Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art
Abstract
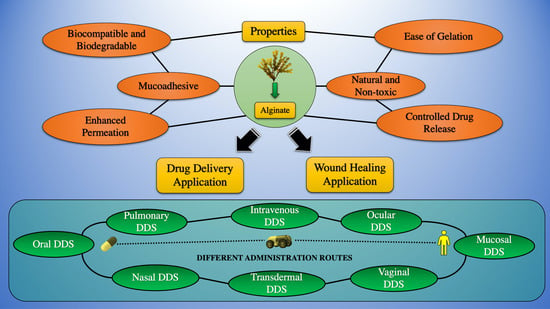
:1. Introduction
2. The Purview of the Present Review
3. Sources of Extraction and Properties of Alginate
4. Hydrogel Formation Methods
4.1. Ionic Cross-Linking
4.2. Covalent Cross-Linking
4.3. Photo Crosslinking
4.4. Click Chemistry Reactions
4.5. Thermal Gelling
4.6. Cell Cross-Linking
5. ALG Particles Formation Methods
6. Current Advancements in ALG Formulations in Drug Delivery
6.1. Oral Drug Delivery
6.2. Ocular Drug Delivery
6.3. Pulmonary Drug Delivery
6.4. Vaginal Drug Delivery
6.5. Nasal Drug Delivery
6.6. Transdermal Drug Delivery
6.7. Mucosal Drug Delivery
6.8. Intravenous Drug Delivery
6.9. Others
7. Recent Advances in ALG Formulations in Wound Healing
| Type of Wound Dressing Materials | ALG-Based Composite Materials | Applications | References |
|---|---|---|---|
| Nanocomposite hydrogel | ALG/Eudragit | Chronic cutaneous wound healing in diabetic mice | [367] |
| Hydrogel | ALG/collagen | Drug delivery for skin wounds in trauma patients | [368] |
| Hydrogel | ALG/Pluronic F127 | Drug release-based bleeding wound healing | [369] |
| Films | ALG | Chronic wound healing | [370] |
| Hydrogel | ALG/gelatin methacrylate | Wound healing | [371] |
| Hydrogel | Oxidized ALG | Chronic wound healing in diabetic mice | [372] |
| Thermoreversible hydrogel | SA/chondroitin sulfate | Drug delivery and diabetic wound healing | [373] |
| Ion exchange responsive film | ALG/hyaluronate | Drug delivery and skin wound healing | [374] |
| Films | ALG/pectin | Wound healing for moderate exudates | [375] |
| Hydrogel | SA/poly(N-isopropyl acrylamide) | Drug delivery and wound healing | [376] |
| Xerogel | ALG/g-polyethylene glycol methacrylate | Wound healing | [377] |
| Film | SA/K-carrageenan | Sustained drug release-based topical wound dressing | [378] |
| Electrospun mat | ALG/polyvinyl alcohol | Wound dressing and real-time evaluation of healing | [379] |
| Sponges | ALG/CS/HA | Wound healing in full-thickness wounds in rats | [380] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| AAC | antioxidant activity coefficient |
| Aah | Androctonus australis hector |
| AGM | agomelatine |
| AGMPs | sodium alginate(AG)based microparticles |
| ALG | Alginate |
| Alg-APBA | 3-aminophenylboronic acid-modified alginate |
| ALG-CNC NPs | Alginate cellulose nanocrystals nanoparticles |
| AmpB | Amphotericin B |
| ARPE-18 | Human retinal pigmented epithelium cells |
| ATO | Arsenic trioxide |
| BALB/c | Bagg Albino mouse strain |
| BBSA | Bifurcaria bifurcata |
| BCG | Bacille Calmette–Guérin |
| BET | Brunauer–Emmett–Teller |
| BSA | Bovine serum albumin |
| CA | Calcium alginate |
| CAC | composite alginate collagen |
| CaG | calcium gluconate |
| CANPs | chitosan/alginate nanoparticles |
| CBSA | C. barbata sodium alginate |
| CD | Crohn’s disease |
| CDD | Curcumin diethyl disuccinate |
| CFX | Cefixime |
| CMC | carboxymethyl cellulose |
| CNCs | Cellulose nanocrystals |
| COPD | Chronic obstructive pulmonary disease |
| CpG ODN | CpG oligodeoxynucleotides |
| CS | Chitosan |
| CTFR | cystic fibrosis transmembrane conductance regulator |
| DDS | Drug delivery system |
| DLS | Dynamic light scattering |
| Dox | Doxorubicin |
| DPPH | 1,1-Diphenyl-2 picrylhydrazyl |
| DTZ | diltiazem |
| ECT | Encapsulated-cell therapy |
| EE | Entrapment efficiency |
| ERG | electroretinogram |
| F–ERG | Flash electroretinogram |
| Fe3O4-SA-DOX | Fe3O4-sodium alginate–doxorubicin |
| FITC | fluorescein isothiocyanate |
| FSSA | Fucus spiralis L. |
| G | 4-α-L-guluronic acid |
| GA | gallic acid |
| GDNF | glial-derived neurotropic factor |
| GL | glycyrrhizin |
| GO | graphene oxide |
| GSM | glyceryl monostearate |
| HA | Hyaluronic acid |
| HaCat | Cultured Human Keratinocyte |
| HAI | Haemagglutinin inhibition |
| HAp | hydroxyapatite |
| HB-CNPs | HbsAg-loaded chitosan nanoparticles |
| HbsAg | Hepatitis B surface antigen |
| HEK293 | Human Embryonic Kidney cells |
| HEMA | (Hydroxyethyl)methacrylate |
| HPBCD | Hydroxypropyl beta cyclodextrin |
| HPMC | Hydroxypropyl methylcellulose |
| HP-β-CD | hydroxypropyl-beta-cyclodextrin |
| i.m. | intramuscular |
| IL | Interleukins |
| KF | ketotifen fumarate |
| KGM | Konjac glucomannan |
| M | 1,4-linked-β-D-mannuronic acid |
| MA | Methyl acrylate |
| MAPTAC | Methacryolyl aminopropyl trimethyl ammonium chloride |
| m-CNC | magnetic cellulose nanocrystal |
| MFS | Miltefosine |
| MIC | Minimum inhibitory concentration |
| MMP-2 | Matrix metalloproteinase-2 |
| M-MSNs | magnetic mesoporous silica nanoparticles |
| MNs | Microneedles |
| MPs | Microparticles |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| NLC | nanostructured lipid carriers |
| nMBA | N, N′-methylenebisacrylamide |
| OA | Oxidized alginate |
| PAH | poly(allylamine hydrochloride) |
| PBMC | Peripheral blood mononuclear cell |
| PCL | Polycaprolactone |
| PECs | polyelectrolyte complexes |
| PEG | Polyethylene glycol |
| PEG-co-PCL | poly (ethylene glycol)-co-poly(-caprolactone) |
| PEG@VTMSg-CS | Polyethylene glycol coated vinyl trimethoxy silane-g-chitosan |
| PEM | polyelectrolyte multilayers |
| PF | Pluronic |
| PFD | pirfenidone |
| P-gp shRNA | P-glycoprotein short hairpin RNA |
| PGX | Pressurized gas expanded liquid |
| PLC/PEG | poly(caprolactone)/polyethylene glycol |
| PLGA | poly (lactic-co-glycolic acid) |
| PLL | Polylysine |
| PMX | Polymyxin B |
| PR8-ALG | PR8 influenza virus sodium alginate |
| PR8-CHT | PR8 influenza virus chitosan |
| PR8-TMC-ALG | PR8 influenza virus sodium alginate-coated chitosan and trimethyl chitosan |
| PSSCMA | poly(4-styrenesulfonic acid-co-maleic acid) sodium salt |
| RBA | Relative Bioavailability |
| RCS | Royal College of Surgeons |
| RPA | Relative Pharmacologic Availability |
| s.c. | subcutaneous |
| SA | sodium alginate |
| SA-ATO-NPs | make ATO-loaded sodium alginate nanoparticles |
| SCC | squamous cell carcinoma |
| SD rat | Sprague Dawley rat |
| SGF | simulated gastric fluid |
| SH | sulfhydryl |
| SpBMP-9 | Small peptide Bone Morphogenetic Protein 9 |
| SRG | Sargassum sp |
| SS | salbutamol sulfate |
| SWCNT-GI | single-walled carbon nanotube modified by glucose |
| TA | Tannic acid |
| TCS | thiolated-chitosan coated sodium alginate |
| TEM | Transmission electron microscopy |
| THSG | 2,3,5,40 -tetrahydroxystilbene 2-O-β-D-glucoside |
| TRB | Turbinaria sp |
| TSA | thiolated sodium alginate |
| UTI | Urinary tract infection |
| VEP | visual evoked potential |
| VLF-AG-NPs | venlafaxine alginate nanoparticles |
| XRD | X-ray Diffraction |
References
- Jain, K.K. Drug Delivery Systems—An Overview. Methods Mol. Biol. 2008, 437, 1–50. [Google Scholar]
- Ansari, M.J.; Ahmed, M.M.; Anwer, M.K.; Jamil, S.; Alshetaili, A.S.; Ali, R.; Shakeel, F. Formulation and Characterization of Fluconazole Loaded Olive Oil Nanoemulsions. Indo Am. J. Pharm. Sci. 2017, 4, 852–860. [Google Scholar]
- Agrawal, P. Significance of Polymers in Drug Delivery System. J. Pharmacovigil. 2015, 3, 10–12. [Google Scholar] [CrossRef]
- Bhowmik, D.; Gopinath, H.; Kumar, B.P.; Duraivel, S.; Kumar, K.P.S. Controlled Release Drug Delivery Systems. Pharma Innov. J. 2012, 1, 24–32. [Google Scholar]
- Abourehab, M.A.S.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an Emerging Platform for Drug Delivery: A State-of-the-Art Review. J. Mater. Chem. B 2022, 10, 2781–2819. [Google Scholar] [CrossRef]
- Ansari, M.J. An Overview of Techniques for Multifold Enhancement in Solubility of Poorly Soluble Drugs. Curr. Issues Pharm. Med. Sci. 2019, 32, 203–209. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ansari, M.J.; Das, S.S.; Alshahrani, S.M. Development and Characterization of Ethyl Cellulose Nanosponges for Sustained Release of Brigatinib for the Treatment of Non-Small Cell Lung Cancer. J. Polym. Eng. 2020, 40, 823–832. [Google Scholar] [CrossRef]
- Grund, S.; Bauer, M.; Fischer, D. Polymers in Drug Delivery-State of the Art and Future Trends. Adv. Eng. Mater. 2011, 13, 61–87. [Google Scholar] [CrossRef]
- Zhuo, F.; Abourehab, M.A.S.; Hussain, Z. Hyaluronic Acid Decorated Tacrolimus-Loaded Nanoparticles: Efficient Approach to Maximize Dermal Targeting and Anti-Dermatitis Efficacy. Carbohydr. Polym. 2018, 197, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Polymeric Delivery Systems for Controlled Drug Release. Chem. Eng. Commun. 1980, 6, 1–48. [Google Scholar] [CrossRef]
- Mady, F.M.; Ibrahim, S.; Abourehab, M. Development and Evaluation of Alginate-Gum Blend Mucoadhesive Microspheres for Controlled Release of Metformin Hydrochloride. J. Adv. Biomed. Pharm. Sci. 2021, 4, 111–118. [Google Scholar] [CrossRef]
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M.; et al. Cell Membrane Cloaked Nanomedicines for Bio-Imaging and Immunotherapy of Cancer: Improved Pharmacokinetics, Cell Internalization and Anticancer Efficacy. J. Control. Release 2021, 335, 130–157. [Google Scholar] [CrossRef]
- Pramanik, S.; Sali, V. Connecting the Dots in Drug Delivery: A Tour d’horizon of Chitosan-Based Nanocarriers System. Int. J. Biol. Macromol. 2021, 169, 103–121. [Google Scholar] [CrossRef]
- Abourehab, M.A.; Ahmed, O.A.; Balata, G.F.; Almalki, W.H. Self-Assembled Biodegradable Polymeric Micelles to Improve Dapoxetine Delivery across the Blood-Brain Barrier. Int. J. Nanomed. 2018, 13, 3679–3687. [Google Scholar] [CrossRef]
- Dong, J.; Tao, L.; Abourehab, M.A.S.; Hussain, Z. Design and Development of Novel Hyaluronate-Modified Nanoparticles for Combo-Delivery of Curcumin and Alendronate: Fabrication, Characterization, and Cellular and Molecular Evidences of Enhanced Bone Regeneration. Int. J. Biol. Macromol. 2018, 116, 1268–1281. [Google Scholar] [CrossRef]
- Fatima, I.; Rasul, A.; Shah, S.; Saadullah, M.; Islam, N.; Khames, A.; Salawi, A.; Ahmed, M.M.; Almoshari, Y.; Abbas, G.; et al. Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections. Molecules 2022, 27, 2936. [Google Scholar] [CrossRef]
- Ashfaq, M.; Shah, S.; Rasul, A.; Hanif, M.; Khan, H.U.; Khames, A.; Abdelgawad, M.A.; Ghoneim, M.M.; Ali, M.Y.; Abourehab, M.A.S.; et al. Enhancement of the Solubility and Bioavailability of Pitavastatin through a Self-Nanoemulsifying Drug Delivery System (SNEDDS). Pharmaceutics 2022, 14, 482. [Google Scholar] [CrossRef]
- Abdel-Kader, M.; Al-Shdefat, R. Evaluation of Antifungal Activity of Olive Oil Based Nanoemulsions. Bull. Env. Pharmacol. Life Sci. 2016, 5, 1–4. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef]
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and Synthetic Polymers as Drug Carriers for Delivery of Therapeutic Proteins. Polym. Rev. 2015, 55, 371–406. [Google Scholar] [CrossRef]
- Pramanik, S.; Shrivastav, P.; Pramanik, S.; Vaidya, G.; Abdelgawad, M.A.; Ghoneim, M.M.; Singh, A.; Abualsoud, B.M.; Amaral, L.S.; Abourehab, M.A.S. Bacterial Cellulose as a Potential Biopolymer in Biomedical Applications: A State-of-the-Art Review. J. Mater. Chem. B 2022, 10, 3199–3241. [Google Scholar] [CrossRef]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial Alginate Production, Modification and Its Applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Remminghorst, U.; Rehm, B.H.A. Bacterial Alginates: From Biosynthesis to Applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Sachan, N.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium Alginate: The Wonder Polymer for Controlled Drug Delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Tavakoli, J.; Laisak, E.; Gao, M.; Tang, Y. AIEgen Quantitatively Monitoring the Release of Ca2+ during Swelling and Degradation Process in Alginate Hydrogels. Mater. Sci. Eng. C 2019, 104, 109951. [Google Scholar] [CrossRef] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.Y.; Ward, K. Multistage Extraction and Purification of Waste Sargassum Natans to Produce Sodium Alginate: An Optimization Approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y. Alginate Fibres: An Overview of the Production Processes and Applications in Wound Management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Satheeshababu, B.K.; Mohamed, I. Synthesis and Characterization of Sodium Alginate Conjugate and Study of Effect of Conjugation on Drug Release from Matrix Tablet. Indian J. Pharm. Sci. 2015, 77, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, Mannitol, Phenolic Compounds and Biological Activities of Two Range-Extending Brown Algae, Sargassum Mangarevense and Turbinaria Ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J. Appl. Phycol. 2008, 20, 1033–1043. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.A.; Silva Filho, E.A.T.; Melo, D.F.; Feitosa, J.P.A.; de Paula, R.C.M.; Lima, M.G.S. Extraction and Physicochemical Characterization of Sargassum Vulgare Alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and Characterization of an Alginate from the Brown Seaweed Sargassum Turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Montero, M.P.; Gómez-Guillén, M.C. Antioxidant Film Development from Unrefined Extracts of Brown Seaweeds Laminaria Digitata and Ascophyllum Nodosum. Food Hydrocoll. 2014, 37, 100–110. [Google Scholar] [CrossRef]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, Physicochemical and Antioxidant Properties of Sodium Alginate Isolated from a Tunisian Brown Seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef]
- Gomez, C.G.; Pérez Lambrecht, M.V.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the Extraction-Purification Conditions on Final Properties of Alginates Obtained from Brown Algae (Macrocystis Pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- González-lópez, N.; Moure, A.; Domínguez, H. Hydrothermal Fractionation of Sargassum Muticum Biomass. J. Appl. Phycol. 2012, 24, 1569–1578. [Google Scholar] [CrossRef]
- Pascaline, M.; Andriamanantoanina, H.; Heyraud, A.; Rinaudo, M. Food Hydrocolloids Structure and Properties of Three Alginates from Madagascar Seacoast Algae. Food Hydrocoll. 2013, 32, 143–146. [Google Scholar] [CrossRef]
- Mazumder, A.; Holdt, S.L.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of Alginate from Sargassum Muticum: Process Optimization and Study of Its Functional Activities. J. Appl. Phycol. 2016, 28, 3625–3634. [Google Scholar] [CrossRef]
- Vauchel, P.; Arhaliass, A.; Legrand, J.; Kaas, R.; Baron, R. Decrease in Dynamic Viscosity and Average Molecular Weight of Alginate from Laminaria Digitata during Alkaline Extraction1. J. Phycol. 2008, 44, 515–517. [Google Scholar] [CrossRef]
- Vauchel, P.; Kaas, R.; Arhaliass, A.; Baron, R.; Legrand, J. A New Process for Extracting Alginates from Laminaria Digitata: Reactive Extrusion. Food Bioprocess Technol. 2008, 1, 297–300. [Google Scholar] [CrossRef]
- Peteiro, C. Alginate Production from Marine Macroalgae, with Emphasis on Kelp Farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 27–66. ISBN 978-981-10-6910-9. [Google Scholar]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles–A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; montassir Dahmane, E.; Taourirte, M.; Brouillette, F. Extraction and Characterization of Sodium Alginate from Moroccan Laminaria Digitata Brown Seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Jain, D.; Bar-Shalom, D. Alginate Drug Delivery Systems: Application in Context of Pharmaceutical and Biomedical Research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Haugstad, K.E.; Håti, A.G.; Nordgård, C.T.; Adl, P.S.; Maurstad, G.; Sletmoen, M.; Draget, K.I.; Dias, R.S.; Stokke, B.T. Direct Determination of Chitosan–Mucin Interactions Using a Single-Molecule Strategy: Comparison to Alginate–Mucin Interactions. Polymers 2015, 7, 161–185. [Google Scholar] [CrossRef]
- Taylor, C.; Pearson, J.P.; Draget, K.I.; Dettmar, P.W.; Smidsrød, O. Rheological Characterisation of Mixed Gels of Mucin and Alginate. Carbohydr. Polym. 2005, 59, 189–195. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B.; et al. Technology Alginate-Based Composites for Environmental Applications: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Biomaterials Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Spasojevic, M.; Bhujbal, S.; Paredes, G.; de Haan, B.J.; Schouten, A.J.; de Vos, P. Considerations in Binding Diblock Copolymers on Hydrophilic Alginate Beads for Providing an Immunoprotective Membrane. J. Biomed. Mater. Res. Part. A 2014, 102, 1887–1896. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.; Gao, Y.; Cai, N.; Jiang, Z.; Xu, X. Anti-Inflammatory Activity of Guluronate Oligosaccharides Obtained by Oxidative Degradation from Alginate in Lipopolysaccharide-Activated Murine Macrophage RAW 264.7 Cells. J. Agric. Food Chem. 2015, 63, 160–168. [Google Scholar] [CrossRef]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant Activities of Sulfated Polysaccharides from Brown and Red Seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- Maciel, D.; Figueira, P.; Xiao, S.; Hu, D.; Shi, X.; Rodrigues, J.; Tomás, H.; Li, Y. Redox-Responsive Alginate Nanogels with Enhanced Anticancer Cytotoxicity. Biomacromolecules 2013, 14, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological Interactions between Polysaccharides and Divalent Cations: The Egg-Box Model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Crow, B.B.; Nelson, K.D. Release of Bovine Serum Albumin from a Hydrogel-Cored Biodegradable Polymer Fiber. Biopolymers 2006, 81, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically Crosslinked Alginate Hydrogels as Scaffolds for Tissue Engineering: Part 1. Structure, Gelation Rate and Mechanical Properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The Tensile Properties of Alginate Hydrogels. Biomaterials 2004, 25, 3187–3199. [Google Scholar] [CrossRef] [PubMed]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Cytotoxicity and Antibacterial Activity of Alginate Hydrogel Containing Nitric Oxide Donor and Silver Nanoparticles for Topical Applications. ACS Biomater. Sci. Eng. 2020, 6, 2117–2134. [Google Scholar] [CrossRef]
- Bruchet, M.; Melman, A. Fabrication of Patterned Calcium Cross-Linked Alginate Hydrogel Films and Coatings through Reductive Cation Exchange. Carbohydr. Polym. 2015, 131, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gattás-Asfura, K.M.; Fraker, C.A.; Stabler, C.L. Covalent Stabilization of Alginate Hydrogel Beads via Staudinger Ligation: Assessment of Poly(Ethylene Glycol) and Alginate Cross-Linkers. J. Biomed. Mater. Res. A 2011, 99, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Kulkarni, G.T.; Ramana, M.V.; de Jesus Andreoli Pinto, T.; Kikuchi, I.S.; Molim Ghisleni, D.D.; de Souza Braga, M.; De Bank, P.; Dua, K. Dual Crosslinked Pectin–Alginate Network as Sustained Release Hydrophilic Matrix for Repaglinide. Int. J. Biol. Macromol. 2017, 97, 721–732. [Google Scholar] [CrossRef]
- Eiselt, P.; Lee, K.Y.; Mooney, D.J. Rigidity of Two-Component Hydrogels Prepared from Alginate and Poly(Ethylene Glycol)-Diamines. Macromolecules 1999, 32, 5561–5566. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling Mechanical and Swelling Properties of Alginate Hydrogels Independently by Cross-Linker Type and Cross-Linking Density. Macromolecules 2000, 33, 4291–4294. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, X. Dual Crosslinked Methacrylated Alginate Hydrogel Micron Fibers and Tissue Constructs for Cell Biology. Mar. Drugs 2019, 17, 557. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Pacelli, S.; Paul, A. Self-Healing DNA-Based Injectable Hydrogels with Reversible Covalent Linkages for Controlled Drug Delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently Polysaccharide-Based Alginate/Chitosan Hydrogel Embedded Alginate Microspheres for BSA Encapsulation and Soft Tissue Engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Smeds, K.A.; Pfister-Serres, A.; Miki, D.; Dastgheib, K.; Inoue, M.; Hatchell, D.L.; Grinstaff, M.W. Photocrosslinkable Polysaccharides for in Situ Hydrogel Formation. J. Biomed. Mater. Res. 2001, 54, 115–121. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Berglund, C.M.; Lee, J.Y.; Polacheck, W.J.; Tsui, Y.; Bonassar, L.J.; Kirby, B.J. Methods for Photocrosslinking Alginate Hydrogel Scaffolds with High Cell Viability. Tissue Eng.-Part C Methods 2011, 17, 173–179. [Google Scholar] [CrossRef]
- Bonino, C.A.; Samorezov, J.E.; Jeon, O.; Alsberg, E.; Khan, S.A. Real-Time in Situ Rheology of Alginate Hydrogel Photocrosslinking. Soft Matter 2011, 7, 11510–11517. [Google Scholar] [CrossRef]
- Jeon, O.; Samorezov, J.E.; Alsberg, E. Single and Dual Crosslinked Oxidized Methacrylated Alginate/PEG Hydrogels for Bioadhesive Applications. Acta Biomater. 2014, 10, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked Alginate Hydrogels with Tunable Biodegradation Rates and Mechanical Properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Buono, P.; Duval, A.; Averous, L.; Habibi, Y. Lignin-Based Materials Through Thiol–Maleimide “Click” Polymerization. ChemSusChem 2017, 10, 984–992. [Google Scholar] [CrossRef]
- Jain, E.; Neal, S.; Graf, H.; Tan, X.; Balasubramaniam, R.; Huebsch, N. Copper-Free Azide–Alkyne Cycloaddition for Peptide Modification of Alginate Hydrogels. ACS Appl. Bio Mater. 2021, 4, 1229–1237. [Google Scholar] [CrossRef]
- Anugrah, D.S.B.; Ramesh, K.; Kim, M.; Hyun, K.; Lim, K.T. Near-Infrared Light-Responsive Alginate Hydrogels Based on Diselenide-Containing Cross-Linkage for on Demand Degradation and Drug Release. Carbohydr. Polym. 2019, 223, 115070. [Google Scholar] [CrossRef]
- García-Astrain, C.; Avérous, L. Synthesis and Evaluation of Functional Alginate Hydrogels Based on Click Chemistry for Drug Delivery Applications. Carbohydr. Polym. 2018, 190, 271–280. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, J.; Chen, X.; Dong, H.; Li, Q.; Zeng, L.; Cao, X. Alginate Based Antimicrobial Hydrogels Formed by Integrating Diels-Alder “Click Chemistry” and the Thiol-Ene Reaction. RSC Adv. 2018, 8, 11036–11042. [Google Scholar] [CrossRef]
- Lueckgen, A.; Garske, D.S.; Ellinghaus, A.; Desai, R.M.; Stafford, A.G.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Hydrolytically-Degradable Click-Crosslinked Alginate Hydrogels. Biomaterials 2018, 181, 189–198. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Shaw, J.E.; Arno, M.C.; Hoyland, J.A.; Richardson, S.M.; Dove, A.P. Robust Alginate/Hyaluronic Acid Thiol-Yne Click-Hydrogel Scaffolds with Superior Mechanical Performance and Stability for Load-Bearing Soft Tissue Engineering. Biomater. Sci. 2020, 8, 405–412. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future Perspectives and Recent Advances in Stimuli-Responsive Materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Zhao, S.; Cao, M.; Li, H.; Li, L.; Xu, W. Synthesis and Characterization of Thermo-Sensitive Semi-IPN Hydrogels Based on Poly(Ethylene Glycol)-Co-Poly($ε$-Caprolactone) Macromer, N-Isopropylacrylamide, and Sodium Alginate. Carbohydr. Res. 2010, 345, 425–431. [Google Scholar] [CrossRef]
- Bezerra, I.C.S.; de Freitas, E.D.; da Silva, M.G.C.; Vieira, M.G.A. Synthesis and Characterization of Furosemide-Loaded Sericin/Alginate Beads Subjected to Thermal or Chemical Cross-Linking for Delayed and Sustained Release. Polym. Adv. Technol. 2021, 32, 461–473. [Google Scholar] [CrossRef]
- Yu, J.; Du, K.T.; Fang, Q.; Gu, Y.; Mihardja, S.S.; Sievers, R.E.; Wu, J.C.; Lee, R.J. The Use of Human Mesenchymal Stem Cells Encapsulated in RGD Modified Alginate Microspheres in the Repair of Myocardial Infarction in the Rat. Biomaterials 2010, 31, 7012–7020. [Google Scholar] [CrossRef]
- Fonseca, K.B.; Bidarra, S.J.; Oliveira, M.J.; Granja, P.L.; Barrias, C.C. Molecularly Designed Alginate Hydrogels Susceptible to Local Proteolysis as Three-Dimensional Cellular Microenvironments. Acta Biomater. 2011, 7, 1674–1682. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. PH-Sensitive Sodium Alginate/Poly(Vinyl Alcohol) Hydrogel Beads Prepared by Combined Ca2+ Crosslinking and Freeze-Thawing Cycles for Controlled Release of Diclofenac Sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Tkalec, G.; Kranvogl, R.; Perva Uzunalić, A.; Knez, Ž.; Novak, Z. Optimisation of Critical Parameters during Alginate Aerogels’ Production. J. Non-Cryst. Solids 2016, 443, 112–117. [Google Scholar] [CrossRef]
- Mattiasson, B.; Kumar, A.; Galeaev, I.Y. Macroporous Polymers: Production Properties and Biotechnological/Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2009; ISBN 1420084623. [Google Scholar]
- Subrahmanyam, R.; Gurikov, P.; Meissner, I.; Smirnova, I. Preparation of Biopolymer Aerogels Using Green Solvents. J. Vis. Exp. 2016, 113, e54116. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Non-Conventional Methods for Gelation of Alginate. Gels 2018, 4, 14. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Torras, J.; Casanovas, J.; Häring, M.; Alemán, C.; Díaz, D.D. Paradigm Shift for Preparing Versatile M2+-Free Gels from Unmodified Sodium Alginate. Biomacromolecules 2017, 18, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Király, G.; Egu, J.C.; Hargitai, Z.; Kovács, I.; Fábián, I.; Kalmár, J.; Szemán-Nagy, G. Mesoporous Aerogel Microparticles Injected into the Abdominal Cavity of Mice Accumulate in Parathymic Lymph Nodes. Int. J. Mol. Sci. 2021, 22, 9756. [Google Scholar] [CrossRef] [PubMed]
- Vasvári, G.; Kalmár, J.; Veres, P.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Haimhoffer, Á.; Rusznyák, Á.; Fenyvesi, F.; et al. Matrix Systems for Oral Drug Delivery: Formulations and Drug Release. Drug Discov. Today Technol. 2018, 27, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Veres, P.; Sebők, D.; Dékány, I.; Gurikov, P.; Smirnova, I.; Fábián, I.; Kalmár, J. A Redox Strategy to Tailor the Release Properties of Fe(III)-Alginate Aerogels for Oral Drug Delivery. Carbohydr. Polym. 2018, 188, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lovskaya, D.; Menshutina, N. Alginate-Based Aerogel Particles as Drug Delivery Systems: Investigation of the Supercritical Adsorption and In Vitro Evaluations. Materials 2020, 13, 329. [Google Scholar] [CrossRef]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Gurikov, P.; Smirnova, I.; Leopold, C.S. Pulmonary Drug Delivery with Aerogels: Engineering of Alginate and Alginate–Hyaluronic Acid Microspheres. Pharm. Dev. Technol. 2021, 26, 509–521. [Google Scholar] [CrossRef]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and Hybrid Alginate-Hyaluronic Acid Aerogel Microspheres as Potential Carrier for Pulmonary Drug Delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- Sugiura, S.; Oda, T.; Izumida, Y.; Aoyagi, Y. Size Control of Calcium Alginate Beads Containing Living Cells Using Micro-Nozzle Array. Biomaterials 2005, 26, 3327–3331. [Google Scholar] [CrossRef]
- Silva, C.M.; Ribeiro, J.; Vit, I. Alginate Microspheres Prepared by Internal Gelation: Development and Effect on Insulin Stability. Int. J. Pharm. 2006, 311, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ouerghi, O.; Geesi, M.H.; Ibnouf, E.O.; Ansari, M.J.; Alam, P.; Elsanousi, A.; Kaiba, A.; Riadi, Y. Sol-Gel Synthesized Rutile TiO2 Nanoparticles Loaded with Cardamom Essential Oil: Enhanced Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2021, 64, 102581. [Google Scholar] [CrossRef]
- Ansari, M.J. Factors Affecting Preparation and Properties of Nanoparticles by Nanoprecipitation Method. Indo Am. J. Pharm. Sci. 2017, 4, 4854–4858. [Google Scholar]
- Fundueanu, G.; Nastruzzi, C.; Carpov, A.; Desbrieres, J.; Rinaudo, M. Physico-Chemical Characterization of Ca-Alginate Microparticles Produced with Different Methods. Biomaterials 1999, 20, 1427–1435. [Google Scholar] [CrossRef]
- Seifert, D.B.; Phillips, J.A. Production of Small, Monodispersed Alginate Beads for Cell Immobilization. Biotechnol. Prog. 2008, 13, 562–568. [Google Scholar] [CrossRef]
- Andriola, A.K.; Brun-graeppi, S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O. Cell Microcarriers and Microcapsules of Stimuli-Responsive Polymers. J. Control. Release 2011, 149, 209–224. [Google Scholar] [CrossRef]
- Zimmermann, H.; Shirley, S.G.; Zimmermann, U. Alginate-Based Encapsulation of Cells: Past, Present, and Future. Curr. Diab. Rep. 2007, 7, 314–320. [Google Scholar] [CrossRef]
- Reis, C.P.; Neufeld, R.J.; Vilela, S.; Ribeiro, A.J.; Veiga, F. Review and Current Status of Emulsion/Dispersion Technology Using an Internal Gelation Process for the Design of Alginate Particles. J. Microencapsul. 2006, 23, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, A.; Agrawal, S.S.; Ray, A.R. Controlled Delivery of Drugs from Alginate Matrix. J. Macromol. Sci.-Polym. Rev. 2003, 43, 187–221. [Google Scholar] [CrossRef]
- Patel, M.A.; AbouGhaly, M.H.H.; Schryer-Praga, J.V.; Chadwick, K. The Effect of Ionotropic Gelation Residence Time on Alginate Cross-Linking and Properties. Carbohydr. Polym. 2017, 155, 362–371. [Google Scholar] [CrossRef]
- Poncelet, D.; Lencki, R.; Beaulieu, C.; Halle, J.P.; Neufeld, R.J.; Fournier, A. Production of Alginate Beads by Emulsification/Internal Gelation. I. Methodology. Appl. Microbiol. Biotechnol. 1992, 38, 39–45. [Google Scholar] [CrossRef]
- Poncelet, D. Production of Alginate Beads by Emulsification/Internal Gelation. Ann. N. Y. Acad. Sci. 2001, 944, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sæther, H.V.; Holme, H.K.; Maurstad, G.; Smidsrød, O.; Stokke, B.T. Polyelectrolyte Complex Formation Using Alginate and Chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar] [CrossRef]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a New Drug Carrier Made from Alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmed, M.M.; Fatima; Anwer, M.K.; Jamil, S.; Al-Shdefat, R.; Ali, B. Solubility and Stability Enhancement of Curcumin through Cyclodextrin Complexation. Int. J. Biol. Pharm. Allied Sci. 2014, 3, 2668–2675. [Google Scholar]
- Dawoud, M.; Abourehab, M.A.S.; Abdou, R. Monoolein Cubic Nanoparticles as Novel Carriers for Docetaxel. J. Drug Deliv. Sci. Technol. 2020, 56, 101501. [Google Scholar] [CrossRef]
- El-aziz, E.A.; Elgayar, S.F.; Mady, F.M.; Abourehab, M.A.S.; Hasan, O.A.; Reda, L.M.; Alaaeldin, E. The Potential of Optimized Liposomes in Enhancement of Cytotoxicity and Apoptosis of Encapsulated Egyptian Propolis on Hep-2 Cell Line. Pharmaceutics 2021, 13, 2184. [Google Scholar] [CrossRef]
- Ansari, M.J.; Alshetaili, A.; Aldayel, I.A.; Alablan, F.M.; Alsulays, B.; Alshahrani, S.; Alalaiwe, A.; Ansari, M.N.; Ur Rehman, N.; Shakeel, F. Formulation, Characterization, in Vitro and in Vivo Evaluations of Self-Nanoemulsifying Drug Delivery System of Luteolin. J. Taibah Univ. Sci. 2020, 14, 1386–1401. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, M.C.; Kang, S.M.; Montemagno, C.D. The Osmotic Stress Response of Split Influenza Vaccine Particles in an Acidic Environment. Arch. Pharm. Res. 2014, 37, 1607–1616. [Google Scholar] [CrossRef]
- Araújo, F.; das Neves, J.; Martins, J.P.; Granja, P.L.; Santos, H.A.; Sarmento, B. Functionalized Materials for Multistage Platforms in the Oral Delivery of Biopharmaceuticals. Prog. Mater. Sci. 2017, 89, 306–344. [Google Scholar] [CrossRef]
- Ansari, M.J. Oral Delivery of Insulin for Treatment of Diabetes: Classical Challenges and Current Opportunities. J. Med. Sci. 2015, 15, 209–220. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan Nanoparticles for Oral Drug and Gene Delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, H.; Ozay, O. Synthesis and Characterization of PH Responsive Alginate Based-Hydrogels as Oral Drug Delivery Carrier. J. Polym. Res. 2020, 27, 251. [Google Scholar]
- Ayub, A.D.; Chiu, H.I.; Yusuf, S.N.A.M.; Kadir, E.A.; Ngalim, S.H.; Lim, V. Biocompatible Disulphide Cross-Linked Sodium Alginate Derivative Nanoparticles for Oral Colon-Targeted Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Cong, A.Z.; Shi, Y.; Wang, Y. A Novel Controlled Drug Delivery System Based on Alginate Hydrogel / Chitosan Micelle Composites. Int. J. Biol. Macromol. 2017, 107, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Van De Ven, H.; Paulussen, C.; Feijens, P.B.; Matheeussen, A.; Rombaut, P.; Kayaert, P.; Mooter, G. Van Den PLGA Nanoparticles and Nanosuspensions with Amphotericin B: Potent in Vitro and in Vivo Alternatives to Fungizone and AmBisome. J. Control. Release 2012, 161, 795–803. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Khames, A.; Genedy, S.; Mostafa, S.; Khaleel, M.A.; Omar, M.M.; El Sisi, A.M. Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection. Pharmaceutics 2021, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Barradas, N.; Cardoso, S.; Castiglione, T.C.; Michael, J.; Gyselle, K.; Holanda, D.; Regina, C.; Mansur, E. Dual Alginate-Lipid Nanocarriers as Oral Delivery Systems for Amphotericin B. Colloids Surf. B Biointerfaces 2018, 166, 187–194. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Ooi, S.Y.; Ahmad, I.; Cairul, M.; Mohd, I. Cellulose Nanocrystals Extracted from Rice Husks as a Reinforcing Material in Gelatin Hydrogels for Use in Controlled Drug Delivery Systems. Ind. Crop. Prod. 2015, 93, 227–234. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Colloids and Surfaces B: Biointerfaces Effect of Polysaccharide Nanocrystals on Structure, Properties, and Drug Release Kinetics of Alginate-Based Microspheres. Colloids Surf. B Biointerfaces 2011, 85, 270–279. [Google Scholar] [CrossRef]
- Thomas, D.; Latha, M.S.; Thomas, K.K. Journal of Drug Delivery Science and Technology Synthesis and in Vitro Evaluation of Alginate-Cellulose Nanocrystal Hybrid Nanoparticles for the Controlled Oral Delivery of Rifampicin. J. Drug Deliv. Sci. Technol. 2018, 46, 392–399. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Matshaya, T.; Werner, R.; Krause, M. Cationic Cyclodextrin/Alginate Chitosan Nano Fl Owers as 5- Fl Uorouracil Drug Delivery System. Mater. Sci. Eng. C 2017, 70, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pitchaya Treenate, P.M. In Vitro Drug Release Profiles of PH-Sensitive Hydroxyethylacryl Chitosan/Sodium Alginate Hydrogels Using Paracetamol as a Soluble Model Drug. Int J Biol Macromol 2017, 99, 71–78. [Google Scholar] [CrossRef]
- Agili, F.A.; Aly, S.F.M. Physicochemical Characterization and Release Properties of Oral Drug Delivery: A PH-Sensitive Nanocomposite Based on Sodium Alginate–Pectin–Tannic Acid–Silver. Polym. Polym. Compos. 2019, 28, 598–608. [Google Scholar] [CrossRef]
- Olayemi, O.J.; Apeji, Y.E.; Isimi, C.Y. Formulation and Evaluation of Cyperus Esculentus (Tiger Nut) Starch-Alginate Microbeads in the Oral Delivery of Ibuprofen. J. Pharm. Innov. 2020, 17, 366–375. [Google Scholar] [CrossRef]
- Chegeni, M.; Rozbahani, Z.S.; Ghasemian, M.; Mehri, M. International Journal of Biological Macromolecules Synthesis and Application of the Calcium Alginate / SWCNT-Gl as a Bio-Nanocomposite for the Curcumin Delivery. Int. J. Biol. Macromol. 2020, 156, 504–513. [Google Scholar] [CrossRef]
- Mao, X.; Li, X.; Zhang, W.; Yuan, L.; Deng, L.; Ge, L.; Mu, C.; Li, D. Development of Microspheres Based on Thiol-Modi Fi Ed Sodium Alginate for Intestinal-Targeted Drug Delivery. ACS Appl. Bio Mater. 2019, 2, 5810–5818. [Google Scholar] [CrossRef]
- Long, L.; Lai, M.; Ke, Z.; Yang, L. Investigation Of Vitamin B 12 -Modi Fi Ed Amphiphilic Sodium Alginate Derivatives For Enhancing The Oral Delivery Ef Fi Cacy Of Peptide Drugs. Int. J. Nanomed. 2019, 14, 7743–7758. [Google Scholar] [CrossRef]
- Chiu, H.I.; Ayub, A.D.; Nur, S.; Mat, A.; Yahaya, N. Docetaxel-Loaded Disulfide Cross-Linked Nanoparticles Derived from Thiolated Sodium Alginate for Colon Cancer Drug Delivery. Pharmaceutics 2020, 12, 38. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Ibrahim, M.A.; Amin, M.A. Cetuximab Conjugated with Octreotide and Entrapped Calcium Alginate-Beads for Targeting Somatostatin Receptors. Sci. Rep. 2020, 10, 4736. [Google Scholar] [CrossRef]
- Kahya, N.; Gölcü, A.; Erim, F.B. Barium Ion Cross-Linked Alginate-Carboxymethyl Cellulose Composites for Controlled Release of Anticancer Drug Methotrexate. J. Drug Deliv. Sci. Technol. 2019, 54, 101324. [Google Scholar] [CrossRef]
- Shamekhi, F.; Tamjid, E.; Khajeh, K. Development of Chitosan Coated Calcium-Alginate Nanocapsules for Oral Delivery of Liraglutide to Diabetic Patients. Int. J. Biol. Macromol. 2018, 120, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Karzar Jeddi, M.; Mahkam, M. Magnetic Nano Carboxymethyl Cellulose-Alginate/Chitosan Hydrogel Beads as Biodegradable Devices for Controlled Drug Delivery. Int. J. Biol. Macromol. 2019, 135, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Urtti, A. Challenges and Obstacles of Ocular Pharmacokinetics and Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- De, M.; Raviña, M.; Paolicelli, P.; Sanchez, A.; Seijo, B.; Jose, M. Chitosan-Based Nanostructures: A Delivery Platform for Ocular Therapeutics . Adv. Drug Deliv. Rev. 2010, 62, 100–117. [Google Scholar] [CrossRef]
- Rupenthal, I.D.; O’Rourke, M. Ocular Drug Delivery—Eye on Innovation. Drug Deliv. Transl. Res. 2016, 6, 631–633. [Google Scholar] [CrossRef]
- Pedraz, L.; Wahlberg, L.U.; De Vos, P.; Emerich, D. Cell Encapsulation: Technical and Clinical Advances. Trends Pharmacol. Sci. 2015, 36, 537–546. [Google Scholar] [CrossRef]
- Siu, F.; Wong, Y.; Kin, K.; Man, A.; Chu, W.; Pui, B.; Ming, K.; Cheuk, A.; Lo, Y. Biomaterials Injectable Cell-Encapsulating Composite Alginate-Collagen Platform with Inducible Termination Switch for Safer Ocular Drug Delivery. Biomaterials 2019, 201, 53–67. [Google Scholar] [CrossRef]
- Patel, A. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Shelley, H.; Rodriguez-Galarza, R.M.; Duran, S.H.; Abarca, E.M.; Babu, R.J. In Situ Gel Formulation for Enhanced Ocular Delivery of Nepafenac. J. Pharm. Sci. 2018, 107, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Nagarwal, R.C.; Kumar, R.; Pandit, J.K. Chitosan Coated Sodium Alginate-Chitosan Nanoparticles Loaded with 5-FU for Ocular Delivery: In Vitro Characterization and in Vivo Study in Rabbit Eye. Eur. J. Pharm. Sci. 2012, 47, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Ghumman, S.A.; Batool, F.; Ijaz, B.; Basharat, M.; Noureen, S.; Kausar, T.; Iqbal, S. Terminalia Arjuna Gum/Alginate in Situ Gel System with Prolonged Retention Time for Ophthalmic Drug Delivery. Int. J. Biol. Macromol. 2020, 152, 1056–1067. [Google Scholar] [CrossRef]
- Polat, H.K.; Pehlivan, S.B.; Özkul, C.; Çalamak, S.; Aytekin, E.; Fırat, A.; Ulubayram, K.; Kocabeyoğlu, S. Development of Besifloxacin HCl Loaded Nanofibrous Ocular Inserts for the Treatment of Bacterial Keratitis: In Vitro, Ex Vivo and in Vivo Evaluation. Int. J. Pharm. 2020, 585, 119552. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Pinto, L.F.V.; Bozukova, D.; Santos, L.F.; Serro, A.P.; Saramago, B. Chitosan/Alginate Based Multilayers to Control Drug Release from Ophthalmic Lens. Colloids Surf. B Biointerfaces 2016, 147, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Potential Chitosan-Coated Alginate Nanoparticles for Ocular Delivery of Daptomycin. Eur. J. Clin. Microbiol. 2015, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chauhan, N.; Gopal, M.; Kumar, R. International Journal of Biological Macromolecules Development and Evaluation of Alginate—Chitosan Nanocapsules for Controlled Release of Acetamiprid. Int. J. Biol. Macromol. 2015, 81, 631–637. [Google Scholar] [CrossRef]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A. An Alternative in Situ Gel-Formulation of Levofloxacin Eye Drops for Prolong Ocular Retention. J. Pharm. Bioallied Sci. 2015, 7, 9–14. [Google Scholar] [CrossRef]
- Gökçe, E.H.; Okur, N.Ü. Novel Nanostructured Lipid Carrier Based Flurbiprofen Loaded Sodium Alginate Inserts for Ocular Drug Delivery. Lat. Am. J. Pharm. 2016, 35, 972–979. [Google Scholar]
- Mokhtari, S.; Jafari, S.M.; Assadpour, E. Development of a Nutraceutical Nano-Delivery System through Emulsification/Internal Gelation of Alginate. Food Chem. 2017, 229, 286–295. [Google Scholar] [CrossRef]
- Khan, N.; Aqil, M.; Ameeduzzafar; Imam, S.S.; Ali, A. Development and Evaluation of a Novel in Situ Gel of Sparfloxacin for Sustained Ocular Drug Delivery: In Vitro and Ex Vivo Characterization. Pharm. Dev. Technol. 2015, 20, 662–669. [Google Scholar] [CrossRef]
- Reed, K.; Lankford, M. Calcium Gluconatee Mediated In-Situ Gelling of Alginates for Ocular Drug Delivery. J. Am. Pharm. Assoc. 2018, 58, e49. [Google Scholar]
- Xu, W.; Liu, K.; Li, T.; Zhang, W.; Dong, Y.; Lv, J.; Wang, W.; Sun, J.; Li, M.; Wang, M.; et al. An in Situ Hydrogel Based on Carboxymethyl Chitosan and Sodium Alginate Dialdehyde for Corneal Wound Healing after Alkali Burn. J. Biomed. Mater. Res. Part A 2019, 107, 742–754. [Google Scholar] [CrossRef]
- Wafa, H.G.; Essa, E.A.; El-Sisi, A.E.; El Maghraby, G.M. Ocular Films versus Film-Forming Liquid Systems for Enhanced Ocular Drug Delivery. Drug Deliv. Transl. Res. 2021, 11, 1084–1095. [Google Scholar] [CrossRef]
- Shinde, U.A.; Shete, J.N.; Nair, H.A.; Singh, K.H. Design and Characterization of Chitosan-Alginate Microspheres for Ocular Delivery of Azelastine. Pharm. Dev. Technol. 2014, 19, 813–823. [Google Scholar] [CrossRef]
- Sadeghi, A.M.; Farjadian, F.; Alipour, S. Sustained Release of Linezolid in Ocular Insert Based on Lipophilic Modified Structure of Sodium Alginate. Iran. J. Basic Med. Sci. 2021, 24, 331–340. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Elaissari, A.; Fessi, H. Lipid-Based Carriers: Manufacturing and Applications for Pulmonary Route. Expert Opin. Drug Deliv. 2012, 9, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for Drug Delivery to the Lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.R.; Kumaran, S.; Banerjee, A.; Berlinski, A. A Hybrid in Vitro in Silico Framework for Albuterol Delivery through an Adult Ventilator Circuit to a Patient-Specific Lung Airway Model. J. Aerosol Sci. 2021, 158, 105844. [Google Scholar] [CrossRef]
- Rajendran, R.R.; Banerjee, A. Mucus Transport and Distribution by Steady Expiration in an Idealized Airway Geometry. Med. Eng. Phys. 2019, 66, 26–39. [Google Scholar] [CrossRef]
- Rajendran, R.R.; Banerjee, A. Effect of Non-Newtonian Dynamics on the Clearance of Mucus From Bifurcating Lung Airway Models. J. Biomech. Eng. 2020, 143, 021011. [Google Scholar] [CrossRef]
- Dolovich, M.B.; Dhand, R. Aerosol Drug Delivery: Developments in Device Design and Clinical Use. Lancet 2011, 377, 19–25. [Google Scholar] [CrossRef]
- Yhee, J.; Im, J.; Nho, R. Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. J. Clin. Med. 2016, 5, 82. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elkasabgy, N.A.; Abdelkhalek, A.A. Design and Characterization of Emulsified Spray Dried Alginate Microparticles as a Carrier for the Dually Acting Drug Roflumilast. Eur. J. Pharm. Sci. 2018, 122, 64–76. [Google Scholar] [CrossRef]
- Alsmadi, M.M.; Obaidat, R.M.; Alnaief, M.; Albiss, B.A.; Hailat, N. Development, In Vitro Characterization, and In Vivo Toxicity Evaluation of Chitosan-Alginate Nanoporous Carriers Loaded with Cisplatin for Lung Cancer Treatment. AAPS PharmSciTech 2020, 21, 191. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the Printer to the Lungs: Inkjet-Printed Aerogel Particles for Pulmonary Delivery. Chem. Eng. J. 2019, 357, 559–566. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.M.; Alsmadi, M.M. Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers 2020, 12, 2223. [Google Scholar] [CrossRef]
- Dulhanty, A.M.; Riordan, J.R. Mutation of Potential Phosphorylation Sites in the Recombinant R Domain of the Cystic Fibrosis Transmembrane Conductance Regulator Has Significant Effects on Domain Conformation. Biochem. Biophys. Res. Commun. 1995, 206, 207–214. [Google Scholar] [CrossRef]
- Haley, C.L.; Colmer-Hamood, J.A.; Hamood, A.N. Characterization of Biofilm-like Structures Formed by Pseudomonas Aeruginosa in a Synthetic Mucus Medium. BMC Microbiol. 2012, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- May, T.B.; Shinabarger, D.; Maharaj, R.; Kato, J.; Chu, L.; Devault, J.D.; Roychoudhury, S.; Zielinski, N.A.; Berry, A.; Rothmel, R.K.; et al. Alginate Synthesis by Pseudomonas Aeruginosa: A Key Pathogenic Factor in Chronic Pulmonary Infections of Cystic Fibrosis Patients. Clin. Microbiol. Rev. 1991, 4, 191–206. [Google Scholar] [CrossRef]
- Selimoglu, E. Aminoglycoside-Induced Ototoxicity. Curr. Pharm. Des. 2007, 13, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kumin, G.D. Clinical Nephrotoxicity of Tobramycin and Gentamicin: A Prospective Study. JAMA 1980, 244, 1808–1810. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Twigg, M.; Sheridan, E.A.; Hardy, J.G.; Elborn, J.S.; Taggart, C.C.; Scott, C.J.; Migaud, M.E. Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications. Pharmaceutics 2019, 11, 379. [Google Scholar] [CrossRef]
- Scolari, I.R.; Páez, P.L.; Musri, M.M.; Petiti, J.P.; Torres, A.; Granero, G.E. Rifampicin Loaded in Alginate/Chitosan Nanoparticles as a Promising Pulmonary Carrier against Staphylococcus Aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Cheaburu-Yilmaz, C.N.; Lupuşoru, C.E.; Vasile, C. New Alginate/PNIPAAm Matrices for Drug Delivery. Polymers 2019, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Hendradi, E.; Kurniawan, T.D. Alginate Microspheres Encapsulating Ciprofloxacin HCl: Characteristics, Release and Antibacterial Activity. Int. J. Pharma Res. Health Sci. 2019, 7, 3020–3027. [Google Scholar] [CrossRef]
- Longuinho, M.M.; Leitão, S.G.; Silva, R.S.F.; Silva, P.E.A.; Rossi, A.L.; Finotelli, P. V Lapazine Loaded Alginate/Chitosan Microparticles: Enhancement of Anti-Mycobacterium Activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101292. [Google Scholar] [CrossRef]
- Islan, G.A.; Ruiz, M.E.; Morales, J.F.; Sbaraglini, M.L.; Enrique, A.V.; Burton, G.; Talevi, A.; Bruno-Blanch, L.E.; Castro, G.R. Hybrid Inhalable Microparticles for Dual Controlled Release of Levofloxacin and DNase: Physicochemical Characterization and in Vivo Targeted Delivery to the Lungs. J. Mater. Chem. B 2017, 5, 3132–3144. [Google Scholar] [CrossRef]
- Tao, L.; Jiang, J.; Gao, Y.; Wu, C.; Liu, Y. Biodegradable Alginate-Chitosan Hollow Nanospheres for Codelivery of Doxorubicin and Paclitaxel for the Effect of Human Lung Cancer A549 Cells. Biomed. Res. Int. 2018, 2018, 4607945. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.; Parumasivam, T.; Pang, A.; Roediger, B.; Tang, P.; Jahn, K.; Britton, W.J.; Chan, H.K. Alginate Modified-PLGA Nanoparticles Entrapping Amikacin and Moxifloxacin as a Novel Host-Directed Therapy for Multidrug-Resistant Tuberculosis. J. Drug Deliv. Sci. Technol. 2019, 52, 642–651. [Google Scholar] [CrossRef]
- Nagpal, P.S.; Kesarwani, A.; Sahu, P.; Upadhyay, P. Aerosol Immunization by Alginate Coated Mycobacterium (BCG/MIP) Particles Provide Enhanced Immune Response and Protective Efficacy than Aerosol of Plain Mycobacterium against M.Tb. H37Rv Infection in Mice. BMC Infect. Dis. 2019, 19, 568. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, F.; Wang, D.; Yang, Z.; Yao, C.; Ye, Y.; Wang, X. Chitosan-Alginate BSA-Gel-Capsules for Local Chemotherapy against Drug-Resistant Breast Cancer. Drug Des. Devel. Ther. 2018, 12, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, S.; Cardozo, L. The Vagina as a Route for Drug Delivery: A Review. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2013, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Vermani, K.; Garg, S. The Scope and Potential of Vaginal Drug Delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364. [Google Scholar] [CrossRef]
- Deshpande, A.A.; Rhodes, C.T.; Danish, M. Intravaginal Drug Delivery. Drug Dev. Ind. Pharm. 1992, 18, 1225–1279. [Google Scholar] [CrossRef]
- Meng, J.; Agrahari, V.; Ezoulin, M.J.; Purohit, S.S.; Zhang, T.; Molteni, A.; Dim, D.; Oyler, N.A.; Youan, B.B.C. Spray-Dried Thiolated Chitosan-Coated Sodium Alginate Multilayer Microparticles for Vaginal HIV Microbicide Delivery. AAPS J. 2017, 19, 692–702. [Google Scholar] [CrossRef]
- Maestrelli, F.; Jug, M.; Cirri, M.; Kosalec, I.; Mura, P. Characterization and Microbiological Evaluation of Chitosan-Alginate Microspheres for Cefixime Vaginal Administration. Carbohydr. Polym. 2018, 192, 176–183. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Perez, T.A.; Pedreiro, L.N.; Prezotti, F.G.; Boni, F.I.; Cardoso, V.M.d.O.; Venâncio, T.; Gremião, M.P.D. A Novel PH-Responsive Hydrogel-Based on Calcium Alginate Engineered by the Previous Formation of Polyelectrolyte Complexes (PECs) Intended to Vaginal Administration. Drug Dev. Ind. Pharm. 2017, 43, 1656–1668. [Google Scholar] [CrossRef]
- Jadhav, K.R.; Kadam, V.J.; Pisal, S.S. Formulation and Evaluation of Lecithin Organogel for Topical Delivery of Fluconazole. Curr. Drug. Deliv. 2009, 6, 174–183. [Google Scholar] [CrossRef]
- Bayomi, M.A. Role of Chitosan on Controlling the Characteristics and Antifungal Activity of Bioadhesive Fluconazole Vaginal Tablets. Saudi. Pharm. J. 2017, 26, 151–161. [Google Scholar] [CrossRef]
- Darwesh, B.; Aldawsari, H.M.; Badr-Eldin, S.M. Optimized Chitosan/Anion Polyelectrolyte Complex Based Inserts for Vaginal Delivery of Fluconazole: In Vitro/in Vivo Evaluation. Pharmaceutics 2018, 10, 227. [Google Scholar] [CrossRef]
- Soliman, G.M.; Fetih, G.; Abbas, A.M. Thermosensitive Bioadhesive Gels for the Vaginal Delivery of Sildenafil Citrate: In Vitro Characterization and Clinical Evaluation in Women Using Clomiphene Citrate for Induction of Ovulation. Drug. Dev. Ind. Pharm. 2017, 43, 399–408. [Google Scholar] [CrossRef]
- Hassan, A.; Soliman, G.; Ali, M.; El-Gindy, G. Mucoadhesive Tablets for the Vaginal Delivery of Progesterone: In Vitro Evaluation and Pharmacokinetics/Pharmacodynamics in Female Rabbits. Drug. Dev. Ind. Pharm. 2017, 44, 224–232. [Google Scholar] [CrossRef]
- Kamel, R.; Abbas, H. A Multi-Microcarrier of Metronidazole-Biopolymers Complexes as a Potential Vaginal Delivery System. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 239–246. [Google Scholar] [CrossRef]
- Yang, T.-T.; Cheng, Y.-Z.; Qin, M.; Wang, Y.-H.; Yu, H.-L.; Wang, A.-L.; Zhang, W.-F. Thermosensitive Chitosan Hydrogels Containing Polymeric Microspheres for Vaginal Drug Delivery. Biomed. Res. Int. 2017, 2017, 3564060. [Google Scholar] [CrossRef]
- Gafitanu, C.A.; Filip, D.; Cernatescu, C.; Rusu, D.; Tuchilus, C.G.; Macocinschi, D.; Zaltariov, M. Design, Preparation and Evaluation of HPMC-Based PAA or SA Freeze-Dried Scaffolds for Vaginal Delivery of Fluconazole. Pharm. Res. 2017, 34, 2185–2196. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Scuota, S.; Bazzucchi, V.; Mura, P. Development and Microbiological Evaluation of Chitosan and Chitosan-Alginate Microspheres for Vaginal Administration of Metronidazole. Int. J. Pharm. 2021, 598, 120375. [Google Scholar] [CrossRef]
- Ghosal, K.; Ranjan, A.; Brata, B. A Novel Vaginal Drug Delivery System: Anti-HIV Bioadhesive Film Containing Abacavir. J. Mater. Sci. Mater. Med. 2014, 25, 1679–1689. [Google Scholar] [CrossRef]
- Nematpour, N.; Moradipour, P.; Mahdi, M.; Arkan, E. Materials Science & Engineering C The Application of Nanomaterial Science in the Formulation a Novel Antibiotic: Assessment of the Antifungal Properties of Mucoadhesive Clotrimazole Loaded Nano Fi Ber versus Vaginal Fi Lms. Mater. Sci. Eng. C 2020, 110, 110635. [Google Scholar] [CrossRef]
- Gnaman, K.C.N.G.; Bouttier, S.; Yeo, A.; Any-grah, A.A.S.A.; Geiger, S. Characterization and in Vitro Evaluation of a Vaginal Gel Containing Lactobacillus Crispatus for the Prevention of Gonorrhea. Int. J. Pharm. 2020, 588, 119733. [Google Scholar] [CrossRef]
- da Silva Campelo, M.; Melo, E.O.; Arrais, S.P.; do Nascimento, F.B.S.A.; Gramosa, N.V.; de Aguiar Soares, S.; Ribeiro, M.E.N.P.; da Silva, C.R.; Júnior, H.V.N.; Ricardo, N.M.P.S. Clove Essential Oil Encapsulated on Nanocarrier Based on Polysaccharide: A Strategy for the Treatment of Vaginal Candidiasis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125732. [Google Scholar] [CrossRef]
- Tentor, F.; Siccardi, G.; Sacco, P.; Demarchi, D.; Marsich, E.; Almdal, K. Long Lasting Mucoadhesive Membrane Based on Alginate and Chitosan for Intravaginal Drug Delivery. J. Mater. Sci. Mater. Med. 2020, 31, 25. [Google Scholar] [CrossRef]
- Kissel, T.; Werner, U. Nasal Delivery of Peptides: An in Vitro Cell Culture Model for the Investigation of Transport and Metabolism in Human Nasal Epithelium. J. Control. Release 1998, 53, 195–203. [Google Scholar] [CrossRef]
- Illum, L. Nasal Drug Delivery-Recent Developments and Future Prospects. J. Control. Release 2012, 161, 254–263. [Google Scholar] [CrossRef]
- Fortuna, A.; Alves, G.; Serralheiro, A.; Sousa, J.; Falcão, A. Intranasal Delivery of Systemic-Acting Drugs: Small-Molecules and Biomacromolecules. Eur. J. Pharm. Biopharm. 2014, 88, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Dukovski, B.J.; Plantić, I.; Čunčić, I.; Krtalić, I.; Juretić, M.; Pepić, I.; Lovrić, J.; Hafner, A. Lipid/Alginate Nanoparticle-Loaded in Situ Gelling System Tailored for Dexamethasone Nasal Delivery. Int. J. Pharm. 2017, 533, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, S.; Kheiri, M.T.; Abnous, K.; Eskandari, M.; Tafaghodi, M. Preparation, Characterization and Immunological Evaluation of Alginate Nanoparticles Loaded with Whole Inactivated Influenza Virus: Dry Powder Formulation for Nasal Immunization in Rabbits. Microb. Pathog. 2017, 115, 74–85. [Google Scholar] [CrossRef]
- Rao, M.; Agrawal, D.K.; Shirsath, C. Thermoreversible Mucoadhesive in Situ Nasal Gel for Treatment of Parkinson’s Disease. Drug Dev. Ind. Pharm. 2017, 43, 142–150. [Google Scholar] [CrossRef]
- Youssef, N.A.H.A.; Kassem, A.A.; Farid, R.M.; Ismail, F.A.; EL-Massik, M.A.E.; Boraie, N.A. A Novel Nasal Almotriptan Loaded Solid Lipid Nanoparticles in Mucoadhesive in Situ Gel Formulation for Brain Targeting: Preparation, Characterization and in Vivo Evaluation. Int. J. Pharm. 2018, 548, 609–624. [Google Scholar] [CrossRef]
- Amorij, J.P.; Hinrichs, W.L.J.; Frijlink, H.W.; Wilschut, J.C.; Huckriede, A. Needle-Free Influenza Vaccination. Lancet Infect. Dis. 2010, 10, 699–711. [Google Scholar] [CrossRef]
- Saluja, V.; Amorij, J.P.; Van Roosmalen, M.L.; Leenhouts, K.; Huckriede, A.; Hinrichs, W.L.J.; Frijlink, H.W. Intranasal Delivery of Influenza Subunit Vaccine Formulated with GEM Particles as an Adjuvant. AAPS J. 2010, 12, 109–116. [Google Scholar] [CrossRef]
- Amin, M.; Jaafari, M.R.; Tafaghodi, M. Impact of Chitosan Coating of Anionic Liposomes on Clearance Rate, Mucosal and Systemic Immune Responses Following Nasal Administration in Rabbits. Colloids Surf. B Biointerfaces 2009, 74, 225–229. [Google Scholar] [CrossRef]
- Tafaghodi, M.; Jaafari, M.; Sajadi Tabassi, S. Nasal Immunization Studies by Cationic, Fusogenic and Cationic-Fusogenic Liposomes Encapsulated with Tetanus Toxoid. Curr. Drug Deliv. 2008, 5, 108–113. [Google Scholar] [CrossRef]
- Slütter, B.; Hagenaars, N.; Jiskoot, W. Rational Design of Nasal Vaccines. J. Drug Target. 2008, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Köping-Höggård, M.; Sánchez, A.; Alonso, M.J. Nanoparticles as Carriers for Nasal Vaccine Delivery. Expert Rev. Vaccines 2005, 4, 185–196. [Google Scholar] [CrossRef]
- Brayden, D.J.; Baird, A.W. Microparticle Vaccine Approaches to Stimulate Mucosal Immunisation. Microbes Infect. 2001, 3, 867–876. [Google Scholar] [CrossRef]
- Mosafer, J.; Sabbaghi, A.H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, Characterization and in Vivo Evaluation of Alginate-Coated Chitosan and Trimethylchitosan Nanoparticles Loaded with PR8 Influenza Virus for Nasal Immunization. Asian J. Pharm. Sci. 2019, 14, 216–221. [Google Scholar] [CrossRef]
- Chen, W.; Li, R.; Zhu, S.; Ma, J.; Pang, L.; Ma, B.; Du, L.; Jin, Y. Nasal Timosaponin BII Dually Sensitive in Situ Hydrogels for the Prevention of Alzheimer’s Disease Induced by Lipopolysaccharides. Int. J. Pharm. 2020, 578, 119115. [Google Scholar] [CrossRef]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Agomelatine-Based in Situ Gels for Brain Targeting via the Nasal Route: Statistical Optimization, in Vitro, and in Vivo Evaluation. Drug Deliv. 2017, 24, 1077–1085. [Google Scholar] [CrossRef]
- Karimi, S.; Bagher, Z.; Najmoddin, N.; Simorgh, S.; Pezeshki-Modaress, M. Alginate-Magnetic Short Nanofibers 3D Composite Hydrogel Enhances the Encapsulated Human Olfactory Mucosa Stem Cells Bioactivity for Potential Nerve Regeneration Application. Int. J. Biol. Macromol. 2021, 167, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Corbo, F.; Agrimi, G.; Ditaranto, N.; Cioffi, N.; Perna, F.; Quivelli, A.; Stefàno, E.; Lunetti, P.; Muscella, A.; et al. Oxidized Alginate Dopamine Conjugate: In Vitro Characterization for Nose-to-Brain Delivery Application. Materials 2021, 14, 3495. [Google Scholar] [CrossRef]
- Haque, S.; Md, S.; Sahni, J.K.; Ali, J.; Baboota, S. Development and Evaluation of Brain Targeted Intranasal Alginate Nanoparticles for Treatment of Depression. J. Psychiatr. Res. 2014, 48, 1–12. [Google Scholar] [CrossRef]
- Caetano, L.A.; Almeida, A.J.; Gonçalves, L.M.D. Effect of Experimental Parameters on Alginate/Chitosan Microparticles for BCG Encapsulation. Mar. Drugs 2016, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Amokrane, F.; Mohamed, N.; Laraba-djebari, F. Development and Characterization of a New Carrier for Vaccine Delivery Based on Calcium-Alginate Nanoparticles: Safe Immunoprotective Approach against Scorpion Envenoming. Vaccine 2016, 34, 2692–2699. [Google Scholar] [CrossRef]
- Lauzon, M.-A.; Marcos, B.; Faucheux, N. Characterization of Alginate/Chitosan-Based Nanoparticles and Mathematical Modeling of Their SpBMP-9 Release Inducing Neuronal Differentiation of Human SH-SY5Y Cells. Carbohydr. Polym. 2018, 181, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, W.; Cruz, J.G.; Marasini, N.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Skwarczynski, M.; Toth, I. Development of Polyelectrolyte Complexes for the Delivery of Peptide-Based Subunit Vaccines Against Group A Streptococcus. Bioorg. Med. Chem. 2020, 10, 823. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Omura, M.H.; Cedran, M.F.; Dekker, R.F.H.; Barbosa-dekker, A.M.; Garcia, S. Effect of natural polymers on the survival ofLac tobace Pt Ed illus caseiencapsulated in alginate micr Tospheres. J. Microencapsul. 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ibnouf, E.O.; Kalam, M.A.; Alshamsan, A.; Aldawsari, M.F.; Alalaiwe, A.; Ansari, M.J. Formulation and in Vitro Evaluation of Topical Nanosponge-Based Gel Containing Butenafine for the Treatment of Fungal Skin Infection. Saudi Pharm. J. 2021, 29, 467–477. [Google Scholar] [CrossRef]
- Badri, W.; Eddabra, R.; Fessi, H.; Elaissari, A. Biodegradable Polymer Based Nanoparticles: Dermal and Transdermal Drug Delivery. J. Colloid Sci. Biotechnol. 2015, 3, 141–149. [Google Scholar] [CrossRef]
- Tewes, F.; Gobbo, O.L.; Ehrhardt, C.; Healy, A.M. Amorphous Calcium Carbonate Based-Microparticles for Peptide Pulmonary Delivery. ACS Appl. Mater. Interfaces 2016, 8, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Du, Z.; Tang, C.; Guan, Y.X.; Yao, S.J. Formulation of Insulin-Loaded N-Trimethyl Chitosan Microparticles with Improved Efficacy for Inhalation by Supercritical Fluid Assisted Atomization. Int. J. Pharm. 2016, 505, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Fang, Y.; Kan, Q.; Zhao, J.; Gan, Y.; Liu, Z. Surface Functional Modification of Self-Assembled Insulin Nanospheres for Improving Intestinal Absorption. Int. J. Biol. Macromol. 2015, 74, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Warner, K.S.; Zhang, J.; Sharma, S.; Gale, B.K. Evaluation Needle Length and Density of Microneedle Arrays in the Pretreatment of Skin for Transdermal Drug Delivery. Int. J. Pharm. 2010, 391, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, A.; McGrath, M.G.; Carey, J.B.; Draper, S.J.; Hill, A.V.S.; O’Mahony, C.; Crean, A.M.; Moore, A.C. Coated Microneedle Arrays for Transcutaneous Delivery of Live Virus Vaccines. J. Control. Release 2012, 159, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, G.; Zhang, Y.; Liu, D.; Xu, B.; Zhou, J. Polymer Microneedles Fabricated from Alginate and Hyaluronate for Transdermal Delivery of Insulin. Mater. Sci. Eng. C 2017, 80, 187–196. [Google Scholar] [CrossRef]
- Lefnaoui, S.; Moulai-Mostefa, N.; Yahoum, M.M.; Gasmi, S.N. Design of Antihistaminic Transdermal Films Based on Alginate–Chitosan Polyelectrolyte Complexes: Characterization and Permeation Studies. Drug Dev. Ind. Pharm. 2018, 44, 432–443. [Google Scholar] [CrossRef]
- Abebe, M.W.; Appiah-Ntiamoah, R.; Kim, H. Gallic Acid Modified Alginate Self-Adhesive Hydrogel for Strain Responsive Transdermal Delivery. Int. J. Biol. Macromol. 2020, 163, 147–155. [Google Scholar] [CrossRef]
- Sgalla, G.; Biffi, A.; Richeldi, L. Idiopathic Pulmonary Fibrosis: Diagnosis, Epidemiology and Natural History. Respirology 2016, 21, 427–437. [Google Scholar] [CrossRef]
- McLean-Tooke, A.; Moore, I.; Lake, F. Idiopathic and Immune-Related Pulmonary Fibrosis: Diagnostic and Therapeutic Challenges. Clin. Transl. Immunol. 2019, 8, e1086. [Google Scholar] [CrossRef]
- Abnoos, M.; Mohseni, M.; Mousavi, S.A.J.; Ashtari, K.; Ilka, R.; Mehravi, B. Chitosan-Alginate Nano-Carrier for Transdermal Delivery of Pirfenidone in Idiopathic Pulmonary Fibrosis. Int. J. Biol. Macromol. 2018, 118, 1319–1325. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, A.S.; Ss, G. The Role of Biopolymer Matrix Films Derived from Carboxymethyl Cellulose, Sodium Alginate and Polyvinyl Alcohol on the Sustained Transdermal Release of Diltiazem. Int. J. Biol. Macromol. 2017, 107, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Kataria, K.; Gupta, A.; Rath, G.; Mathur, R.B.; Dhakate, S.R. In Vivo Wound Healing Performance of Drug Loaded Electrospun Composite Nanofibers Transdermal Patch. Int. J. Pharm. 2014, 469, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Aktar, B.; Erdal, M.S.; Sagirli, O.; Güngör, S.; Özsoy, Y. Optimization of Biopolymer Based Transdermal Films of Metoclopramide as an Alternative Delivery Approach. Polymers 2014, 6, 1350–1365. [Google Scholar] [CrossRef]
- El-Houssiny, A.S.; Ward, A.A.; Mostafa, D.M.; Abd-El-Messieh, S.L.; Abdel-Nour, K.N.; Darwish, M.M.; Khalil, W.A. Sodium Alginate Nanoparticles as a New Transdermal Vehicle of Glucosamine Sulfate for Treatment of Osteoarthritis. Eur. J. Nanomedicine 2017, 9, 105–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, G.; Yu, W.; Liu, D.; Xu, B. Microneedles Fabricated from Alginate and Maltose for Transdermal Delivery of Insulin on Diabetic Rats. Mater. Sci. Eng. C 2018, 85, 18–26. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Say, K.M. Development of Alginate-Reinforced Chitosan Nanoparticles Utilizing W/O Nanoemulsification/Internal Crosslinking Technique for Transdermal Delivery of Rabeprazole. Life Sci. 2014, 110, 35–43. [Google Scholar] [CrossRef]
- Lai, W.; Tang, R.; Wong, W. Ionically Crosslinked Complex Gels Loaded with Oleic Acid-Containing Vesicles for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef]
- Giacone, D.V.; Dartora, V.F.M.C.; De Matos, J.K.R.; Passos, J.S.; Miranda, D.A.G.; De Oliveira, E.A.; Silveira, E.R.; Costa-lotufo, L.V.; Maria-engler, S.S.; Lopes, L.B. International Journal of Biological Macromolecules Effect of Nanoemulsion Modi Fi Cation with Chitosan and Sodium Alginate on the Topical Delivery and Ef Fi Cacy of the Cytotoxic Agent Piplartine in 2D and 3D Skin Cancer Models. Int. J. Biol. Macromol. 2020, 165, 1055–1065. [Google Scholar] [CrossRef]
- Esposito, L.; Barbosa, A.I.; Moniz, T.; Lima, S.C.; Costa, P.; Celia, C.; Reis, S. Design and Characterization of Sodium Alginate and Poly ( Vinyl ) Alcohol Hydrogels for Enhanced Skin Delivery of Quercetin. Pharmaceutics 2020, 12, 1149. [Google Scholar] [CrossRef]
- Ng, S.F.; Tan, L.S.; Buang, F. Transdermal Anti-Inflammatory Activity of Bilayer Film Containing Olive Compound Hydroxytyrosol: Physical Assessment, in Vivo Dermal Safety and Efficacy Study in Freund’s Adjuvant-Induced Arthritic Rat Model. Drug Dev. Ind. Pharm. 2017, 43, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Sadashivaiah, R.; Babu, B.K.S. Role of Sodium L-Cysteine Alginate Conjugate and Isopropyl Myristate to Enhance the Permeation Enhancing Activity of BCS Class III Drug from TDDS.; Optimization by Central Composite Design and in Vivo Pharmacokinetics Study. Drug Dev. Ind. Pharm. 2020, 46, 1427–1442. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, A.S.; Bino, S.J. Nanoparticle Assisted Solvent Selective Transdermal Combination Therapy of Curcumin and 5-Flurouracil for Efficient Cancer Treatment. Carbohydr. Polym. 2017, 173, 131–142. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic Hydrocolloids for the Intestinal Delivery of Protein Drugs: Alginate and Chitosan-a Review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Shah, J.P.; Gil, Z. Current Concepts in Management of Oral Cancer-Surgery. Oral Oncol. 2009, 45, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; Abu El-Naaj, I.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive Alginate Pastes with Embedded Liposomes for Local Oral Drug Delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, S.; Sahoo, R.N.; Mallick, S. Lipopolysaccharide Derived Alginate Coated Hepatitis B Antigen Loaded Chitosan Nanoparticles for Oral Mucosal Immunization. Int. J. Biol. Macromol. 2020, 154, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Akram, S.; Noreen, S. International Journal of Biological Macromolecules Linum Usitatissimum Seed Mucilage-Alginate Mucoadhesive Microspheres of Metformin HCl: Fabrication, Characterization and Evaluation. Int. J. Biol. Macromol. 2020, 155, 358–368. [Google Scholar] [CrossRef]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. European Journal of Pharmaceutical Sciences Preparation and Evaluation of Clindamycin Phosphate Loaded Chitosan / Alginate Polyelectrolyte Complex Fi Lm as Mucoadhesive Drug Delivery System for Periodontal Therapy. Eur. J. Pharm. Sci. 2018, 123, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.S.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.M.; Smirnova, I. Alginate-Based Hybrid Aerogel Microparticles for Mucosal Drug Delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef]
- Kaur, I.P.; Kakkar, S. Topical Delivery of Antifungal Agents. Expert Opin. Drug Deliv. 2010, 7, 1303–1327. [Google Scholar] [CrossRef]
- Pemán, J.; Salavert, M. Epidemiología General de La Enfermedad Fúngica Invasora. Enferm. Infecc. Y Microbiol. Clínica 2012, 30, 90–98. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. The Effects of Pluronic Block Copolymers on the Aggregation State of Nystatin. J. Control. Release 2004, 95, 161–171. [Google Scholar] [CrossRef]
- Martín-Villena, M.J.; Fernández-Campos, F.; Calpena-Campmany, A.C.; Bozal-De Febrer, N.; Ruiz-Martínez, M.A.; Clares-Naveros, B. Novel Microparticulate Systems for the Vaginal Delivery of Nystatin: Development and Characterization. Carbohydr. Polym. 2013, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.J.; Calpena, A.C.; Fernández, F.; Mallandrich, M.; Gálvez, P.; Clares, B. Development of Alginate Microspheres as Nystatin Carriers for Oral Mucosa Drug Delivery. Carbohydr. Polym. 2015, 117, 140–149. [Google Scholar] [CrossRef]
- Gennari, C.G.M.; Sperandeo, P.; Polissi, A.; Minghetti, P.; Cilurzo, F. Lysozyme Mucoadhesive Tablets Obtained by Freeze-Drying. J. Pharm. Sci. 2019, 108, 3667–3674. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Wang, L.; Han, J.; Yang, Y.; Fu, T.; Qiao, H.; Wang, Z.; Li, J. Colloids and Surfaces B: Biointerfaces Mucoadhesive Nanocrystal-in-Microspheres with High Drug Loading Capacity for Bioavailability Enhancement of Silybin. Colloids Surf. B Biointerfaces 2020, 198, 111461. [Google Scholar] [CrossRef]
- El-feky, G.S.; Abdulmaguid, R.F.; Zayed, G.M.; Kamel, R.; Abdulmaguid, R.F.; Zayed, G.M.; Kamel, R. Mucosal Co-Delivery of Ketorolac and Lidocaine Using Polymeric Wafers for Dental Application. Drug Deliv. 2018, 25, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Okeke, O.C.; Boateng, J.S. Composite HPMC and Sodium Alginate Based Buccal Formulations for Nicotine Replacement Therapy. Int. J. Biol. Macromol. 2016, 91, 31–44. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Schroeder, A.; Bianco-Peled, H. Alginate Modified with Maleimide-Terminated PEG as Drug Carriers with Enhanced Mucoadhesion; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 175, ISBN 0014350580. [Google Scholar]
- Jang, C.H.; Ahn, S.H.; Kim, G.H. Antifibrotic Effect of Dexamethasone/Alginate-Coated Silicone Sheet in the Abraded Middle Ear Mucosa. Int. J. Biol. Macromol. 2016, 93, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A. Rohit Synthesis and Characterization of Alginate and Sterculia Gum Based Hydrogel for Brain Drug Delivery Applications. Int. J. Biol. Macromol. 2020, 148, 248–257. [Google Scholar] [CrossRef]
- Bera, H.; Ippagunta, S.R.; Kumar, S.; Vangala, P. Core-Shell Alginate-Ghatti Gum Modified Montmorillonite Composite Matrices for Stomach-Specific Flurbiprofen Delivery. Mater. Sci. Eng. C 2017, 76, 715–726. [Google Scholar] [CrossRef]
- Boppana, R.; Yadaorao Raut, S.; Krishna Mohan, G.; Sa, B.; Mutalik, S.; Reddy, K.R.; Das, K.K.; Biradar, M.S.; Kulkarni, R.V. Novel PH-Sensitive Interpenetrated Network Polyspheres of Polyacrylamide-g-Locust Bean Gum and Sodium Alginate for Intestinal Targeting of Ketoprofen: In Vitro and in Vivo Evaluation. Colloids Surf. B Biointerfaces 2019, 180, 362–370. [Google Scholar] [CrossRef]
- Ivanovska, T.P.; Mladenovska, K.; Zhivikj, Z.; Pavlova, M.J.; Gjurovski, I.; Ristoski, T.; Petrushevska-Tozi, L. Synbiotic Loaded Chitosan-Ca-Alginate Microparticles Reduces Inflammation in the TNBS Model of Rat Colitis. Int. J. Pharm. 2017, 527, 126–134. [Google Scholar] [CrossRef]
- Saralkar, P.; Dash, A.K. Alginate Nanoparticles Containing Curcumin and Resveratrol: Preparation, Characterization, and In Vitro Evaluation Against DU145 Prostate Cancer Cell Line. AAPS Pharmscitech 2017, 18, 2814–2823. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Chen, Z.; Geng, Y.; Xie, X.; Shen, X.; Li, T.; Li, S.; Wu, C.; Liu, Y. Chemo-Photodynamic Combined Gene Therapy and Dual-Modal Cancer Imaging Achieved by PH-Responsive Alginate/Chitosan Multilayer-Modified Magnetic Mesoporous Silica Nanocomposites. Biomater. Sci. 2017, 5, 1001–1013. [Google Scholar] [CrossRef]
- Wang, Q.S.; Gao, L.N.; Zhu, X.N.; Zhang, Y.; Zhang, C.N.; Xu, D.; Cui, Y.L. Co-Delivery of Glycyrrhizin and Doxorubicin by Alginate Nanogel Particles Attenuates the Activation of Macrophage and Enhances the Therapeutic Efficacy for Hepatocellular Carcinoma. Theranostics 2019, 9, 6239–6255. [Google Scholar] [CrossRef]
- Lian, Y.; Wang, X.; Guo, P.; Li, Y.; Raza, F.; Su, J.; Qiu, M. Erythrocyte Membrane-Coated Arsenic Trioxide-Loaded Sodium Alginate Nanoparticles for Tumor Therapy. Pharmaceutics 2020, 12, 21. [Google Scholar] [CrossRef]
- Karabasz, A.; Lachowicz, D.; Karewicz, A.; Mezyk-Kopec, R.; Stalińska, K.; Werner, E.; Cierniak, A.; Dyduch, G.; Bereta, J.; Bzowska, M. Analysis of Toxicity and Anticancer Activity of Micelles of Sodium Alginate-Curcumin. Int. J. Nanomed. 2019, 14, 7249–7262. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Guo, X.; Zhong, R.; Wang, C.; Liu, H.; Li, H.; Ma, L.; Guan, J.; You, C.; Tian, M. Interactions of Alginate-Deferoxamine Conjugates With Blood Components and Their Antioxidation in the Hemoglobin Oxidation Model. Front. Bioeng. Biotechnol. 2020, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berrios, M.P.; Aponte-Reyes, L.M.; Diaz-Figueroa, L.; Vivero-Escoto, J.; Johnston, A.; Sanchez-Rodriguez, D. Preparation and in Vitro Evaluation of Alginate Microparticles Containing Amphotericin B for the Treatment of Candida Infections. Int. J. Biomater. 2020, 2020, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Nune, K.C.; Misra, R.D.K. Alginate/Poly(Amidoamine) Injectable Hybrid Hydrogel for Cell Delivery. J. Biomater. Appl. 2018, 33, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, A.H.; Azodi-Deilami, S.; Abdouss, M.; Payravand, H.; Farzaneh, S. Synthesis and Evaluation of Hydroponically Alginate Nanoparticles as Novel Carrier for Intravenous Delivery of Propofol. J. Mater. Sci. Mater. Med. 2015, 26, 145. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Nie, W.; He, C.; Zhou, X.; Chen, L.; Qiu, K.; Wang, W.; Yin, Z. Effect of PH-Responsive Alginate/Chitosan Multilayers Coating on Delivery Efficiency, Cellular Uptake and Biodistribution of Mesoporous Silica Nanoparticles Based Nanocarriers. ACS Appl. Mater. Interfaces 2014, 6, 8447–8460. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Mizuno, K.; Ikeuchi-Takahashi, Y.; Hattori, Y. Targeting Potential of Alginate-Glycyl-Prednisolone Conjugate Nanogel to Inflamed Joints in Rats with Adjuvant-Induced Arthritis. J. Drug Target. 2021, 29, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A.K. Buccal Bioadhesive Drug Delivery—A Promising Option for Orally Less Efficient Drugs. J. Control. Release 2006, 114, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Küppers, P. Adhesive Alginate for Buccal Delivery in Aphthous Stomatitis. Carbohydr. Res. 2019, 477, 51–57. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, T.S.; Venkataprasanna, K.S.; Niranjan, R.; Kaushik, M.; Samal, D.B.; Venkatasubbu, G.D. PVA/Alginate/Hydroxyapatite Films for Controlled Release of Amoxicillin for the Treatment of Periodontal Defects. Appl. Surf. Sci. 2019, 495, 143543. [Google Scholar] [CrossRef]
- Shim, B.-S.; Choi, Y.; Cheon, I.S.; Song, M.K. Sublingual Delivery of Vaccines for the Induction of Mucosal Immunity. Immune Netw. 2013, 13, 81. [Google Scholar] [CrossRef]
- Hanson, S.M.; Singh, S.; Tabet, A.; Sastry, K.J.; Michael Barry, C.W. Mucoadhesive Wafers Composed of Binary Polymer Blends for Sublingual Delivery and Preservation of Protein Vaccines. J. Control. Release 2021, 330, 427–437. [Google Scholar] [CrossRef]
- Hussein, N.; Omer, H.; Ismael, A.; Albed Alhnan, M.; Elhissi, A.; Ahmed, W. Spray-Dried Alginate Microparticles for Potential Intranasal Delivery of Ropinirole Hydrochloride: Development, Characterization and Histopathological Evaluation. Pharm. Dev. Technol. 2020, 25, 290–299. [Google Scholar] [CrossRef]
- Di Martino, A.; Trusova, M.E.; Postnikov, P.S.; Sedlarik, V. Folic Acid-Chitosan-Alginate Nanocomplexes for Multiple Delivery of Chemotherapeutic Agents. J. Drug Deliv. Sci. Technol. 2018, 47, 67–76. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Zhu, W.; Liang, Z.; Zeng, Q. Self-Assembly PH-Sensitive Chitosan/Alginate Coated Polyelectrolyte Complexes for Oral Delivery of Insulin. J. Microencapsul. 2019, 36, 96–107. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Chen, B. Retinoic Acid-Loaded Alginate Microspheres as a Slow Release Drug Delivery Carrier for Intravitreal Treatment. Biomed. Pharmacother. 2018, 97, 722–728. [Google Scholar] [CrossRef]
- Faidi, A.; Lassoued, M.A.; Becheikh, M.E.H.; Touati, M.; Stumbé, J.F.; Farhat, F. Application of Sodium Alginate Extracted from a Tunisian Brown Algae Padina Pavonica for Essential Oil Encapsulation: Microspheres Preparation, Characterization and in Vitro Release Study. Int. J. Biol. Macromol. 2019, 136, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sohail, R.; Abbas, S.R. Evaluation of Amygdalin-Loaded Alginate-Chitosan Nanoparticles as Biocompatible Drug Delivery Carriers for Anticancerous Efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef]
- Kassem, A.A.; Ahmed, D.; Issa, E.; Kotry, G.S. Thiolated Alginate-Based Multiple Layer Mucoadhesive Films of Metformin Forintra-Pocket Local Delivery: In Vitro Characterization and Clinical Assessment. Drug Dev. Ind. Pharm. 2017, 43, 120–131. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate Coated Chitosan Core-Shell Nanoparticles for Efficient Oral Delivery of Naringenin in Diabetic Animals—An in Vitro and in Vivo Approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- de Castro Spadari, C.; Lopes, L.B.; Ishida, K. Alginate Nanoparticles as Non-Toxic Delivery System for Miltefosine in the Treatment of Candidiasis and Cryptococcosis. Int. J. Nanomed. 2019, 14, 5187–5199. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Gong, J.; Mu, R.; Li, Y.; Wu, C.; Pang, J. Fabrication of Chitosan-Coated Konjac Glucomannan/Sodium Alginate/Graphene Oxide Microspheres with Enhanced Colon-Targeted Delivery. Int. J. Biol. Macromol. 2019, 131, 209–217. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-Alginate Nanoparticles as Effective Oral Carriers to Improve the Stability, Bioavailability, and Cytotoxicity of Curcumin Diethyl Disuccinate. Carbohydr. Polym. 2020, 256, 117426. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Sodium Alginate–Polyvinyl Alcohol–Bovin Serum Albumin Coated Fe3O4 Nanoparticles as Anticancer Drug Delivery Vehicle: Doxorubicin Loading and in Vitro Release Study and Cytotoxicity to HepG2 and L02 Cells. Mater. Sci. Eng. C 2017, 79, 410–422. [Google Scholar] [CrossRef]
- Thanh Uyen, N.T.; Abdul Hamid, Z.A.; Thi, L.A.; Ahmad, N.B. Synthesis and Characterization of Curcumin Loaded Alginate Microspheres for Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 58, 101796. [Google Scholar] [CrossRef]
- Yu, X.; Wen, T.; Cao, P.; Shan, L.; Li, L. Alginate-Chitosan Coated Layered Double Hydroxide Nanocomposites for Enhanced Oral Vaccine Delivery. J. Colloid Interface Sci. 2019, 556, 258–265. [Google Scholar] [CrossRef]
- Doniparthi, J.; B, J.J. Novel Tamarind Seed Gum-Alginate Based Multi-Particulates for Sustained Release of Dalfampridine Using Response Surface Methodology. Int. J. Biol. Macromol. 2020, 144, 725–741. [Google Scholar] [CrossRef]
- Wang, Q.S.; Wang, G.F.; Zhou, J.; Gao, L.N.; Cui, Y.L. Colon Targeted Oral Drug Delivery System Based on Alginate-Chitosan Microspheres Loaded with Icariin in the Treatment of Ulcerative Colitis. Int. J. Pharm. 2016, 515, 176–185. [Google Scholar] [CrossRef]
- Abo El-Ela, F.I.; Hussein, K.H.; El-Banna, H.A.; Gamal, A.; Rouby, S.; Menshawy, A.M.S.; EL-Nahass, E.L.S.; Anwar, S.; Zeinhom, M.M.A.; Salem, H.F.; et al. Treatment of Brucellosis in Guinea Pigs via a Combination of Engineered Novel PH-Responsive Curcumin Niosome Hydrogel and Doxycycline-Loaded Chitosan–Sodium Alginate Nanoparticles: An In Vitro and In Vivo Study. AAPS PharmSciTech 2020, 22, 12. [Google Scholar] [CrossRef]
- Balanč, B.; Trifković, K.; Đorđević, V.; Marković, S.; Pjanović, R.; Nedović, V.; Bugarski, B. Novel Resveratrol Delivery Systems Based on Alginate-Sucrose and Alginate-Chitosan Microbeads Containing Liposomes. Food Hydrocoll. 2016, 61, 832–842. [Google Scholar] [CrossRef]
- Scholz, M.; Reske, T.; Böhmer, F.; Hornung, A.; Grabow, N.; Lang, H. In Vitro Chlorhexidine Release from Alginate Based Microbeads for Periodontal Therapy. PLoS ONE 2017, 12, e0185562. [Google Scholar] [CrossRef]
- Hamed, S.F.; Hashim, A.F.; Abdel Hamid, H.A.; Abd-Elsalam, K.A.; Golonka, I.; Musiał, W.; El-Sherbiny, I.M. Edible Alginate/Chitosan-Based Nanocomposite Microspheres as Delivery Vehicles of Omega-3 Rich Oils. Carbohydr. Polym. 2020, 239, 116201. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Caetano, B.L.; Boni, F.I.; Sousa, F.; Magnani, M.; Sarmento, B.; Ferreira Cury, B.S.; Daflon Gremião, M.P. Alginate-Based Delivery Systems for Bevacizumab Local Therapy: In Vitro Structural Features and Release Properties. J. Pharm. Sci. 2019, 108, 1559–1568. [Google Scholar] [CrossRef]
- Sepúlveda-Rivas, S.; Fritz, H.F.; Valenzuela, C.; Santiviago, C.A.; Morales, J.O. Development of Novel EE/Alginate Polyelectrolyte Complex Nanoparticles for Lysozyme Delivery: Physicochemical Properties and in Vitro Safety. Pharmaceutics 2019, 11, 103. [Google Scholar] [CrossRef]
- Rassu, G.; Salis, A.; Porcu, E.P.; Giunchedi, P.; Roldo, M.; Gavini, E. Composite Chitosan/Alginate Hydrogel for Controlled Release of Deferoxamine: A System to Potentially Treat Iron Dysregulation Diseases. Carbohydr. Polym. 2016, 136, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, S. Biocompatible and Self-Recoverable Succinoglycan Dialdehyde-Crosslinked Alginate Hydrogels for PH-Controlled Drug Delivery. Carbohydr. Polym. 2020, 250, 116934. [Google Scholar] [CrossRef]
- Khan, S.; Boateng, J.S.; Mitchell, J.; Trivedi, V. Formulation, Characterisation and Stabilisation of Buccal Films for Paediatric Drug Delivery of Omeprazole. AAPS PharmSciTech 2015, 16, 800–810. [Google Scholar] [CrossRef]
- Abdul Rasool, B.K.; Khalifa, A.Z.; Abu-Gharbieh, E.; Khan, R. Employment of Alginate Floating in Situ Gel for Controlled Delivery of Celecoxib: Solubilization and Formulation Studies. Biomed. Res. Int. 2020, 2020, 1879125. [Google Scholar] [CrossRef]
- Supramaniam, J.; Adnan, R.; Haida, N.; Kaus, M.; Bushra, R. Magnetic Nanocellulose Alginate Hydrogel Beads as Potential Drug Delivery System. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J. Materials Science & Engineering C Magnetic and Self-Healing Chitosan-Alginate Hydrogel Encapsulated Gelatin Microspheres via Covalent Cross-Linking for Drug Delivery. Mater. Sci. Eng. C 2019, 101, 619–629. [Google Scholar] [CrossRef]
- Sheng, Y.; Gao, J.; Yin, Z.-Z.; Kang, J.; Kong, Y. Dual-Drug Delivery System Based on the Hydrogels of Alginate and Sodium Carboxymethyl Cellulose for Colorectal Cancer Treatment. Carbohydr. Polym. 2021, 269, 118325. [Google Scholar] [CrossRef] [PubMed]
- Criado-Gonzalez, M.; Fernandez-Gutierrez, M.; San Roman, J.; Mijangos, C.; Hernández, R. Local and Controlled Release of Tamoxifen from Multi (Layer-by-Layer) Alginate/Chitosan Complex Systems. Carbohydr. Polym. 2019, 206, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Haldar, J. Direct Synthesis of Dextran-Based Antibacterial Hydrogels for Extended Release of Biocides and Eradication of Topical Biofilms. ACS Appl. Mater. Interfaces 2017, 9, 15975–15985. [Google Scholar] [CrossRef] [PubMed]
- Kujath, P. Wounds–From Physiology to Wound Dressing: In Reply. Dtsch. Arztebl. Int. 2008, 105, 239–248. [Google Scholar] [CrossRef]
- Fatima, F.; Aldawsari, M.F.; Ahmed, M.M.; Anwer, M.K.; Naz, M.; Ansari, M.J.; Hamad, A.M.; Zafar, A.; Jafar, M. Green Synthesized Silver Nanoparticles Using Tridax Procumbens for Topical Application: Excision Wound Model and Histopathological Studies. Pharmaceutics 2021, 13, 1754. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and Synthetic Polymers for Wounds and Burns Dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Cavaco-Paulo, A. Wound Dressings for a Proteolytic-Rich Environment. Appl. Microbiol. Biotechnol. 2011, 90, 445–460. [Google Scholar] [CrossRef]
- Qin, Y. Advanced Wound Dressings. J. Text. Inst. 2001, 92, 127–138. [Google Scholar] [CrossRef]
- Anumolu, S.S.; Menjoge, A.R.; Deshmukh, M.; Gerecke, D.; Stein, S.; Laskin, J.; Sinko, P.J. Doxycycline Hydrogels with Reversible Disulfide Crosslinks for Dermal Wound Healing of Mustard Injuries. Biomaterials 2011, 32, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-Based Composite Materials for Wound Dressing Application:A Mini Review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Kong, H.J.; Kaigler, D.; Kim, K.; Mooney, D.J. Controlling Rigidity and Degradation of Alginate Hydrogels via Molecular Weight Distribution. Biomacromolecules 2004, 5, 1720–1727. [Google Scholar] [CrossRef]
- Gong, X.; Dang, G.; Guo, J.; Liu, Y.; Gong, Y. A Sodium Alginate/Feather Keratin Composite Fiber with Skin-Core Structure as the Carrier for Sustained Drug Release. Int. J. Biol. Macromol. 2020, 155, 386–392. [Google Scholar] [CrossRef]
- Ji, M.; Chen, H.; Yan, Y.; Ding, Z.; Ren, H.; Zhong, Y. Effects of Tricalcium Silicate/Sodium Alginate/Calcium Sulfate Hemihydrate Composite Cements on Osteogenic Performances in Vitro and in Vivo. J. Biomater. Appl. 2020, 34, 1422–1436. [Google Scholar] [CrossRef]
- Choe, G.; Oh, S.; Seok, J.M.; Park, S.A.; Lee, J.Y. Graphene Oxide/Alginate Composites as Novel Bioinks for Three-Dimensional Mesenchymal Stem Cell Printing and Bone Regeneration Applications. Nanoscale 2019, 11, 23275–23285. [Google Scholar] [CrossRef]
- Qin, Y.; Hu, H.; Luo, A. The Conversion of Calcium Alginate Fibers into Alginic Acid Fibers and Sodium Alginate Fibers. J. Appl. Polym. Sci. 2006, 101, 4216–4221. [Google Scholar] [CrossRef]
- Miranda, M.E.S.; Marcolla, C.; Rodrígues, C.A.; Wilhelm, H.M.; Sierakowski, M.R.; Bresolin, T.M.B.; de Freitas, R.A. Chitosan and N-Carboxymethylchitosan: I. The Role of N-Carboxymethylation of Chitosan in the Thermal Stability and Dynamic Mechanical Properties of Its Films. Polym. Int. 2006, 55, 961–969. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, Y.; Shi, Y.; Zhao, L. PH-Responsive Calcium Alginate Hydrogel Laden with Protamine Nanoparticles and Hyaluronan Oligosaccharide Promotes Diabetic Wound Healing by Enhancing Angiogenesis and Antibacterial Activity. Drug. Deliv. Transl. Res. 2019, 9, 227–239. [Google Scholar] [CrossRef]
- Luiza, A.; Pires, R.; De Azevedo, L.; Dias, A.M.A.; De Sousa, H.C.; Moraes, Â.M.; Braga, M.E.M. Materials Science & Engineering C Towards Wound Dressings with Improved Properties: E Ff Ects of Poly (Dimethylsiloxane) on Chitosan-Alginate Fi Lms Loaded with Thymol and Beta- Carotene. Mater. Sci. Eng. C 2018, 93, 595–605. [Google Scholar] [CrossRef]
- Bergonzi, C.; D’Ayala, G.G.; Elviri, L.; Laurienzo, P.; Bandiera, A.; Catanzano, O. Alginate/Human Elastin-like Polypeptide Composite Films with Antioxidant Properties for Potential Wound Healing Application. Int. J. Biol. Macromol. 2020, 164, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, X.; Li, S.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Preparation of Aminated Fish Scale Collagen and Oxidized Sodium Alginate Hybrid Hydrogel for Enhanced Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2020, 164, 626–637. [Google Scholar] [CrossRef]
- Choudhary, M.; Chhabra, P.; Tyagi, A.; Singh, H. Scar Free Healing of Full Thickness Diabetic Wounds: A Unique Combination of Silver Nanoparticles as Antimicrobial Agent, Calcium Alginate Nanoparticles as Hemostatic Agent, Fresh Blood as Nutrient/Growth Factor Supplier and Chitosan as Base Matrix. Int. J. Biol. Macromol. 2021, 178, 41–52. [Google Scholar] [CrossRef]
- Sharma, A.; Puri, V.; Kumar, P.; Singh, I. Rifampicin-Loaded Alginate-Gelatin Fibers Incorporated within Transdermal Films as a Fiber-in-Film System for Wound Healing Applications. Membranes 2021, 11, 7. [Google Scholar] [CrossRef]
- Fahmy, H.M. Hyaluronic Acid / Na-Alginate Fi Lms as Topical Bioactive Wound Dressings. Eur. Polym. J. 2018, 109, 101–109. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, A.; Puri, V.; Kumar, P.; Singh, I. Curcumin-Loaded, Alginate–Gelatin Composite Fibers for Wound Healing Applications. 3 Biotech. 2020, 10, 464. [Google Scholar] [CrossRef]
- Pagano, C.; Puglia, D.; Luzi, F.; Di Michele, A.; Scuota, S.; Primavilla, S.; Ceccarini, M.R.; Beccari, T.; Iborra, C.A.V.; Ramella, D.; et al. Development and Characterization of Xanthan Gum and Alginate Based Bioadhesive Film for Pycnogenol Topical Use in Wound Treatment. Pharmaceutics 2021, 13, 324. [Google Scholar] [CrossRef]
- Akbar, M.U.; Zia, K.M.; Akash, M.S.H.; Nazir, A.; Zuber, M.; Ibrahim, M. In-Vivo Anti-Diabetic and Wound Healing Potential of Chitosan/Alginate/Maltodextrin/Pluronic-Based Mixed Polymeric Micelles: Curcumin Therapeutic Potential. Int. J. Biol. Macromol. 2018, 120, 2418–2430. [Google Scholar] [CrossRef]
- Shahzad, A.; Khan, A.; Afzal, Z.; Umer, M.F.; Khan, J.; Khan, G.M. Formulation Development and Characterization of Cefazolin Nanoparticles-Loaded Cross-Linked Films of Sodium Alginate and Pectin as Wound Dressings. Int. J. Biol. Macromol. 2019, 124, 255–269. [Google Scholar] [CrossRef]
- anne Johnson, K.; Muzzin, N.; Toufanian, S.; Slick, R.A.; Lawlor, M.W.; Seifried, B.; Moquin, P.; Latulippe, D.; Hoare, T. Drug-Impregnated, Pressurized Gas Expanded Liquid-Processed Alginate Hydrogel Scaffolds for Accelerated Burn Wound Healing. Acta Biomater. 2020, 112, 101–111. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Z.; Zuo, J.; Wu, C.; Zha, L.; Xu, Y.; Wang, S.; Shi, J.; Liu, X.H.; Zhang, J.; et al. Novel Cannabidiol−carbamate Hybrids as Selective BuChE Inhibitors: Docking-Based Fragment Reassembly for the Development of Potential Therapeutic Agents against Alzheimer’s Disease. Eur. J. Med. Chem. 2021, 223, 113735. [Google Scholar] [CrossRef]
- Rezaei, M.; Nikkhah, M.; Mohammadi, S.; Bahrami, S.H.; Sadeghizadeh, M. Nano-Curcumin/Graphene Platelets Loaded on Sodium Alginate/Polyvinyl Alcohol Fibers as Potential Wound Dressing. J. Appl. Polym. Sci. 2021, 138, 50884. [Google Scholar] [CrossRef]
- Asadi, L.; Mokhtari, J.; Abbasi, M. An Alginate–PHMB–AgNPs Based Wound Dressing Polyamide Nanocomposite with Improved Antibacterial and Hemostatic Properties. J. Mater. Sci. Mater. Med. 2021, 32, 7. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, W.; Dong, Z.; Ji, Y.; Li, M.; Hao, Y.; Zhang, D.; Yuan, C.; Deng, J.; Zhao, P.; et al. A Biodegradable Antibacterial Alginate/Carboxymethyl Chitosan/Kangfuxin Sponges for Promoting Blood Coagulation and Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2021, 167, 182–192. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, W.; Lei, Y.; Gaucher, C.; Pei, S.; Zhang, J.; Xia, X. Edaravone-Loaded Alginate-Based Nanocomposite Hydrogel Accelerated Chronic Wound Healing in Diabetic Mice. Mar Drugs. 2019, 17, 285. [Google Scholar] [CrossRef]
- Mobaraki, M.; Bizari, D.; Soltani, M.; Khshmohabat, H.; Raahemifar, K.; Amirdehi, M.A. The Effects of Curcumin Nanoparticles Incorporated into Collagen-Alginate Scaffold on Wound Healing of Skin Tissue in Trauma Patients. Polymers 2021, 13, 4291. [Google Scholar] [CrossRef] [PubMed]
- Buyana, B.; Aderibigbe, B.A.; Ndinteh, D.T.; Fonkui, Y.T.; Kumar, P. Alginate-Pluronic Topical Gels Loaded with Thymol, Norfloxacin and ZnO Nanoparticles as Potential Wound Dressings. J. Drug. Deliv. Sci. Technol. 2020, 60, 101960. [Google Scholar] [CrossRef]
- Tan, W.S.; Arulselvan, P.; Ng, S.F.; Taib, C.N.M.; Sarian, M.N.; Fakurazi, S. Healing Effect of Vicenin-2 (VCN-2) on Human Dermal Fibroblast (HDF) and Development VCN-2 Hydrocolloid Film Based on Alginate as Potential Wound Dressing. Biomed. Res. Int. 2020, 2020, 4730858. [Google Scholar] [CrossRef]
- Ansari, S.; Pouraghaei Sevari, S.; Chen, C.; Sarrion, P.; Moshaverinia, A. RGD-Modified Alginate-GelMA Hydrogel Sheet Containing Gingival Mesenchymal Stem Cells: A Unique Platform for Wound Healing and Soft Tissue Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 3774–3782. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Santos-Vizcaino, E.; Sanchez, P.; Gutierrez, F.B.; Aguirre, J.J.; Hernandez, R.M.; Igartua, M. Bioactive and Degradable Hydrogel Based on Human Platelet-Rich Plasma Fibrin Matrix Combined with Oxidized Alginate in a Diabetic Mice Wound Healing Model. Mater. Sci. Eng. C 2022, 135, 112695. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.A.; Kousar, M. Improved Drug Delivery and Accelerated Diabetic Wound Healing by Chondroitin Sulfate Grafted Alginate-Based Thermoreversible Hydrogels. Mater. Sci. Eng. C 2021, 126, 112169. [Google Scholar] [CrossRef]
- Sanchez, M.F.; Guzman, M.L.; Apas, A.L.; del Lujan Alovero, F.; Olivera, M.E. Sustained Dual Release of Ciprofloxacin and Lidocaine from Ionic Exchange Responding Film Based on Alginate and Hyaluronate for Wound Healing. Eur. J. Pharm. Sci. 2021, 161, 105789. [Google Scholar] [CrossRef]
- Chin, C.Y.; Jalil, J.; Ng, P.Y.; Ng, S.F. Development and Formulation of Moringa Oleifera Standardised Leaf Extract Film Dressing for Wound Healing Application. J. Ethnopharmacol. 2018, 212, 188–199. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-Incorporated Crosslinked Sodium Alginate-g-Poly (N-Isopropyl Acrylamide) Thermo-Responsive Hydrogel as an in-Situ Forming Injectable Dressing for Wound Healing: In Vitro Characterization and in Vivo Evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Rajalekshmy, G.P.; Rekha, M.R. Strontium Ion Cross-Linked Alginate-g-Poly (PEGMA) Xerogels for Wound Healing Applications: In Vitro Studies. Carbohydr. Polym. 2021, 251, 117119. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aly, A.A.; Sayed, S.M.; Abou-Okeil, A. K-Carrageenan/Na-Alginate Wound Dressing with Sustainable Drug Delivery Properties. Polym. Adv. Technol. 2021, 32, 1793–1801. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.; Osman, B.; Göktalay, G.; Özer, E.T.; Şahan, Y.; Becerir, B.; Karaca, E. Design and in Vivo Evaluation of Alginate-Based PH-Sensing Electrospun Wound Dressing Containing Anthocyanins. J. Polym. Res. 2021, 28, 50. [Google Scholar] [CrossRef]
- Meng, X.; Lu, Y.; Gao, Y.; Cheng, S.; Tian, F.; Xiao, Y.; Li, F. Chitosan/Alginate/Hyaluronic Acid Polyelectrolyte Composite Sponges Crosslinked with Genipin for Wound Dressing Application. Int. J. Biol. Macromol. 2021, 182, 512–523. [Google Scholar] [CrossRef] [PubMed]
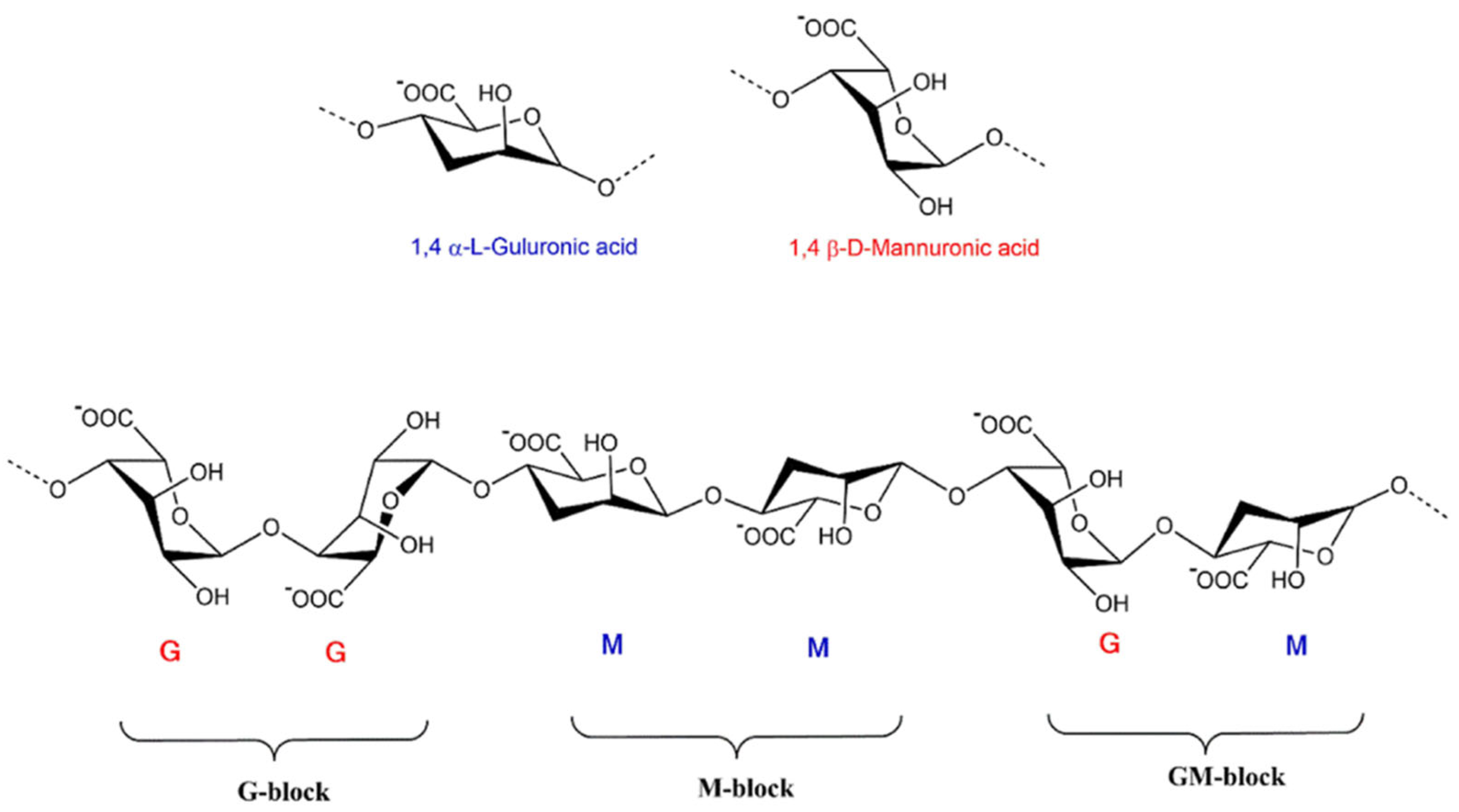
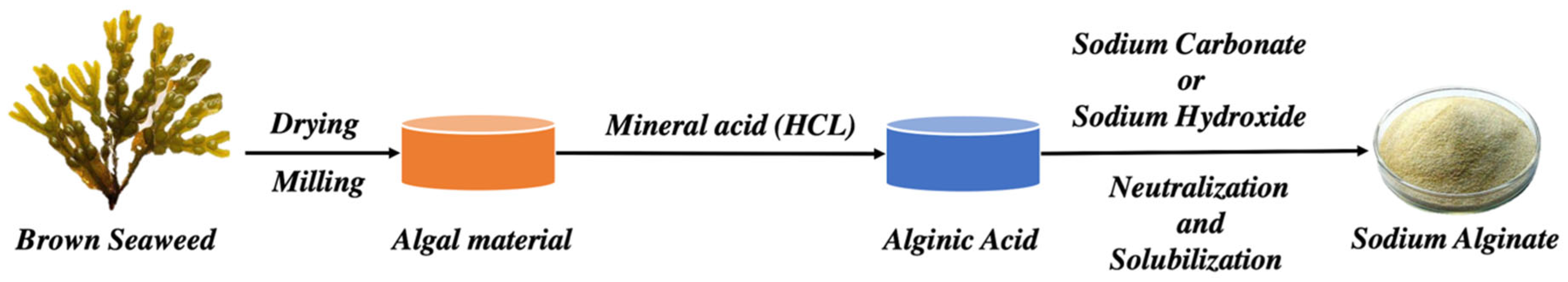



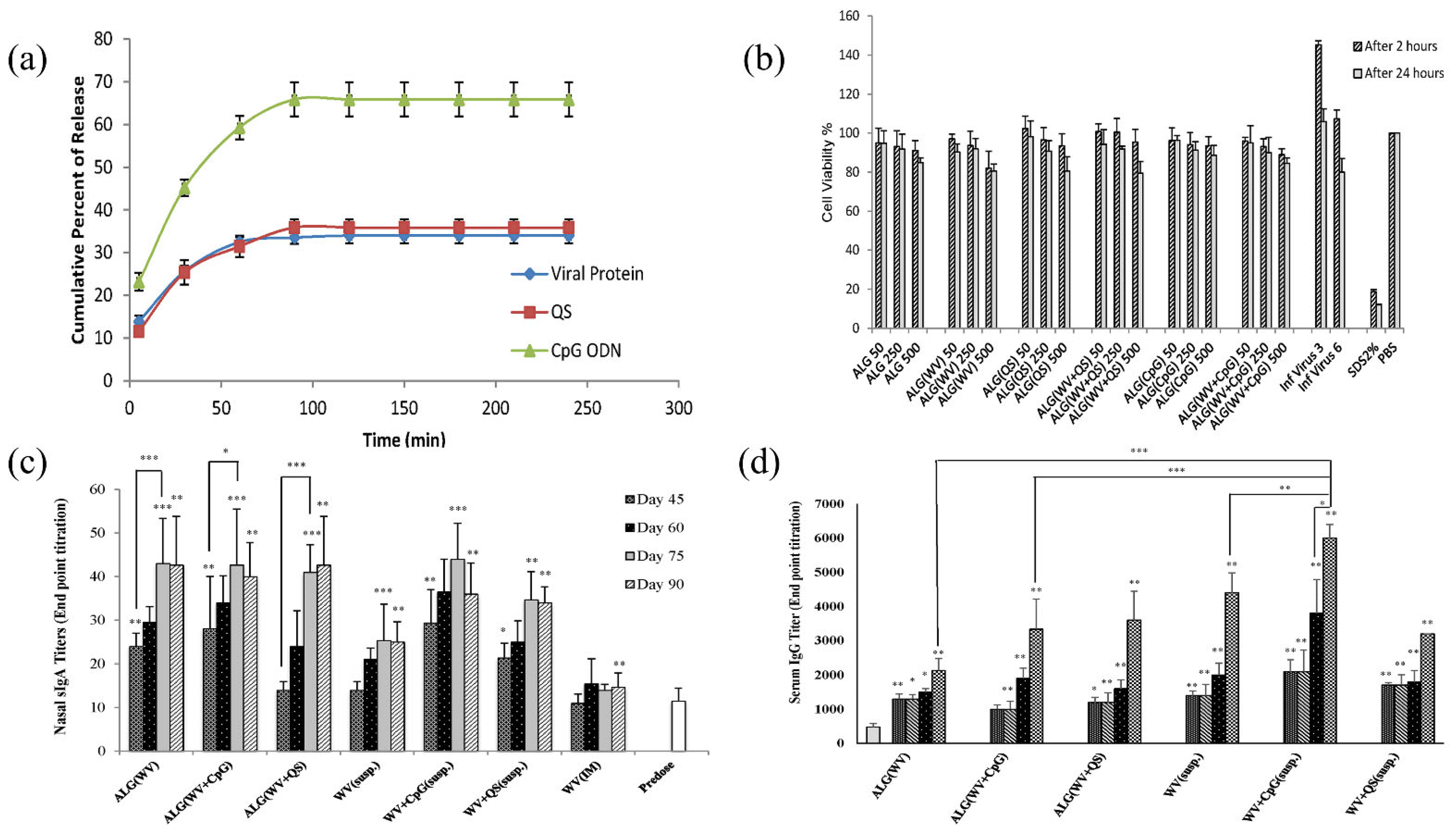
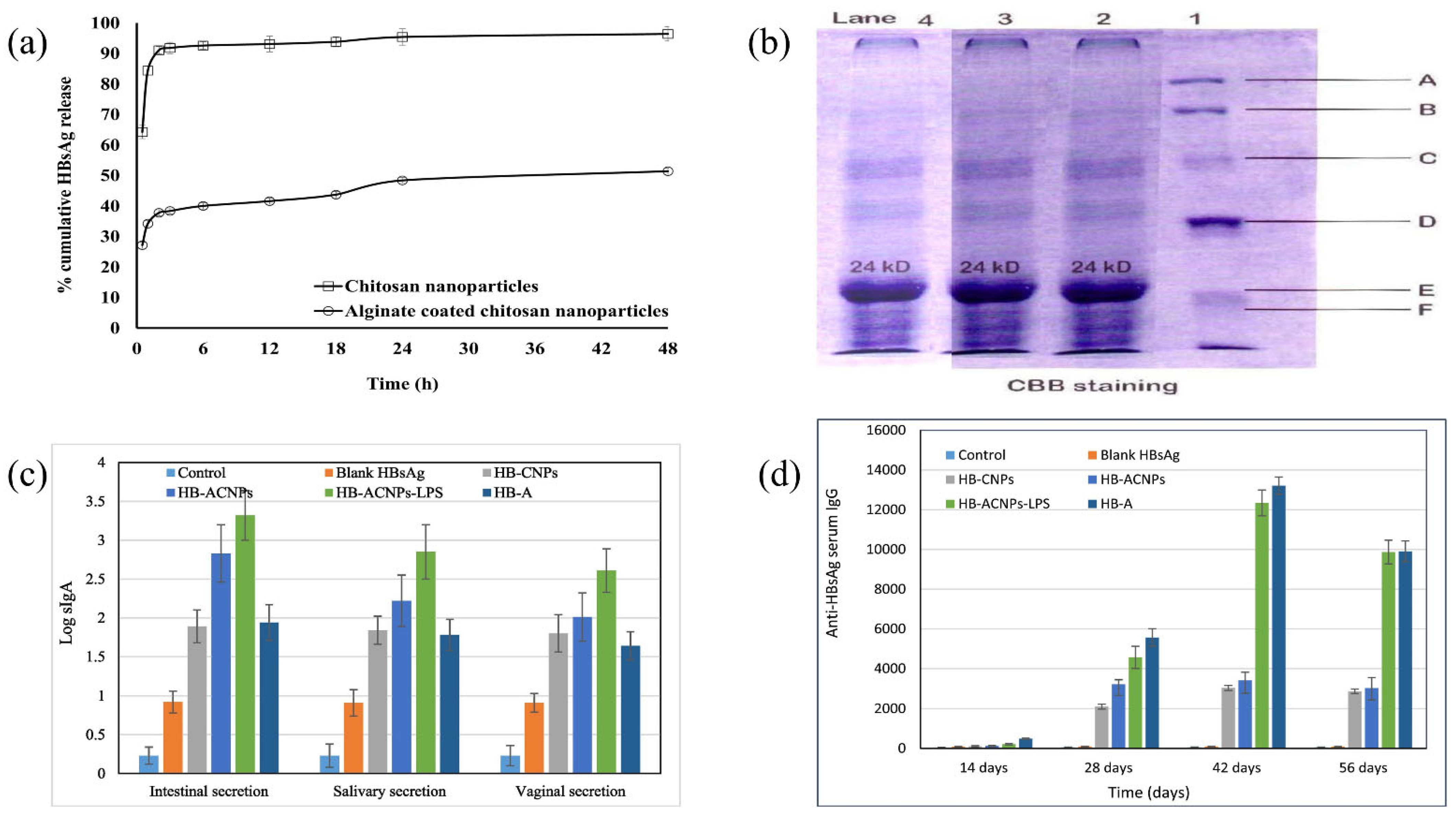
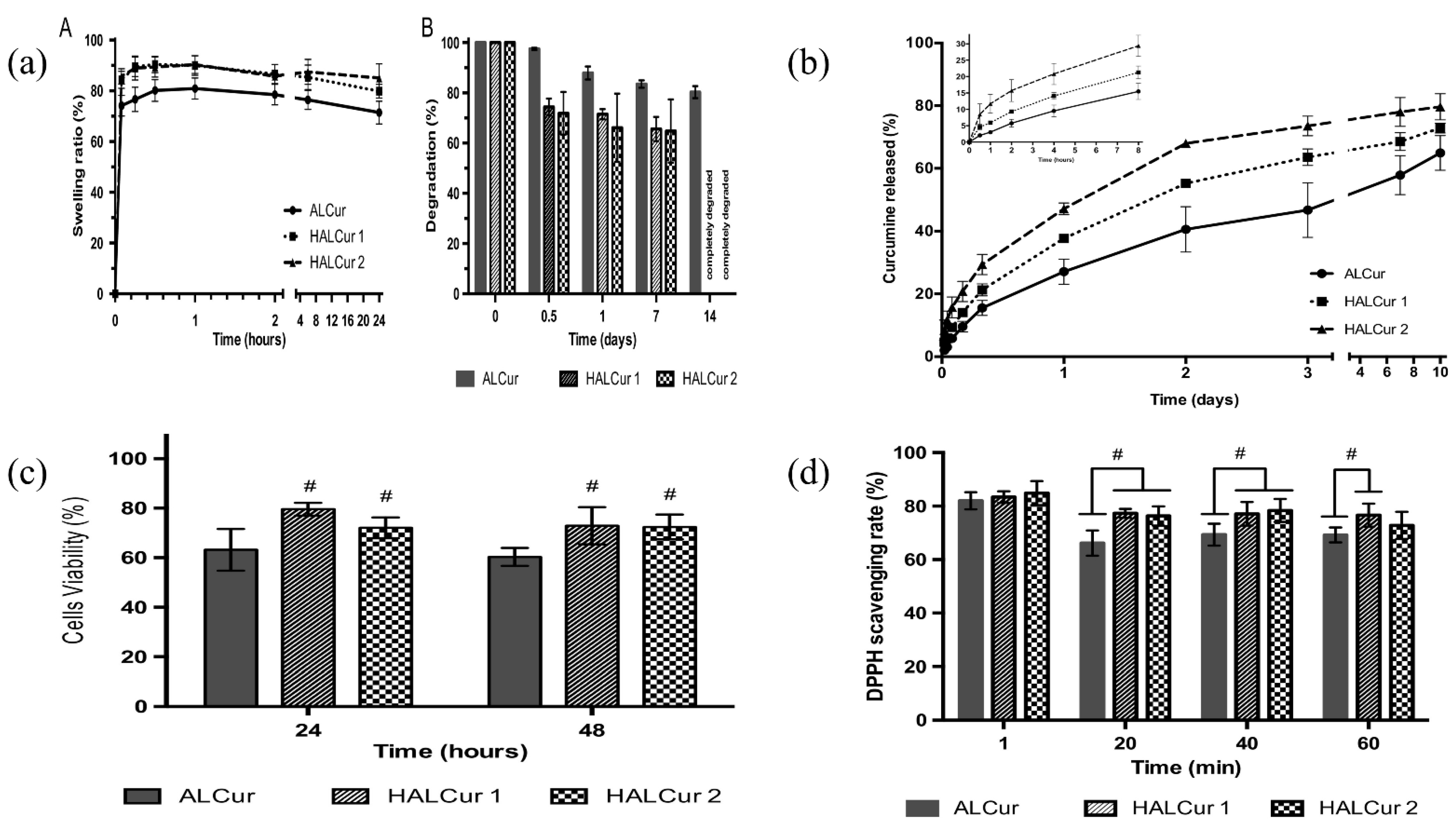
| Seaweed Species | Components Used for Extraction | Extraction Yield (% Dry Weight—d.w.) | Characteristics | References |
|---|---|---|---|---|
| Sargassum mangarevense and Turbinaria ornata | Formaldehyde-acidification-Na2CO3-ethanol | Sargassum mangarevense (6.0–12.4% d.w.); T. ornata (16.8–21.1% d.w.) | T. ornata demonstrated a greater viscosity and yield than S. mangarevense. Both the species showed a high M:G ratio (1.25–1.42) compared to the reported literature. No seasonal variation was observed. | [31] |
| Sargassum vulgare | Formaldehyde-HCl-Na2CO3 | 16.9% | M/G ratio for S. vulgaris low density and S. vulgaris high density were higher than most Sargassum species ALG (1.56 and 1.27, respectively). Optimal conditions for extraction of ALG from S. vulgaris were 60 °C and 5 h duration. Newtonian activity seen for a solution fraction was 0.7% for SVLV, whereas, it was 0.5%. for the SVHV sample. | [32] |
| Sargassum turbinarioides Grunow | Formaldehyde-HCl-Na2CO3 | 10% | M/G ratio was 0.94, η < 1, M.W. (5.528 × 105 g mol−1), and polydispersity index were low (1.43). | [33] |
| Laminaria digitata and Ascophyllum nodosum | Na2CO3 or NaOH after different acid pre-treatments (H2SO4 and HCl) at different temperatures | 28.65 ± 0.92% to 78.02 ± 16.81% | Unrefined extracts produced films with appropriate mechanical characteristics without cationic complexation. The treatment with sodium carbonate resulted in extracts with good plasticizing capacity, while sodium hydroxide extraction guided to polymer chains with enhanced cross-linking ability. Ascophyllum films possessed radical scavenging property. | [34] |
| Tunisian seaweed (Cystoseira barbata) | high-temperature alkaline extraction | 9.9% | M/G ratio was 0.59, pseudoplastic flow behavior. The emulsion formed was highly stable at acidic pH and less affected by temperature. CBSA exhibited DPPH radical scavenging activity (74% 33 inhibition at a concentration of 0.5 mg/mL). Excellent hydroxyl-radical scavenging activity, ferric reducing potential, and protection against DNA breakage were observed. | [35] |
| Macrocystis pyrifera | Ethanol route, HCl route, CaCl2 route | 25–33% | Direct polymer precipitation with ethanol gave the best yield. The precipitation step with calcium and cation exchange gave an ALG with poor viscoelastic properties. A pH higher than 3.5 in the acid pre-treatment step amended the ethanol route, thereby preventing the ethanol linkages from being ruptured. | [36] |
| Sargassum muticum | conventional alkaline extraction and hydrothermal fractionation | 5.04–10.09% | EC50 values for DPPH radical scavenging (0.72 and 1.18 g L−1 at 190 °C than at 220 °C, respectively) were comparable with synthetic antioxidants. However, at the minimum tested value (0.2 g L−1), the manufactured extracts at 190 °C appeared to be prooxidant. The AAC values that reached the maximum tested concentration at (0.5 g L−1) were similar to those for BHA and BHT. | [37] |
| Sargassum sp. (SRG) (genus Sargassum), Turbinaria sp. (TRB) (genus Turbinaria), Hormophysa sp. (RHT) (genus Hormophysa) | HCl-Na2CO3-EDTA | SRG 31 RHT 31 TRB 30 | M/G ratio by 1H NMR 0.7–1.0, while after hydrolysis was 0.52–1.1. TRG with M/G <1 Gave a softer gel than SRG, while RHT, rich in mannuronic acid, gave the softest gel. | [38] |
| Sargassum muticum | Formaldehyde-HCl-Na2CO3 | 13.57 ± 0.13% | Optimum conditions for extraction are 86 °C temperature, 3% alkali, and 93% ethanol for 3 h. M/G was 1.08. | [39] |
| Devices | Model Drug/Drug | Composition | Preparation Technique | Delivery Site | Route of Administration | Key Features | References |
|---|---|---|---|---|---|---|---|
| Microparticles | Ropinirole hydrochloride | ALG | Spray-drying | Nasal epithelium | Intranasal | Excellent drug loading efficiency, in vitro rapid drug release (>95% in 30 min), negative zeta potential (−39.82 to and 70.07 mV), and no detrimental impact on the nasal mucosa. | [305] |
| Nanocomplexes | Doxorubicin and Temozolamide | Folic acid-CS-ALG | Complexation | - | - | Spherical diameter between 70–120 nm, Zeta potential ranging from 30–35 mV. In-vitro research on human cervical carcinoma cells and mouse fibroblasts showed more significant cytotoxicity or the dual-drug loaded formulation as compared to single-drug and free-drug formulations. | [306] |
| Nanoparticles | Insulin | CS-ALG | Self-assembly | Oral | The %EE of ALG-coated and CS-coated NPs were 81.5 ± 7.4% and 55.2 ± 7.0%, respectively. Effective plasma glucose reduction and prolonged insulin release after oral delivery to diabetic rats. | [307] | |
| Microspheres | Retinoic acid | SA | One-pot method | eye | Intravitreal | Average particle size was 95.7 ± 9.6 μm. Stable and controlled release, no harm to the optic nerve, and physiological function assessed by VEP 5b and ERG b wave. | [308] |
| Microspheres | Clove essential oil | SA | Modified emulsification | Oral | % Yield of microspheres, loading capacity, encapsulation efficiency, and in vitro release were found to be 72.73%, 0.99 ± 0.3%, 24.77 ± 7.47%, and 48.64 ± 3.00% after 4 h, respectively. The microspheres showed a controlled in vitro release profile of the clove oil. | [309] | |
| Nanoparticles | Amygdalin | CS-ALG | Ionic cross-linking | Mucosal | Zeta potential (−36 ± 0.88 and +32 ± 4.8 mV), mean size (around 119 nm), encapsulation efficiency (~90.3 ± 0.5%), effective swelling, and sustained release characteristics at pH 3.1, 5.0, 7.1, suitable mucoadhesive property in BioFlux system. | [310] | |
| Multiple layer mucoadhesive films | Metformin | Thiolated SA and CMC sodium | Double casting followed by compression | Intrapocket | Mucosal | Homogeneous, thin, and strong films for effortless insertion into the periodontal cavity. Adequate mucoadhesion and sustained release for 12 h. | [311] |
| Nanoparticles | Naringenin | ALG coated CS | Dual crosslinking using Na2SO4 and CaCl2 | Small intestine | Oral | Characterization by DLS, SEM, FTE, XRD, and pH-dependent dialysis study demonstrated excellent %EE of 91% and sustained flavonoid release. In vivo studies on rats showed significant anti-diabetic responses after oral delivery. | [312] |
| Nanoparticles | Miltefosine | ALG | Emulsification-external gelation method | mucosa | Oral | MFS-Alg NPs exhibited an average size of 279.1 ± 56.7 nm, polydispersity index of 0.42 ± 0.15, the zeta potential of −39.7 ± 5.2 mV, and %EE of 81.70 ± 6.64%. It presented no hemolysis or toxicity in G. mellonella larvae. Histopathological and CFU data show that MFS-Alg nanoparticles decreased the fungal load. | [313] |
| Microspheres | Ciprofloxacin | CS-coated konjac glucomannan/SA/graphene oxide | electrospinning | colon | Oral | The KGM/SA/GO microspheres were evaluated for their swelling rate (290%), drug loading (7.02%) and %EE (19.11%), zeta potential (−10.84, and −10.55 mV), and drug release (52% drug release after 20 h). | [314] |
| Nanoparticles | Curcumin diethyl disuccinate (CDD) | CS-ALG | Emulsification-ionotropic gelation | Human breast cancer | Oral | Encapsulated CDD in CANPs improved the stability and bio accessibility during the digestive stimulation and exhibited higher chemical stability when exposed to UV radiations. Bioavailability was enhanced five-fold. Greater cellular uptake and cytotoxicity in HepG2 cells than free CDD. | [315] |
| Nanocomposites | Doxorubicin | Fe3O4-SA-PVA-BSA | Co-precipitation/Ionotropic gelation | Cancer cells | Oral | The zeta potential ranged from −48.1 ± 2.3 to −22.4 ± 4.1 mV. The %EE varied between 36.2 ± 0.01 and 96.45 ± 2.12%. In vitro cytotoxicity tests using HepG2 and L02 cells demonstrated that DOX-loaded NPs (Fe3O4-SA-DOX-PVA-BSA) showed more significant cytotoxicity to HepG2 cells and non-toxic to L02 cells as compared to unloaded nanocomposites. | [316] |
| Devices | Model Drug/Drug | Composition | Preparation Method | Delivery Site | Applications | References |
| Microspheres | Curcumin | ALG | Emulsion-gelation | Mucosa | Parenteral drug delivery | [317] |
| Layered double hydroxide Nanocomposites | Bovine serum albumin | ALG/CS | Ionic gelation | Intestine | Oral vaccine drug delivery | [318] |
| Multi-particulates | Dalfampridine | Tamarind seed gum/ALG | Ionotropic gelation | Intestine | Oral drug delivery | [319] |
| Microspheres | Icariin | CS/SA | Emulsification-internal gelation | Colon | Oral drug delivery | [320] |
| Nanoparticles | Doxycycline | CS/SA | Coacervation method | Spleen, blood | Oral drug delivery | [321] |
| Microbeads | Resveratrol | CS/ALG and ALG/sucrose | Ionotropic gelation | Intestine | Oral drug delivery | [322] |
| Microbeads | Chlorhexidine | CA | Internal gelation | Oral cavity | Periodontal drug delivery | [323] |
| Microspheres | Omega-3 rich oils (fish liver oil) | ALG/CS | Oil-in-water (o/w) emulsification, gelation, and microencapsulation | Intestine | Oral drug delivery | [324] |
| PECs/hydrogels | Bevacizumab | ALG | Dispersion | - | Local drug delivery | [325] |
| Nanoparticles | Lysozyme | Polymethacrylate/ALG | Coacervation | - | Delivery system | [326] |
| Hydrogels | Deferoxamine | CS/ALG with poly(d,l-lactide-co-glycolide) | Mixing | Intestine | Oral drug delivery | [327] |
| Hydrogels | 5-Fluorouracil | Succinoglycan dialdehyde cross-linked hydrazine functionalized ALG | Ionic cross-linking | pH-controlled drug delivery | [328] | |
| Films | Omeprazole | Hydroxypropyl methyl cellulose (HPMC)/Methyl cellulose (MC)/SA/carrageenan(CA)/metolose(MET) | Film casting | Stomach | Buccal drug delivery | [329] |
| Floating In situ gel | Celecoxib | SA/PEG | Ionic cross-linking | Site of inflammation/edema | Oral sustained drug delivery | [330] |
| Hydrogel beads | Ibuprofen | ALG-magnetic nitrocellulose (m-CNC) | Ionic cross-linking | - | Drug delivery | [331] |
| Hydrogel encapsulated microspheres | 5-Fluorouracil | CS/ALG/gelatin | Emulsion cross-linking | - | Drug delivery | [332] |
| Microspheres entrapped hydrogels | Methotrexate, loaded Calcium Carbonate, and Aspirin | ALG and sodium CMC crosslinked with Ca2+ ions | Co-precipitation | - | Drug delivery | [333] |
| Films | Tamoxifen | ALG/CS | Spray-assisted LbL technique | - | Drug delivery | [334] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. https://doi.org/10.3390/ijms23169035
Abourehab MAS, Rajendran RR, Singh A, Pramanik S, Shrivastav P, Ansari MJ, Manne R, Amaral LS, Deepak A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. International Journal of Molecular Sciences. 2022; 23(16):9035. https://doi.org/10.3390/ijms23169035
Chicago/Turabian StyleAbourehab, Mohammad A. S., Rahul R. Rajendran, Anshul Singh, Sheersha Pramanik, Prachi Shrivastav, Mohammad Javed Ansari, Ravi Manne, Larissa Souza Amaral, and A. Deepak. 2022. "Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art" International Journal of Molecular Sciences 23, no. 16: 9035. https://doi.org/10.3390/ijms23169035
APA StyleAbourehab, M. A. S., Rajendran, R. R., Singh, A., Pramanik, S., Shrivastav, P., Ansari, M. J., Manne, R., Amaral, L. S., & Deepak, A. (2022). Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. International Journal of Molecular Sciences, 23(16), 9035. https://doi.org/10.3390/ijms23169035