Comparison of the Modulating Effect of Anthocyanin-Rich Sour Cherry Extract on Occludin and ZO-1 on Caco-2 and HUVEC Cultures
Abstract
:1. Introduction
2. Results
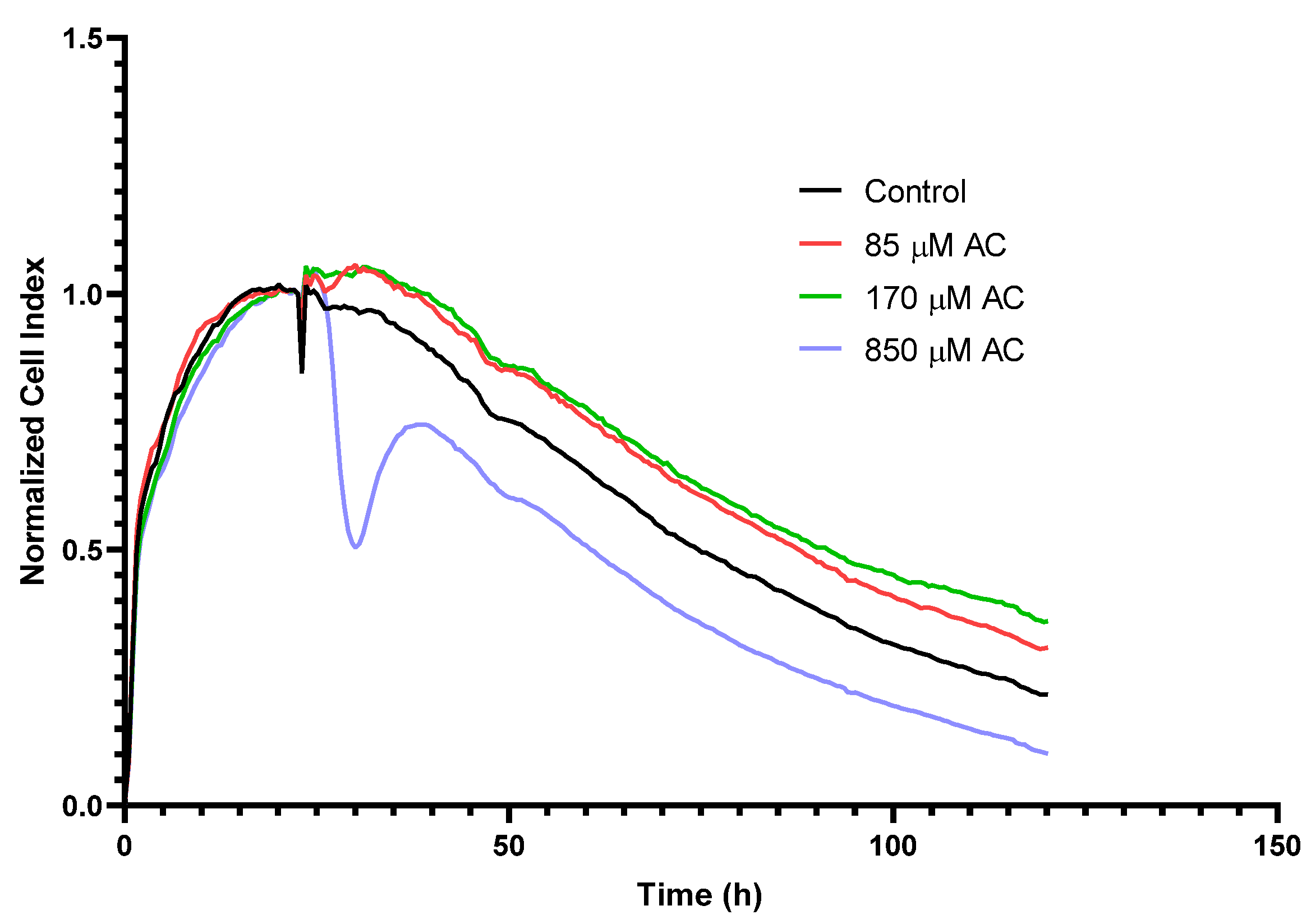
2.1. Real-Time Monitoring of Cell Index (RTCA) in HUVEC
2.2. Permeability Assay on HUVECs
2.3. Immunofluorescence
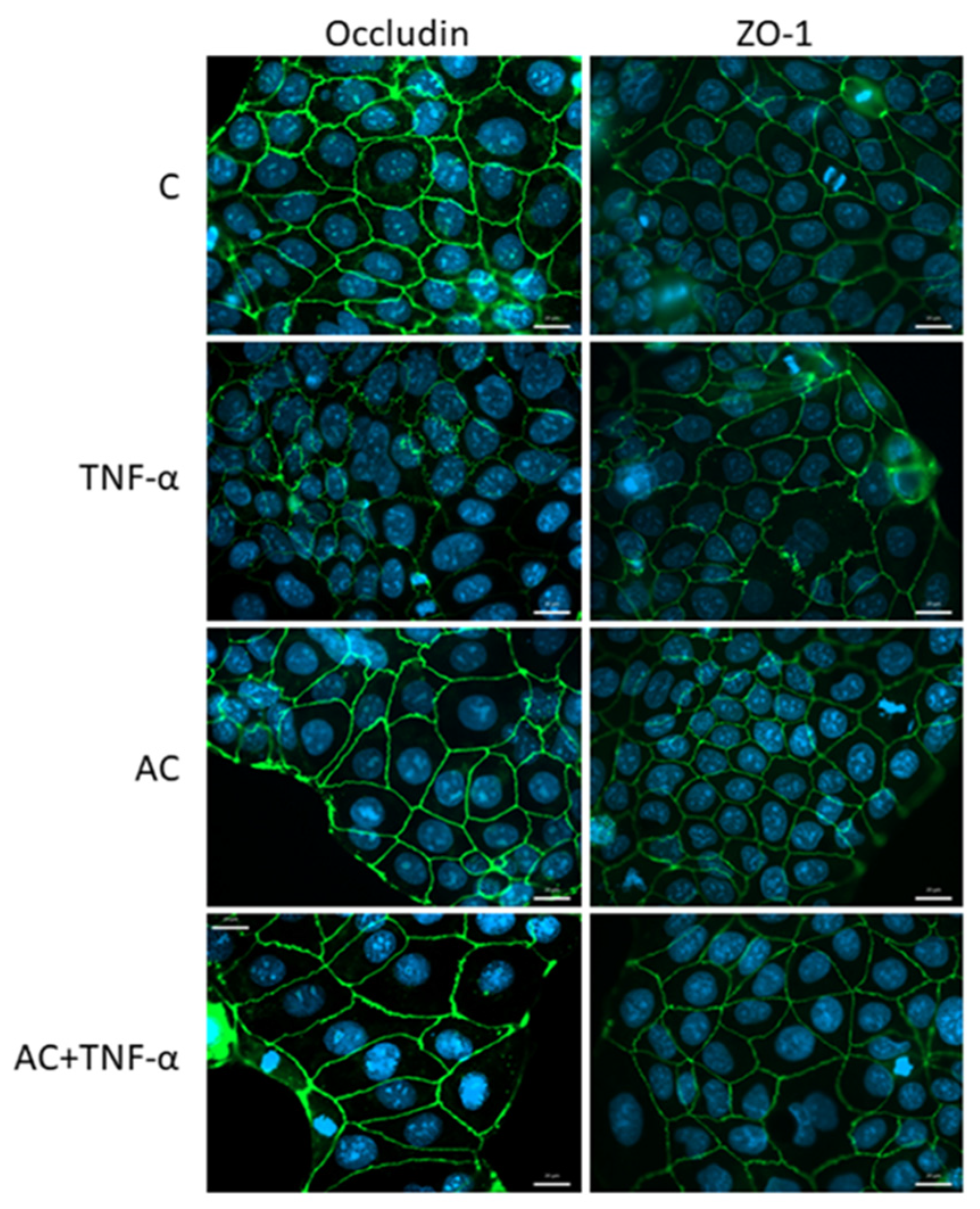
2.3.1. Immunohistochemical Staining of Occludin and ZO-1
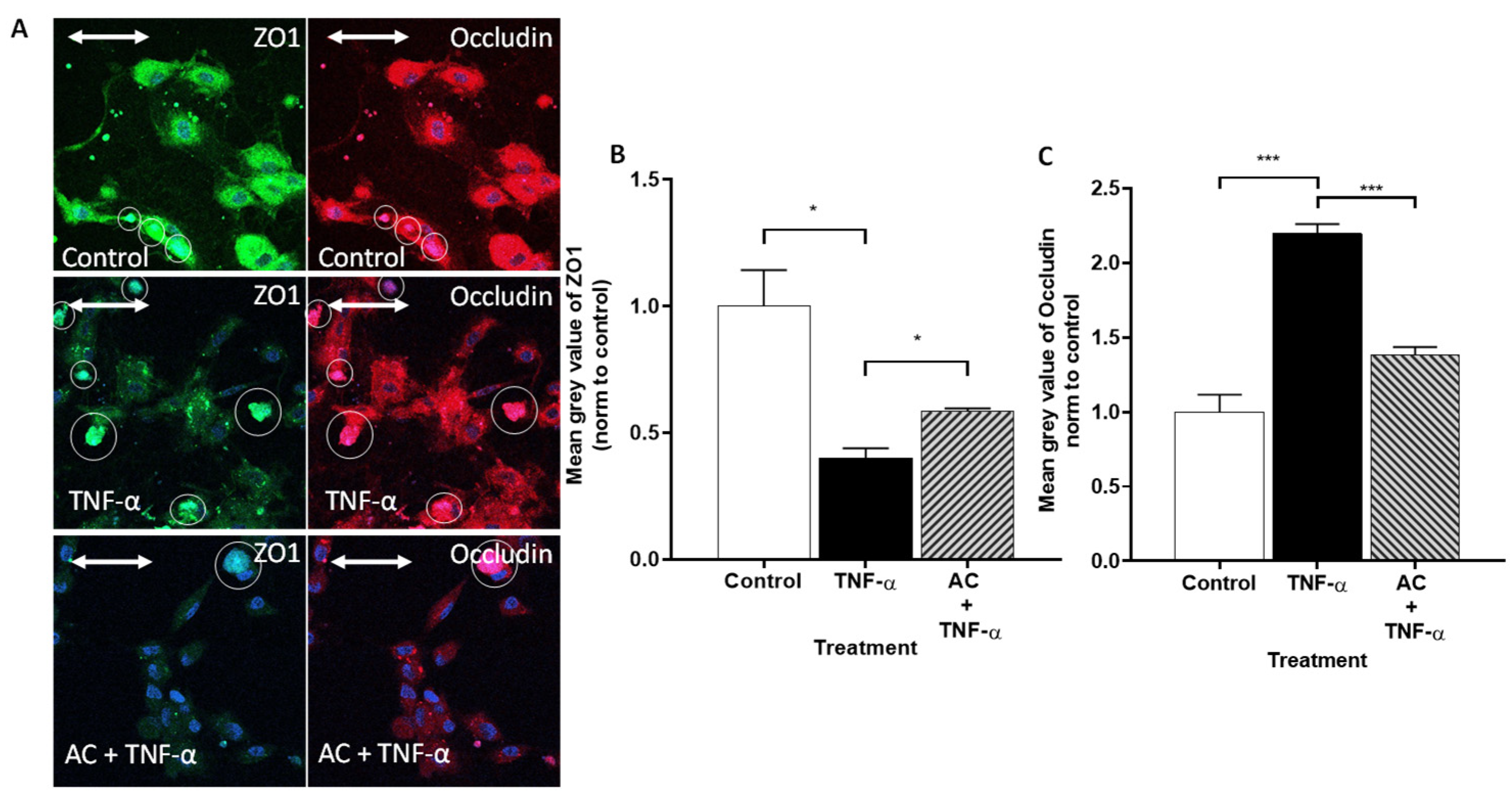
2.3.2. Immunofluorescence Determination of Occludin and ZO-1 Proteins by Flow Cytometry
2.4. Quantitative Real-Time PCR (qPCR)
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Permeability Assay on HUVECs
4.3. Immunofluorescence
4.4. Flow Cytometry
4.5. Real-Time Monitoring of Cell Index (RTCA)
4.6. qPCR
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Van Meerveld, B.G.; Verne, G.N. Intestinal Barrier Function in Health and Gastrointestinal Disease. Neurogastroenterol. Motil. J. Eur. Gastrointest. Motil. Soc. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Alon, R. Leukocyte Migration into Inflamed Tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Milton, S.G.; Knutson, V.P. Comparison of the Function of the Tight Junctions of Endothelial Cells and Epithelial Cells in Regulating the Movement of Electrolytes and Macromolecules across the Cell Monolayer. J. Cell. Physiol. 1990, 144, 498–504. [Google Scholar] [CrossRef]
- Wolburg, H.; Lippoldt, A. Tight Junctions of the Blood-Brain Barrier: Development, Composition and Regulation. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases. Int. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Garberg, P.; Ball, M.; Borg, N.; Cecchelli, R.; Fenart, L.; Hurst, R.D.; Lindmark, T.; Mabondzo, A.; Nilsson, J.E.; Raub, T.J.; et al. In Vitro Models for the Blood-Brain Barrier. Toxicol. Vitr. 2005, 19, 299–334. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood–Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Tight Junctions and the Molecular Basis for Regulation of Paracellular Permeability. Am. J. Physiol. 1995, 269, G467–G475. [Google Scholar] [CrossRef]
- Madara, J.L. Loosening Tight Junctions. Lessons from the Intestine. J. Clin. Investig. 1989, 83, 1089–1094. [Google Scholar] [CrossRef]
- Hollander, D. Crohn’s Disease—A Permeability Disorder of the Tight Junction? Gut 1988, 29, 1621–1624. [Google Scholar] [CrossRef]
- Ma, T.Y. Intestinal Epithelial Barrier Dysfunction in Crohn’s Disease. Proc. Soc. Exp. Biol. Med. 1997, 214, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.K.; Bamba, P.S.; Perkins, B.N.; Luscinskas, F.W. Real-Time Imaging of Vascular Endothelial-Cadherin during Leukocyte Transmigration across Endothelium. J. Immunol. 2001, 167, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.X.; Liu, Z.J. Potential Roles of Neutrophils in Regulating Intestinal Mucosal Inflammation of Inflammatory Bowel Disease. J. Dig. Dis. 2017, 18, 495–503. [Google Scholar] [CrossRef]
- Neurath, M.F.; Fuss, I.; Pasparakis, M.; Alexopoulou, L.; Haralambous, S.; Meyer zum Büschenfelde, K.H.; Strober, W.; Kollias, G. Predominant Pathogenic Role of Tumor Necrosis Factor in Experimental Colitis in Mice. Eur. J. Immunol. 1997, 27, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Ricart, E.; Panaccione, R.; Loftus, E.V.; Tremaine, W.J.; Sandborn, W.J. Infliximab for Crohn’s Disease in Clinical Practice at the Mayo Clinic: The First 100 Patients. Am. J. Gastroenterol. 2001, 96, 722–729. [Google Scholar] [CrossRef]
- Mullin, J.M.; Snock, K. V Effect of Tumor Necrosis Factor on Epithelial Tight Junctions and Transepithelial Permeability. Cancer Res. 1990, 50, 2172–2176. [Google Scholar]
- McKay, D.M.; Singh, P.K. Superantigen Activation of Immune Cells Evokes Epithelial (T84) Transport and Barrier Abnormalities via IFN-Gamma and TNF Alpha: Inhibition of Increased Permeability, but Not Diminished Secretory Responses by TGF-Beta2. J. Immunol. 1997, 159, 2382–2390. [Google Scholar]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells--A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef]
- Cunningham, K.E.; Turner, J.R. Myosin Light Chain Kinase: Pulling the Strings of Epithelial Tight Junction Function. Ann. N. Y. Acad. Sci. 2012, 1258, 34–42. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3- O -Glucoside Inhibits NF-KB Signalling in Intestinal Epithelial Cells Exposed to TNF-α and Exerts Protective Effects via Nrf2 Pathway Activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
- Le Phuong Nguyen, T.; Fenyvesi, F.; Remenyik, J.; Homoki, J.R.; Gogolák, P.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Vasvári, G.; Vecsernyés, M.; et al. Protective Effect of Pure Sour Cherry Anthocyanin Extract on Cytokine-Induced Inflammatory Caco-2 Monolayers. Nutrients 2018, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, K.D.; Collins, L.E.; McLoughlin, A.; Cummins, P.M. Tumour Necrosis Factor-α-Mediated Disruption of Cerebrovascular Endothelial Barrier Integrity in Vitro Involves the Production of Proinflammatory Interleukin-6. J. Neurochem. 2016, 136, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Teng, T.; Li, R.; Simonyi, A.; Sun, G.Y.; Lee, J.C. TNFα Alters Occludin and Cerebral Endothelial Permeability: Role of P38MAPK. PLoS ONE 2017, 12, e0170346. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lee, C.; Yang, S.; Park, E.K.; Ku, S.-K.; Bae, J.-S. Suppressive Effects of Pelargonidin on Lipopolysaccharide-Induced Inflammatory Responses. Chem.-Biol. Interact. 2019, 302, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ershad, M.; Shigenaga, M.K.; Bandy, B. Differential Protection by Anthocyanin-Rich Bilberry Extract and Resveratrol against Lipid Micelle-Induced Oxidative Stress and Monolayer Permeability in Caco-2 Intestinal Epithelial Cells. Food Funct. 2021, 12, 2950–2961. [Google Scholar] [CrossRef] [PubMed]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin Composition, Antioxidant Efficiency, and α-Amylase Inhibitor Activity of Different Hungarian Sour Cherry Varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef]
- Varga, B.; Priksz, D.; Lampé, N.; Bombicz, M.; Kurucz, A.; Szabó, A.M.; Pósa, A.; Szabó, R.; Kemény-Beke, Á.; Remenyik, J.; et al. Protective Effect of Prunus Cerasus (Sour Cherry) Seed Extract on the Recovery of Ischemia/Reperfusion-Induced Retinal Damage in Zucker Diabetic Fatty Rat. Molecules 2017, 22, 1782. [Google Scholar] [CrossRef]
- Homoki, J.; Gyémánt, G.; Balogh, P.; Stündl, L.; Bíró-Molnár, P.; Paholcsek, M.; Váradi, J.; Ferenc, F.; Kelentey, B.; Nemes, J.; et al. Sour Cherry Extract Inhibits Human Salivary α-Amylase and Growth of Streptococcus Mutans (a Pilot Clinical Study). Food Funct. 2018, 9, 4008–4016. [Google Scholar] [CrossRef]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedűs, C.; Kovács, D.; Peitl, B.; Gál, F.; Stündl, L.; Szilvássy, Z.; Remenyik, J. Effect of Anthocyanin-Rich Tart Cherry Extract on Inflammatory Mediators and Adipokines Involved in Type 2 Diabetes in a High Fat Diet Induced Obesity Mouse Model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef]
- Nemes, A.; Szőllősi, E.; Stündl, L.; Biró, A.; Homoki, J.R.; Szarvas, M.M.; Balogh, P.; Cziáky, Z.; Remenyik, J. Determination of Flavonoid and Proanthocyanidin Profile of Hungarian Sour Cherry. Molecules 2018, 23, 3278. [Google Scholar] [CrossRef]
- Biro, A.; Markovich, A.; Homoki, J.R.; Szőllősi, E.; Hegedűs, C.; Tarapcsák, S.; Lukács, J.; Stündl, L.; Remenyik, J. Anthocyanin-Rich Sour Cherry Extract Attenuates the Lipopolysaccharide-Induced Endothelial Inflammatory Response. Molecules 2019, 24, 3427. [Google Scholar] [CrossRef] [PubMed]
- Csernus, B.; Biró, S.; Babinszky, L.; Komlósi, I.; Jávor, A.; Stündl, L.; Remenyik, J.; Bai, P.; Oláh, J.; Pesti-Asbóth, G.; et al. Effect of Carotenoids, Oligosaccharides and Anthocyanins on Growth Performance, Immunological Parameters and Intestinal Morphology in Broiler Chickens Challenged with Escherichia Coli Lipopolysaccharide. Animals 2020, 10, 347. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Cong, X.; Kong, W. Endothelial Tight Junctions and Their Regulatory Signaling Pathways in Vascular Homeostasis and Disease. Cell. Signal. 2020, 66, 109485. [Google Scholar] [CrossRef] [PubMed]
- Markovics, A.; Biró, A.; Kun-Nemes, A.; Fazekas, M.É.; Rácz, A.A.; Paholcsek, M.; Lukács, J.; Stündl, L.; Remenyik, J. Effect of Anthocyanin-Rich Extract of Sour Cherry for Hyperglycemia-Induced Inflammatory Response and Impaired Endothelium-Dependent Vasodilation. Nutrients 2020, 12, 3373. [Google Scholar] [CrossRef]
- Matter, K.; Balda, M.S. Signalling to and from Tight Junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Shi, Y.; Li, R.; Yang, J.; Li, X. No Tight Junctions in Tight Junction Protein-1 Expressing HeLa and Fibroblast Cells. Int. J. Physiol. Pathophysiol. Pharmacol. 2020, 12, 70–78. [Google Scholar]
- Yuan, X.; Chen, Y.; Chen, G.; Liu, G.; Hang, M.; Wang, P.; Luo, Y.; Guo, D.; Xu, L. The Heat Shock Protein 70 Plays a Protective Role in Sepsis by Maintenance of the Endothelial Permeability. BioMed Res. Int. 2020, 2020, 2194090. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Predescu, D.; Palade, G.E. Plasmalemmal Vesicles Represent the Large Pore System of Continuous Microvascular Endothelium. Am. J. Physiol.-Heart Circ. Physiol. 1993, 265, H725–H733. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Aijaz, S.; Tsapara, A.; Balda, M.S. Mammalian Tight Junctions in the Regulation of Epithelial Differentiation and Proliferation. Curr. Opin. Cell Biol. 2005, 17, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of Intestinal Epithelial Permeability by Tight Junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remenyik, J.; Biró, A.; Klusóczki, Á.; Juhász, K.Z.; Szendi-Szatmári, T.; Kenesei, Á.; Szőllősi, E.; Vasvári, G.; Stündl, L.; Fenyvesi, F.; et al. Comparison of the Modulating Effect of Anthocyanin-Rich Sour Cherry Extract on Occludin and ZO-1 on Caco-2 and HUVEC Cultures. Int. J. Mol. Sci. 2022, 23, 9036. https://doi.org/10.3390/ijms23169036
Remenyik J, Biró A, Klusóczki Á, Juhász KZ, Szendi-Szatmári T, Kenesei Á, Szőllősi E, Vasvári G, Stündl L, Fenyvesi F, et al. Comparison of the Modulating Effect of Anthocyanin-Rich Sour Cherry Extract on Occludin and ZO-1 on Caco-2 and HUVEC Cultures. International Journal of Molecular Sciences. 2022; 23(16):9036. https://doi.org/10.3390/ijms23169036
Chicago/Turabian StyleRemenyik, Judit, Attila Biró, Ágnes Klusóczki, Krisztián Zoltán Juhász, Tímea Szendi-Szatmári, Ádám Kenesei, Erzsébet Szőllősi, Gábor Vasvári, László Stündl, Ferenc Fenyvesi, and et al. 2022. "Comparison of the Modulating Effect of Anthocyanin-Rich Sour Cherry Extract on Occludin and ZO-1 on Caco-2 and HUVEC Cultures" International Journal of Molecular Sciences 23, no. 16: 9036. https://doi.org/10.3390/ijms23169036
APA StyleRemenyik, J., Biró, A., Klusóczki, Á., Juhász, K. Z., Szendi-Szatmári, T., Kenesei, Á., Szőllősi, E., Vasvári, G., Stündl, L., Fenyvesi, F., Váradi, J., & Markovics, A. (2022). Comparison of the Modulating Effect of Anthocyanin-Rich Sour Cherry Extract on Occludin and ZO-1 on Caco-2 and HUVEC Cultures. International Journal of Molecular Sciences, 23(16), 9036. https://doi.org/10.3390/ijms23169036










