Advanced Maternal Age Impairs Uterine Artery Adaptations to Pregnancy in Rats
Abstract
:1. Introduction
2. Results
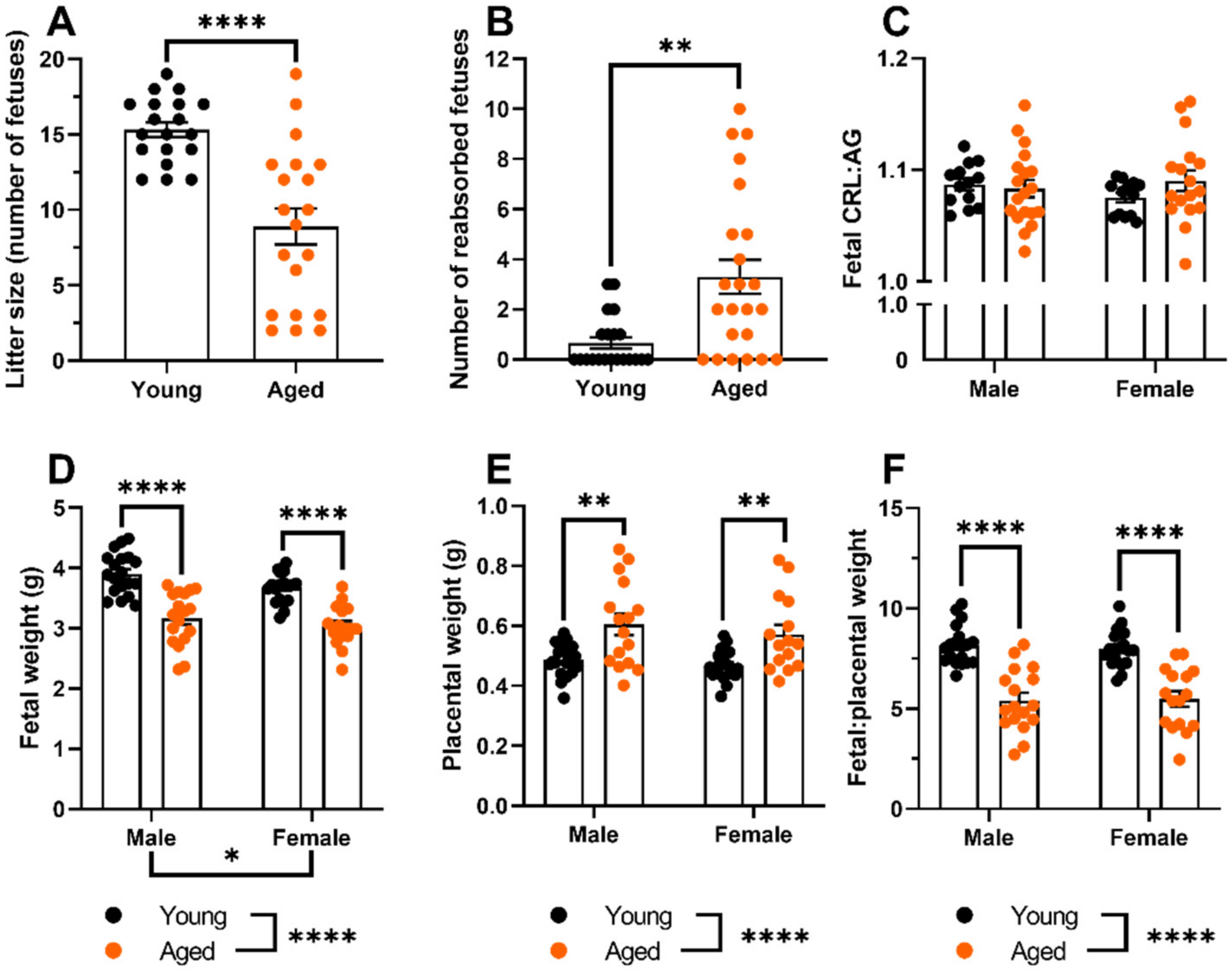
2.1. Aged Dams Had Worse Pregnancy Outcomes Than Young Dams
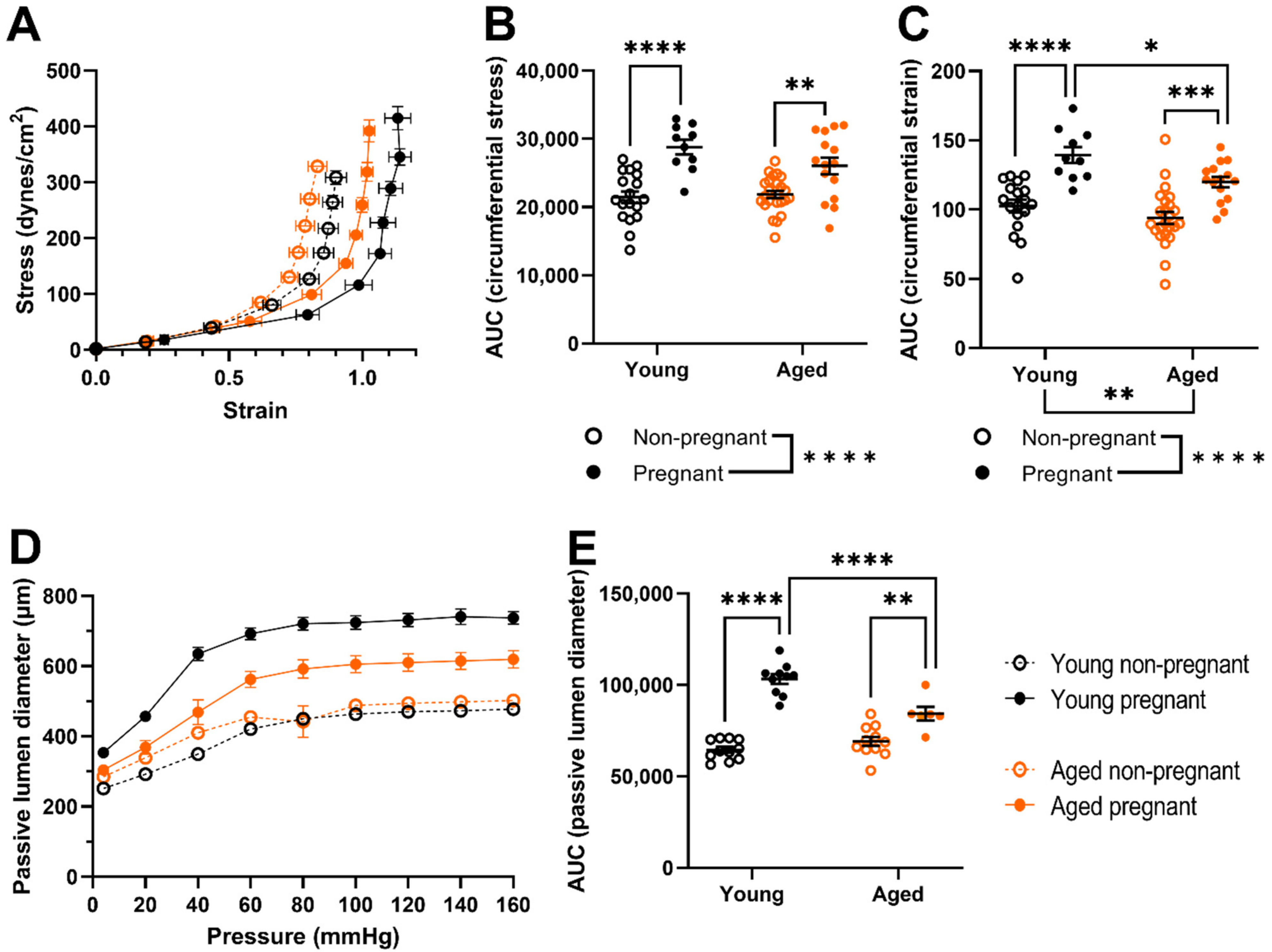
2.2. Myogenic Tone Was Reduced Only the Uterine Arteries of Young, but Not Aged, Pregnant Dams; Which Showed Forced Vasodilation at High Intraluminal Pressures
2.3. Uterine Arteries of Aged Pregnant Dams Had Reduced Circumferential Strain and Smaller Diameters Than Uterine Arteries of Young Pregnant Dams
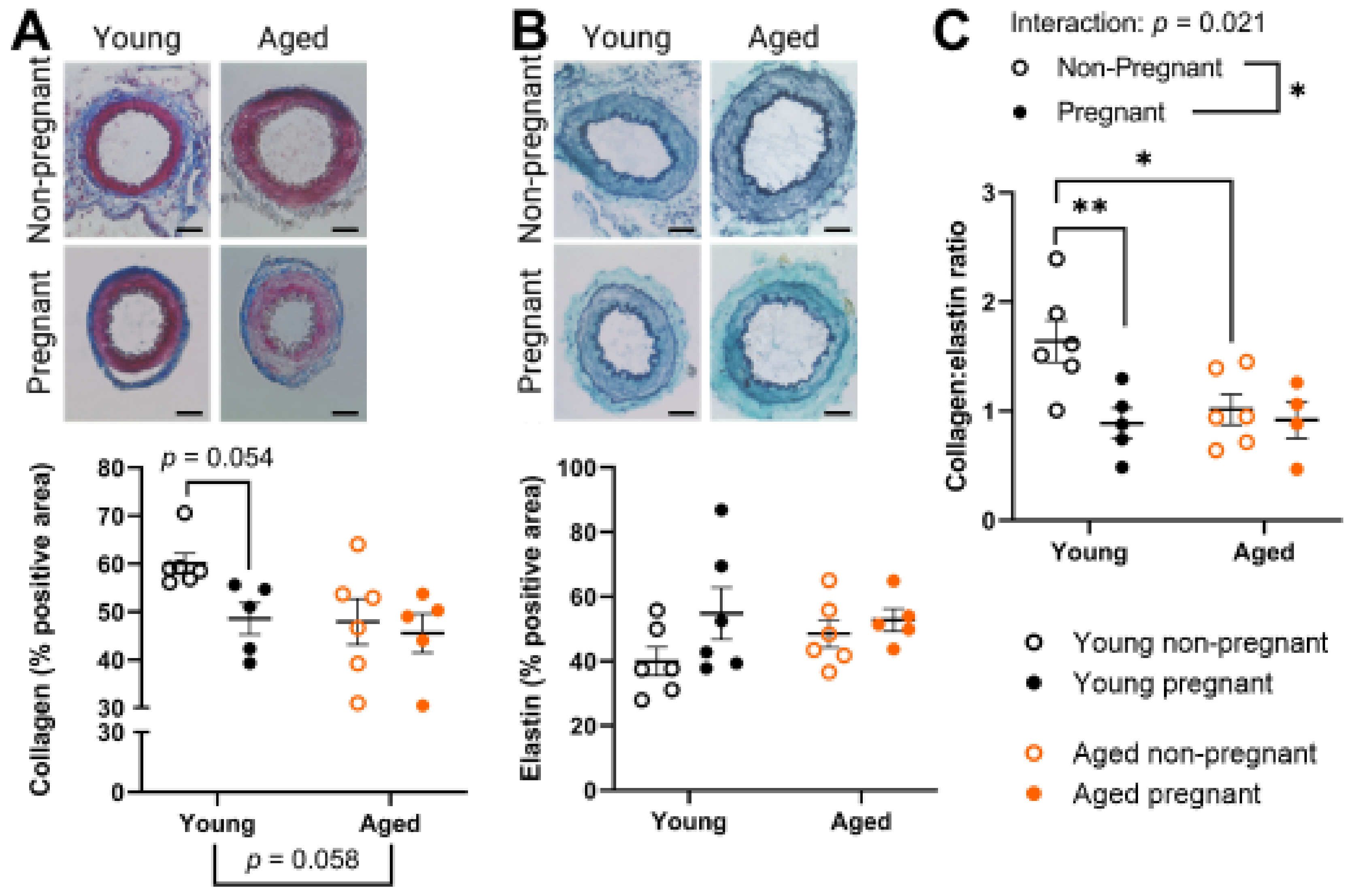
2.4. Aged Rats Had a Lower Collagen:Elastin Ratio Prior to Pregnancy, Which Was Not Affected by Pregnancy
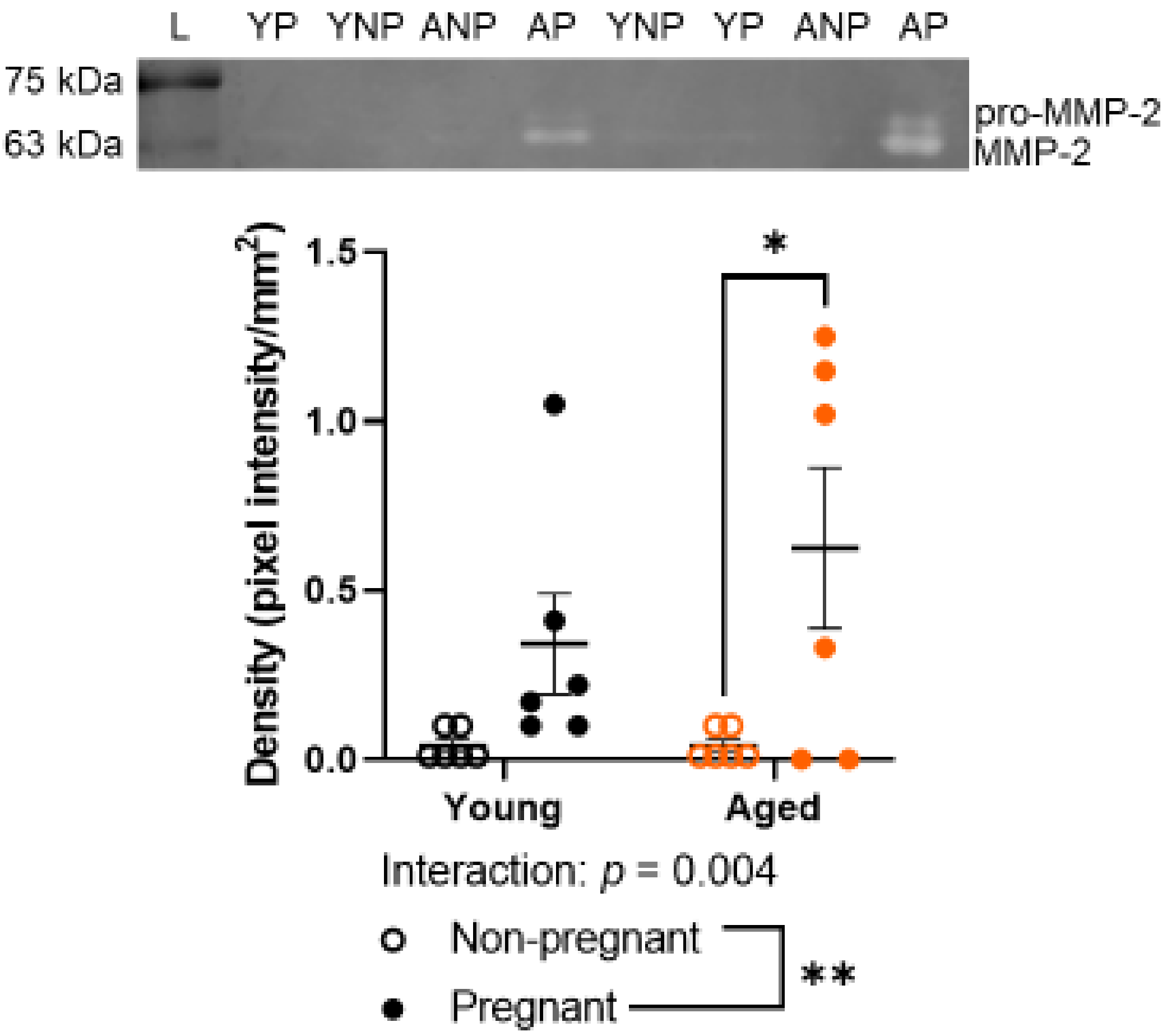
2.5. Advanced Maternal Age Did Not Detectably Alter Gelatinase Activity by MMP-2
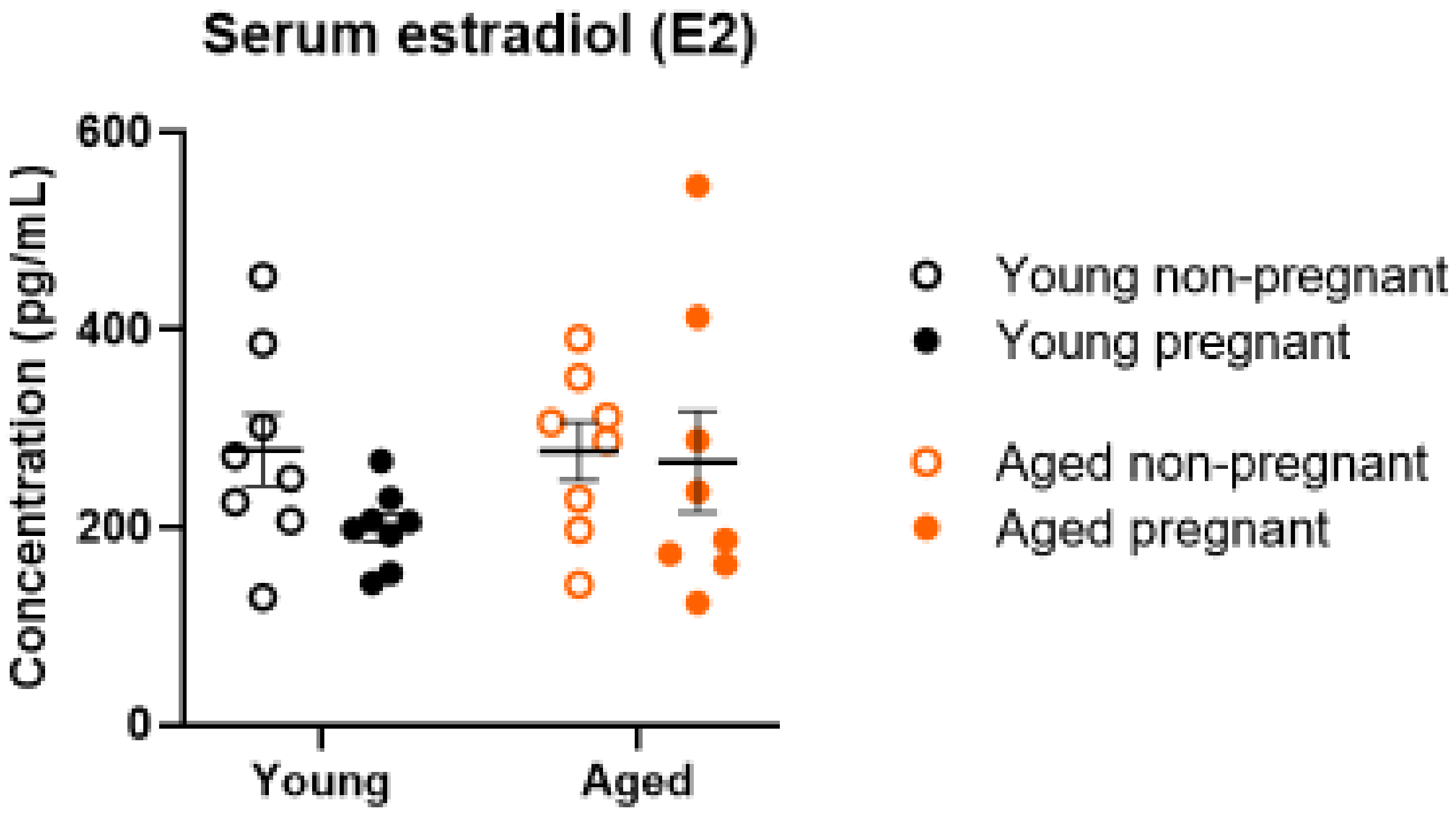
2.6. Serum Estrogen Levels Were Not Altered by Age or Pregnancy
3. Discussion
4. Materials and Methods
4.1. Advanced Maternal Age Rat Model
4.2. Pregnancy Outcomes and Tissue Collection
4.3. Ex Vivo Assessment of Uterine Artery Vascular Function Using Pressure Myography
4.4. Histological Assessment of Arterial Remodeling
4.5. Zymography Assessment of MMP-2 and MMP-9
4.6. Assessment of Serum Estradiol Levels
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Canada. Live Births, by Age of Mother. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310041601 (accessed on 1 November 2021).
- Jacobsson, B.; Ladfors, L.; Milsom, I. Advanced Maternal Age and Adverse Perinatal Outcome. Obstet. Gynecol. 2004, 104, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Odibo, A.O.; Nelson, D.; Stamilio, D.M.; Sehdev, H.M.; Macones, G.A. Advanced Maternal Age Is an Independent Risk Factor for Intrauterine Growth Restriction. Am. J. Perinatol. 2006, 23, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.-L.M.; Davidge, S.T. Advanced maternal age and the impact on maternal and offspring cardiovascular health. Am. J. Physiol. Circ. Physiol. 2019, 317, H387–H394. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, K.L.; Jacobson, S.-L.; Giraud, G.D.; Morton, M.J. Hemodynamic changes in pregnancy. Semin. Perinatol. 2000, 24, 11–14. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [PubMed]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and Mechanisms of Intrauterine Hypoxia and Its Impact on the Fetal Cardiovascular System: A Review. Int. J. Pediatr. 2010, 2010, 401323. [Google Scholar] [CrossRef]
- Velauthar, L.; Plana, M.N.; Kalidindi, M.; Zamora, J.; Thilaganathan, B.; Illanes, S.E.; Khan, K.S.; Aquilina, J.; Thangaratinam, S. First-trimester uterine artery Doppler and adverse pregnancy outcome: A meta-analysis involving 55 974 women. Ultrasound Obstet. Gynecol. 2014, 43, 500–507. [Google Scholar] [CrossRef]
- Kauffenstein, G.; Laher, I.; Matrougui, K.; Guerineau, N.; Henrion, D. Emerging role of G protein-coupled receptors in microvascular myogenic tone. Cardiovasc. Res. 2012, 95, 223–232. [Google Scholar] [CrossRef]
- Eckman, D.M.; Gupta, R.; Rosenfeld, C.R.; Morgan, T.M.; Charles, S.M.; Mertz, H.; Moore, L.G. Pregnancy increases myometrial artery myogenic tone via NOS- or COX-independent mechanisms. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R368–R375. [Google Scholar] [CrossRef]
- Mukhtarova, N.; Ko, N.L.; Gokina, N.I.; Mandalá, M.; Osol, G. Enhanced Vascular Smooth Muscle Calcium Sensitivity and Loss of Endothelial Vasodilator Influence Contribute to Myogenic Tone Development in Rat Radial Uterine Arteries during Gestation. J. Vasc. Res. 2020, 57, 126–135. [Google Scholar] [CrossRef]
- Osol, G.; Cipolla, M. Interaction of myogenic and adrenergic mechanisms in isolated, pressurized uterine radial arteries from late-pregnant and nonpregnant rats. Am. J. Obstet. Gynecol. 1993, 168, 697–705. [Google Scholar] [CrossRef]
- Gokina, N.I.; Kuzina, O.Y.; Fuller, R.; Osol, G. Local Uteroplacental Influences are Responsible for the Induction of Uterine Artery Myogenic Tone during Rat Pregnancy. Reprod. Sci. 2009, 16, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Barron, C.; Mandala, M.; Osol, G. Effects of Pregnancy, Hypertension and Nitric Oxide Inhibition on Rat Uterine Artery Myogenic Reactivity. J. Vasc. Res. 2010, 47, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.; Taggart, M.; Baker, P.; Austin, C. Responses of Isolated Pressurised Rat Uterine Arteries to Changes in Pressure: Effects of Pre-constriction, Endothelium and Pregnancy. Placenta 2009, 30, 529–535. [Google Scholar] [CrossRef]
- Mateev, S.N.; Mouser, R.; Young, D.A.; Mecham, R.P.; Moore, L.G. Chronic hypoxia augments uterine artery distensibility and alters the circumferential wall stress-strain relationship during pregnancy. J. Appl. Physiol. 2006, 100, 1842–1850. [Google Scholar] [CrossRef]
- Marshall, S.A.; Senadheera, S.N.; Jelinic, M.; O’Sullivan, K.; Parry, L.J.; Tare, M. Relaxin Deficiency Leads to Uterine Artery Dysfunction During Pregnancy in Mice. Front. Physiol. 2018, 9, 255. [Google Scholar] [CrossRef]
- Hilgers, R.H.; Bergaya, S.; Schiffers, P.M.; Meneton, P.; Boulanger, C.M.; Henrion, D.; Leévy, B.I.; De Mey, J.G.R. Uterine Artery Structural and Functional Changes During Pregnancy in Tissue Kallikrein–Deficient Mice. Arter. Thromb. Vasc. Biol. 2003, 23, 1826–1832. [Google Scholar] [CrossRef]
- Mandala, M.; Osol, G. Physiological Remodelling of the Maternal Uterine Circulation during Pregnancy. Basic Clin. Pharmacol. Toxicol. 2011, 110, 12–18. [Google Scholar] [CrossRef]
- Cipolla, M.; Osol, G. Hypertrophic and hyperplastic effects of pregnancy on the rat uterine arterial wall. Am. J. Obstet. Gynecol. 1994, 171, 805–811. [Google Scholar] [CrossRef]
- Palmer, S.K.; Zamudio, S.; Coffin, C.; Parker, S.; Stamm, E.; Moore, L. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet. Gynecol. 1992, 80, 1000–1006. [Google Scholar]
- Griendling, K.K.; Fuller, E.O.; Cox, R. Pregnancy-induced changes in sheep uterine and carotid arteries. Am. J. Physiol. Circ. Physiol. 1985, 248, H658–H665. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.E.; Conley, A.J.; Van Orden, D.E.; Farley, D.B.; Ford, S.P. Structural and Mechanical Changes of Uterine Arteries during Pregnancy in the Pig. J. Anim. Sci. 1988, 66, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, O.W.H.; Essers, Y.P.G.; Simkens, L.H.J.; Teunissen, Q.G.A.; Peeters, L.L.H.; De Mey, J.G.R.; Van Eys, G.J.J.M. Aging Blunts Remodeling of the Uterine Artery During Murine Pregnancy. J. Soc. Gynecol. Investig. 2004, 11, 304–310. [Google Scholar] [CrossRef]
- McNulty, M.; Spiers, P.; McGovern, E.; Feely, J. Aging is associated with increased matrix metalloproteinase-2 activity in the human aorta. Am. J. Hypertens. 2005, 18, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Khalil, R.A. Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. Prog. Mol. Biol. Transl. Sci. 2017, 148, 87–165. [Google Scholar]
- Wang, M.; Kim, S.H.; Monticone, R.E.; Lakatta, E.G. Matrix Metalloproteinases Promote Arterial Remodeling in Aging, Hypertension, and Atherosclerosis. Hypertension 2015, 65, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Galewska, Z.; Bańkowski, E.; Romanowicz, L.; Jaworski, S. Pre-eclampsia (EPH-gestosis)-induced decrease of MMP-s content in the umbilical cord artery. Clin. Chim. Acta 2003, 335, 109–115. [Google Scholar] [CrossRef]
- Montagnana, M.; Lippi, G.; Albiero, A.; Scevarolli, S.; Salvagno, G.L.; Franchi, M.; Guidi, G.C. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J. Clin. Lab. Anal. 2009, 23, 88–92. [Google Scholar] [CrossRef]
- Schafer-Somi, S.; Aksoy, O.A.; Patzl, M.; Findik, M.; Erunal-Maral, N.; Beceriklisoy, H.B.; Polat, B.; Aslan, S. The Activity of Matrix Metalloproteinase-2 and -9 in Serum of Pregnant and Non-Pregnant Bitches. Reprod. Domest. Anim. 2005, 40, 46–50. [Google Scholar] [CrossRef]
- Barrett, E.S.; Mbowe, O.; Thurston, S.W.; Butts, S.; Wang, C.; Nguyen, R.; Bush, N.; Redmon, J.B.; Sheshu, S.; Swan, S.H.; et al. Predictors of Steroid Hormone Concentrations in Early Pregnancy: Results from a Multi-Center Cohort. Matern. Child Health J. 2019, 23, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Hoover, R.N.; Thadhani, R.; Hsieh, C.-C.; Sluss, P.; Ballard-Barbash, R.; Potischman, N. Maternal, prenatal and perinatal characteristics and first trimester maternal serum hormone concentrations. Br. J. Cancer 2008, 99, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.; Vääräsmäki, M.; Lehtinen, M.; Zeleniuch-Jacquotte, A.; Lundin, E.; Rodgers, K.-G.; Lakso, H.-A.; Chen, T.; Schock, H.; Hallmans, G.; et al. Determinants of Maternal Sex Steroids During the First Half of Pregnancy. Obstet. Gynecol. 2011, 118, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, C.S.; Garr, E.; Godsland, I.F.; Stevenson, J.C. 17β-Oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1998, 1406, 169–174. [Google Scholar] [CrossRef]
- Babischkin, J.S.; Bonagura, T.W.; Udoff, L.C.; Vergara, C.O.; Johnson, H.W.; Atlas, R.O.; Pepe, G.J.; Albrecht, E.D. Estrogen stimulates the human endometrium to express a factor(s) that promotes vascular smooth muscle cell migration as an early step in microvessel remodeling. Endocrine 2008, 35, 81–88. [Google Scholar] [CrossRef]
- Sprague, B.J.; Phernetton, T.M.; Magness, R.R.; Chesler, N.C. The effects of the ovarian cycle and pregnancy on uterine vascular impedance and uterine artery mechanics. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144 (Suppl. 1), S170–S178. [Google Scholar] [CrossRef]
- Care, A.S.; Bourque, S.L.; Morton, J.S.; Hjartarson, E.P.; Davidge, S.T. Effect of Advanced Maternal Age on Pregnancy Outcomes and Vascular Function in the Rat. Hypertension 2015, 65, 1324–1330. [Google Scholar] [CrossRef]
- Merchant, S.J.; Davidge, S.T. The role of matrix metalloproteinases in vascular function: Implications for normal pregnancy and pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 931–939. [Google Scholar] [CrossRef]
- Dang, Y.; Li, W.; Tran, V.; Khalil, R.A. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem. Pharmacol. 2013, 86, 734–747. [Google Scholar] [CrossRef]
- Lekontseva, O.; Jiang, Y.; Davidge, S.T. Estrogen replacement increases matrix metalloproteinase contribution to vasoconstriction in a rat model of menopause. J. Hypertens. 2009, 27, 1602–1608. [Google Scholar] [CrossRef]
- Lekontseva, O.N.; Rueda-Clausen, C.; Morton, J.S.; Davidge, S.T. Ovariectomy in aged versus young rats augments matrix metalloproteinase-mediated vasoconstriction in mesenteric arteries. Menopause 2010, 17, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Osol, G.; Brekke, J.F.; McElroy-Yaggy, K.; Gokina, N.I. Myogenic tone, reactivity, and forced dilatation: A three-phase model of in vitro arterial myogenic behavior. Am. J. Physiol. Circ. Physiol. 2002, 283, H2260–H2267. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J. Myogenic response gradient in an arteriolar network. Am. J. Physiol. Circ. Physiol. 1993, 264, H2168–H2179. [Google Scholar] [CrossRef] [PubMed]
- Bouskela, E.; Wiederhielm, C.A. Microvascular myogenic reaction in the wing of the intact unanesthetized bat. Am. J. Physiol. Circ. Physiol. 1979, 237, H59–H65. [Google Scholar] [CrossRef]
- Jones-Muhammad, M.; Warrington, J.P. Cerebral Blood Flow Regulation in Pregnancy, Hypertension, and Hypertensive Disorders of Pregnancy. Brain Sci. 2019, 9, 224. [Google Scholar] [CrossRef]
- Morris, E.A.; Mandalà, M.; Ko, N.L.; Osol, G. Postpartum Persistence of Maternal Uterine Vascular Gestational Adaptation in Rodents. Reprod. Sci. 2020, 27, 611–620. [Google Scholar] [CrossRef]
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, C.A. Age-related vascular stiffening: Causes and consequences. Front. Genet. 2015, 6, 112. [Google Scholar] [CrossRef]
- Mahmud, A.; McNulty, M.; McGovern, E.; Spiers, J.; Kavanagh, P.; Tolan, M.; Young, V.; Feely, J.; Silke, B. Arterial stiffness is associated with elastin fragmentation and medial collagen content in the human aorta. Eur. Heart J. 2020, 41, ehaa946-2706. [Google Scholar] [CrossRef]
- Schlatmann, T.J.; Becker, A.E. Histologic changes in the normal aging aorta: Implications for dissecting aortic aneurysm. Am. J. Cardiol. 1977, 39, 13–20. [Google Scholar] [CrossRef]
- Kelly, B.; Bond, B.; Poston, L. Gestational profile of matrix metalloproteinases in rat uterine artery. Mol. Hum. Reprod. 2003, 9, 351–358. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, L.; Monticone, R.E.; Lakatta, E.G. Proinflammation: The key to arterial aging. Trends Endocrinol. Metab. 2014, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G. The reality of aging viewed from the arterial wall. Artery Res. 2013, 7, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Meschiari, C.A.; Ero, O.K.; Pan, H.; Finkel, T.; Lindsey, M.L. The impact of aging on cardiac extracellular matrix. GeroScience 2017, 39, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, J.E.; Mecham, R.P. Elastin in Large Artery Stiffness and Hypertension. J. Cardiovasc. Transl. Res. 2012, 5, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, Z.; Zhu, M.; Khalil, R.A. Decreased homodimerization and increased TIMP-1 complexation of uteroplacental and uterine arterial matrix metalloproteinase-9 during hypertension-in-pregnancy. Biochem. Pharmacol. 2017, 138, 81–95. [Google Scholar] [CrossRef]
- Lin, C.; He, H.; Cui, N.; Ren, Z.; Zhu, M.; Khalil, R.A. Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix metalloproteinase-2 and -9 in hypertensive pregnancy. Am. J. Physiol. Circ. Physiol. 2020, 318, H165–H180. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Sharma, V.; Riddle, A.; Mason, B.A.; Pampiglione, J.; Campbell, S. An analysis of factors influencing the establishment of a clinical pregnancy in an ultrasound-based ambulatory in vitro fertilization program. Fertil. Steril. 1988, 49, 468–478. [Google Scholar] [CrossRef]
- Phelps, J.Y.; Levine, A.S.; Hickman, T.N.; Zacur, H.A.; Wallach, E.E.; Hinton, E.L. Day 4 Estradiol Levels Predict Pregnancy Success in Women Undergoing Controlled Ovarian Hyperstimulation for IVF. Fertil. Steril. 1998, 69, 1015–1019. [Google Scholar] [CrossRef]
- Fisher, S.; Grin, A.; Paltoo, A.; Shapiro, H. Falling estradiol levels as a result of intentional reduction in gonadotrophin dose are not associated with poor IVF outcomes, whereas spontaneously falling estradiol levels result in low clinical pregnancy rates. Hum. Reprod. 2005, 20, 84–88. [Google Scholar] [CrossRef]
- Anadol, E.; Kanca, H.; Yar, A.S.; Helvacioğlu, F.; Menevşe, S.; Çalgüner, E.; Erdoğan, D. Prostaglandin F receptor expression in intrauterine tissues of pregnant rats. J. Veter-Sci. 2014, 15, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.-L.M.; Shah, A.; Kirschenman, R.D.; Quon, A.L.; Morton, J.S.; Care, A.S.; Davidge, S.T. Increased susceptibility to cardiovascular disease in offspring born from dams of advanced maternal age. J. Physiol. 2018, 596, 5807–5821. [Google Scholar] [CrossRef]
- Napso, T.; Hung, Y.-P.; Davidge, S.T.; Care, A.; Sferruzzi-Perri, A.N. Advanced maternal age compromises fetal growth and induces sex-specific changes in placental phenotype in rats. Sci. Rep. 2019, 9, 16916. [Google Scholar] [CrossRef] [PubMed]
- Pasha, M.; Wooldridge, A.L.; Kirschenman, R.; Spaans, F.; Davidge, S.T.; Cooke, C.-L.M. Altered Vascular Adaptations to Pregnancy in a Rat Model of Advanced Maternal Age. Front. Physiol. 2021, 12, 718568. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.S.; Rueda-Clausen, C.F.; Davidge, S.T. Flow-mediated vasodilation is impaired in adult rat offspring exposed to prenatal hypoxia. J. Appl. Physiol. 2011, 110, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Schjørring, O.L.; Carlsson, R.; Simonsen, U. Pressure Myography to Study the Function and Structure of Isolated Small Arteries. In Methods in Mouse Atherosclerosis; Andrés, V., Dorado, B., Eds.; Springer: New York, NY, USA, 2015; pp. 277–295. [Google Scholar]
- Verhoeff, F.H. Some new staining methods of wide applicability: Including a rapid differential stain for elastic tissue. J. Am. Med Assoc. 1908, 50, 876–877. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wooldridge, A.L.; Pasha, M.; Chitrakar, P.; Kirschenman, R.; Quon, A.; Spaans, F.; Sáez, T.; Cooke, C.-L.M.; Davidge, S.T. Advanced Maternal Age Impairs Uterine Artery Adaptations to Pregnancy in Rats. Int. J. Mol. Sci. 2022, 23, 9191. https://doi.org/10.3390/ijms23169191
Wooldridge AL, Pasha M, Chitrakar P, Kirschenman R, Quon A, Spaans F, Sáez T, Cooke C-LM, Davidge ST. Advanced Maternal Age Impairs Uterine Artery Adaptations to Pregnancy in Rats. International Journal of Molecular Sciences. 2022; 23(16):9191. https://doi.org/10.3390/ijms23169191
Chicago/Turabian StyleWooldridge, Amy L., Mazhar Pasha, Palehswan Chitrakar, Raven Kirschenman, Anita Quon, Floor Spaans, Tamara Sáez, Christy-Lynn M. Cooke, and Sandra T. Davidge. 2022. "Advanced Maternal Age Impairs Uterine Artery Adaptations to Pregnancy in Rats" International Journal of Molecular Sciences 23, no. 16: 9191. https://doi.org/10.3390/ijms23169191





