NH2-Functionalized Magnetic Nanoparticles for the N-Glycomic Analysis of Patients with Multiple Sclerosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Amine-Functionalized Ferrite Nanoparticles
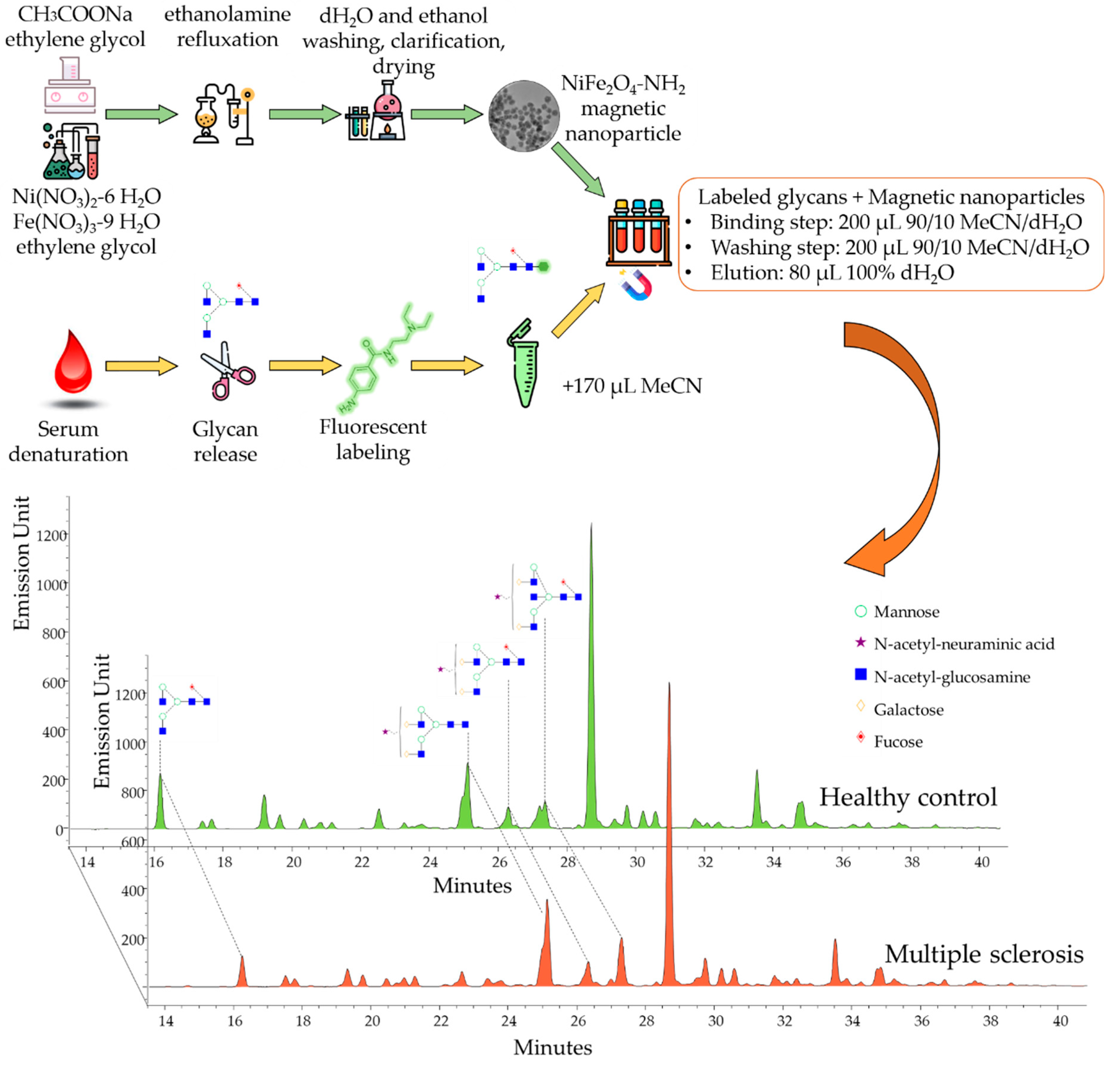
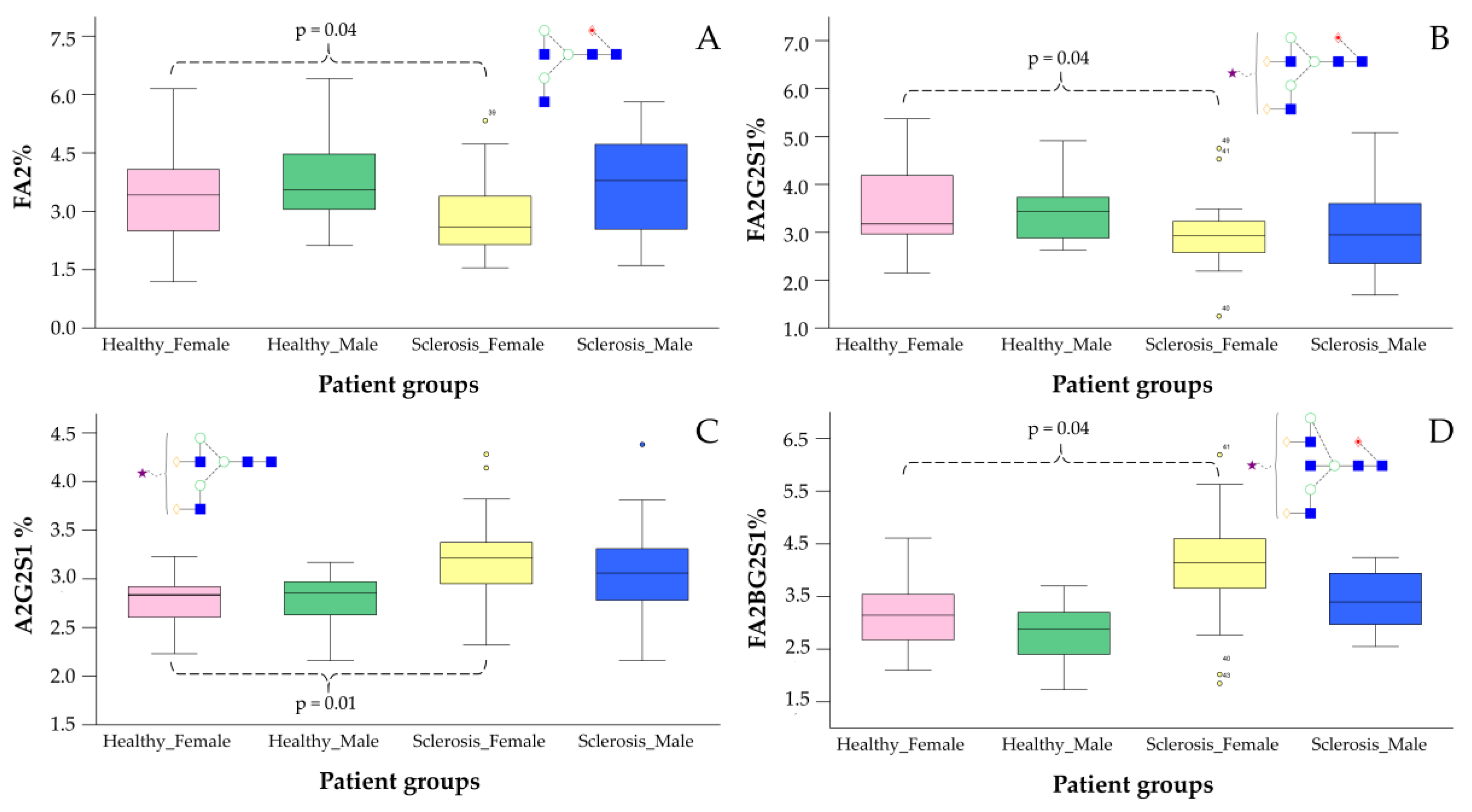
2.2. The Application of NH2-Functionalized Magnetic Nanoparticles for Glyco-Analytical Sample Preparation
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of the Amine-Functionalized Ferrite Nanoparticles
3.3. Characterization of the Amine-Functionalized Ferrite Nanoparticles
3.4. Patient Samples
3.5. N-Glycan Release, Labeling and Clean-Up
3.6. UPLC-FLR-MS Analysis
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Koch, L.C.; Rumrill, P.D.; Chan, F. Multiple sclerosis in the COVID-19 era: Vocational rehabilitation strategies to meet the needs of a growing and immune-vulnerable clientele. J. Vocat. Rehabil. 2021, 54, 175–183. [Google Scholar] [CrossRef]
- Murray, T.J. The history of multiple sclerosis: From the age of description to the age of therapy. In Primer on Multiple Sclerosis; UCLA: Los Angeles, CA, USA, 2016; pp. 3–10. [Google Scholar]
- Pakenham, K.I. Multiple sclerosis. In The Oxford Handbook of Rehabilitation Psychology; Oxford University Press: New York, NY, USA, 2012; pp. 211–234. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ömerhoca, S.; Akkaş, S.Y.; İçen, N.K. Multiple sclerosis: Diagnosis and differential diagnosis. Noro Psikiyatr. Ars. 2018, 55, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular biomarkers in multiple sclerosis. J. Neuroinflamm. 2019, 16, 272. [Google Scholar] [CrossRef]
- Ragusa, A.; Romano, P.; Lenucci, M.S.; Civino, E.; Vergara, D.; Pitotti, E.; Neglia, C.; Distante, A.; Romano, G.D.; Di Renzo, N.; et al. Differential glycosylation levels in saliva from patients with lung or breast cancer: A preliminary assessment for early diagnostic purposes. Metabolites 2021, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Mkhikian, H.; Grigorian, A.; Li, C.F.; Chen, H.-L.; Newton, B.; Zhou, R.W.; Beeton, C.; Torossian, S.; Tatarian, G.G.; Lee, S.-U.; et al. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat. Commun. 2011, 2, 334. [Google Scholar] [CrossRef]
- Dewald, J.H.; Colomb, F.; Bobowski-Gerard, M.; Groux-Degroote, S.; Delannoy, P. Role of cytokine-induced glycosylation changes in regulating cell interactions and cell signaling in inflammatory diseases and cancer. Cells 2016, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Guindolet, D.; Woodward, A.M.; Gabison, E.E.; Argüeso, P. Glycogene expression profile of human limbal epithelial cells with distinct clonogenic potential. Cells 2022, 11, 1575. [Google Scholar] [CrossRef] [PubMed]
- Cvetko, A.; Kifer, D. Glycosylation alterations in multiple sclerosis show increased proinflammatory potential. Biomedicines 2020, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; Graner, M.; Pointon, T.; Li, X.; Tanimoto, K.; Dennison, K.; Im, G.; Fringuello, A.; Zhou, W.; Graner, A.; et al. Aberrant immunoglobulin G glycosylation in multiple sclerosis. J. Neuroimmune Pharmacol. 2021, 1–10. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Huhn, C.; Waterreus, W.-J.; de Boer, A.R.; Neusüss, C.; Hokke, C.H.; Deelder, A.M.; Wuhrer, M. Hydrophilic interaction chromatography-based high-throughput sample preparation method for N-glycan analysis from total human plasma glycoproteins. Anal. Chem. 2008, 80, 6119–6126. [Google Scholar] [CrossRef]
- Tarentino, A.L.; Plummer, T.H. [4] Enzymatic deglycosylation of asparagine-linked glycans: Purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1994; Volume 230, pp. 44–57. [Google Scholar]
- Ruhaak, L.R.; Zauner, G.; Huhn, C.; Bruggink, C.; Deelder, A.M.; Wuhrer, M. Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 2010, 397, 3457–3481. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H. Solid-phase glycan isolation for glycomics analysis. Proteom. Clin. Appl. 2012, 6, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Feng, X.; Liu, B.-F.; Liu, X. Purification of derivatized oligosaccharides by solid phase extraction for glycomic analysis. PLoS ONE 2014, 9, e94232. [Google Scholar] [CrossRef]
- Olajos, M.; Hajós, P.; Bonn, G.K.; Guttman, A. Sample preparation for the analysis of complex carbohydrates by multicapillary gel electrophoresis with light-emitting diode induced fluorescence detection. Anal. Chem. 2008, 80, 4241–4246. [Google Scholar] [CrossRef]
- Váradi, C.; Mittermayr, S.; Millán-Martín, S.; Bones, J. Quantitative twoplex glycan analysis using 12C6 and 13C6 stable isotope 2-aminobenzoic acid labelling and capillary electrophoresis mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 8691–8700. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Tieu, M.V.; Hwang, S.Y.; Lee, M.-H. Magnetic particles: Their applications from sample preparations to biosensing platforms. Micromachines 2020, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.L.; Stine, K.J.; Demchenko, A.V. Immobilization of glycans on solid surfaces for application in glycomics. J. Carbohydr. Chem. 2018, 37, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Váradi, C.; Sikora, E.; Vanyorek, L.; Viskolcz, B. Purification of Fluorescently Derivatized N-Glycans by Magnetic Iron Nanoparticles. Nanomaterials 2019, 9, 1480. [Google Scholar] [CrossRef]
- Harvey, D.J.; Merry, A.H.; Royle, L.; Campbell, M.P.; Dwek, R.A.; Rudd, P.M. Proposal for a standard system for drawing structural diagrams of N- and O-linked carbohydrates and related compounds. PROTEOMICS 2009, 9, 3796–3801. [Google Scholar] [CrossRef]



| NiFe2O4-NH2 | MnFe2O4-NH2 | MgFe2O4-NH2 | CoFe2O4-NH2 | |
|---|---|---|---|---|
| C content (wt%) | 6.3 | 1.7 | 6.4 | 2.5 |
| N content (wt%) | 1.4 | 0.3 | 0.6 | 0.4 |
| Surface area (m2/g) | 93.8 | 155 | 86.3 | 279.4 |
| Particle size (nm) | 6 ± 2 | 8 ± 1 | 6 ± 1 | 4 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dojcsák, D.; Ilosvai, Á.M.; Vanyorek, L.; Gilányi, I.; Oláh, C.; Horváth, L.; Váradi, C. NH2-Functionalized Magnetic Nanoparticles for the N-Glycomic Analysis of Patients with Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 9095. https://doi.org/10.3390/ijms23169095
Dojcsák D, Ilosvai ÁM, Vanyorek L, Gilányi I, Oláh C, Horváth L, Váradi C. NH2-Functionalized Magnetic Nanoparticles for the N-Glycomic Analysis of Patients with Multiple Sclerosis. International Journal of Molecular Sciences. 2022; 23(16):9095. https://doi.org/10.3390/ijms23169095
Chicago/Turabian StyleDojcsák, Dalma, Ágnes Mária Ilosvai, László Vanyorek, Ibolya Gilányi, Csaba Oláh, László Horváth, and Csaba Váradi. 2022. "NH2-Functionalized Magnetic Nanoparticles for the N-Glycomic Analysis of Patients with Multiple Sclerosis" International Journal of Molecular Sciences 23, no. 16: 9095. https://doi.org/10.3390/ijms23169095
APA StyleDojcsák, D., Ilosvai, Á. M., Vanyorek, L., Gilányi, I., Oláh, C., Horváth, L., & Váradi, C. (2022). NH2-Functionalized Magnetic Nanoparticles for the N-Glycomic Analysis of Patients with Multiple Sclerosis. International Journal of Molecular Sciences, 23(16), 9095. https://doi.org/10.3390/ijms23169095







