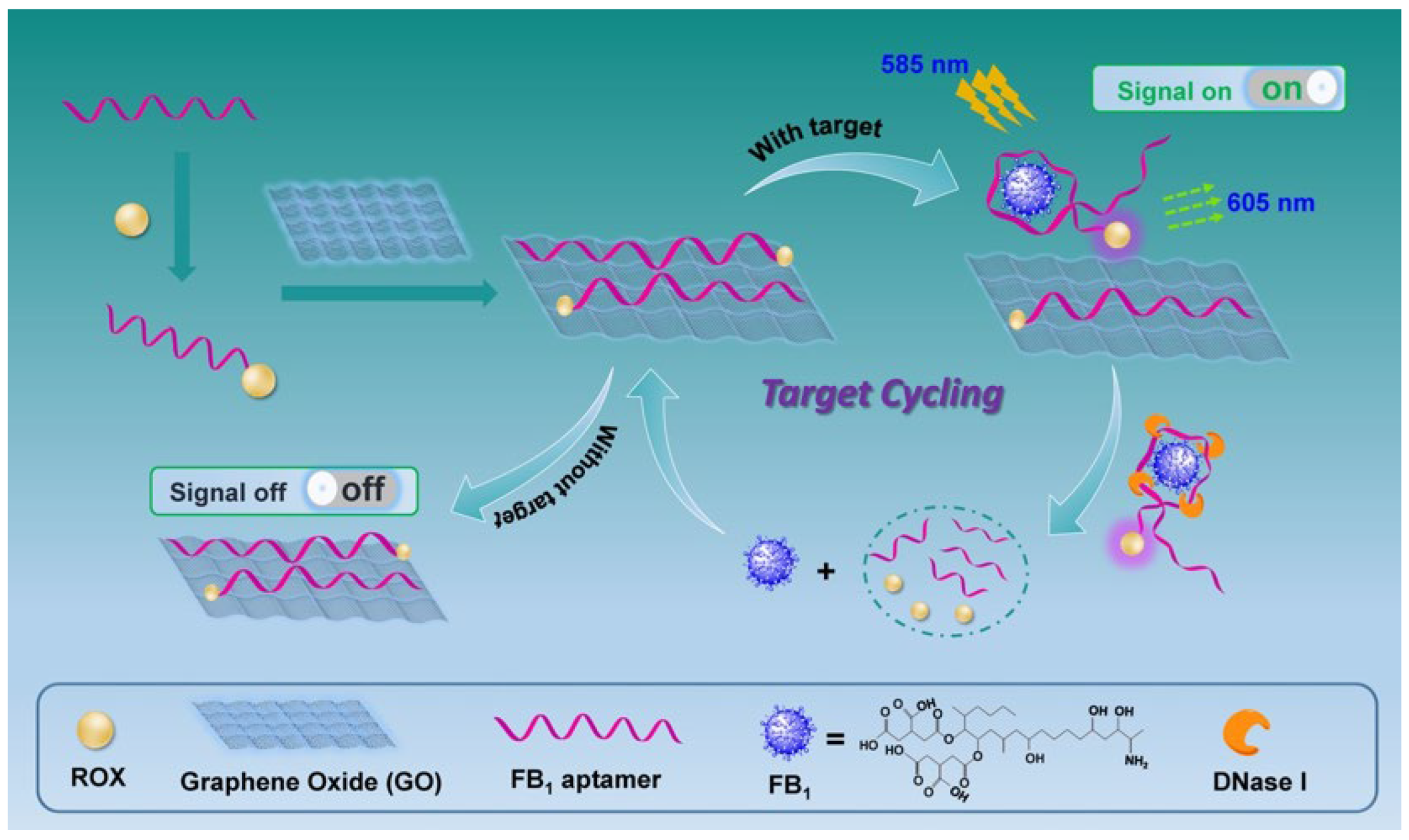
2.1. Sensing Strategy for FB1 Detection
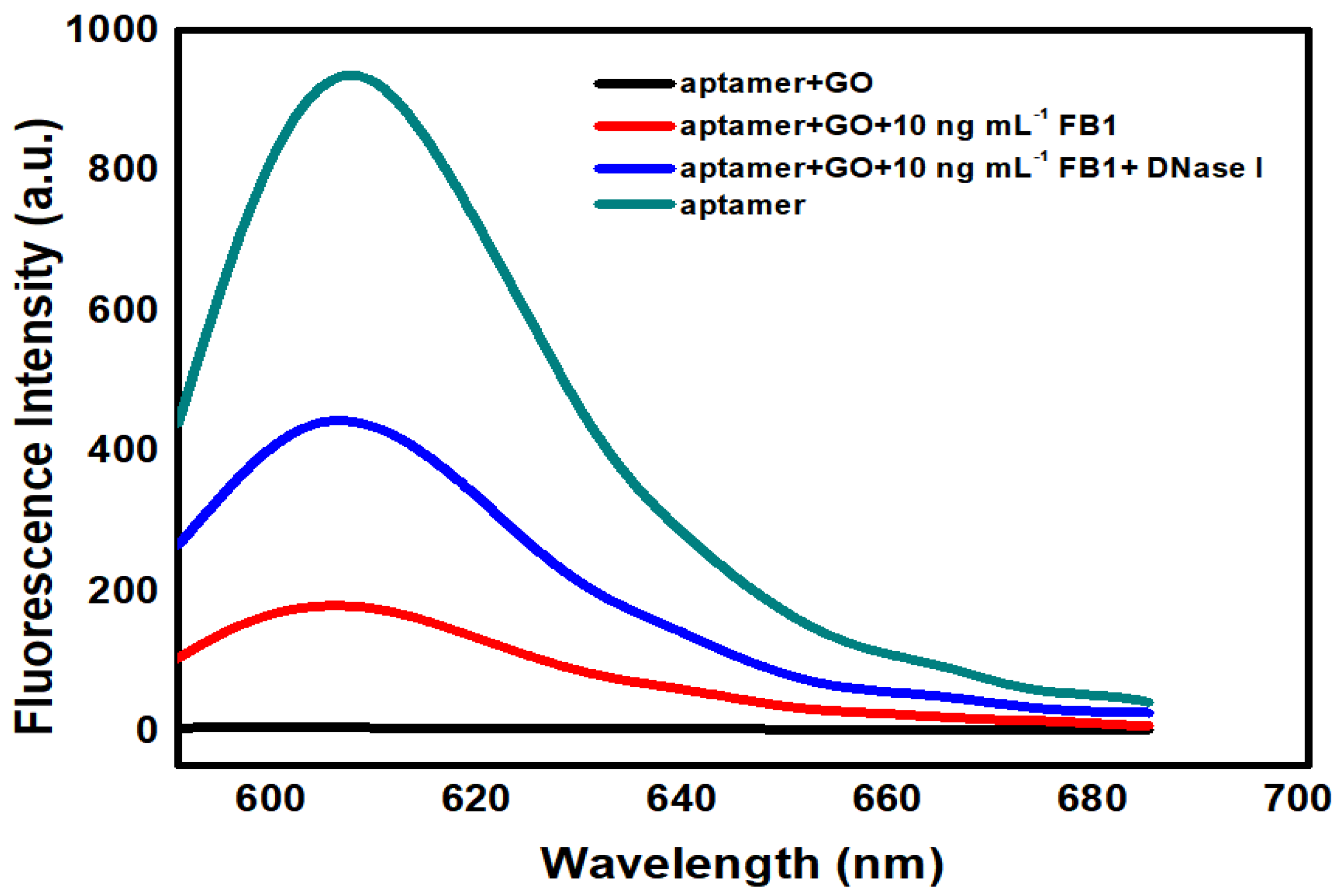
2.2. Signal Enhancement Sensing of FB1 with Nuclease
2.3. Detection Performance of the Aptasensor
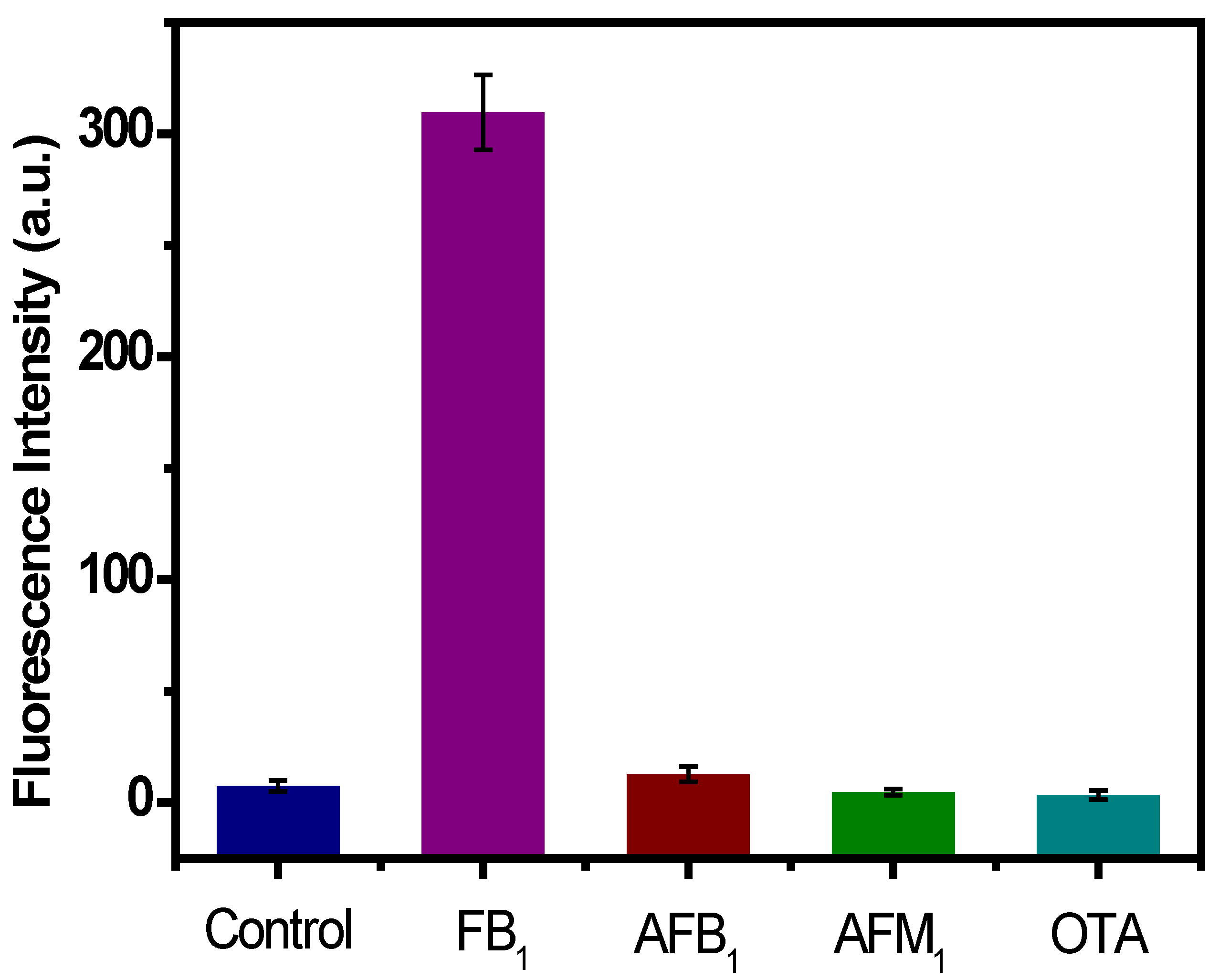
2.4. Selectivity Analysis of the Aptasensor
2.5. Method Validation of This Method
3. Materials and Methods
3.1. Materials and Reagents
3.2. Fluorescent Response for Aptasensing of FB1
3.3. Specificity Analysis
3.4. Practicability Analysis of This Aptasensing Platform
3.5. Statistical Analysis of the Experiment Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atar, N.; Eren, T.; Yola, M.L. A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice. Food Chem. 2015, 184, 7–11. [Google Scholar] [CrossRef] [PubMed]
- González-Jartín, J.M.; Alfonso, A.; Rodríguez, I.; Sainz, M.J.; Vieytes, M.R.; Botana, L.M. A QuEChERS based extraction procedure coupled to UPLC-MS/MS detection for mycotoxins analysis in beer. Food Chem. 2019, 275, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Pomastowski, P.; Rafińska, K.; Raileanplugaru, V.; Zloch, M.; Walczak, J.; Buszewski, B. A study of zearalenone biosorption and metabolisation by prokaryotic and eukaryotic cells. Toxicon 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Liu, B.; Zhang, D.; Zhang, C.; Guo, Y.; Chu, X.; Wang, W.; Yan, X.; Li, Z. Signal enhancing strategies in aptasensors for the detection of smallmolecular contaminants by nanomaterials and nucleic acid amplification. Talanta 2022, 236, 122866. [Google Scholar] [CrossRef]
- Cendoya, E.; Chiotta, M.L.; Zachetti, V.; Chulze, S.N.; Ramirez, M.L. Fumonisins and fumonisin-producing Fusarium occurrence in wheat and wheat by products: A review. J. Cereal Sci. 2018, 80, 158–166. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Zhang, Q.; Zha, Y.; Lu, S.; Yang, Y.; Li, P.; Zhou, Y. Colorimetric immunoassay via smartphone based on Mn2+-Mediated aggregation of AuNPs for convenient detection of fumonisin B1. Food Control 2022, 132, 108481. [Google Scholar] [CrossRef]
- Martins, H.; Almeida, I.; Camacho, C.; Santos, S.; Costa, J.; Bernardo, F. Occurrence of fumonisins in feed for swine and horses. Rev. Iberoam. Micol. 2012, 29, 175–177. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Wan, D.; Liu, Q.; Chen, D.; Liu, Z.; Martinez-Larranaga, M.R.; Martinez, M.A.; Anadon, A.; Yuan, Z. Fumonisins: Oxidative stress-mediated toxicity and metabolism in vivo and in vitro. Arch. Toxicol. 2016, 90, 81–101. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Zhang, W.; Leng, Y.; Xiong, Y. A colorimetric immunoassay based on glucose oxidase-induced AuNP aggregation for the detection of fumonisin B1. Talanta 2018, 186, 29–35. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, B.; Sheng, P.; Liao, X.; Shi, L.; Fang, L.; Zhou, L.; Kong, W. Aptasensors for mycotoxins in foods: Recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2032–2073. [Google Scholar] [CrossRef]
- Hu, W.J.; Yan, J.X.; You, K.H.; Wei, T.L.; Li, Y.P.; He, Q.H. Streptococcal protein G based fluorescent universal probes and biosynthetic mimetics for Fumonisin B1 immunochromatographic assay. Food Control 2020, 118, 107442. [Google Scholar] [CrossRef]
- Acuña-Gutiérrez, C.; Schock, S.; Jiménez, V.M.; Müller, J. Detecting fumonisin B1 in black beans (Phaseolus vulgaris L.) by near-infrared spectroscopy (NIRS). Food Control 2021, 130, 108335. [Google Scholar] [CrossRef]
- Martins, F.; Ferreira, F.; Bando, É.; Nerilo, S.; Hirooka, E.; Machinski, J. Daily intake estimates of fumonisins in corn-based food products in the population of Parana, Brazil. Food Control 2012, 26, 614–618. [Google Scholar] [CrossRef]
- Muscarella, M.; Magro, S.; Nardiello, D.; Palermo, C.; Centonze, D. Development of a new analytical method for the determination of fumonisins B1 and B2 in food products based on high performance liquid chromatography and fluorimetric detection with post-column derivatization. J. Chromatogr. A 2008, 1203, 88–93. [Google Scholar] [CrossRef]
- Gazzotti, T.; Lugoboni, B.; Zironi, E.; Barbarossa, A.; Serraino, A.; Pagliuca, G. Determination of fumonisin B1 in bovine milk by LC-MS/MS. Food Control 2009, 20, 1171–1174. [Google Scholar] [CrossRef]
- Ediage, E.; Mavungu, J.; Song, S.; Wu, A.; Peteghem, C.; Saeger, S. A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2012, 741, 58–69. [Google Scholar] [CrossRef]
- Souto, P.C.; Jager, A.V.; Tonin, F.G.; Petta, T.; Di Gregório, M.C.; Cossalter, A.-M.; Pinton, P.; Oswald, I.P.; Rottinghaus, G.E.; Oliveira, C.A. Determination of fumonisin B1 levels in body fluids and hair from piglets fed fumonisin B1-contaminates diets. Food. Chem. Toxicol. 2017, 108, 1–9. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. Quantum dot nanobead-based fluorescent immunochromatographic assay for simultaneous quantitative detection of fumonisin B1, dexyonivalenol, and zearalenone in grains. Food Control 2020, 117, 107331. [Google Scholar] [CrossRef]
- Masikini, M.; Williams, A.; Sunday, C.; Waryo, T.; Nxusani, E.; Wilson, L.; Iwuoha, E. Label free poly(2,5-dimethoxyaniline)-multi-walled carbon nanotubes impedimetric immunosensor for fumonisin B1 detection. Materials 2016, 9, 273. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, X.; Zhang, X.; Qing, Y.; Luo, M.; Liu, X.; Li, C.; Li, Y.; Xia, H.; Qiu, J. A highly sensitive electrochemical immunosensor for fumonisin B1 detection in corn using single-walled carbon nanotubes/chitosan. Electroanalysis 2015, 27, 2679–2687. [Google Scholar] [CrossRef]
- Milua, M.; Stephen, N.; Abebaw, T.; Njagi, N.; Kerileng, M.; Chinwe, O.; Emmanuel, I. A fumonisins immunosensor based on polyanilino-carbon nanotubes doped with palladium telluride quantum dots. Sensors 2015, 15, 529–546. [Google Scholar]
- Guo, X.; Wen, F.; Zheng, N.; Luo, Q.; Wang, H.; Wang, H.; Li, S.; Wang, J. Development of an ultrasensitive aptasensor for the detection of aflatoxin B1. Biosens. Bioelectron. 2014, 56, 340–344. [Google Scholar] [CrossRef]
- Qiao, Q.; Guo, X.; Wen, F.; Chen, L.; Xu, Q.; Zheng, N.; Cheng, J.; Xue, X.; Wang, J. Aptamer-Based Fluorescence Quenching Approach for Detection of Aflatoxin M1 in Milk. Front. Chem. 2021, 9, 653869. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X. Gold/platinum bimetallic nanomaterials for immunoassay and immunosensing. Coord. Chem. Rev. 2022, 465, 214578. [Google Scholar] [CrossRef]
- Molinero-Fernandez, A.; Moreno-Guzman, M.; Angel Lopez, M.; Escarpa, A. Biosensing strategy for simultaneous and accurate quantitative analysis of mycotoxins in food samples using unmodified graphene micromotors. Anal. Chem. 2017, 89, 10850–10857. [Google Scholar] [CrossRef]
- Niazi, S.; Khan, I.; Yan, L.; Khan, M.; Mohsin, A.; Duan, N.; Wang, Z. Simultaneous detection of fumonisin B1 and ochratoxin A using dual-color, time-resolved luminescent nanoparticles (NaYF4: Ce, Tb and NH2-Eu/DPA@SiO2) as labels. Anal. Bioanal. Chem. 2019, 411, 1453–1465. [Google Scholar] [CrossRef]
- Rao, C.; Sood, A.; Subrahmanyam, K.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Zahra, Q.U.A.; Fang, X.; Luo, Z.; Ullah, S.; Fatima, S.; Batool, S.; Qiu, B.; Shahzad, F. Graphene Based Nanohybrid Aptasensors in Environmental Monitoring: Concepts, Design and Future Outlook. Crit. Rev. Anal. Chem. 2022, 1–22. [Google Scholar] [CrossRef]
- Danesh, N.M.; Bostan, H.B.; Abnous, K.; Ramezani, M.; Youssefi, K.; Taghdisi, S.M.; Karimi, G. Ultrasensitive detection of aflatoxin B1 and its major metabolite aflatoxin M1 using aptasensors: A review. TrAC-Trend. Anal. Chem. 2018, 99, 117–128. [Google Scholar] [CrossRef]
- Guo, X.D.; Wen, F.; Zheng, N.; Saive, M.; Fauconnier, M.L.; Wang, J.Q. Aptamer-based biosensor for detection of mycotoxins. Front. Chem. 2020, 8, 195. [Google Scholar] [CrossRef]
- Lu, C.; Lim, J.; Lin, M.; Wang, Y.; Yang, H.; Chen, X.; Chen, G. Amplified Aptamer-Based Assay through Catalytic Recycling of the Analyte. Angew. Chem. Ed. 2010, 122, 8632–8635. [Google Scholar] [CrossRef]
- Tang, D.; Tang, J.; Li, Q.; Su, B.; Chen, G. Ultrasensitive aptamer-based multiplexed electrochemical detection by coupling distinguishable signal tags with catalytic recycling of DNase I. Anal. Chem. 2011, 83, 7255–7259. [Google Scholar] [CrossRef]
- Sun, A.; Zhang, Y.; Sun, G.; Wang, X.; Tang, D. Homogeneous electrochemical detection of ochratoxin A in foodstuff using aptamer-graphene oxide nanosheets and DNase I-based target recycling reaction. Biosens. Bioelectron. 2017, 89, 659–665. [Google Scholar] [CrossRef]
- Guo, X.; Wen, F.; Qiao, Q.; Zheng, N.; Saive, M.; Fauconnier, M.L.; Wang, J. A Novel Graphene Oxide-Based Aptasensor for Amplified Fluorescent Detection of Aflatoxin M1 in Milk Powder. Sensors 2019, 19, 3840. [Google Scholar] [CrossRef]
- Setlem, S.K.; Mondal, B.; Ramlal, S. A fluorescent aptasensor for the detection of Aflatoxin B1 by graphene oxide mediated quenching and release of fluorescence. J. Microbiol. Methods 2022, 193, 106414. [Google Scholar] [CrossRef]
- Shu, M.; Xu, Y.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Hammock, B. Anti-idiotypic nanobody-alkaline phosphatase fusion proteins: Development of a one-step competitive enzyme immunoassay for fumonisin B1 detection in cereal. Anal. Chim. Acta 2016, 924, 53–59. [Google Scholar] [CrossRef]
- Jodra, A.; Ángel López, M.; Escarpa, A. Disposable and reliable electrochemical magnetoimmunosensor for fumonisins simplified determination in maize-based foodstuffs. Biosens. Bioelectron. 2015, 64, 633–638. [Google Scholar] [CrossRef]
- Shu, M.; Xu, Y.; Wang, D.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Qiu, Y.; Ji, Y.; Wang, X. Anti-idiotypic nanobody: A strategy for development of sensitive and green immunoassay for Fumonisin B1. Talanta 2015, 143, 388–393. [Google Scholar] [CrossRef]
- Li, L.; Xia, L.; Zhao, Y.; Wang, H. Development of immune-affinity 96 spots monolith array for multiple mycotoxins detection in food samples. J. Chromatogr. B 2016, 1029–1030, 72–80. [Google Scholar] [CrossRef]
- Jie, M.; Yu, S.; Yu, F.; Liu, L.; He, L.; Li, Y.; Zhang, H.; Qu, L.; Harrington, P.D.B.; Wu, Y. An ultrasensitive chemiluminescence immunoassay for fumonisin B1 detection in cereals based on gold-coated magnetic nanoparticles. J. Sci. Food Agric. 2018, 98, 3384–3390. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Q.; Liu, X.; Yang, Y.; Yang, Y.; Liu, M.; Li, P.; Zhou, Y. Antibody-biotin-streptavidin-horseradish peroxidase (HRP) sensor for rapid and ultra-sensitive detection of fumonisins. Food Chem. 2020, 316, 126356. [Google Scholar] [CrossRef] [PubMed]
- McKeague, M.; Bradley, C.; De Girolamo, A.; Visconti, A.; Miller, J.; DeRosa, M. Screening and initial binding assessment of fumonisin B1 aptamers. Int. J. Mol. Sci. 2010, 11, 4864–4881. [Google Scholar] [CrossRef] [PubMed]


| Method | Detection Time (min) | Linear Range (ng mL−1) | LOD (ng mL−1) | Reference |
|---|---|---|---|---|
| Chemiluminescence ELISA | 60 | 0.93–7.73 | 0.12 | [37] |
| Electrochemical | 180 | 0.01–1000 | 0.002 | [20] |
| Amperometric | 60 | 0.73–11.2 | 0.33 | [38] |
| ELISA | ~60 | 0.27–5.92 | 0.15 | [39] |
| Chemiluminescence | 60 | 0.01–0.1 | 0.0017 | [40] |
| Chemiluminescence | 150 | 0.05–25 | 0.027 | [41] |
| Colorimetric immunoassay | 120 | 3.125–25 | 12.5 | [9] |
| Antibody-based HRP sensor | 22 | 0.31–162.42 | 0.21 | [42] |
| Fluorescent aptasensor | 15 | 1–10,000 | 0.4 | [25] |
| Fluorescent aptasensor | 5 | 0.5–20 | 0.15 | Current work |
| Sample | Spiked Concentration (ng mL−1) | Current Aptasensor Method | Classic ELISA Method | ||
|---|---|---|---|---|---|
| Detected Concentrations Mean a ± SD b (ng mL−1) | Recovery (%) | Detected Concentrations Mean a ± SD b (ng mL−1) | Recovery (%) | ||
| Wheat flour | 0 | ND c | - | ND c | - |
| 1.5 | 1.67 ± 0.02 | 111 | 1.71 ± 0.08 | 114 | |
| 8 | 7.93 ± 0.56 | 99 | 8.02 ± 0.52 | 100 | |
| 15 | 15.47 ± 0.68 | 103 | 16.22 ± 0.84 | 108 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Qiao, Q.; Zhang, M.; Fauconnier, M.-L. Nuclease Triggered “Signal-On” and Amplified Fluorescent Sensing of Fumonisin B1 Incorporating Graphene Oxide and Specific Aptamer. Int. J. Mol. Sci. 2022, 23, 9024. https://doi.org/10.3390/ijms23169024
Guo X, Qiao Q, Zhang M, Fauconnier M-L. Nuclease Triggered “Signal-On” and Amplified Fluorescent Sensing of Fumonisin B1 Incorporating Graphene Oxide and Specific Aptamer. International Journal of Molecular Sciences. 2022; 23(16):9024. https://doi.org/10.3390/ijms23169024
Chicago/Turabian StyleGuo, Xiaodong, Qinqin Qiao, Mengke Zhang, and Marie-Laure Fauconnier. 2022. "Nuclease Triggered “Signal-On” and Amplified Fluorescent Sensing of Fumonisin B1 Incorporating Graphene Oxide and Specific Aptamer" International Journal of Molecular Sciences 23, no. 16: 9024. https://doi.org/10.3390/ijms23169024
APA StyleGuo, X., Qiao, Q., Zhang, M., & Fauconnier, M.-L. (2022). Nuclease Triggered “Signal-On” and Amplified Fluorescent Sensing of Fumonisin B1 Incorporating Graphene Oxide and Specific Aptamer. International Journal of Molecular Sciences, 23(16), 9024. https://doi.org/10.3390/ijms23169024






