Dissection of Crop Metabolome Responses to Nitrogen, Phosphorus, Potassium, and Other Nutrient Deficiencies
Abstract
:1. Introduction
2. Metabolisms Responsive to Nutrient Deficiencies in Crops
2.1. N Deficiency
| Crop Species | Tissue | Duration of Treatment (days) | Method | Number of DAMs | Main Changes in Metabolites or Metabolic Pathways | Reference |
|---|---|---|---|---|---|---|
| Maize (Zea mays) a | Leaves | 20/30 | GC–MS | 70 (in total) | Decreasing most amino acids; increasing starch and secondary metabolites. | [25] |
| Tomato (Solanum lycopersicum) | Leaves | 5/15 | LC/GC–MS | 28/34 | Decreasing amino acids and organic acids; increasing Fru-6-P, Glc-6-P, and sedoheptulose-7-P. | [26] |
| Roots | 5/15 | LC/GC–MS | 28/34 | Decreasing amino acids and organic acids; increasing Fru-6-P, glucose, Glc-6-P, glycerate, pyruvate, ribulose, fructose, and sucrose. | [26] | |
| Rice (Oryza sativa) | Shoots | 5/15 | CE–TOF MS | 49/65 | Decreasing l-aspartate, l-phenylalanine, GABA, guanosine, adenine, and cytidine. | [28] |
| Roots | 5/15 | CE–TOF MS | 59/73 | Decreasing nicotinamide, sorbitol-6P, glycero-3P, l-phenylalanine, GABA, citrulline, acetylserine, and histidinol. | [28] | |
| Root exudates | 5/15 | CE–TOF MS | 17/24 | Increasing glutarate, adipate, 2-hydroxyisobutyrate, succinate, 2-isopropylmalate, raffinose, and abscisate. | [28] | |
| Leaves | 30 | LC–ESI-MS/MS | 432 | Promoting TCA cycle to produce more energy and α-ketoglutarate. | [29] | |
| Barley (Hordeum vulgare) a | Leaves | 1/3/6/9/12/15/18 | GC–MS | 51 (in total) | Decreasing all major amino acids. | [27] |
| Roots | 1/3/6/9/12/15/18 | GC–MS | 51 (in total) | Increasing both minor and major amino acids at late stage. | [27] | |
| Shoots | 20 | GC–MS | 51 | Decreasing amino acids (glycine, asparagine, aspartic acid, glutamine, lysine, and threonine); increasing sugars (maltose, glucose, fructose, galactose, and psicose). | [35] | |
| Barley (Hordeum vulgare) a | Roots | 20 | GC–MS | 49 | Decreasing amino acids (lysine, tyrosine, threonine, ornithine, and glutamine) | [35] |
| Soybean (Glycine max) a | Roots | 14 | GC–MS | 36/40 | Increasing soluble sugars and organic acids. | [36] |
| Wheat (Triticum aestivum) | Grains | 25 days post anthesis | GC–MS | 77 | Increasing ornithine, cysteine, aspartate, and tyrosine; promoting sugar accumulation. | [31] |
| Rapeseed (Brassica napus) | Leaves | 14 | LC–ESI-MS/MS | 175 | Decreasing aspartic acid; increasing l-alanine. | [32] |
| Rapeseed (Brassica napus) | Roots | 14 | LC–ESI-MS/MS | 166 | Increasing aspartic acid. | [32] |
| Apple (Malus pumila) | Leaves | 30 | LC–ESI-MS/MS | 527 | Increasing ornithine, arginine, and asparagine. | [33] |
| Roots | 30 | LC–ESI-MS/MS | 477 | Decreasing cinnamic acid, cyanidin-3-O-glucoside, and pelargonidin-3-O-glucoside | [33] |

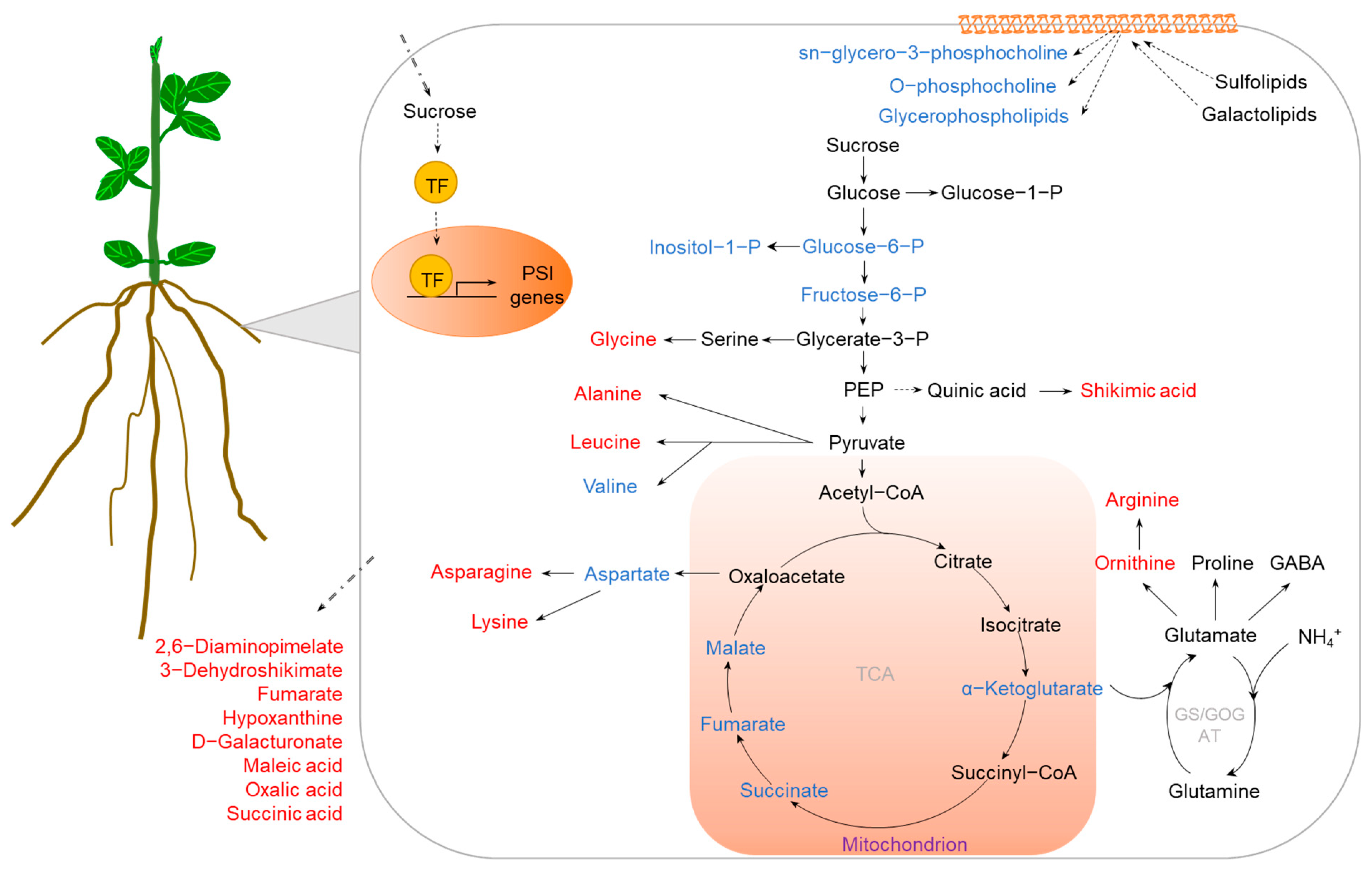
2.2. P Deficiency
| Crop Species | Tissue | Duration of Treatment (days) | Method | Number of DAMs | Main Changes in Metabolites or Metabolic Pathways | Reference |
|---|---|---|---|---|---|---|
| Tomato (Solanum lycopersicum) | Leaves | 5/15 | LC/GC–MS | 17/30 | Decreasing soluble sugars. | [26] |
| Roots | 5/15 | LC/GC–MS | 29/30 | Decreasing soluble sugars; increasing amino acids and organic acids. | [26] | |
| Rice (Oryza sativa) | Shoots | 5/15 | CE–TOF MS | 26/38 | Decreasing l-aspartate, l-phenylalanine, GABA, guanosine, adenine, and cytidine. | [28] |
| Roots | 5/15 | CE–TOF MS | 33/8 | Decreasing trans-zeatin, citrate, and d-glucosamine. | [28] | |
| Root exudates | 5/15 | CE–TOF MS | 18/12 | Increasing cytosine, hypoxanthine, nicotinate, choline, 1,4-butanediamine, creatine, 2,6-diaminopimelate, 3-dehydroshikimate, galactosamine, fumarate, glycerate, and glutamate. | [28] | |
| Common bean (Phaseolus vulgaris) | Roots | 21 | GC–MS | 42 | Increasing polyols and sugars. | [51] |
| Nodules | 21 | GC–MS | 45 | Increasing organic and polyhydroxy acids. | [52] | |
| Oats (Avena sativa) | Roots | 10 | GC–MS | 30 | Decreasing phosphorylated metabolites; increasing citric acid and malic acid. | [53] |
| Soybean (Glycine max) | Roots | 12 | LC–ESI-MS/MS | 155 | Decreasing phosphorylated lipids and nucleic acids. | [54] |
| Quinoa (Chenopodium quinoa) a | Shoots | 30 | UPLC–MS/MS | 149 | Decreasing dihydroxyacetone phosphate, 3-phospho-d-glyceric acid, glucose-1-phosphate, and uridine diphospho-d-glucose | [55] |
| Barley (Hordeum vulgare) | Shoots | 20 | GC–MS | 51 | Decreasing phosphorus-containing compounds (glucose-6-phosphate, mannose-6-phosphate, and glycerol-3-phosphate). | [35] |
| Roots | 20 | GC–MS | 49 | Increasing sugars (fructose, glucose, and sucrose) and organic acids (citric acid and malic acid). | [35] | |
| Shoots | 10/17 | GC–MS | 22/38 | Decreasing glucose-6-P, fructose-6-P, glycerol-3-P, and inositol-1-P. | [58] | |
| Roots | 10/17 | GC–MS | 7/42 | Decreasing succinic acid and fumaric acid. | [58] | |
| Wheat (Triticum aestivum) | Leaves/roots | 28 | GC–MS | nd | Decreasing glycerol-3-P in roots; increasing raffinose and 1-kestose in roots and aspartate, glutamine, and alanine in leaves. | [61] |
| White lupin (Lupinus albus) | Shoots | 14/22 | GC–MS | nd | Decreasing fructose, glucose, and sucrose after 14 days of treatment. | [63] |
| Non-cluster roots | 14/22 | GC–MS | nd | Decreasing phosphorylated metabolites; increasing organic acids and several shikimate pathway products. | [63] |
2.3. K Deficiency
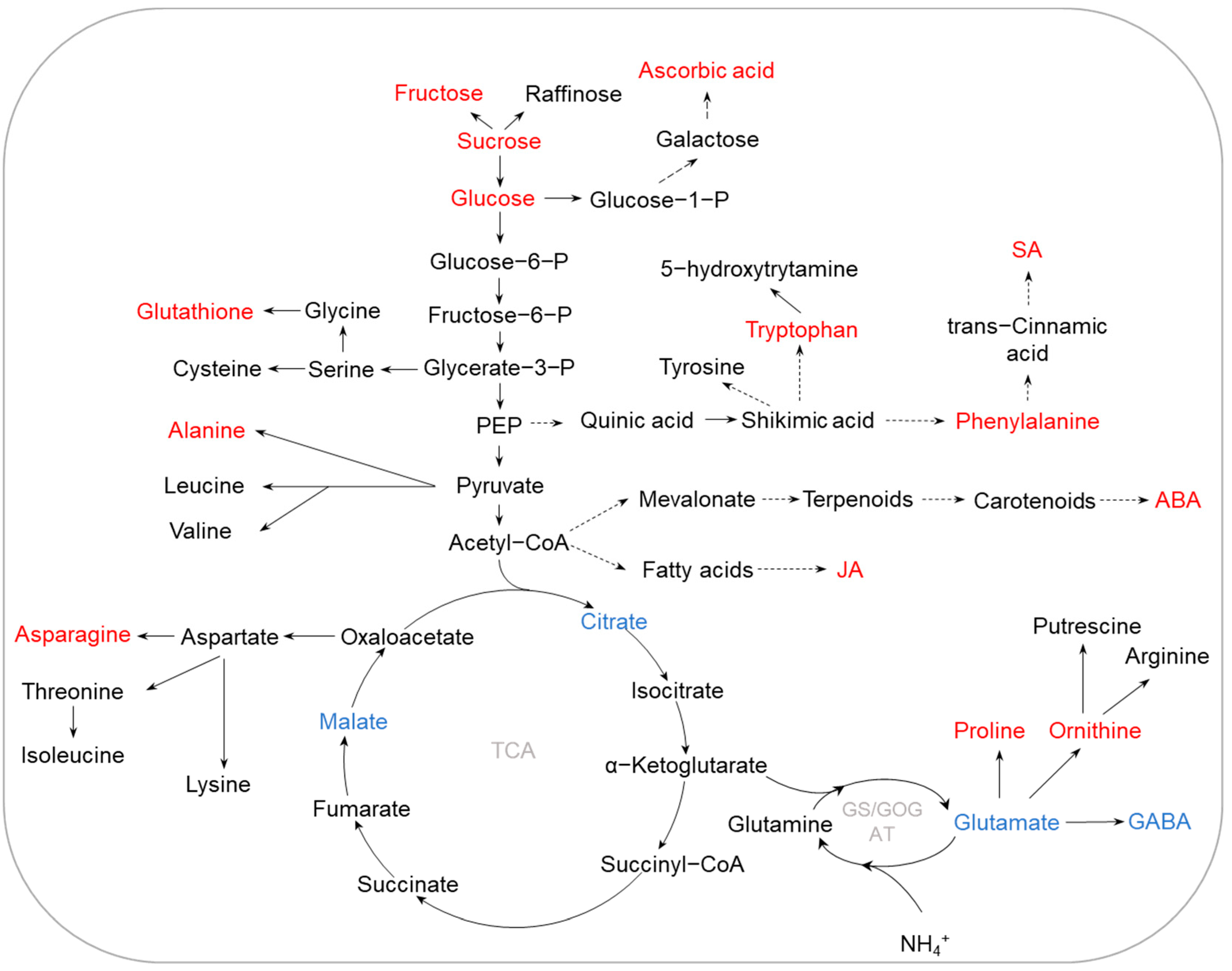
2.4. Other Nutrient Deficiencies
| Nutrient | Crop Species | Tissue | Duration of Treatment (d) | Method | Number of DAMs | Main Changes of Metabolites or Metabolic Pathways | Reference |
|---|---|---|---|---|---|---|---|
| Potassium | Tomato (Solanum lycopersicum) | Leaves | 5/15 | LC/GC–MS | 30/28 | Decreasing organic acids and amino acids. | [26] |
| Roots | 5/15 | LC/GC–MS | 32/29 | Accumulating soluble sugars and amino acids. | [26] | ||
| Barley (Hordeum vulgare) a | Shoots | 20 | GC–MS | 51 | Increasing monosaccharides (fructose, galactose, and glucose), disaccharides (sucrose and maltose), and polysaccharide (psicose). | [35] | |
| Roots | 20 | GC–MS | 49 | Increasing putrescine and 5-hydroxytryptamine. | [35] | ||
| Leaves/roots | 16 | GC–MS | 57 (in total) | Decreasing negatively charged amino acids (Asp and Glu) and most organic acids; increasing positively charged amino acids (Lys and Gln). | [70] | ||
| Sunflower (Helianthus annuus) | Leaves/roots | 14 | GC–MS | nd | Decreasing glycerol 3-phosphate and fructose 6-phosphate; increasing citrate, aconitate, malate, fumarate, and putrescine. | [69] | |
| Rapeseed (Brassica napus) b | Leaves | 45 | LC–MS | nd | Increasing citric acid, arginine, and asparagine. | [71] | |
| Peanut (Arachis hypogaea) | Leaves/roots | 15 | GC–MS | nd | Decreasing aspartic acid and glutamic acid; increasing lysine, histidine, and arginine | [72] | |
| Wheat (Triticum aestivum) a | Roots | 14 | UPLC–ESI-MS/MS | 162 | Decreasing more amino acids in K-sensitive genotype BN207; increasing more amino acids in K-tolerant genotype KN9204. | [77] | |
| Magnesium | Soybean (Glycine max) | Leaves | 4/8 | GC–MS | 5/26 | Decreasing methylmalonic acid; increasing phenylalanine, carbon allocation, and respiration metabolism (e.g., sucrose, glucose, and fructose). | [89] |
| Roots | 4/8 | GC–MS | 3/16 | Decreasing urea and TCA cycle; increasing glutamine and allantoic acid. | [89] | ||
| Iron | Rice (Oryza sativa) | Roots | 7 | LC–MS | nd | Increasing amino acids related to α-ketoglutarate family (proline, histidine, and glutamine). | [91] |
| Betel palm (Areca catechu) | Leaves | 28 | LC–MS | 106 | Increasing organic acids and flavonoids. | [92] | |
| Zinc | Tea (Camellia sinensis) | Leaves | 120 | LC–MS | 10 | Decreasing fructose-6-phosphate, digalactosylglycerol, and 2-O-glycerol-beta-d-galactopyranoside; increasing caffeine and catechin gallate. | [93] |
| Sulfur | Lettuce (Lactuca sativa) a | Leaves | 42 | LC–MS | 14 | Increasing caffeoyl derivatives, caffeic acid hexose, 5-caffeoylquinic acid (5-OCQA), quercetin, and luteolin glucoside derivatives. | [94] |
| Boron | Alfalfa (Medicago sativa) | Flowers | 7 | GC–MS | 19 | Increasing large sugars. | [95] |
| Seeds | Until harvest | GC–MS | 13 | Increasing sugars and phenolic compounds. | [95] |
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Vance, C.P. Update on the state of nitrogen and phosphorus nutrition: Symbiotic nitrogen fixation and phosphorus acquisition. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, M.; Folberth, C.; Balkovič, J.; Ciais, P.; Fritz, S.; Janssens, I.A.; Obersteiner, M.; See, L.; Skalský, R.; Xiong, W.; et al. African crop yield reductions due to increasingly unbalanced nitrogen and phosphorus consumption. Glob. Change Biol. 2014, 20, 1278–1288. [Google Scholar] [CrossRef]
- Tayefeh, M.; Sadeghi, S.M.; Noorhosseini, S.A.; Bacenetti, J.; Damalas, C.A. Environmental impact of rice production based on nitrogen fertilizer use. Environ. Sci. Pollut. Res. Int. 2018, 25, 15885–15895. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. Int. 2017, 24, 26983–26987. [Google Scholar] [CrossRef]
- Ju, X.; Kou, C.; Christie, P.; Dou, Z.; Zhang, F. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, K.R.; Ahmad, A.; Iqbal, M.; Gucel, S.; Ozturk, M. Nitrogen-efficient rice cultivars can reduce nitrate pollution. Environ. Sci. Pollut. Res. 2011, 18, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Tian, J.; Liao, H. Proteomics dissection of plant responses to mineral nutrient deficiency. Proteomics 2013, 13, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, S.; Mo, X.; Guo, Q.; Li, Y.; Tian, J.; Liang, C. Proteomic analysis dissects molecular mechanisms underlying plant responses to phosphorus deficiency. Cells 2022, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Metabolite-based genome-wide association studies in plants. Curr. Opin. Plant Biol. 2015, 24, 31–38. [Google Scholar] [CrossRef]
- Weckwerth, W. Metabolomics in systems biology. Annu. Rev. Plant Biol. 2003, 54, 669–689. [Google Scholar] [CrossRef]
- Windsor, A.J.; Reichelt, M.; Figuth, A.; Svatos, A.; Kroymann, J.; Kliebenstein, D.J.; Gershenzon, J.; Mitchell-Olds, T. Geographic and evolutionary diversification of glucosinolates among near relatives of Arabidopsis thaliana (Brassicaceae). Phytochemistry 2005, 66, 1321–1333. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 2000, 151, 135–143. [Google Scholar] [CrossRef]
- Cruz, J.L.; Mosquim, P.R.; Pelacani, C.R.; Araujo, W.L.; DaMatta, F.M. Photosynthesis impairment in cassava leaves in response to nitrogen deficiency. Plant Soil 2003, 257, 417–423. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Chu, C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef]
- Kant, S.; Bi, Y.M.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Xuan, H.; Yang, Y.; Wang, L.; Wei, L.; Wang, Y.; Kang, G. Transcription analysis of genes encoding the wheat root transporter NRT1 and NRT2 families during nitrogen starvation. J. Plant Growth Regul. 2014, 33, 837–848. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Hu, L.; Zhang, Y.; Zhang, B.; Xia, H.; Du, W.; Fan, S.; Kong, L. Low nitrogen stress stimulates lateral root initiation and nitrogen assimilation in wheat: Roles of phytohormone signaling. J. Plant Growth Regul. 2020, 40, 436–450. [Google Scholar] [CrossRef]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef]
- Schlüter, U.; Mascher, M.; Colmsee, C.; Scholz, U.; Brautigam, A.; Fahnenstich, H. Maize source leaf adaptation to nitrogen deficiency affects not only nitrogen and carbon metabolism but also control of phosphate homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Quan, X.; Qian, Q.; Ye, Z.; Zeng, J.; Han, Z.; Zhang, G. Metabolic analysis of two contrasting wild barley genotypes grown hydroponically reveals adaptive strategies in response to low nitrogen stress. J. Plant Physiol. 2016, 206, 59–67. [Google Scholar] [CrossRef]
- Tawaraya, K.; Horie, R.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of shoot extract, root extract, and root exudate of rice under nitrogen and phosphorus deficiency. Soil Sci. Plant Nutr. 2018, 64, 312–322. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Li, Z.; Zhao, Y.; Peng, C.; Zhao, C. An integrated analysis of the rice transcriptome and metabolome reveals differential regulation of carbon and nitrogen metabolism in response to nitrogen availability. Int. J. Mol. Sci. 2019, 20, 2349. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Y.; Xie, B.; Zhu, S.; Lu, X.; Liang, C.; Tian, J. Complex gene regulation between young and old soybean leaves in responses to manganese toxicity. Plant Physiol. Biochem. 2020, 155, 231–242. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Tao, Z.; Yang, Y.; Gao, Z.; Zhao, G.; Chang, X. Impacts of nitrogen deficiency on wheat (Triticum aestivum L.) grain during the medium filling stage: Transcriptomic and metabolomic comparisons. Front. Plant Sci. 2021, 12, 674433. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yang, L.; Han, P.; Gu, C.; Li, Y.; Liao, X.; Qin, L. Metabolic profiles reveal changes in the leaves and roots of rapeseed (Brassica napus L.) seedlings under nitrogen deficiency. Int. J. Mol. Sci. 2022, 23, 5784. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Integrative physiological, transcriptome, and metabolome analysis reveals the effects of nitrogen sufficiency and deficiency conditions in apple leaves and roots. Environ. Exp. Bot. 2021, 192, 104633. [Google Scholar] [CrossRef]
- Sugiura, D.; Betsuyaku, E.; Terashima, I. Interspecific differences in how sink-source imbalance causes photosynthetic downregulation among three legume species. Ann. Bot. 2019, 123, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ni, S.; Cai, S.; Zhang, G. Comprehensive dissection of primary metabolites in response to diverse abiotic stress in barley at seedling stage. Plant Physiol. Biochem. 2021, 161, 54–64. [Google Scholar] [CrossRef]
- Li, M.X.; Xu, J.S.; Wang, X.X.; Fu, H.; Zhao, M.L.; Wang, H.; Shi, L.X. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef]
- Urbanczyk-Wochniak, E.; Fernie, A.R. Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2005, 56, 309–321. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Shi, H.; Chan, Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 2014, 56, 114–121. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Xu, C.Y. Advance of studies on the mechanism of effect of light on carbon and nitrogen allocation in plants. J. Jilin Agr. Sci. 2014, 39, 18–22. [Google Scholar]
- Touraine, B.; Muller, B.; Grignon, C. Effect of phloem-translocated malate on NO3− uptake by roots of intact soybean plants. Plant Physiol. 1992, 99, 1118–1123. [Google Scholar] [CrossRef]
- Singh, P.K.; Chaturvedi, V.K. Effects of salicylic acid on seedling growth and nitrogen use efficiency in cucumber (Cucumis sativus L.). Giorn. Bot. Ital. 2010, 146, 302–308. [Google Scholar]
- Dai, H.; Xiao, C.N.; Liu, H.B.; Hao, F.H.; Tang, H.R. Combined NMR and LC-DAD-MS analysis reveals comprehensive metabonomic variations for three phenotypic cultivars of Salvia miltiorrhiza bunge. J. Proteome Res. 2010, 9, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant Sci. 2015, 6, 837. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.J.; Lin, S.I. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 2011, 62, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Ajmera, I.; Hodgman, T.C.; Lu, C. An integrative systems perspective on plant phosphate research. Genes 2019, 10, 139. [Google Scholar] [CrossRef]
- Mo, X.; Liu, G.; Zhang, Z.; Lu, X.; Liang, C.; Tian, J. Mechanisms underlying soybean response to phosphorus deficiency through integration of omics analysis. Int. J. Mol. Sci. 2022, 23, 4592. [Google Scholar] [CrossRef]
- Hernández, G.; Ramírez, M.; Valdés-López, O.; Tesfaye, M.; Graham, M.A.; Czechowski, T.; Schlereth, A.; Wandrey, M.; Erban, A.; Cheung, F.; et al. Phosphorus stress in common bean: Root transcript and metabolic responses. Plant Physiol. 2007, 144, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Valdés-López, O.; Ramírez, M.; Goffard, N.; Weiller, G.; Aparicio-Fabre, R.; Fuentes, S.I.; Erban, A.; Kopka, J.; Udvardi, M.K.; et al. Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol. 2009, 151, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lysøe, E.; Armarego-Marriott, T.; Erban, A.; Paruch, L.; van Eerde, A.; Bock, R.; Liu-Clarke, J. Transcriptome and metabolome analyses provide insights into root and root-released organic anion responses to phosphorus deficiency in oat. J. Exp. Bot. 2018, 69, 3759–3771. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Zhang, M.; Liang, C.; Cai, L.; Tian, J. Integration of metabolome and transcriptome analyses highlights soybean roots responding to phosphorus deficiency by modulating phosphorylated metabolite processes. Plant Physiol. Biochem. 2019, 139, 697–706. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Huang, T.; Zhang, X.; Zhang, P.; Xie, H.; Liu, J.; Li, L.; Kong, Z.; Qin, P. Transcriptome and metabolome analyses revealed the response mechanism of quinoa seedlings to different phosphorus stresses. Int. J. Mol. Sci. 2022, 23, 4704. [Google Scholar] [CrossRef]
- Wu, P.; Ma, L.; Hou, X.; Wang, M.; Wu, Y.; Liu, F.; Deng, X.W. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 2003, 132, 1260–1271. [Google Scholar] [CrossRef]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef]
- Huang, C.Y.; Roessner, U.; Eickmeier, I.; Genc, Y.; Callahan, D.L.; Shirley, N.; Langridge, P.; Bacic, A. Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficient barley plants (Hordeum vulgare L.). Plant Cell Physiol. 2008, 49, 691–703. [Google Scholar] [CrossRef]
- Liu, J.; Samac, D.A.; Bucciarelli, B.; Allan, D.L.; Vance, C.P. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant J. 2005, 41, 257–268. [Google Scholar] [CrossRef]
- Dong, D.; Peng, X.; Yan, X. Organic acid exudation induced by phosphorus deficiency and/or aluminum toxicity in two contrasting soybean genotypes. Physiol. Plant. 2004, 122, 190–199. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Palmer, L.; Roessner, U.; Stangoulis, J. Genotypic variation in the root and shoot metabolite profiles of wheat (Triticum aestivum L.) indicate sustained, preferential carbon allocation as a potential mechanism in phosphorus efficiency. Front. Plant Sci. 2019, 10, 995. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Müller, J.; Gödde, V.; Niehaus, K.; Zörb, C. Metabolic adaptations of white lupin roots and shoots under phosphorus deficiency. Front. Plant Sci. 2015, 6, 1014. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Ohnishi, N.; Kurita, Y.; Iwamura, S.; Ohnishi, M.; Kusaba, M.; Mimura, T.; Sakamoto, W. Organelle DNA degradation contributes to the efficient use of phosphate in seed plants. Nat. Plants 2018, 4, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium deficiency in plants: Effects and signaling cascades. Acta Physiol. Plant 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Genetic approaches for improvement of the crop potassium acquisition and utilization efficiency. Curr. Opin. Plant Biol. 2015, 25, 46–52. [Google Scholar] [CrossRef]
- Cui, J.; Abadie, C.; Carroll, A.; Lamade, E.; Tcherkez, G. Responses to K deficiency and waterlogging interact via respiratory and nitrogen metabolism. Plant Cell Environ. 2019, 42, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Quan, X.; He, X.; Cai, S.; Ye, Z.; Chen, G.; Zhang, G. Root and leaf metabolite profiles analysis reveals the adaptive strategies to low potassium stress in barley. BMC Plant Biol. 2018, 18, 187. [Google Scholar] [CrossRef]
- Hu, W.; Lu, Z.; Meng, F.; Li, X.; Cong, R.; Ren, T.; Lu, J. Potassium modulates central carbon metabolism to participate in regulating CO2 transport and assimilation in Brassica napus leaves. Plant Sci. 2021, 307, 110891. [Google Scholar] [CrossRef]
- Patel, M.; Fatnani, D.; Parida, A.K. Potassium deficiency stress tolerance in peanut (Arachis hypogaea) through ion homeostasis, activation of antioxidant defense, and metabolic dynamics: Alleviatory role of silicon supplementation. Plant Physiol. Biochem. 2022, 182, 55–75. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars-metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Sulpice, R.; Miller, A.J.; Stitt, M.; Amtmann, A.; Gibon, Y. Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots. Plant Physiol. 2009, 150, 772–785. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Karley, A.J. Potassium. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.R., Eds.; Springer: Berlin, Germany, 2010; pp. 199–224. [Google Scholar]
- Zhao, Y.; Sun, R.; Liu, H.; Liu, X.; Xu, K.; Xiao, K.; Xue, C. Multi-omics analyses reveal the molecular mechanisms underlying the adaptation of wheat (Triticum aestivum L.) to potassium deprivation. Front. Plant Sci. 2020, 11, 588994. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Hernandez, M.; Fernandez-Garcia, N.; Garcia-Garma, J.; Rubio-Asensio, J.S.; Rubio, F.; Olmos, E. Potassium starvation induces oxidative stress in Solanum lycopersicum L. roots. J. Plant Physiol. 2012, 169, 1366–1374. [Google Scholar] [CrossRef]
- Khan, M.N.; AlSolami, M.A.; Basahi, R.A.; Siddiqui, M.H.; Al-Huqail, A.A.; Abbas, Z.K.; Siddiqui, Z.H.; Ali, H.M.; Khan, F. Nitric oxide is involved in nano-titanium dioxide-induced activation of antioxidant defense system and accumulation of osmolytes under water-deficit stress in Vicia faba L. Ecotoxicol. Environ. Saf. 2020, 190, 110152. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Shalata, A.; Neumann, P.M. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J. Exp. Bot. 2001, 52, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [PubMed]
- Peuke, A.D.; Jeschke, W.D.; Hartung, W. Flows of elements, ions and abscisic acid in Ricinus communis and site of nitrate reduction under potassium limitation. J. Exp. Bot. 2002, 53, 241–250. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Shim, J.K.; Kim, D.H.; Lee, K.Y.; Lee, I.J. Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J. Plant Growth Regul. 2014, 33, 137–149. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Function of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil. 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Yang, N.; Jiang, J.; Xie, H.; Bai, M.; Xu, Q.; Wang, X.; Yu, X.; Chen, Z.; Guan, Y. Metabolomics reveals distinct carbon and nitrogen metabolic responses to magnesium deficiency in leaves and roots of soybean [Glycine max (Linn.) Merr.]. Front. Plant Sci. 2017, 8, 2091. [Google Scholar] [CrossRef]
- Kim, S.A.; Guerinot, M.L. Mining iron: Iron uptake and transport in plants. FEBS Lett. 2007, 581, 2273–2280. [Google Scholar] [CrossRef]
- Selby-Pham, J.; Lutz, A.; Moreno-Moyano, L.T.; Boughton, B.A.; Roessner, U.; Johnson, A.A.T. Diurnal changes in transcript and metabolite levels during the iron deficiency response of rice. Rice 2017, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, X.; Jia, X.; Liu, L.; Cao, H.; Qin, W.; Li, M. Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front. Plant Sci. 2021, 12, 710093. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ding, Z.; Wang, H.; Song, L.; Jia, S.; Ma, D. Zinc stress affects ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 111, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Li, F.; Wenzel-Storjohann, A.; Sulieman, S.; Tasdemir, D.; Mühling, K.H. Comparative metabolite profile, biological activity and overall quality of three lettuce (Lactuca sativa L., Asteraceae) cultivars in response to sulfur nutrition. Pharmaceutics 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xia, F.; Wang, M.; Wang, W.; Mao, P. Metabolomic analyses of alfalfa (Medicago sativa L. cv. ‘Aohan’) reproductive organs under boron deficiency and surplus conditions. Ecotoxicol. Environ. Saf. 2020, 202, 111011. [Google Scholar] [CrossRef]
- Feng, Z.; Ding, C.; Li, W.; Wang, D.; Cui, D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020, 310, 125914. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xue, M.; Liu, H.; Fernie, A.R.; Chen, W. Exploring the genic resources underlying metabolites through mGWAS and mQTL in wheat: From large-scale gene identification and pathway elucidation to crop improvement. Plant Commun. 2021, 2, 100216. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.; Shi, T.; Yin, H.; Sun, D.; Hao, Y.; Xia, X.; Luo, J.; Fernie, A.R.; He, Z.; et al. Metabolite-based genome-wide association study enables dissection of the flavonoid decoration pathway of wheat kernels. Plant Biotechnol. J. 2020, 18, 1722–1735. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, P.; Du, Q.; Quan, M.; Li, L.; Xiao, L.; Song, F.; Lu, W.; Fang, Y.; Zhang, D. Genetic architecture underlying the metabolites of chlorogenic acid biosynthesis in Populus tomentosa. Int. J. Mol. Sci. 2021, 22, 2386. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yuan, H.; Dong, X.; Peng, M.; Jing, X.; Xu, Q.; Tang, T.; Wang, Y.; Zha, S.; Gao, M.; et al. Genome-wide dissection of co-selected UV-B responsive pathways in the UV-B adaptation of qingke. Mol. Plant 2020, 13, 112–127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhu, S.; Schultze-Kraft, R.; Liu, G.; Chen, Z. Dissection of Crop Metabolome Responses to Nitrogen, Phosphorus, Potassium, and Other Nutrient Deficiencies. Int. J. Mol. Sci. 2022, 23, 9079. https://doi.org/10.3390/ijms23169079
Xue Y, Zhu S, Schultze-Kraft R, Liu G, Chen Z. Dissection of Crop Metabolome Responses to Nitrogen, Phosphorus, Potassium, and Other Nutrient Deficiencies. International Journal of Molecular Sciences. 2022; 23(16):9079. https://doi.org/10.3390/ijms23169079
Chicago/Turabian StyleXue, Yingbin, Shengnan Zhu, Rainer Schultze-Kraft, Guodao Liu, and Zhijian Chen. 2022. "Dissection of Crop Metabolome Responses to Nitrogen, Phosphorus, Potassium, and Other Nutrient Deficiencies" International Journal of Molecular Sciences 23, no. 16: 9079. https://doi.org/10.3390/ijms23169079
APA StyleXue, Y., Zhu, S., Schultze-Kraft, R., Liu, G., & Chen, Z. (2022). Dissection of Crop Metabolome Responses to Nitrogen, Phosphorus, Potassium, and Other Nutrient Deficiencies. International Journal of Molecular Sciences, 23(16), 9079. https://doi.org/10.3390/ijms23169079






