Controllable Nitric Oxide Storage and Release in Cu-BTC: Crystallographic Insights and Bioactivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Cu-BTC and NO⊂Cu-BTCs
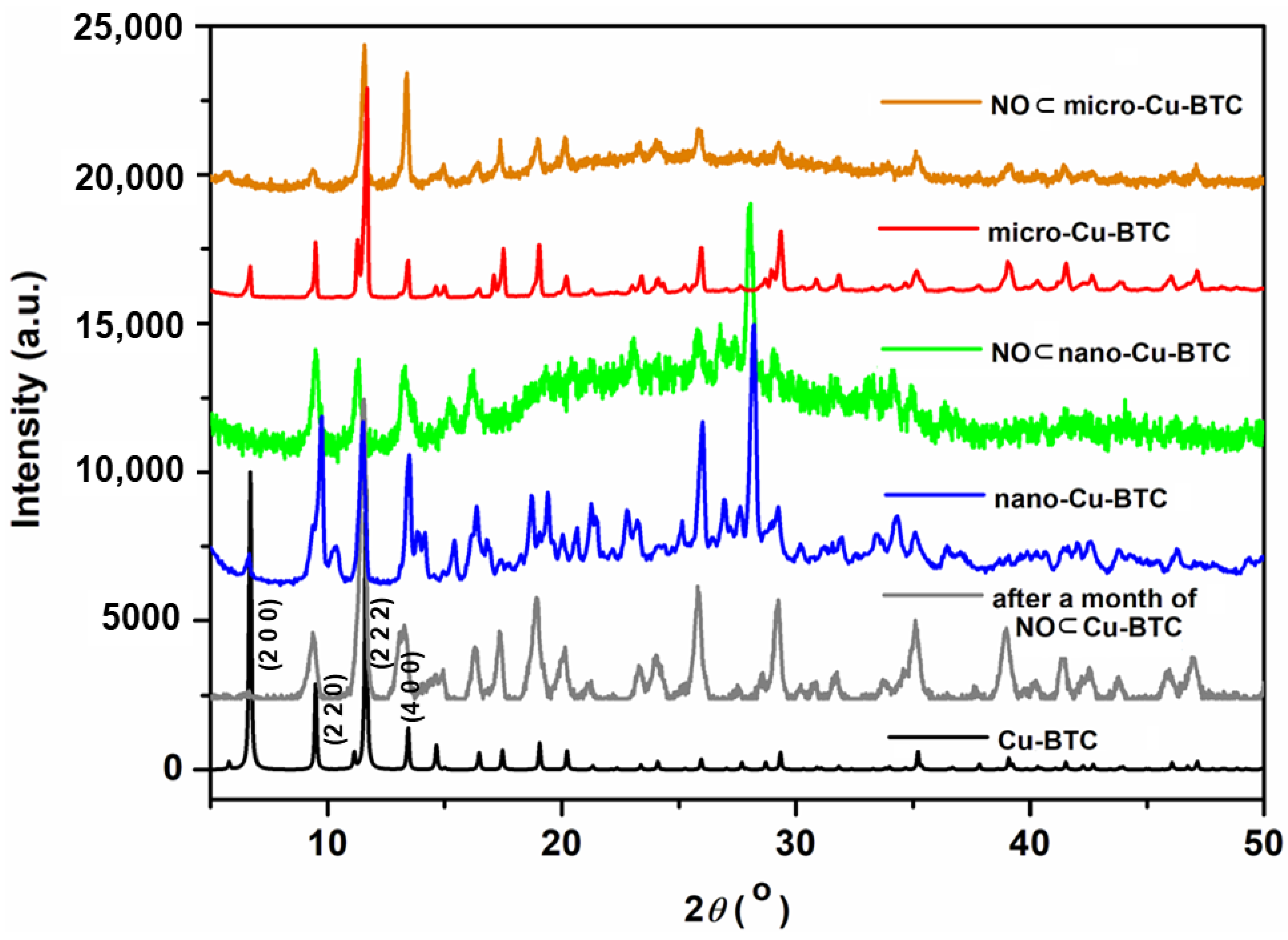
2.2. X-ray Crystallography and PXRD
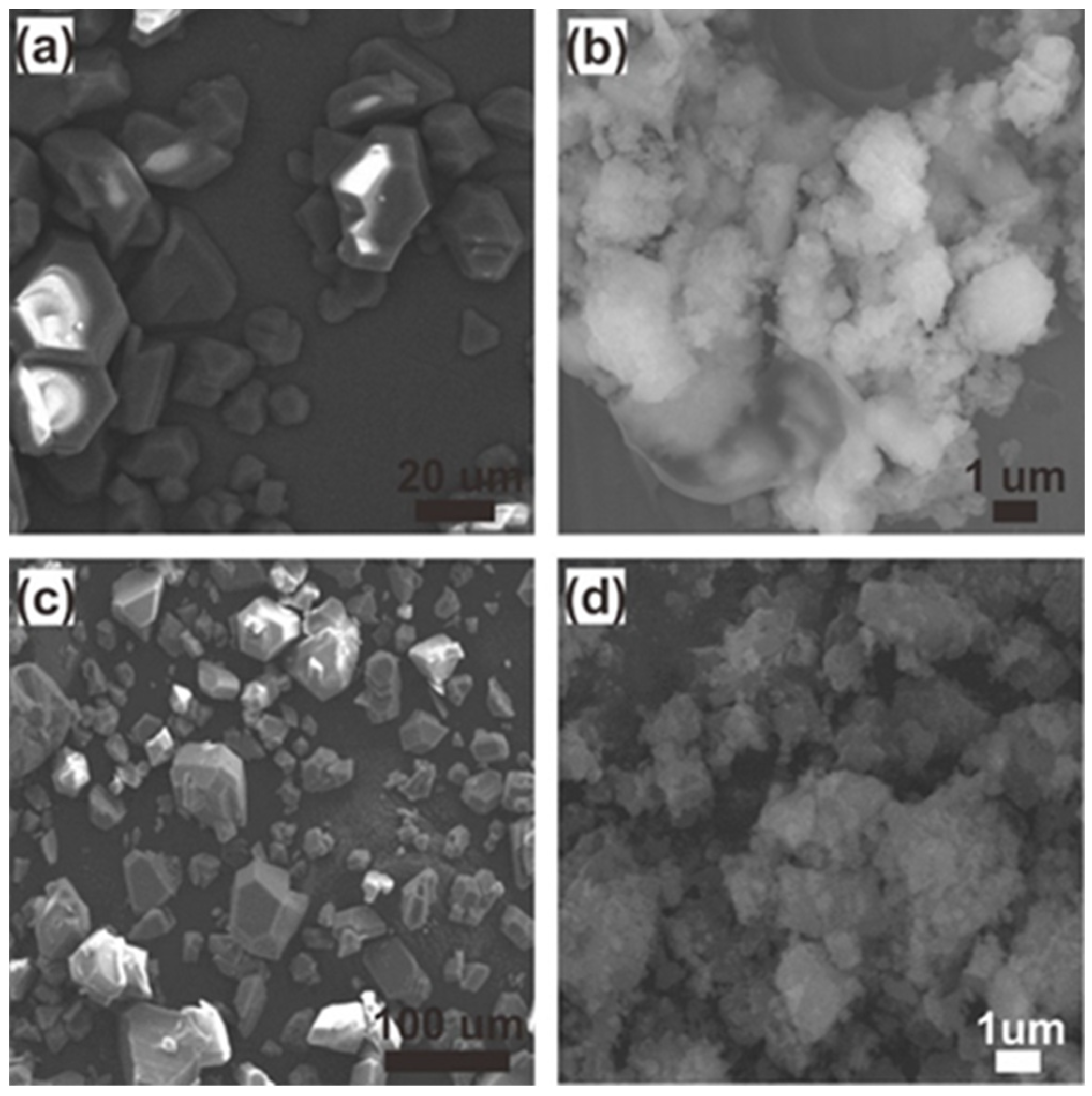
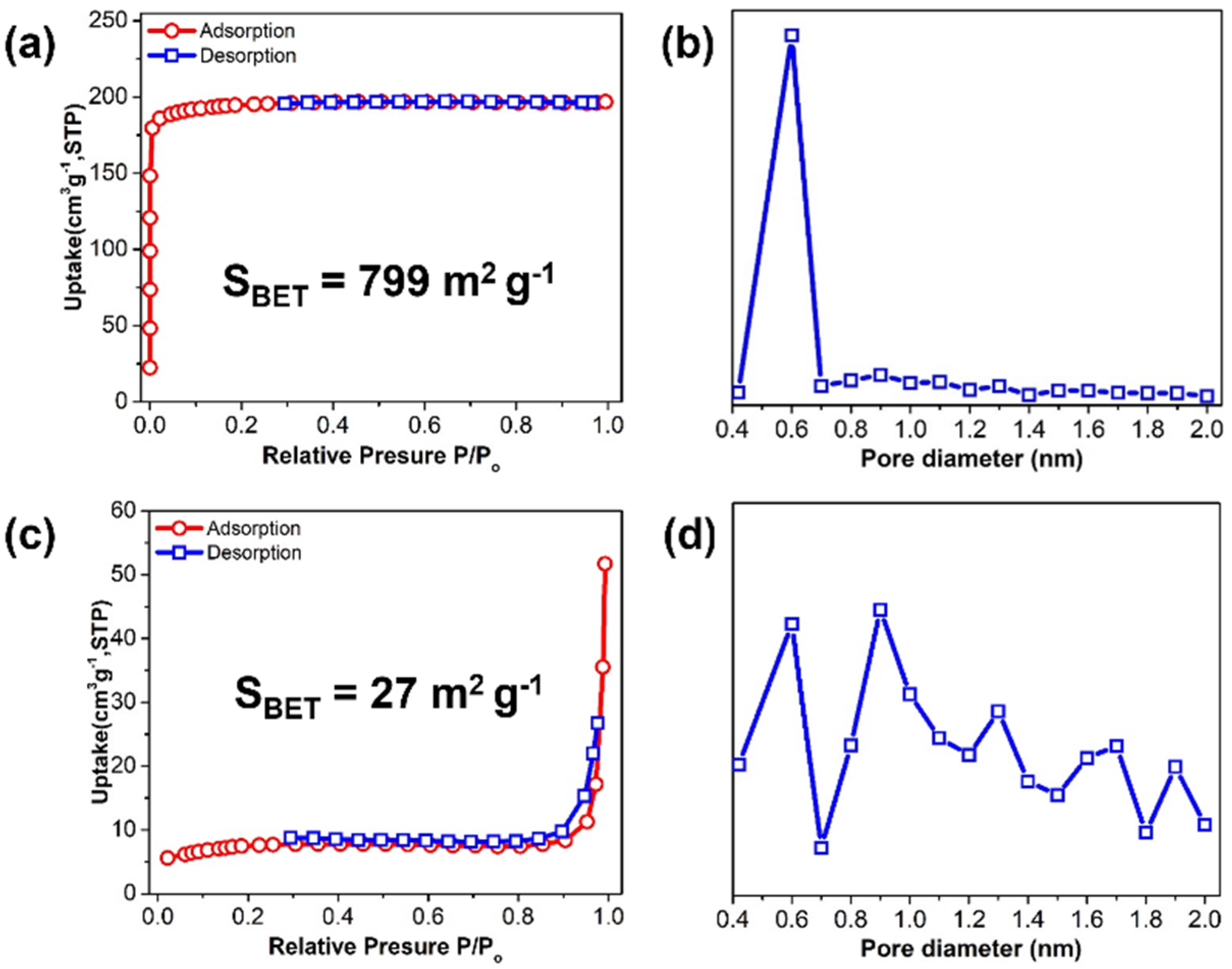
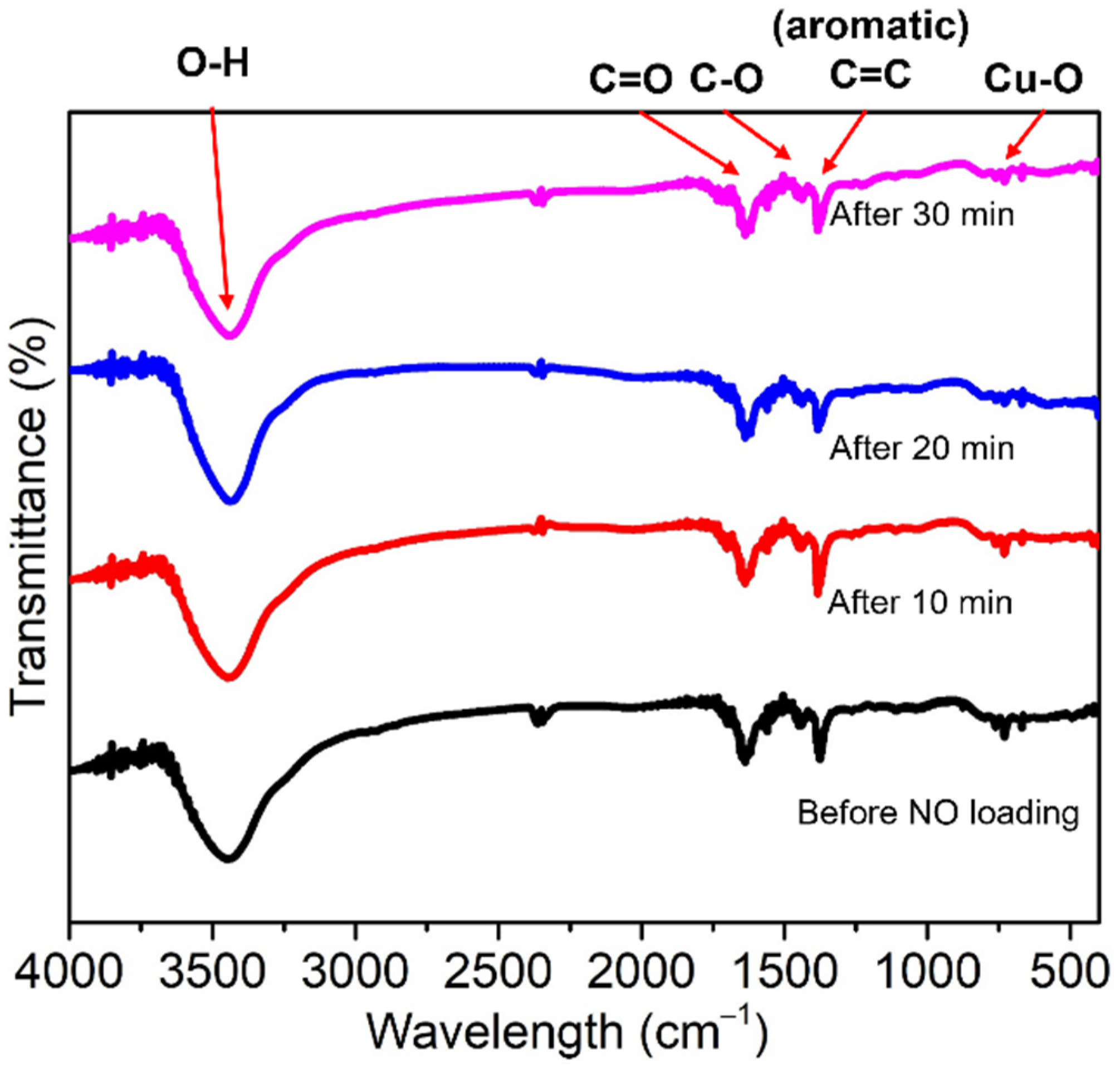
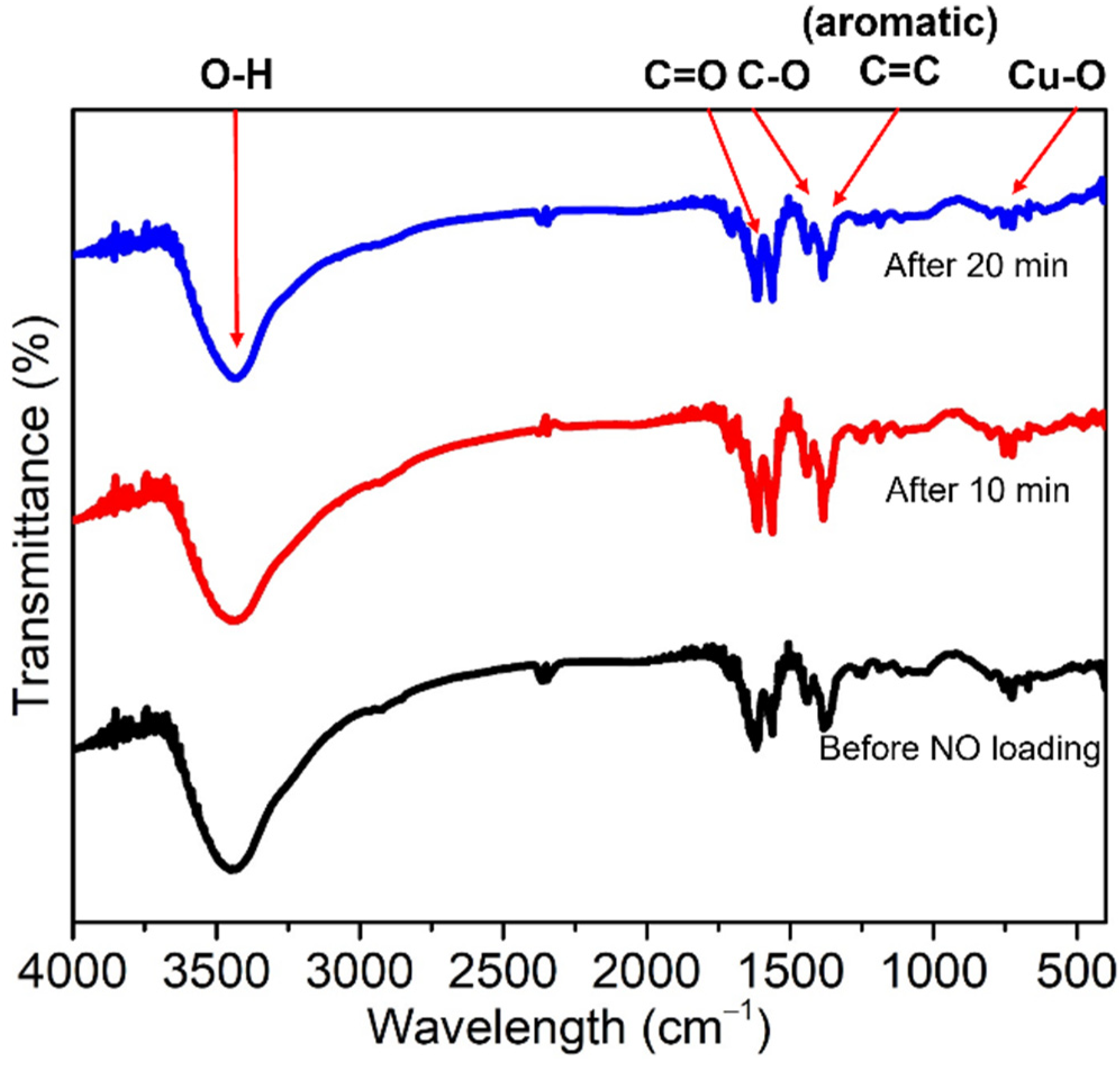
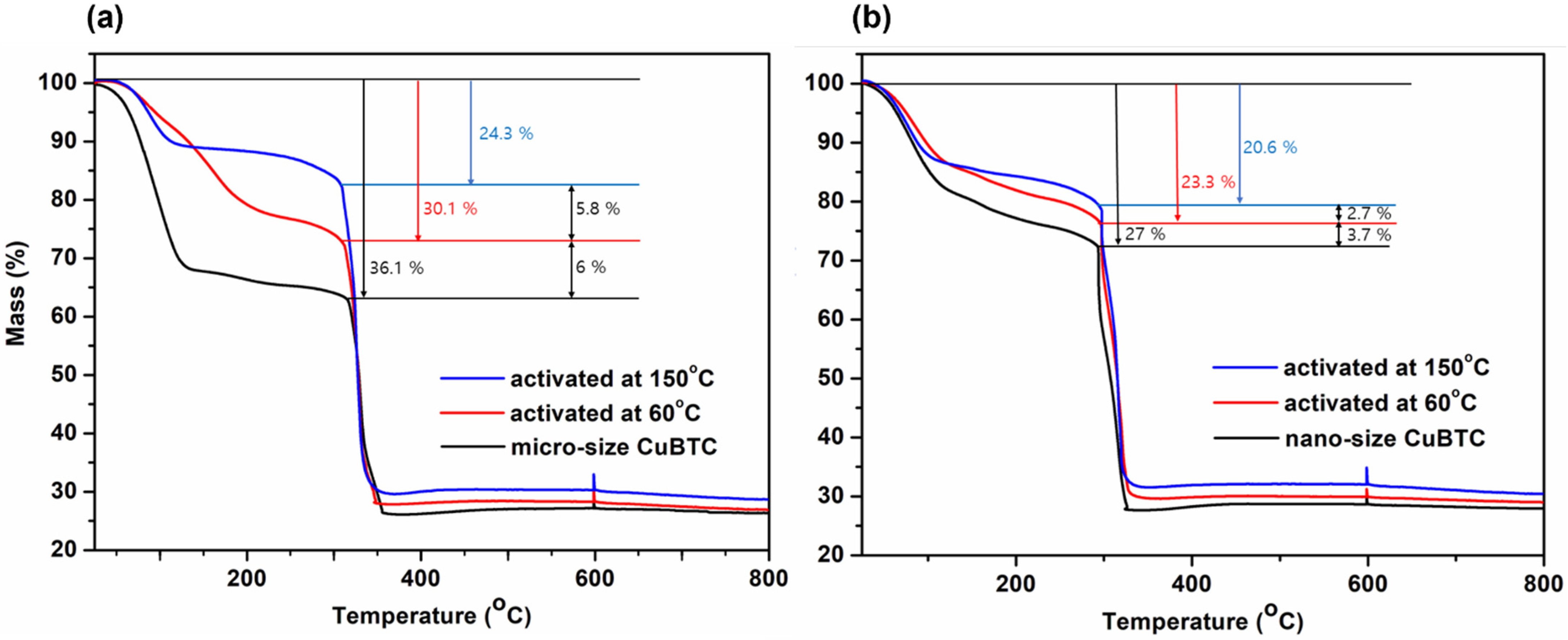
2.3. SEM, BET Measurements, FTIR, and TGA
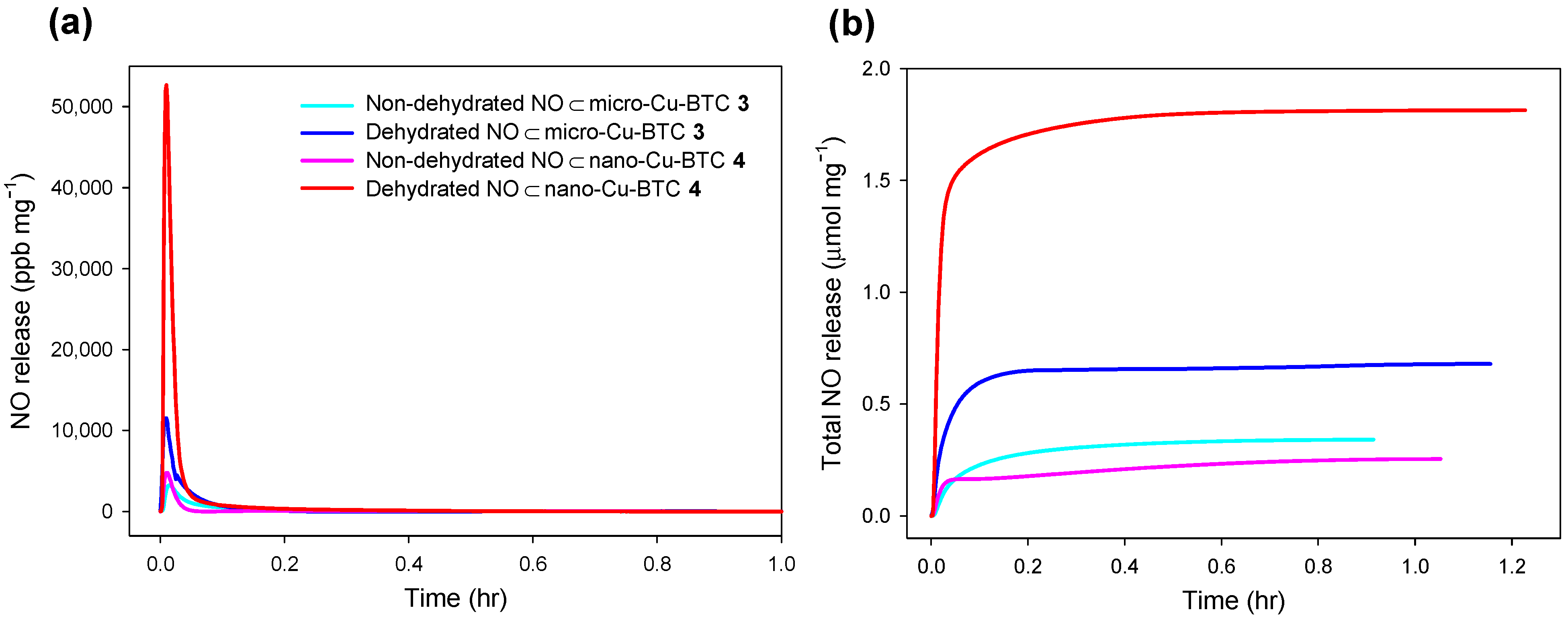
2.4. NO Release from NO⊂Cu-BTC
2.5. Antibacterial Properties
3. Materials and Methods
3.1. Preparation of Cu-BTC 1 and 2
3.2. X-ray Crystallography
3.3. NO Storage/Release of Cu-BTC
3.4. Antibacterial Test
3.5. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furchgott, R.F.; Khan, M.T.; Jothianandan, D. Similarities on behavior of nitric oxide and endothelium derived relaxing factor in a perfusion cascade bioassay system. Fed. Proc. 1987, 46, 385. [Google Scholar]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Arnold, W.P.; Mittal, C.K.; Katsuki, S.; Murad, F. Nitric oxide activates guanylate cyclase and increases guanosine 3’:5’-cyclic monophosphate levels in various tissue preparations. Proc. Natl Acad. Sci. USA 1977, 74, 3203–3207. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Perspectives Series: Host/Pathogen Interactions, Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.D.; Chen, A.F. Nitric Oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. [Google Scholar] [CrossRef]
- Mocellin, S.; Bronte, V.; Nitti, D. Nitric, a double edged sword in cancer biology: Searching for therapeutic opportunities. Med. Res. Rev. 2007, 27, 317–352. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Finelli, I.; Tortora, M.; Mozetic, P.; Chiessi, E.; Polizio, F.; Brismar, T.B.; Paradossi, G. Polymer microbubbles as diagnostic and therapeutic gas delivery device. Chem. Mater. 2008, 20, 3254–3258. [Google Scholar] [CrossRef]
- Riccio, D.A.; Schoenfisch, M.H. Nitric oxide release: Part I. Macromolecular scaffolds. Chem. Soc. Rev. 2012, 41, 3731–3741. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Huxford, R.C.; Rocca, J.D.D.; Lin, W. Metal-organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Hrabie, J.A.; Keefer, L.K. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem. Rev. 2002, 102, 1135–1154. [Google Scholar] [CrossRef] [PubMed]
- Lyn, D.L.H.; Williams, H. The Chemistry of S-Nitrosothiols. Acc. Chem. Res. 1999, 32, 869–876. [Google Scholar] [CrossRef]
- Parzuchowski, P.G.; Frost, M.C.; Meyerhoff, M.E. Synthesis and characterization of polymethacrylate-based nitric oxide donors. J. Am. Chem. Soc. 2002, 124, 12182–12191. [Google Scholar] [CrossRef]
- Jeong, H.; Park, K.; Yoo, J.C.; Hong, J. Structural heterogeneity in polymeric nitric oxide donor nanoblended coatings for controlled release behaviors. RSC Adv. 2018, 8, 38792–38800. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.G.G. S-Nitrosothiols as platforms for topical nitric oxide delivery. Basic Clin. Pharmacol. Toxicol. 2016, 119 (Suppl. S3), 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane storage in metal–organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon Dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Alavijeh, R.K.K.; Beheshti, S.; Akhbari, K.; Morsali, A. Investigation of reasons for metal–organic framework’s antibacterial activities. Polyhedron 2018, 156, 257–278. [Google Scholar] [CrossRef]
- Chowdhury, P.; Bikkina, C.; Meister, D.; Dreisbach, F.; Gumma, S. Comparison of adsorption isotherms on Cu-BTC metal organic frameworks synthesized from different routes. Micropor. Mesopor. Mater. 2009, 117, 406–413. [Google Scholar] [CrossRef]
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High pressure adsorption of hydrogen, nitrogen, carbon dioxide and methane on the metal–organic framework HKUST-1. Micropor. Mesopor. Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]
- Martín-Calvo, A.; García-Pérez, E.; García-Sánchez, A.; Bueno-Pérez, R.; Hamad, S.; Calero, S. Effect of air humidity on the removal of carbon tetrachloride from air using Cu-BTC metal-organic framework. Phys. Chem. Chem. Phys. 2011, 13, 11165–11174. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Mekala, S.; Dreisbach, F.; Gumma, S. Adsorption of CO, CO2 and CH4 on Cu-BTC and MIL-101 metal organic frameworks: Effect of open metal sites and adsorbate polarity. Micropor. Mesopor. Mater. 2012, 152, 246–252. [Google Scholar] [CrossRef]
- Férey, G.; Latroche, M.; Serre, C.; Millange, F.; Loiseau, T.; Percheron-Guégan, A. Hydrogen adsorption in the nanoporous metal-benzenedicarboxylate M(OH)(O2C-C6H4-CO2) (M = Al3+, Cr3+), MIL-53. Chem. Commun. (Camb) 2003, 2976, 2976–2977. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.J.; Dutour, S. Surble and I. Margiolaki, A. chromiumterephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal-organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. Engl. 2006, 45, 5974–5978. [Google Scholar] [CrossRef]
- Bauer, S.; Serre, C.; Devic, T.; Horcajada, P.; Marrot, J.; Férey, G.; Stock, N. High-throughput assisted rationalization of the formation of metal organic frameworks in the iron(III) aminoterephthalate solvothermal system. Inorg. Chem. 2008, 47, 7568–7576. [Google Scholar] [CrossRef]
- Xiao, B.; Wheatley, P.S.; Zhao, X.; Fletcher, A.J.; Fox, S.; Rossi, A.G.; Megson, I.L.; Bordiga, S.; Regli, L.; Thomas, K.M.; et al. High-capacity hydrogen and nitric oxide adsorption and storage in a metal-organic framework. J. Am. Chem. Soc. 2007, 129, 1203–1209. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Xiao, B.; Wragg, D.S.; Wheatley, P.S.; Megson, I.L.; Morris, R.E. Exceptional behavior over the whole adsorption-storage-delivery cycle for no in porous metal organic frameworks. J. Am. Chem. Soc. 2008, 130, 10440–10444. [Google Scholar] [CrossRef] [PubMed]
- Peikert, K.; McCormick, L.J.; Cattaneo, D.; Duncan, M.J.; Hoffmann, F.; Khan, A.H.; Bertmer, M.; Morris, R.E.; Fröba, M. Tuning the nitric oxide release behavior of amino functionalized HKUST-1. Micropor. Mesopor. Mater. 2015, 216, 118–126. [Google Scholar] [CrossRef]
- Khan, A.H.; Peikert, K.; Hoffmann, F.; Fröba, M.; Bertmer, M. Nitric oxide adsorption in Cu3btc2-type MOFs-physisorption and chemisorption as NONOates. J. Phys. Chem. C 2019, 123, 4299–4307. [Google Scholar] [CrossRef]
- Pinto, R.V.; Antunes, F.; Pires, J.; Graça, V.; Brandão, P.; Pinto, M.L. Vitamin B3 metal-organic frameworks as potential delivery vehicles for therapeutic nitric oxide. Acta Biomater. 2017, 51, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Cohen, S.M. Post synthetic modification of metal-organic frameworks-a progress report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Schindler, B.J.; Killops, K.L.; Browe, M.A.; Mahle, J.J. The effect of water adsorption on the structure of the carboxylate containing metal–organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A 2013, 1, 11922. [Google Scholar] [CrossRef]
- Chen, M.; Ye, Q.; Jiang, S.; Shao, M.; Jin, C.; Huang, Z. Two-step elution recovery of cyanide platinum using functional metal organic resin. Molecules 2019, 24, 2779. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Metal organic framework (MOF) porous octahedral nanocrystals of Cu-BTC: Synthesis, properties and enhanced adsorption properties. Mater. Res. Bull. 2019, 109, 124–133. [Google Scholar] [CrossRef]
- Davydovskaya, P.; Pohle, R.; Tawil, A.; Fleischer, M. Work function based gas sensing with Cu-BTC metal-organic framework for selective aldehyde detection. Sens. Actuators B 2013, 187, 142–146. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Zeinali, S.; Sheikhi, M.H. Fabrication of capacitive sensor based on Cu-BTC (MOF-199) nanoporous film for detection of ethanol and methanol vapors. Sens. Actuators B 2016, 230, 9–16. [Google Scholar] [CrossRef]
- Kidanemariam, A.; Lee, J.; Park, J. Recent innovation of metal-organic frameworks for carbon dioxide photocatalytic reduction. Polymers 2019, 11, 2090. [Google Scholar] [CrossRef]
- Eubank, J.F.; Wheatley, P.S.; Lebars, G.; McKinlay, A.C.; Leclerc, H.; Horcajada, P.; Daturi, M.; Vimont, A.; Morris, R.E.; Serre, C. Porous, rigid metal (III). APL Mater. 2014, 2, 124112. [Google Scholar] [CrossRef]
- Nguyen, J.G.; Tanabe, K.K.; Cohen, S.M. Postsynthetic diazeniumdiolate formation and NO release from MOFs. CrystEngComm 2010, 12, 2335. [Google Scholar] [CrossRef]
- Gallis, D.F.S.S.; Vogel, D.J.; Vincent, G.A.; Rimsza, J.M.; Nenoff, T.M. NOx Adsorption and optical detection in rare earth metal−organic frameworks. ACS Appl. Mater. Interfaces 2019, 11, 43270–43277. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, J.; Hijikata, Y. Trans Influence across a Metal−Metal Bond of a Paddle-Wheel Unit on Interaction with Gases in a Metal−Organic Framework. Inorg. Chem. 2020, 59, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.C.; Samanta, B.; Lee, C.Y.; Farha, O.K.; Hupp, J.T. Coordination-chemistry control of proton conductivity in the iconic metal−organic framework material HKUST-1. J. Am. Chem. Soc. 2012, 134, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Majano, G.; Pérez-Ramírez, J. Room temperature synthesis and size control of HKUST-1. Helv. Chim. Acta 2012, 95, 2278–2286. [Google Scholar] [CrossRef]
- Umemura, A.; Diring, S.; Furukawa, S.; Uehara, H.; Tsuruoka, T.; Kitagawa, S. Morphology design of porous coordination polymer crystals by coordination modulation. J. Am. Chem. Soc. 2011, 133, 15506–15513. [Google Scholar] [CrossRef]
- Emam, H.E.; Darwesh, O.M.; Abdelhameed, R.M. In-growth metal organic framework/synthetic hybrids as antimicrobial fabrics and its toxicity. Colloids Surf. B Biointerfaces 2018, 165, 219–228. [Google Scholar] [CrossRef]
- Song, F.; Zhong, Q.; Zhao, Y. A protophilic solvent-assisted solvothermal approach to Cu-BTC for enhanced CO2 capture. Appl. Organomet. Chem. 2015, 29, 612–617. [Google Scholar] [CrossRef]
- Schlesinger, M.; Schulze, S.; Hietschold, M.; Mehring, M. Evaluation of synthetic methods for microporous metal-organic frameworks exemplified by the competitive formation of [Cu2(btc)3(H2O)3] and [Cu2(btc)(OH)(H2O)]. Micropor. Mesopor. Mater. 2010, 132, 121–127. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Gao, B.; Zou, W.; Dong, L. Facile ball-milling synthesis of CuO/biochar nanocomposites for efficient removal of reactive Red 120. ACS Omega 2020, 5, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. Engl. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Kumar, S.S.; Kulandainathan, M.A. Microporous and mesoporous materials efficient electrosynthesis of highly active Cu3(BTC)2-MOF and its catalytic application to chemical reduction. Micropor. Mesopor. Mater. 2013, 63, 57. [Google Scholar] [CrossRef]
- Kim, K.J.; Li, Y.J.; Kreider, P.B.; Chang, C.H.; Wannenmacher, N.; Thallapally, P.K.; Ahn, H.G. High-rate synthesis of Cu–BTC metal–organic frameworks. Chem. Commun. (Camb) 2013, 49, 11518–11520. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Blecher, K.; Sanchez, D.; Tuckman-Vernon, C.; Gialanella, P.; Friedman, J.M.; Martinez, L.R.; Nosanchuk, J.D. Susceptibility of Gram-positive and -negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence 2011, 2, 217–221. [Google Scholar] [CrossRef]
- Lis, M.J.; Caruzi, B.B.; Gil, G.A.; Samulewski, R.B.; Bail, A.; Scacchetti, F.A.P.; Moisés, M.P.; Bezerra, F.M.M. In-Situ Direct synthesis of HKUST-1 in wool fabric for the improvement of antibacterial properties. Polymers 2019, 11, 713. [Google Scholar] [CrossRef]
- Duncan, M.J.; Wheatley, P.S.; Coghill, E.M.; Vornholt, S.M.; Warrender, S.J.; Megson, I.L.; Morris, R.E. Antibacterial efficacy from NO-releasing MOF–polymer films. Mater. Adv. 2020, 1, 2509. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]




| Empirical Formula | C6H2CuO5 (NO)0.17 |
|---|---|
| Formula weight | 222.62 |
| Temp. (K) | 223(2) |
| Wavelength (Å) | 0.71073 |
| Space group | Fm-3m |
| a (Å) | 26.3068(11) |
| b (Å) | 26.3068(11) |
| c (Å) | 26.3068(11) |
| α (°) | 90.00 |
| β (°) | 90.00 |
| γ (°) | 90.00 |
| Volume (Å3) | 18,206(2) |
| Z | 48 |
| Density (calc.) (Mg m−3) | 0.975 |
| Absorption coeff. (mm−1) | 1.429 |
| Crystal size (mm) | 0.090 × 0.080 × 0.020 |
| Reflections collected | 148,341 |
| Independent reflections | 1177 [R(int) = 0.1842] |
| Data/restraints/parameters | 1177/1/40 |
| Goodness-of-fit on F2 | 1.291 |
| Final R indices [I>2σ(I)] | R1 = 0.1149, wR2 = 0.2110 |
| R indices (all data) | R1 = 0.1264, wR2 = 0.2170 |
| Largest diff. peak and hole (e.Å-3) | 0.697 and −0.564 |
| CCDC | 2,073,930 |
| t[NO] (μmol·mg−1) | tm (s) | [NO]m (ppm·mg−1) | t1/2 (s) | td (h) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |
| Non-dehydrated | 0.34 | 0.25 | 50 | 40 | 3.25 | 4.78 | 185 | 75 | 0.9 | 1.1 |
| Dehydrated | 0.68 | 1.81 | 33 | 39 | 11.5 | 52.7 | 73 | 58 | 1.2 | 1.2 |
| Bacterial Strains * | Inhibition Zone (mm2) | p Value | ||
|---|---|---|---|---|
| Cu-BTC | NO⊂Cu-BTC | |||
| Gram-negative strains | E. coli (ATCC11775) | 712.3 ± 38.6 | 803.4 ± 54.7 | <0.024 |
| P. aeruginosa (ATCC9027) | 47.8 ± 22.2 | 52.3 ± 26.2 | <0.21 | |
| Gram-positive strains | S. aureus (ATCC14458) | 414.2 ± 18.4 | 376.8 ± 21.6 | <0.016 |
| B. cereus (ATCC11706) | 51.5 ± 7.3 | 74 ± 8.8 | <0.003 | |
| Gram-positive MRSA strains | MRSA (KCCM40510) | 271.4 ± 8.1 | 297.4 ± 8.1 | <0.0018 |
| MRSA (Clinical isolation) | 501.1 ± 5.6 | 573.3 ± 6.9 | <0.00057 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.N.; Kim, Y.R.; Yang, S.; Tran, N.M.; Park, B.J.; Lee, S.J.; Kim, Y.; Yoo, H.; Kim, S.-J.; Shin, J.H. Controllable Nitric Oxide Storage and Release in Cu-BTC: Crystallographic Insights and Bioactivity. Int. J. Mol. Sci. 2022, 23, 9098. https://doi.org/10.3390/ijms23169098
Lee DN, Kim YR, Yang S, Tran NM, Park BJ, Lee SJ, Kim Y, Yoo H, Kim S-J, Shin JH. Controllable Nitric Oxide Storage and Release in Cu-BTC: Crystallographic Insights and Bioactivity. International Journal of Molecular Sciences. 2022; 23(16):9098. https://doi.org/10.3390/ijms23169098
Chicago/Turabian StyleLee, Do Nam, Yeong Rim Kim, Sohyeon Yang, Ngoc Minh Tran, Bong Joo Park, Su Jung Lee, Youngmee Kim, Hyojong Yoo, Sung-Jin Kim, and Jae Ho Shin. 2022. "Controllable Nitric Oxide Storage and Release in Cu-BTC: Crystallographic Insights and Bioactivity" International Journal of Molecular Sciences 23, no. 16: 9098. https://doi.org/10.3390/ijms23169098
APA StyleLee, D. N., Kim, Y. R., Yang, S., Tran, N. M., Park, B. J., Lee, S. J., Kim, Y., Yoo, H., Kim, S.-J., & Shin, J. H. (2022). Controllable Nitric Oxide Storage and Release in Cu-BTC: Crystallographic Insights and Bioactivity. International Journal of Molecular Sciences, 23(16), 9098. https://doi.org/10.3390/ijms23169098






