The Chemerin/CMKLR1 Axis Is Involved in the Recruitment of Microglia to Aβ Deposition through p38 MAPK Pathway
Abstract
1. Introduction
2. Results
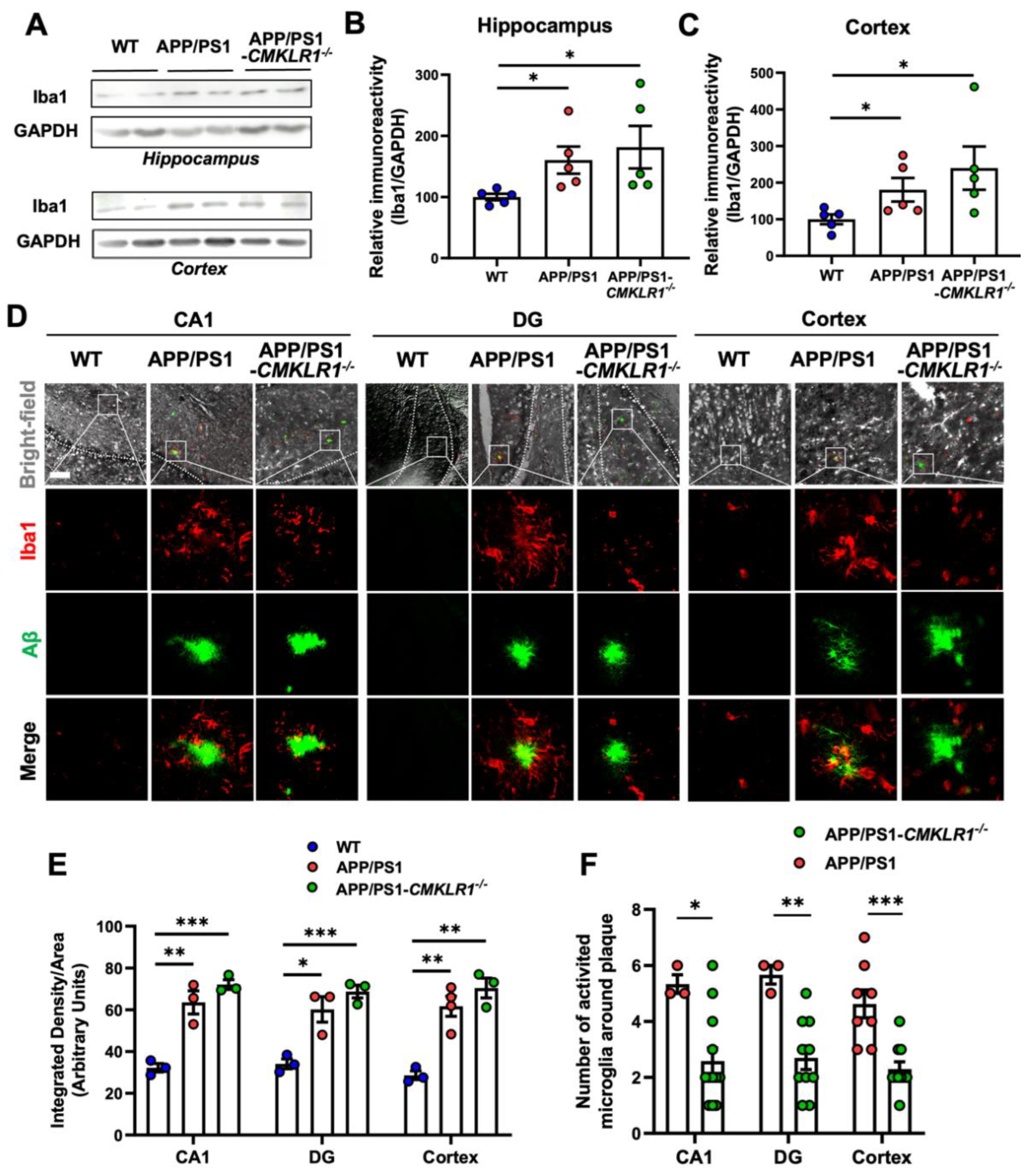
2.1. CMKLR1 Deficiency Decreases Microglia Colocalization with Aβ Plaques in the Brain of APP/PS1 Transgenic Mice
2.2. Chemerin/CMKLR1 Axis Induces the Migration of Microglia
2.3. Chemerin/CMKLR1 Promotes Actin and Microtubule Remodeling, and Golgi Reorientation in Microglia
2.4. p38 MAPK Pathway Is Involved in the Promotion of Chemerin/CMKLR1 on the Migration and Polarization of Microglia
2.5. p38 MAPK Pathway Is Involved in the Suppressive Effects of Chemerin on Aβ-Induced the Aggregation of Microglia
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Animals
4.3. Microglial Cell Cultures
4.4. Western Blot
4.5. Immunofluorescence Staining
4.6. Boyden Chamber Assay
4.7. Scratch-Wound Assay
4.8. Measurement of Microglial Clusters
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Abeta oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Yu, Y.; Ye, R.D. Microglial Abeta receptors in Alzheimer’s disease. Cell Mol. Neurobiol. 2015, 35, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a Novel Adipokine That Regulates Adipogenesis and Adipocyte Metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef]
- Krautbauer, S.; Wanninger, J.; Eisinger, K.; Hader, Y.; Beck, M.; Kopp, A.; Schmid, A.; Weiss, T.S.; Dorn, C.; Buechler, C. Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver. Exp. Mol. Pathol. 2013, 95, 199–205. [Google Scholar] [CrossRef]
- Chamberland, J.P.; Berman, R.L.; Aronis, K.N.; Mantzoros, C.S. Chemerin is expressed mainly in pancreas and liver, is regulated by energy deprivation and lacks day/night variation in humans. Eur. J. Endocrinol. 2013, 169, 453–462. [Google Scholar] [CrossRef]
- El-Sagheer, G.; Gayyed, M.; Ahmad, A.; Abd El-Fattah, A.; Mohamed, M. Expression of chemerin correlates with a poor prognosis in female breast cancer patients. Breast Cancer 2018, 10, 169–176. [Google Scholar] [CrossRef]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Silverio, A.M.; Butcher, E.C. Chemokine-Like Receptor 1 Expression and Chemerin-Directed Chemotaxis Distinguish Plasmacytoid from Myeloid Dendritic Cells in Human Blood. J. Immunol. 2004, 174, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Parolini, S.; Santoro, A.; Marcenaro, E.; Luini, W.; Massardi, L.; Facchetti, F.; Communi, D.; Parmentier, M.; Majorana, A.; Sironi, M.; et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007, 109, 3625–3632. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.-F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Y.; Ling, X.; Qin, H.; Wang, M.; Luo, B. Chemerin/CMKLR1 Axis Promotes Inflammation and Pyroptosis by Activating NLRP3 Inflammasome in Diabetic Cardiomyopathy Rat. Front. Physiol. 2020, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Nakae, S.; Znuñiga, L.; Kim, J.-Y.; Ohyama, T.; Alt, C.; Pan, J.; Suto, H.; Soler, D.; Allen, S.J.; et al. Mast cell–expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 2008, 205, 2207–2220. [Google Scholar] [CrossRef]
- Cash, J.; Hart, R.; Russ, A.; Dixon, J.P.; Colledge, W.H.; Doran, J.; Hendrick, A.; Carlton, M.B.; Greaves, D.R. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008, 205, 767–775. [Google Scholar] [CrossRef]
- Barnea, G.; Strapps, W.; Herrada, G.; Berman, Y.; Ong, J.; Kloss, B.; Axel, R.; Lee, K.J. The genetic design of signaling cascades to record receptor activation. Proc. Natl. Acad. Sci. USA 2008, 105, 64–69. [Google Scholar] [CrossRef]
- Meder, W.; Wendland, M.; Busmann, A.; Kutzleb, C.; Spodsberg, N.; John, H.; Richter, R.; Schleuder, D.; Meyer, M.; Forssmann, W. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 2003, 555, 495–499. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Davenport, A.P. International Union of Basic and Clinical Pharmacology CIII: Chemerin Receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2018, 70, 174–196. [Google Scholar] [CrossRef]
- Peng, L.; Yu, Y.; Liu, J.; Li, S.; He, H.; Ni Cheng, N.; Ye, R.D. The Chemerin Receptor CMKLR1 is a Functional Receptor for Amyloid-β Peptide. J. Alzheimers Dis. 2014, 43, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, N.; Ding, Y.; Zhang, Y.; Li, Q.; Flores, J.; Haghighiabyaneh, M.; Doycheva, D.; Tang, J.; Zhang, J.H. Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav. Immun. 2018, 70, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, A.; Gong, P.; Chen, Y.; Ye, R.D.; Qian, F.; Zhang, Y.; Yu, Y. The chemokine-like receptor 1 deficiency improves cognitive deficits of AD mice and attenuates tau hyperphosphorylation via regulating tau seeding. J. Neurosci. 2020, 40, 6991–7007. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Floyd, R.A.; Zheng, N.Y.; Nael, R.; Robinson, K.A.; Nguyen, X.; Pye, Q.N.; Stewart, C.A.; Geddes, J.; Markesbery, W.R.; et al. p38 kinase is activated in the Alzheimer’s disease brain. J. Neurochem. 1999, 72, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Dixon, S.G.; Hu, W.; Hamlett, E.D.; Jin, J.; Ergul, A.; Wang, G.Y. p38 MAPK Is a Major Regulator of Amyloid Beta-Induced IL-6 Expression in Human Microglia. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ittner, A.; Chua, S.W.; Bertz, J.; Volkerling, A.; van der Hoven, J.; Gladbach, A.; Przybyla, M.; Bi, M.; van Hummel, A.; Stevens, C.H.; et al. Site-specific phosphorylation of tau inhibits amyloid-beta toxicity in Alzheimer’s mice. Science 2016, 354, 904–908. [Google Scholar] [CrossRef]
- Lin, A.; Liu, J.; Gong, P.; Chen, Y.; Zhang, H.; Zhang, Y.; Yu, Y. Serum amyloid A inhibits astrocyte migration via activating p38 MAPK. J. Neuroinflamm. 2020, 17, 254. [Google Scholar] [CrossRef]
- Das, R.; Balmik, A.A.; Chinnathambi, S. Phagocytosis of full-length Tau oligomers by Actin-remodeling of activated microglia. J. Neuroinflamm. 2020, 17, 10. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Malik, A.B.; Tang, H.; Yang, T.; Sun, B.; Wang, G.; Minshall, R.D.; Li, Y.; Zhao, Y.; et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat. Immunol. 2012, 13, 457–464. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Kaur, J.; Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Identification of chemerin receptor (ChemR23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochem. Biophys. Res. Commun. 2010, 391, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, N.; Ding, Y.; Doycheva, D.M.; Zhang, Y.; Li, Q.; Flores, J.; Haghighiabyaneh, M.; Tang, J.; Zhang, J.H. Chemerin reverses neurological impairments and ameliorates neuronal apoptosis through ChemR23/CAMKK2/AMPK pathway in neonatal hypoxic-ischemic encephalopathy. Cell Death Dis. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Mairet-Coello, G.; Courchet, J.; Pieraut, S.; Courchet, V.; Maximov, A.; Polleux, F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron 2013, 78, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Vermi, W.; Riboldi, E.; Wittamer, V.; Gentili, F.; Luini, W.; Marrelli, S.; Vecchi, A.; Franssen, J.-D.; Communi, D.; Massardi, L.; et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J. Exp. Med. 2005, 201, 509–515. [Google Scholar] [CrossRef]
- Luangsay, S.; Wittamer, V.; Bondue, B.; De Henau, O.; Rouger, L.; Brait, M.; Franssen, J.-D.; De Nadai, P.; Huaux, F.; Parmentier, M. Mouse ChemR23 Is Expressed in Dendritic Cell Subsets and Macrophages, and Mediates an Anti-Inflammatory Activity of Chemerin in a Lung Disease Model. J. Immunol. 2009, 183, 6489–6499. [Google Scholar] [CrossRef]
- Wittamer, V.; Grégoire, F.; Robberecht, P.; Vassart, G.; Communi, D.; Parmentier, M. The C-terminal Nonapeptide of Mature Chemerin Activates the Chemerin Receptor with Low Nanomolar Potency. J. Biol. Chem. 2004, 279, 9956–9962. [Google Scholar] [CrossRef]
- Pachynski, R.K.; Zabel, B.A.; Kohrt, H.E.; Tejeda, N.M.; Monnier, J.; Swanson, C.D.; Holzer, A.K.; Gentles, A.J.; Sperinde, G.V.; Edalati, A.; et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J. Exp. Med. 2012, 209, 1427–1435. [Google Scholar] [CrossRef]
- Wen, J.; Wang, J.; Guo, L.; Cai, W.; Wu, Y.; Chen, W.; Tang, X. Chemerin stimulates aortic smooth muscle cell proliferation and migration via activation of autophagy in VSMCs of metabolic hypertension rats. Am. J. Transl. Res. 2019, 11, 1327–1342. [Google Scholar]
- Kumar, J.D.; Aolymat, I.; Tiszlavicz, L.; Reisz, Z.; Garalla, H.M.; Beynon, R.; Simpson, D.; Dockray, G.J.; Varro, A. Chemerin acts via CMKLR1 and GPR1 to stimulate migration and invasion of gastric cancer cells: Putative role of decreased TIMP-1 and TIMP-2. Oncotarget 2019, 10, 98–112. [Google Scholar] [CrossRef][Green Version]
- Peng, L.; Chen, Y.; Li, Y.; Feng, P.; Zheng, Y.; Dong, Y.; Yang, Y.; Wang, R.; Li, A.; Yan, J.; et al. Chemerin Regulates the Proliferation and Migration of Pulmonary Arterial Smooth Muscle Cells via the ERK1/2 Signaling Pathway. Front. Pharmacol. 2022, 13, 767705. [Google Scholar] [CrossRef]
- Van Helvert, S.; Storm, C.; Friedl, P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 2018, 20, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Junior, E.; Leite, G.A.; Carmo-Silva, C.C.; Taira, T.M.; Neves, K.B.; Colon, D.; Ab Da Silva, L.; Salvador, S.L.; Tostes, R.; Cunha, F.Q.; et al. Adipokine Chemerin Bridges Metabolic Dyslipidemia and Alveolar Bone Loss in Mice. J. Bone Miner. Res. 2017, 32, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Tatsuno, K.; Ito, T.; Sakabe, J.-I.; Funakoshi, A.; Tokura, Y. Distinctive downmodulation of plasmacytoid dendritic cell functions by vitamin D3 analogue calcipotriol. J. Dermatol. Sci. 2016, 84, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, H.; Kazama, K.; Takai, M.; Oda, M.; Okada, M.; Yamawaki, H. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am. J. Physiol. Circ. Physiol. 2015, 309, H1017–H1028. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; Christian, A.R.; Greaves, D.R. Chemerin Peptides Promote Phagocytosis in a ChemR23- and Syk-Dependent Manner. J. Immunol. 2010, 184, 5315–5324. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Curran, J.E.; Stocker, C.J.; Zaibi, M.S.; Segal, D.; Konstantopoulos, N.; Morrison, S.; Carless, M.; Dyer, T.D.; Cole, S.A.; et al. Chemerin, a Novel Adipokine in the Regulation of Angiogenesis. J. Clin. Endocrinol. Metab. 2010, 95, 2476–2485. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Li, S.-Q.; Yu, Y.; Ye, R.D. Suppression of LPS-induced tau hyperphosphorylation by serum amyloid A. J. Neuroinflamm. 2016, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, J.; Li, S.-Q.; Peng, L.; Ye, R.D. Serum Amyloid A Differentially Activates Microglia and Astrocytes via the PI3K Pathway. J. Alzheimers Dis. 2013, 38, 133–144. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Li, S.-Q.; Su, N.; Gong, P.; Zhang, H.-B.; Liu, J.; Wang, D.; Sun, Y.-P.; Zhang, Y.; Qian, F.; Zhao, B.; et al. The Expression of Formyl Peptide Receptor 1 is Correlated with Tumor Invasion of Human Colorectal Cancer. Sci. Rep. 2017, 7, 5918. [Google Scholar] [CrossRef]
- Osmani, N.; Vitale, N.; Borg, J.-P.; Etienne-Manneville, S. Scrib Controls Cdc42 Localization and Activity to Promote Cell Polarization during Astrocyte Migration. Curr. Biol. 2007, 16, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S.; Hall, A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 2001, 106, 489–498. [Google Scholar] [CrossRef]
- Huang, W.C.; Yen, F.C.; Shie, F.S.; Pan, C.M.; Shiao, Y.J.; Yang, C.N.; Huang, F.L.; Sung, Y.J.; Tsay, H.J. TGF-beta1 blockade of microglial chemotaxis toward Abeta aggregates involves SMAD signaling and down-regulation of CCL5. J. Neuroinflamm. 2010, 7, 28. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, Z.; Gong, P.; Zhang, H.; Chen, Y.; Yao, S.; Li, W.; Zhang, Y.; Yu, Y. The Chemerin/CMKLR1 Axis Is Involved in the Recruitment of Microglia to Aβ Deposition through p38 MAPK Pathway. Int. J. Mol. Sci. 2022, 23, 9041. https://doi.org/10.3390/ijms23169041
Chen Y, Liu Z, Gong P, Zhang H, Chen Y, Yao S, Li W, Zhang Y, Yu Y. The Chemerin/CMKLR1 Axis Is Involved in the Recruitment of Microglia to Aβ Deposition through p38 MAPK Pathway. International Journal of Molecular Sciences. 2022; 23(16):9041. https://doi.org/10.3390/ijms23169041
Chicago/Turabian StyleChen, Yanqing, Zhen Liu, Ping Gong, Haibo Zhang, Yijun Chen, Songquan Yao, Wei Li, Yan Zhang, and Yang Yu. 2022. "The Chemerin/CMKLR1 Axis Is Involved in the Recruitment of Microglia to Aβ Deposition through p38 MAPK Pathway" International Journal of Molecular Sciences 23, no. 16: 9041. https://doi.org/10.3390/ijms23169041
APA StyleChen, Y., Liu, Z., Gong, P., Zhang, H., Chen, Y., Yao, S., Li, W., Zhang, Y., & Yu, Y. (2022). The Chemerin/CMKLR1 Axis Is Involved in the Recruitment of Microglia to Aβ Deposition through p38 MAPK Pathway. International Journal of Molecular Sciences, 23(16), 9041. https://doi.org/10.3390/ijms23169041





