Anti-Inflammatory Effects of 1,25(OH)2D/Calcitriol in T Cell Immunity: Does Sex Make a Difference?
Abstract
1. Introduction
2. Results
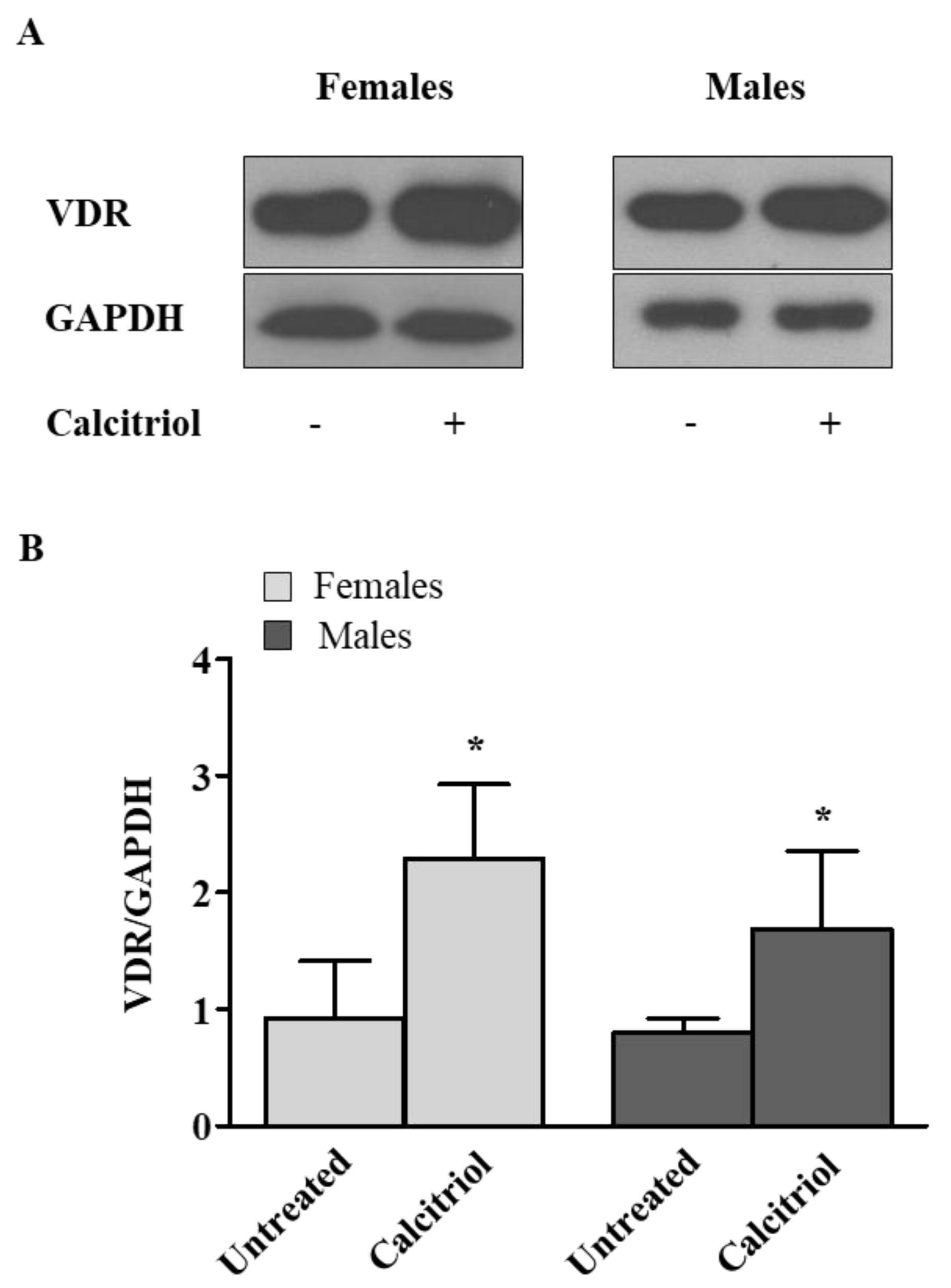
2.1. Calcitriol Upregulates VDR Expression in Activated Peripheral Blood T Lymphocytes from Female and Male Healthy Donors
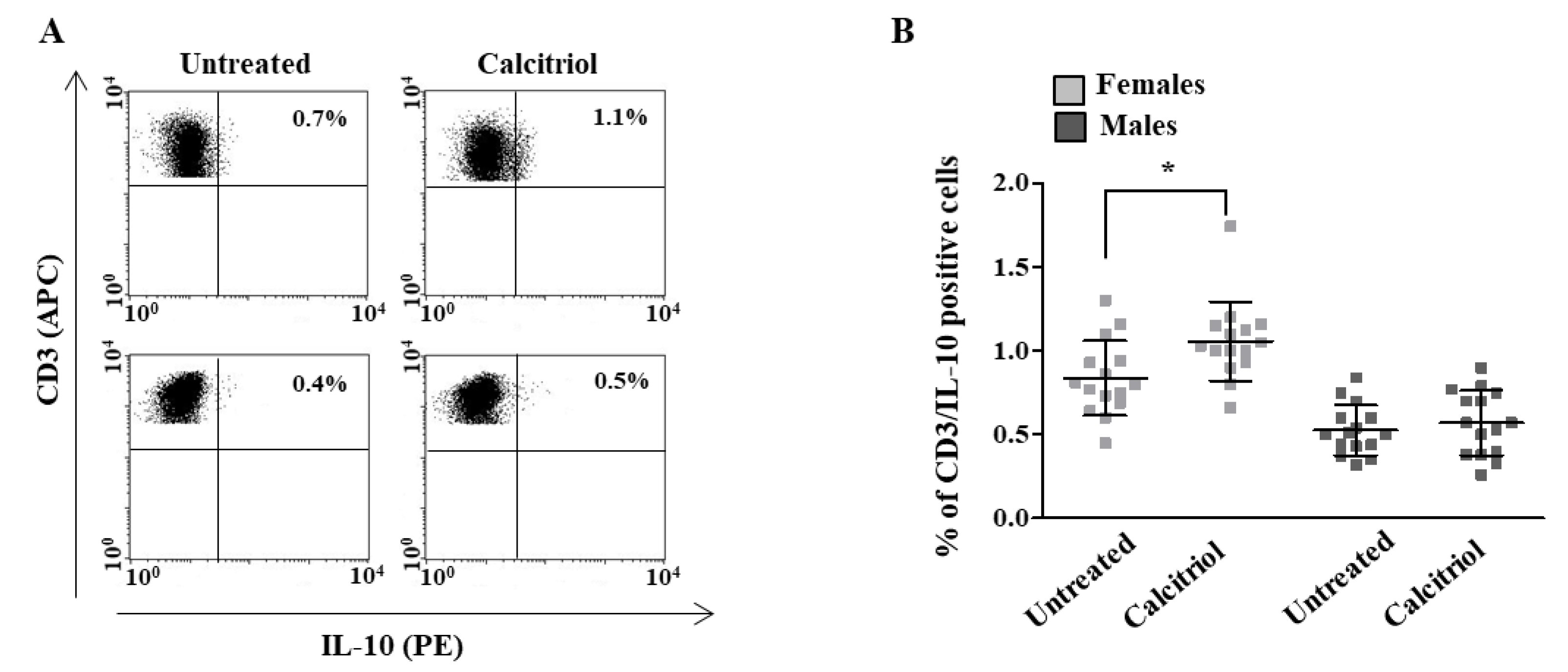
2.2. Calcitriol Effects on Cytokine Production in Peripheral Blood T Lymphocytes from Female and Male Healthy Donors
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Isolation of Peripheral Blood Lymphocytes and Cell Culture Conditions
4.3. SDS-PAGE and Western Blot
4.4. Flow Cytometry
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonucci, R.; Locci, C.; Clemente, M.G.; Chicconi, E.; Antonucci, L. Vitamin D deficiency in childhood: Old lessons and current challenges. J. Pediatr. Endocrinol. Metab. 2018, 31, 247–260. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2005; Available online: https://apps.who.int/iris/handle/10665/42716 (accessed on 22 March 2021).
- Cheng, J.B.; Motola, D.L.; Mangelsdorf, D.J.; Russell, D.W. De-orphanization of cytochrome P450 2R1: A microsomal vitamin D 25-hydroxilase. J. Biol. Chem. 2003, 278, 38084–38093. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D in the Context of Evolution. Nutrients 2022, 14, 3018. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The role of vitamin D in autoimmune diseases: Could sex make the difference? Biol. Sex Differ. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Rochel, N. Vitamin D and Its Receptor from a Structural Perspective. Nutrients 2022, 14, 2847. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Black, P.N.; Scragg, R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest 2005, 128, 3792–3798. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Weiss, S.T. Is vitamin D deficiency to blame for the asthma epidemic? J. Allergy Clin. Immunol. 2007, 120, 1031–1035. [Google Scholar] [CrossRef]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 2019, 106, 58–75. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Weinstein, S.J.; Freedman, D.M.; Helzlsouer, K.; Flanders, W.D.; Koenig, K.; Kolonel, L.; Laden, F.; Le Marchand, L.; Purdue, M.; et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 21–35. [Google Scholar] [CrossRef]
- Hagenau, T.; Vest, R.; Gissel, T.N.; Poulsen, C.S.; Erlandsen, M.; Mosekilde, L.; Vestergaard, P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic meta-regression analysis. Osteoporos. Int. 2009, 20, 133–140. [Google Scholar] [CrossRef]
- Jungert, A.; Neuhauser-Berthold, M. Sex-specific determinants of serum 25-hydroxyvitamin D3 concentrations in an elderly German cohort: A cross-sectional study. Nutr. Metab. 2015, 12, 2. [Google Scholar] [CrossRef]
- Correale, J.; Ysrraelit, M.C.; Gaitan, M.I. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J. Immunol. 2010, 185, 4948–4958. [Google Scholar] [CrossRef]
- Spanier, J.A.; Nashold, F.E.; Mayne, C.G.; Nelson, C.D.; Hayes, C.E. Vitamin D and estrogen synergy in Vdr-expressing CD4(+) T cells is essential to induce Helios(+)FoxP3(+) T cells and prevent autoimmune demyelinating disease. J. Neuroimmunol. 2015, 286, 48–58. [Google Scholar] [CrossRef]
- Cheema, C.; Grant, B.F.; Marcus, R. Effects of estrogen on circulating “free” and total 1,25-dihydroxyvitamin D and on the parathyroid-vitamin D axis in postmenopausal women. J. Clin. Investig. 1989, 83, 537–542. [Google Scholar] [CrossRef]
- Lundqvist, J.; Norlin, M.; Wikvall, K. 1alpha,25-Dihydroxyvitamin D3 exerts tissue-specific effects on estrogen and androgen metabolism. Biochim. Biophys. Acta 2011, 1811, 263–270. [Google Scholar] [CrossRef]
- Takeyama, K.; Masuhiro, Y.; Fuse, H.; Endoh, H.; Murayama, A.; Kitanaka, S.; Suzawa, M.; Yanagisawa, J.; Kato, S. Selective interaction of vitamin D receptor with transcriptional coactivators by a vitamin D analog. Mol. Cell. Biol. 1999, 19, 1049–1055. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Crescioli, C. The Role of Estrogens and Vitamin D in Cardiomyocyte Protection: A Female Perspective. Biomolecules 2021, 11, 1815. [Google Scholar] [CrossRef]
- Norlin, M. Effects of vitamin D in the nervous system: Special focus on interaction with steroid hormone signalling and a possible role in the treatment of brain cancer. J. Neuroendocrinol. 2020, 32, e12799. [Google Scholar] [CrossRef]
- Harmon, Q.E.; Umbach, D.M.; Baird, D.D. Use of Estrogen-Containing Contraception Is Associated with Increased Concentrations of 25-Hydroxy Vitamin D. J. Clin. Endocrinol. Metab. 2016, 101, 3370–3377. [Google Scholar] [CrossRef]
- Zhao, D.; Ouyang, P.; de Boer, I.H.; Lutsey, P.L.; Farag, Y.M.; Guallar, E.; Siscovick, D.S.; Post, W.S.; Kalyani, R.R.; Billups, K.L.; et al. Serum vitamin D and sex hormones levels in men and women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2017, 96, 95–102. [Google Scholar] [CrossRef][Green Version]
- Pierdominici, M.; Maselli, A.; Colasanti, T.; Giammarioli, A.M.; Delunardo, F.; Vacirca, D.; Sanchez, M.; Giovannetti, A.; Malorni, W.; Ortona, E. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol. Lett. 2010, 132, 79–85. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peruzzu, D.; Dupuis, M.L.; Pierdominici, M.; Fecchi, K.; Gagliardi, M.C.; Ortona, E.; Pagano, M.T. Anti-Inflammatory Effects of 1,25(OH)2D/Calcitriol in T Cell Immunity: Does Sex Make a Difference? Int. J. Mol. Sci. 2022, 23, 9164. https://doi.org/10.3390/ijms23169164
Peruzzu D, Dupuis ML, Pierdominici M, Fecchi K, Gagliardi MC, Ortona E, Pagano MT. Anti-Inflammatory Effects of 1,25(OH)2D/Calcitriol in T Cell Immunity: Does Sex Make a Difference? International Journal of Molecular Sciences. 2022; 23(16):9164. https://doi.org/10.3390/ijms23169164
Chicago/Turabian StylePeruzzu, Daniela, Maria Luisa Dupuis, Marina Pierdominici, Katia Fecchi, Maria Cristina Gagliardi, Elena Ortona, and Maria Teresa Pagano. 2022. "Anti-Inflammatory Effects of 1,25(OH)2D/Calcitriol in T Cell Immunity: Does Sex Make a Difference?" International Journal of Molecular Sciences 23, no. 16: 9164. https://doi.org/10.3390/ijms23169164
APA StylePeruzzu, D., Dupuis, M. L., Pierdominici, M., Fecchi, K., Gagliardi, M. C., Ortona, E., & Pagano, M. T. (2022). Anti-Inflammatory Effects of 1,25(OH)2D/Calcitriol in T Cell Immunity: Does Sex Make a Difference? International Journal of Molecular Sciences, 23(16), 9164. https://doi.org/10.3390/ijms23169164






